Abstract
Introduction:
Contacts of pulmonary tuberculosis (TB) cases are at high risk of TB infection and progression to disease. Close and household contacts and those <5 years old have the highest risk. Isoniazid preventive therapy (IPT) can largely prevent TB disease among infected individuals. International and Peruvian recommendations include TB contact investigation and IPT prescription to eligible contacts. We conducted a study in Lima, Peru, to determine the number of close and household contacts who were evaluated, started on IPT, and who completed it, and the factors associated to compliance to national guidelines.
Methods:
We conducted a longitudinal retrospective study including all TB cases diagnosed between January 2015 and July 2016 in 13 health facilities in south Lima. Treatment cards, TB registers and clinical files were reviewed and we extracted data on index cases (sex, age, smear status, TB treatment outcome), contact investigation (sex, age, kinship to the index case, evaluations at month 0, 2 and 6) and health facility (number of TB cases notified per year, proportion of TB cases with treatment success). We tabulated frequencies of contact evaluation by contact and index case characteristics. To investigate determinants of IPT initiation and completion we used generalized linear mixed models.
Results:
A total of 2323 contacts were reported by 662 index cases, the median number of contacts per case was four (IQR, 2-5). Evaluation at month 0 was completed by 99.2% (255/257) contacts < 5 and 98.1% (558/569) contacts 5-19 years old. Of 191 eligible contacts <5 years old, 70.2% (134) started IPT and 31.4% (42) of them completed it. Of 395 contacts 5-19 years old, 36.7% (145) started IPT and 32.4% (47) completed it. Factors associated to not starting IPT among contacts <5 years old, were being a second degree relative to the index case (OR 6.6 95CI% 2.6-16.5), not having received a tuberculin skin test (TST) (OR 3.9 95%CI 1.4-10.8), being contact of a smear negative index case (OR 5.5 95%CI 2.0-15.1), attending a low-caseload health facility (OR 2.8 95%CI 1.3-6.2). Factors associated to not starting IPT among 5-19 years old were age (OR 13.7 95%CI 5.9-32.0 for 16-19 as compared to 5-7 years old), being a second degree relative (OR 3.0 95%CI 1.6-5.6), not having received a TST (OR 5.4, 95%CI 2.5-11.8), being contact of a male index case (OR 2.1 95CI% 1.2-3.5), with smear negative TB (OR 1.9 95%CI 1.0-3.6), attending a high-caseload health facility (OR 2.1 95%CI 1.2-3.6). Factors associated to not completing IPT, amongst contacts who started, were not having received a TST (OR 3.4 95%CI 1.5-7.9 for <5 years old, and OR 4.3 95%CI 1.7-10.8 for 5-19 years old), being contact of an index case with TB treatment outcome other than success (OR 9.3 95%CI 2.6-33.8 for < 5 years old and OR 15.3 95%CI 1.9-125.8 for 5-19 years old), and, only for 5-19 years old, attending a health facility with high caseload (OR 3.2 95%CI 1.4-7.7) and a health facility with low proportion of TB cases with treatment success (OR 4.4 95%CI 1.9-10.2).
Conclusions:
We found partial compliance to TB contact investigation, and identified contact, index case and health facility-related factors associated to IPT start and completion that can guide the TB program in increasing coverage and quality of this fundamental activity.
Keywords: tuberculosis, contact investigation, isoniazid preventive therapy, pediatric tuberculosis, Peru
Introduction
Contacts of tuberculosis (TB) cases are at high risk of TB infection and disease and in low and middle income countries 2.2% to 4.4% of them will develop TB (1). Systematic screening of contacts and high risk groups is a priority of the World Health Organization (WHO) TB elimination strategy – End TB (2). This intervention aims at early diagnosis and treatment of TB cases and the identification of individuals with latent TB infection (LTBI) who can benefit from TB preventive therapy. However, contacts and persons at risk, are lost in each step of the LTBI cascade of care - the process between the identification of eligible individuals to completion of TB preventive therapy (3).
TB preventive therapy is a global priority among children < 5 years old exposed to TB, because they are at high risk of disease progression after a primary infection, and of developing severe forms of TB (WHO 2018,Perez Velez 2012). Despite high quality evidence on the benefits of TB preventive therapy among children and it being included in most National TB Program (NTP) guidelines, the implementation of this intervention at global level is poor. In 2017, only 23% of the estimated 1.3 million eligible <5 years old contacts of bacteriologically confirmed TB cases initiated isoniazid preventive therapy (IPT), far below the 90% global target (5). A mixed-methods systematic review on studies reporting on child TB contact management identified challenges at health system and users level, related to lack of infrastructure, knowledge gaps, attitudes and perceptions, stigma, access to care, competing priorities and treatment (6).
TB incidence in Peru is estimated at 116/100,000 inhabitants (1)(5). National guidelines recommend investigation of all household and close contacts of TB cases (7). With the ultimate aim to guide interventions to improve contact investigation and IPT provision in Peru, this study investigated the adherence to each step of the process of TB contact management and the use of IPT in pediatric contacts and the factors associated to losses at each step of the process in Lima.
Methods
Study setting
Two districts of south Lima with a total population of 865,642 and a TB notification rate of 120 per 100,000 population. TB centres conducting TB screening, diagnosis and treatment are located in the 52 public health facilities of the districts. All patients diagnosed with bacteriologically confirmed TB undergo drug susceptibility testing (DST). Drug-sensitive TB cases are started on six month treatment under facility-based directly observed treatment. All TB cases are requested to list their close and household contacts and pulmonary TB cases are encouraged to tell their contacts to attend the facility for evaluation immediately after diagnosis of the index case and at month two and six. Nursing staff also performs household visits to the index’s case, to deliver health promotion, confirm the contact list and encourage attending the health facility. In practice, contacts of pulmonary TB cases who are adults are symptom-screened for TB and a sputum sample is collected if they have respiratory symptoms. Contacts up to 19 years old are screened for active TB with a clinical examination, a chest X-ray (CXR), a TST and any other test the physician may find necessary (e.g. a sputum obtained by gastric aspiration or induction). In primary health care clinics, IPT is prescribed to contacts <19 years old in whom active TB has been ruled out and whose index cases has pulmonary TB that is sensitive to isoniazid and rifampicin, regardless of smear result. Contacts < 5 years old are started on IPT for six months, regardless of the TST result. Contacts 5-19 years old are eligible for IPT if their tuberculin skin test (TST) is positive (≥10 mm) and if negative, it is repeated at month 2. If TST cannot be done, the physician will decide if the child 5-19 years old would benefit of IPT based on individual risk. Adults with a high risk of progression to TB, that is persons living with HIV or other immunosuppression also receive IPT but this usually occurs at referral hospitals.
Study design, population, data collection
We conducted a longitudinal retrospective study and included all TB cases (all forms, all ages, drug-sensitive and drug resistant TB) registered between January 2015 and July 2016 in 13 health facilities selected so that a spectrum of caseload (based on number of annual TB cases) was represented. Treatment cards and clinical files were reviewed and index case data (sex, age, type of TB, treatment, sputum result, DST), contact investigation data (sex, age, kinship, if the contact was evaluated, presence of symptoms, date and results of CXR, TST, sputum and any other diagnostic test done) IPT indication and dose pick up (the forms have a field for each of the 24 pick ups) were extracted to a case report form by two trained research staff from June until December 2016. Index case treatment outcomes and IPT completion data for individuals still on treatment in December 2016 were updated in August 2017.
Data management and analysis
Data were entered in an Access (Microsoft Redmond, WA, USA) database and analyzed with Stata v.12 (Stata Corp, 12.0, College Station, TX). Contacts were categorized in <5, 5-19 and >19 years old age groups to match IPT guidelines. Under 5 years old contacts were further grouped in ≤1, 2, 3 and 4 years old; 5-19 year old contacts and all index cases were categorized in age groups with cut-offs based on the quartiles of their respective age distribution. Drug-sensitive TB treatment outcomes followed WHO definitions: success (cured or completed treatment), lost to follow up (LTFU), death, failure, not evaluated. DST results are routinely recorded only if the patient is resistant to any drug, hence we considered drug sensitive all those who did not have a record of resistant DST. Health facilities were categorized as low or high caseload if they registered <50 or ≥50 TB cases per year, respectively, and as low or high TB cases treatment success (the cutoff was the median value of the proportion of cases with treatment success in the study health facilities).
The proportion of contacts evaluated at month zero, two and six was calculated –each evaluation is registered in a separate field of the routine registers, - and the associated contact and index case characteristics were described. For IPT initiation and completion, we considered only contacts of pulmonary drug-sensitive TB index cases, with known age and that did not have TB at baseline. Assessment of IPT eligibility was only possible in few of the 5-19 year old contacts because it hinges on a TST result and the TB program had not been able to buy TST due to a shortage in its production which affected all facilities in the country. IPT initiation was defined as the presence of written evidence that the caretaker or contact had picked up the first weekly dose of IPT. IPT full completion was defined as having picked up all 24 weekly doses; ≥80% IPT completion as having picked up ≥20 weekly doses. For contacts <5 years old started on IPT, the frequency distribution of contacts picking up IPT weekly doses at each week was calculated.
Bivariate and multivariate logistic regression analysis was conducted to investigate variables associated with two outcomes among contacts <5 and 5-19 years old: IPT initiation and ≥80% completion. We used a generalized linear mixed model (GLMM) with a binomial distribution and random effects for household/index case to control for clustering . Models were built by entering all variables with a p value <0.2 in bivariate analysis and backward elimination. Variables with the weakest association to the outcome were taken out one by one until a significant difference with the previous model was found. The number of losses at each of the 24 weeks of IPT pick up was analysed with Kaplan Meier for contacts <5 years old started on IPT. Pick up interruptions (if a caretaker missed a pick up and then resumed the pick-ups), were described but not considered in the survival analysis.
Ethical considerations
The institutional review board at Universidad Peruana Cayetano Heredia in Lima (Peru) and the Ethics Committee of the University of Antwerp (Belgium) approved the study. The TB prevention and control programme of the Ministry of Health and at the study districts supported the study and granted access to data at each facility.
Results
Study population
A total of 662 index cases and 2323 contacts were registered during the study period (Table 1). The median number of contacts per case was 4 (interquartile range -IQR- 2-5); 16.5% (109/662) index cases did not report any contact. Index cases not reporting any contact were more frequently male (72.5% 79/109, vs 59.7% (330/553) p=0.01), older (34 (IQR 23-47.5 years vs 27 (20-43) years and had a similar frequency of extrapulmonary TB (20.8%(26/125) vs 82/536 15.3%, respectively, p=0.1), than index cases reporting at least one contact. Treatment outcomes of cases reporting contacts differed from those not reporting contacts: 25/106 (23.6%) were lost to follow up compared to 56 (10.2%) lost to follow up among those reporting contacts. Deaths were slightly higher (7/106 6.6% vs 22/550 4.0%). The proportion with a treatment outcome not evaluated was also higher (22/106 20.8% vs 50/550 9.1%). Among the 553 cases reporting contacts, 59.7% (330) were male, their median age was 27 years (interquartile range – IQR - 20-43), 82.1% (453) had pulmonary TB, 66.7% (369) were smear positive, 88.1% (490) had drug-sensitive TB and 76.3% (422) treatment success as outcome. The majority of contacts (95.5%, 2219) were household contacts and their median age was 26 (IQR 13-45). Seven had already taken IPT in the past: one was <5 years old, four were 5-19 years old and two had unknown age. Table 1 shows the characteristics of contacts per age group.
Table 1.
Characteristics of close and household contacts of pulmonary and extrapulmonary TB cases, by age group, Lima, Peru, 2015-2016.
| Age group, n (%) |
|||||
|---|---|---|---|---|---|
| <5 | 5-19 | >19 | Unknown | All | |
| Pulmonary TB index case | |||||
| Sex | |||||
| Female | 110 (49.3) | 232 (50.5) | 614 (56.3) | 4 (2.5) | 960 (49.7) |
| Male | 110 (49.3) | 226 (49.2) | 476 (43.7) | 11 (6.9) | 823 (42.6) |
| Missing | 3 (1.4) | 1(0.2) | 0 (0.0) | 145 (90.6) | 149 (7.7) |
| Contact type | |||||
| Household | 206 (92.4) | 443 (96.5) | 1028 (94.3) | 156 (97.5) | 1833 (94.9) |
| Non household | 17 (7.6) | 16 (3.5) | 62 (5.7) | 4 (2.5) | 99 (5.1) |
| Kinship | |||||
| Partner | 0 (0.0) | 14 (3.1) | 130 (11.9) | 2 (1.3) | 146 (7.6) |
| Offspring | 87 (39.0) | 125 (27.2) | 103 (9.5) | 1 (0.6) | 316 (16.4) |
| Sibling | 17 (7.6) | 140 (30.5) | 236 (21.7) | 3 (1.9) | 396 (20.5) |
| Parent | 0 (0.0) | 0 (0.0) | 340 (31.2) | 2 (1.3) | 342 (17.7) |
| Other | 115 (51.6) | 163 (35.5) | 190 (17.4) | 7 (4.4) | 475 (24.6) |
| Missing | 4 (1.8) | 17 (3.7) | 91 (8.4) | 145 (90.6) | 257 (13.3) |
| Total, n (%) | 223 | 459 | 1090 | 160 | 1932 |
| Extrapulmonary TB index case | |||||
| Sex | |||||
| Female | 16 (47.1) | 54 49.1) | 117 (54.9) | 0 (0.0) | 187 (47.8) |
| Male | 18 (52.9) | 55 (50.0) | 96 (45.1) | 0 (0.0) | 169 (43.2) |
| Missing | 0 (0.0) | 1(0.9) | 0 (0.0) | 34 (100.0) | 35 (9.0) |
| Contact type | |||||
| Household | 34 (100.0) | 108 (98.2) | 210 (98.6) | 210 (98.6) | 386 (98.7) |
| Non household | 0 (0.0) | 2 (1.8) | 3 (1.4) | 3 (1.4) | 5 (1.3) |
| Kinship | |||||
| Partner | 0 (0.0) | 1 (0.9) | 37 (17.4) | 0 (0.0) | 38 (9.7) |
| Offspring | 21 (61.8) | 49 (44.6) | 25 (11.7) | 0 (0.0) | 95 (24.3) |
| Sibling | 5 (14.7) | 34 (30.9) | 34 (16.0) | 0 (0.0) | 73 (18.7) |
| Parent | 0 (0.0) | 0 (0.0) | 67 (31.5) | 0 (0.0) | 67 (17.4) |
| Other | 7 (20.6) | 22 (20.0) | 45 (21.1) | 0 (0.0) | 74 (18.9) |
| Missing | 1 (2.9) | 4 (3.6) | 5 (2.4) | 34 (100.0) | 44 (11.3) |
| Total, n (%) | 34 | 110 | 213 | 34 | 391 |
Contact investigation
At the first registered contact evaluation, an active TB diagnosis was recorded in 14 (0.6%) contacts. They were all contacts of a smear positive pulmonary TB index case, three were 5-19 years old, seven were > 19 years old and four had unknown age. Whether TB was diagnosed prior to or during the evaluation could not be ascertained from records. Another nine contacts <5 years old presented symptoms suggestive of TB at the first evaluation: TB was definitely ruled out among four and the other five had no recorded outcome of the diagnostic work up.
Table 2 shows the number and proportion of contacts evaluated and their characteristics. Losses in the proportion of contacts evaluated at month 2 were higher among >19 years old than among ≤19 years old contacts (51%, 95%CI 49%-54% vs 44%, 95%CI 41%-48%), non-household vs household contacts (72.5%, 95%CI 63.9%-81.2% vs 47.6%, 95%CI 45.5%-49.7%). Losses were also higher in contacts of female vs male index case (54%, 95%CI 51%-57% vs 45%, 95%CI 42%-48%), with extrapulmonary vs pulmonary TB (56.8%, 95% CI 51.8%-61.8% vs 47.2%, 95%CI 44.9%-49.4%), with smear negative vs smear positive TB (53.3%, 95%CI 49.5%-57.0% vs 46.3%, 95%CI 43.8%-48.8%) and when attending health facilities with high vs low caseload (66.2%, 95%CI 63.7%-68.6% vs 17.2%, 95%CI 14.6%-19.8%) and low vs high proportion of TB cases with treatment success (51.8%, 95%CI 49.0%-54.7% vs 45.4%, 95%CI 42.4%-48.4%).
Table 2.
Proportion of contacts with contact evaluations in function of contact, index case and health facility characteristics. Lima, Peru, 2015-2016.
| Evaluation |
|||
|---|---|---|---|
| Month 0 | Month 2* | Month 6* | |
| Total contacts listed (n=2323), n (%) | 2256 (97.1) | 1153 (50.1) | 116 (5.0) |
| Contacts of pulmonary TB index cases (n=1932) | 1893 (98.0) | 993 (51.8) | 102 (5.3) |
| Contacts of smear positive pulmonary TB index cases (n=1542) | 1512 (98.1) | 802 (52.4) | 78 (5.1) |
| Characteristics, n (%) | |||
| Contact | |||
| Sex | |||
| Female | 1128 (98.3) | 590 (51.7) | 63 (5.5) |
| Male | 965 (97.3) | 480 (48.6) | 45 (4.6) |
| Missing | 177 (96.2) | 86 (47.8) | 8 (4.4) |
| Age | |||
| <5 | 255 (99.2) | 147 (57.2) | 17 (6.6) |
| 5-19 | 558 (98.1) | 303 (53.5) | 20 (3.5) |
| >19 | 1271 (97.5) | 615 (47.5) | 70 (5.4) |
| Missing | 186 (95.9) | 91 (47.9) | 9 (4.7) |
| Kinship | |||
| Partner | 179 (97.3) | 100 (54.4) | 12 (6.5) |
| Offspring | 405 (98.5) | 224 (54.8) | 20 (4.9) |
| Sibling | 457 (97.4) | 235 (50.3) | 28 (6.0) |
| Parent | 393 (96.1) | 201 (49.4) | 31 (7.6) |
| Other | 544 (99.1) | 272 (49.9) | 14 (2.6) |
| Missing | 292 (97.0) | 124 (41.8) | 11 (3.7) |
| Type of contact | |||
| Household | 2168 (97.7) | 1128 (51.2) | 106 (4.8) |
| Non household | 102 (98.1) | 28 (26.9) | 10 (9.6) |
| Index case | |||
| Sex | |||
| Female | 943 (98.6) | 433 (45.6) | 52 (5.5) |
| Male | 1327 (97.1) | 723 (53.2) | 64 (4.7) |
| Age group, years | |||
| ≤18 | 428 (96.8) | 220 (50.2) | 29 (6.6) |
| 19-29 | 837 (97.8) | 424 (49.8) | 38 (4.5) |
| 30-45 | 505 (97.9) | 285 (55.5) | 34 (6.6) |
| >45 | 499 (98.2) | 227 (44.9) | 15 (3.0) |
| Missing | 1 (100) | 0 | 0 |
| TB type | |||
| Pulmonary | 1893 (97.9) | 993 (51.8) | 102 (5.3) |
| Extrapulmonary | 377 (96.4) | 163 (41.7) | 14 (3.6) |
| Smear status | |||
| Positive | 1520 (98.1) | 810 (52.7) | 78 (5.1) |
| Negative | 689 (98.8) | 321 (45.2) | 38 (5.4) |
| Missing | 61 (100.0) | 25 (41.0) | 0 (0) |
| Drug susceptibility | |||
| Susceptible | 1988 (97.7) | 1016 (50.2) | 106 (5.2) |
| MDR/XDR | 254 (97.3) | 135 (52.3) | 10 (3.9) |
| Other resistance | 28 (100.0) | 5 (18.5) | 0 (0) |
| Treatment outcome | |||
| Success | 1747 (98.1) | 889 (50.2) | 112 (6.3) |
| Adverse | 523 (96.3) | 267 (49.5) | 4 (1.4) |
| Health facility | |||
| TB caseload | |||
| Low | 807 (98.7) | 664 (81.7) | 72 (8.9) |
| High | 1463 (97.2) | 492 (32.9) | 44 (2.9) |
| Proportion of TB treatment success | |||
| Low | 1189 (97.2) | 569 (52.0) | 24 (2.2) |
| High | 1081 (98.3) | 587 (49.3) | 92 (7.6) |
TB= tuberculosis; MDR:=Multi drug resistant; XDR = extensively drug resistant; LTFU= Lost to follow-up.
Fourteen contacts with TB at month 0 are excluded from the denominator for month 2 and 6.
Among all contacts, 127 (5.5%) had at least one recorded TST result. Among < 5 years old contacts, 21.5% (55/256) received a TST, which was positive in 12 (21.8%) . Among 5-19 years old contacts, 11.0% (62/564) received a TST, which was positive in 22 (34.5%).
IPT initiation and completion
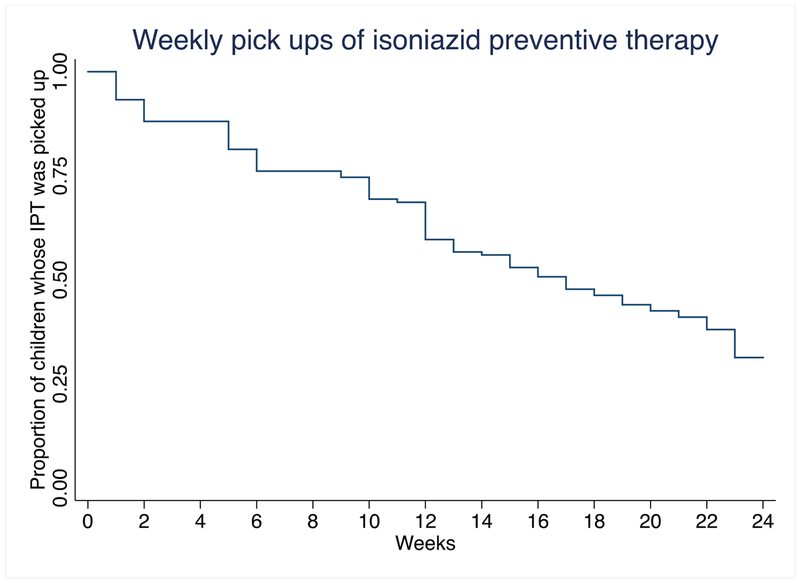
To assess IPT initiation and completion, we excluded 14 contacts with active TB at first evaluation, 391 contacts of index cases with extrapulmonary TB, 250 contacts of MDR/XDR TB index cases, 27 contacts of index cases with TB with other patterns of resistance and 128 contacts with unknown age and analysed 1513 contacts of pulmonary drug sensitive TB. Table 3 shows the initiation and completion by age group. IPT was initiated in 282 (18.6%) contacts and 126 (44.7%) completed ≥80% doses. Of 191 contacts < 5 years old eligible for IPT 13 were non-household contacts; IPT was initiated in 134 (70.2%), 59 (44.0%) of whom had ≥80% IPT completion; of all eligible, 22.0% (42/191) received a full course. Four out of nine contacts < 5 years old that had TB symptoms but in whom active TB was ruled out, were started on IPT, whereas the other five had no records of IPT initiation. One of the seven contacts (an 11 year old) who had already taken IPT before, took a second IPT course. Twenty-five percent of < 5 years old contacts who started IPT picked up less than 9 weekly doses (Figure). Five contacts < 5 and four contacts 5-19 interrupted the weekly pick up. Eight of them interrupted a single week pick up, while one interrupted two continuos pick ups. Fifteen of 32 <5 years old contacts of an index case with any pattern of resistance started IPT, and in 10, it was later discontinued by the clinician in charge; the other five continued taking IPT beyond the date the index case’s drug resistant TB diagnosis was recorded and two completed ≥80% IPT doses. IPT was also started in 27 contacts 5-19 years old of an index case with drug resistance.
Table 3.
Number and proportion of eligible contacts* initiating and completing IPT, by age group. Lima, Peru, 2015-2016.
| Age group of the contacts in years* |
|||
|---|---|---|---|
| < 5 | 5-19** | >19 | |
| N=191 n (%) | N=395 n (%) | N=927 n (%) | |
| Initiation | 134 (70.2) | 145 (36.7) | 3 (0.3) |
| Full completion (24 weeks) | 42/134 (31.4) | 47/145 (32.4) | 1/3 (33.3) |
| > 80% of doses | 59/134 (44.0) | 65/145 (44.8) | 2/3 (66.7) |
Contacts are eligible if they do not have TB and if their index case has drug-sensitive pulmonary TB.
As per Peruvian guidelines, eligibility for 5-19 years old should also be assessed with a positive TST. Due to shortages, TST was not performed in most of them. Only 22 of contacts 5-19 years old had a positive TST and thus were truly eligible (of them, 18 (81.8%) started IPT, 10 (55.6%) completed > 80% doses).”
Figure.
Time to isoniazid preventive therapy pick up discontinuations among 134 contacts < 5 years old started on IPT. Lima, Peru, 2015-2016.
Table 4 and 5 show the contact, index case and health facility characteristics and their association to IPT uptake and ≥80% completion among <5 and 5-19 years old contacts, controlled for the clustering at household level. In <5 years old contacts, being a second degree relative of the index case, not having received a TST, being contact of a smear negative TB case and attending a health facility with low caseload were associated with not starting IPT. In contacts 5-19 years old, older age groups, not having received a TST, being contact of a male index case, with smear negative TB, with TB treatment outcome other than success, attending a health facility with high caseload and a health facility with low proportion of treatment success were associated with not starting IPT. In both age groups not having received a TST and having an index case with treatment outcome other than success were associated with not completing ≥80% IPT doses. Attending a health facility with high caseload and with low proportion of treatment success were associated with not completing ≥80% IPT doses only among 5-19 years old contacts.
Table 4.
Characteristics associated to not starting IPT and not completing ≥80% IPT doses among eligible < 5 years old contacts. Lima, Peru, 2015-2016.
| Characteristics | IPT initiation (N= 191) | IPT completion (N=134) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Started | Did not start | GLMM crude OR (95%CI) | GLMM aOR (95%CI) | Completed | Did not complete | GLMM crude OR (95%CI) | GLMM aOR (95%CI) | ||
| n (%) | n (%) | n (%) | n (%) | ||||||
| Contact | |||||||||
| Sex | Female | 61 (67.0) | 30 (33.0) | 1 | - | 26 (42.6) | 35 (57.4) | 1 | - |
| Male | 73 (72.0) | 27 (27.0) | 0.8 (0.4-1.5) | - | 33 (45.2) | 40 (54.8) | 0.9 (0.4-1.8) | - | |
| Age group, years | ≤1 | 29 (65.9) | 15 (34.1) | 1 | - | 9 (31.0) | 20 (69.0) | 1 | - |
| 2 | 55 (75.3) | 18 (24.7) | 0.6 (0.3-1.4) | - | 24 (43.6) | 31 (56.4) | 0.6 (0.2-1.5) | - | |
| 3 | 21 (61.8) | 13 (38.2) | 1.2 (0.5-3.0) | - | 12 (57.1) | 9 (42.9) | 0.3 (0.1-1.1) | - | |
| 4 | 29 (72.5) | 11 (27.5) | 0.7 (0.5-1.9) | - | 14 (48.3) | 15 (51.7) | 0.5 (0.2-1.4) | - | |
| Kinship | Offspring | 65 (84.4) | 12 (15.6) | 1 | 1 | 29 (44.6) | 36 (55.4) | 1 | - |
| Sibling | 12 (80.0) | 3 (20.0) | 1.2 (0.3-5.1) | 1. 9 (0.4-9.0) | 8 (66.7) | 4 (33.3) | 0.4 (0.1-1.5) | - | |
| Other | 54 (56.3) | 42 (43.8) | 4.3 (2.0-9.4) | 6.6 (2.6-16.5) | 21 (38.9) | 33 (61.1) | 1.3 (0.6-2.6) | - | |
| Missing | 3 (100) | 0 (0) | - | - | 1 (33.3) | 2 (66.7) | - | - | |
| Type of contact | HH | 127 (71.4) | 51 (28.7) | 1 | - | 55 (43.3) | 72 (56.7) | 1 | - |
| Non HH | 7 (53.9) | 6 (46.2) | 2.1 (0.7-6.7) | - | 4 (57.1) | 3 (42.9) | 0.6 (0.1-2.7) | - | |
| Use of TST | Yes | 40 (85.1) | 7 (14.9) | 1 | 1 | 26 (65.0) | 14 (35.0) | 1 | 1 |
| No | 94 (65.3) | 50 (34.7) | 3.0 (1.3-7.3) | 3.9 (1.4-10.8) | 33 (35.1) | 61 (64.9) | 3.4 (1.6-7.5) | 3.4 (1.5-7.9) | |
| Index case | |||||||||
| Sex | Female | 65 (73.0) | 24 (26.9) | 1 | - | 31 (47.7) | 34 (52.3) | 1 | - |
| Male | 69 (67.7) | 33 (32.4) | 1.2 (0.6-2.3) | - | 28 (40.6) | 41 (59.4) | 1.3 (0.7-2.7) | - | |
| Age group, years | ≤18 | 16 (64.0) | 9 (36.0) | 1 | - | 10 (62.5) | 6 (37.5) | 1 | - |
| 19-29 | 73 (78.5) | 20 (21.5) | 0.5 (0.2-1.4) | - | 34 (46.6) | 39 (53.4) | 1.9 (0.6-5.8) | - | |
| 30-45 | 27 (65.9) | 14 (34.2) | 0.9 (0.3-2.7) | - | 10 (37.0) | 17 (63) | 2.8 (0.8-10.2) | - | |
| >45 | 18 (56.3 | 14 (43.8) | 1.3 (0.5-4.0) | - | 5 (27.8) | 13 (72.3) | 4.3 (1.0-18.4) | - | |
| Smear status | Smear (+) | 121 (75.2) | 40 (24.8) | 1 | 1 | 55 (45.5) | 66 (54.6) | 1 | - |
| Smear (−) | 13 (48.2) | 17 (56.6) | 4.2 (1.7-10.1) | 5.5 (2.0-15.1) | 4 (23.1) | 9 (69.2) | 2.2 (0.6-8.8) | - | |
| Treatment outcome | Success | 106 (72.1) | 41 (27.9) | 1 | - | 56 (52.8) | 50 (47.2) | 1 | 1 |
| Adverse | 28 (63.6) | 16 (36.4) | 1.6 (0.7-3.4) | - | 3 (10.7) | 25 (89.3) | 9.3 (2.7-32.8) | 9.3 (2.6-33.8) | |
| Health facility | |||||||||
| TB caseload | High | 100 (74.1) | 35 (25.9) | 1 | 1 | 42 (42.0) | 58 (58.0) | 1 | - |
| Low | 34 (60.7) | 22 (38.3) | 2.0 (1.0-3.9) | 2.8 (1.3-6.2) | 17 (50.0) | 17 (50.0) | 0.7 (0.3-1.6) | - | |
| Treatment success | Low | 48 (62.3) | 29 (37.7) | 2.0 (1.0-3.8) | 1.9 (0.9-4.2) | 22 (45.8) | 26 (54.2) | 0.9 (0.4-1.8) | - |
| High | 86 (75.4) | 28 (24.6) | 1 | 1 | 37 (43.0) | 49 (56.9) | 1 | - | |
GLMM= Generalized linear mixed models; IPT=Isoniazid preventive therapy; HH=Household; OR= Odds Ratio; aOR=adjusted OR; CI= Confidence Interval; TB= Tuberculosis: TST= Tuberculin skin test; LTFU= Lost to follow-up. Eligible contacts were those with no active TB and whose index case had drug-sensitive pulmonary TB index cases.
Table 5.
Characteristics associated to not starting and not completing IPT in eligible 5-19 years old contacts. Lima, Peru, 2015-2016.
| Characteristics | IPT initiation (N= 395) | IPT completion (N=145) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Started | Did no start | GLMM crude OR (95%CI) | GLMM aOR (95%CI) | Completed | Did not complete | GLMM crude OR (95%CI) | GLMM aOR (95%CI) | ||
| n (%) | n (%) | n (%) | n (%) | ||||||
| Contact | |||||||||
| Sex | Female | 67 (34.0) | 130 (66.0) | 1 | - | 34 (50.8) | 33 (49.3) | 1 | - |
| Male | 78 (39.3) | 120 (60.6) | 0.8 (0.5-1.2) | - | 31 (39.7) | 47 (60.3) | 1.6 (0.8-3.2) | - | |
| Age group, years | 5-7 | 57 (60.0) | 38 (40.0) | 1 | 1 | 23 (40.4) | 34 (59.7) | 1 | - |
| 8-11 | 46 (47.4) | 51 (52.6) | 1.7 (0.9-2.9) | 1.9 (1.0-3.7) | 24 (52.2) | 33 (47.8) | 0.6 (0.3-1.3) | - | |
| 12-15 | 29(29.9) | 68 (70.1) | 3.5 (1.9-6.4) | 5.1 (2.5-10.5) | 12 (41.4) | 17 (58.6) | 0.9 (0.4-2.4) | - | |
| 16-19 | 13 (12.3) | 93 (87.7) | 10.7 (5.3-21.8) | 13.7 (5.9-32.0) | 6 (46.2) | 7 (53.9) | 0.7 (0.2-2.5) | - | |
| Kinship | Offspring | 56 (52.3) | 51 (47.6) | 1 | 1 | 20 (35.7) | 36 (64.3) | 1 | - |
| Sibling | 39 (30.2) | 90 (69.3) | 2.5 (1.5-4.3) | 1.8 (0.9-3.3) | 21 (53.9) | 18 (46.2) | 0.5 (0.2-1.1) | - | |
| Other | 47 (32.4) | 98 (67.6) | 2.0 (1.2-3.4) | 3.0 (1.6-5.6) | 22 (46.8) | 25 (53.2) | 0.7 (0.3-1.5) | - | |
| Partner | 1 (7.1) | 13 (92.9) | 14.3 (1.8-113.0) | 1.7 (0.2-15.0) | 1 (100.0) | 0 (0) | - | - | |
| Missing | 3(21.4) | 11 (78.6) | - | - | 2 (66.7) | 1 (33.3) | - | - | |
| Type of contact | HH | 142 (37.7) | 238 (62.6) | 1 | - | 63 (44.4) | 79 (55.6) | 1 | - |
| Non HH | 3 (20.0) | 12(80.0) | 2.4 (0.7-8.6) | - | 2 (66.7) | 1 (33.3) | 0.4 (0.04-4.5) | - | |
| Use of TST | Yes | 37 (75.5) | 12 (24.5) | 6.9 (3.4-13.7) | 5.4 (2.5-11.8) | 25 (67.6) | 12 (32.4) | 1 | 1 |
| No | 108 (31.2) | 238 (68.8) | 1 | 1 | 40 (37.0) | 68 (62.9) | 3.7 (1.7-8.3) | 4.3 (1.7-10.8) | |
| Index case | |||||||||
| Sex | Female | 78 (43.8) | 100 (56.2) | 1 | 1 | 40 (51.3) | 38 (48.7) | 1 | - |
| Male | 67 (30.9) | 150 (69.5) | 1.8 (1.2-2.6) | 2.1 (1.2-3.5) | 25 (37.3) | 42 (62.7) | 1.8 (0.9-3.5) | - | |
| Age group, years | ≤18 | 19 (26.0) | 54 (74.0) | 1 | - | 9 (47.4) | 10 (52.6) | 1 | - |
| 19-29 | 56 (39.1) | 87 (60.8) | 0.5 (0.3-1.0) | - | 27 (48.2) | 29 (51.8) | 1.0 (0.3-2.8) | - | |
| 30-45 | 41 (42.3) | 56 (57.7) | 0.5 (0.2-0.9) | - | 17 (41.5) | 24 (58.5) | 1.3 (0.4-4.1) | - | |
| >45 | 29 (35.4) | 53 (64.6) | 0.6 (0.3-1.3) | - | 12 (41.4) | 17 (58.6) | 1.3 (0.4-4.1) | - | |
| Smear status | Smear (+) | 116 (39.7) | 176 (60.3) | 1 | 1 | 56 (48.3) | 60 (51.7) | 1 | - |
| Smear (−) | 29 (27.9) | 74 (71.2) | 1.9 (1.2-3.3) | 1.9 (1.0-3.6) | 9 (31.3) | 20 (69.0) | 1.6 (0.6-3.9) | - | |
| Treatment outcome | Success | 130 (40.0) | 195 (60.0) | 1 | 1 | 64 (49.2) | 66 (50.8) | 1 | 1 |
| Adverse | 15 (21.4) | 55 (78.6) | 2.4 (1.3-4.5) | 1.8 (0.9-3.6) | 1 (6.7) | 14 (93.3) | 13.6 (1.7-106.7) | 15.3 (1.9-125.8) | |
| Health facility | |||||||||
| TB caseload | High | 82 (32.3) | 172 (67.7) | 1.7 (1.1-2.5) | 2.1 (1.2-3.6) | 33 (40.2) | 49 (59.8) | 1.6 (0.8-3.2) | 3.2 (1.4-7.7) |
| Low | 63 (44.7) | 78 (55.3) | 1 | 1 | 32 (50.8) | 31 (49.2) | 1 | 1 | |
| Treatment success | Low | 1.4 (0.9-2) | 1.8 (1.1-3.1) | 2.6 (1.3-5.1) | 4.4 (1.9-10.2) | ||||
| High | 1 | 1 | 1 | 1 | |||||
GLMM= Generalized linear mixed models; IPT=Isoniazid preventive therapy; HH=Household; OR= Odds Ratio; CI= Confidence Interval; aOR= adjusted OR; GLMM= Generalized Linear Mixed Models; TB= Tuberculosis, TST= Tuberculin skin test; LTFU= Lost to follow-up. Eligible contacts were those with no active TB and whose index case had drug-sensitive pulmonary TB index cases.
Discussion
In this study, we found partial compliance to contact investigation guidelines, a limited uptake of TB preventive therapy among children and a low completion of it. Most household and close contacts were screened after the index case was diagnosed with TB but only half of them had a month 2 follow up evaluation and less than 10% one at month 6. Few household contacts were found to have TB, none of them were < 5 years old. Seventy percent of children < 5 years old eligible for IPT were started on it and 31% completed it. Of all < 5 years old eligible for IPT, only 22% received a full course. Thirty seven percent of children 5-19 years old were started on IPT and 32% completed it. Kinship, smear status of the index case, use of TST in the contact and health facility characteristics were all associated to IPT initiation in both <5 and 5-19 years old. The age of the contact was associated to IPT initiation in 5-19 years old group. Index case’s TB treatment outcome and use of TST were associated to IPT completion in both <5 and 5-19 years old. Health facility’s characteristics were associated to IPT completion among 5-19 years old.
We found that 70% of <5 years old contacts started IPT, which is similar to reports from diverse settings for this age group: 64% in Ethiopia (10,11), 84% in South India (20) and 79% in The Gambia (21). The pooled proportion of children of all ages starting TB preventive treatment in 8 studies was 69.1% in a systematic review (3). In our study, <5 years old contacts that were second degree relatives of index cases and contacts of a smear negative index case were less likely to start IPT. This is in line with a systematic review of child contact management that found children who were offspring of the index case and those who slept in the same room of the index case to be more likely to start IPT (6). The risk of TB transmission is related to contact intensity and infectiousness of the index case, and both health staff and parents may have a lower risk perception in the above mentioned instances. However, the risk for developing active TB in young children with any household exposure is higher than in the general population of the same age.
We also found health service factors associated to IPT initiation among children. Contacts who had not received a TST were less likely to start IPT. A shortage of TST was recorded during the study period, possibly reducing health staff’s confidence in ruling out TB in <5 years old contacts (for whom a TST result is not needed to start IPT) and making it impossible to assess and comply with a formal eligibility criterium for IPT in 5-19 years old contacts. Contacts <5 attending health facilities with lower caseload were less likely to start IPT, while contacts 5-19 attending such health facilities were more likely to start. The reason for this discrepancy is unclear and may be related to the study not being powered to detect interaction effects that might cause such a difference.
Completion of TB preventive therapy among children and adults is low worldwide (3,22,23). Across 8 studies, the pooled proportion of children completing TB preventive therapy out of those started on it was 18.3% (CI95% 6-31%) (3), lower than the third of children exposed to TB completing IPT in our study setting. In our study, in both age groups the strongest risk factor for non-completion of IPT was being contact of an index case with treatment outcome other than success. This could be a spurious finding related to index case and contact moving to a new address, which was a frequent cause of contact evaluation non-completion in a study conducted in Guinea Bissau (24) and elsewhere too (3). Yet, competing priorities in the household, or other factors impacting on discontinuation of TB care in the household, such as substance abuse, could also explain this (25). Enhanced tracing of TB cases, could increase the proportion of TB cases successfully treated as well as IPT completion among their contacts. Other factors negatively associated to completion were not having a TST placed in both age groups and, only in the 5-19 years old group, attending health facilities with a larger caseload, and with lower proportions of treatment success compared to other facilities in the area. These health service determinants suggest that performance of the TB service may affect both index case and contact management. In Uganda, health staff behavior and health service environment facilitated compliance to TB contact investigation (23). A patient-centered approach, including expanding opening hours of the clinics or delivering IPT at home, and counseling caretakers on the risk of discontinuing IPT, could increase IPT completion (11,24,26). In our study, children < 5 years old stopped picking up IPT throughout the 24 weeks, with larger numbers stopping at week 6 and week 13. Shorter regimens such as a weekly dose of rifapentine and isoniazid for three months (3HP), recently recommended by WHO, have been found to enhance treatment completion as compared to a daily dose of isoniazid (27,28)(29).
In an individual patient data meta-analysis of over 100,000 children from all over the world, including Peru, 2.9% (95% CI: 1.8-4.7%), children were diagnosed with TB within 90 days of the index case diagnosis (Martinez L, personal communication). The lower proportion of TB among children we found, may reflect underlying epidemiological differences or different study designs and data sources. High coverage of the first contact evaluation favors the detection of prevalent TB, while incident TB cases, occurring weeks to months after exposure, may be missed. In our study population, only half of all contacts (and 57% of < 5 years old) were evaluated at month 2. Routine registers from primary care clinics do not include recording of pediatric contacts diagnosed with TB in referral hospitals. Furthermore, ruling out TB in young children, especially in the <2 years old - the age group at highest risk of developing TB after exposure (12,13) - involves the recognition of warning signs and symptoms by parents and physicians, including the revision of growth charts (10,14) These are not routinely recorded in TB contact investigation, hampering follow up. Training primary care staff on pediatric TB screening and diagnosis, strengthening communication between referral and primary care facilities, and designing forms to facilitate the appropriate recording and follow up of contacts may increase TB detection in and provision of preventive treatment to eligible contacts (15–19).
Our study has several limitations. Using routine data may have underestimated the actual number of household and close contacts and active TB among them. Children diagnosed with TB at referral hospitals may not have been included leading to an underestimation of TB cases among children. Completion of IPT was measured by the weekly pick-ups marked on the back of the TB treatment card, which does not necessarily imply that the contact daily took the treatment. Also, factors possibly associated to starting and completing IPT, both related to the index case, the contact, the health facility and the regimen remained unmeasured , which limited our in depth understanding of the poor compliance to NTP guidelines for child contact management . However, using routine data allowed us to evaluate the actual programmatic implementation of contact investigation and IPT use.
The largest gap between estimated and notified TB cases in the world is among children <5 years old (5), who are also at highest risk of death and sequelae (13,30). Recent WHO guidelines stress the priority of treating LTBI among close TB cases contacts in that age group (29,31). Investing in TB programs and staff in order to fully comply with guidelines and evaluate all child contacts throughout the risk period and not only when the index case starts treatment, the provision of patient-centered preventive treatment to all eligible contacts, as well as the use of shorter regimens and pediatric formulations, could reduce TB incidence and mortality (6,16,32,33). A modelling study, estimated that full implementation of household contact investigation would avert 108 400 deaths worldwide in children < 15 years and that giving 48 children a preventive therapy course would prevent one death (34). The contact, index case and health facility factors associated to sub-optimal contact management and IPT provision identified in this study can guide the Peruvian TB control program to increase coverage and quality of this fundamental activity.
Acknowledgements:
We thank all health staff in the study districts that facilitated access to the data for their collaboration. We thank Maribel Reyes and Viviana Quintana for collecting the data.
Funding: this study was funded by the Belgian Cooperation through a project of Institutional collaboration between the Institute of Tropical Medicine in Antwerp, Belgium and the Instituto de Medicina Tropical Alexander von Humboldt in Lima, Peru. LO is supported by an Emerging Global Leader Award from the Fogarty International Center at the National Institutes of Health (K43TW011137). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013. Jan;41(1):140–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, et al. WHO’s new End TB Strategy. Lancet. 2015. March 23;385(9979):1799–801. [DOI] [PubMed] [Google Scholar]
- 3.Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016; [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Guidelines on the management of latent tuberculosis infection. Geneva, Switzerland: World Health Organization; 2015. [PubMed] [Google Scholar]
- 5.World Health Organization. Global Tuberculosis Report 2018 [Internet]. 2018. Available from: https://www.who.int/tb/publications/global_report/en/
- 6.Szkwarko D, Hirsch-Moverman Y, Du Plessis L, Du Preez K, Carr C, Mandalakas AM. Child contact management in high tuberculosis burden countries: A mixed- methods systematic review. PLoS ONE. 2017;12(8):e0182185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estrategia Sanitaria Nacional para la Prevención y Control de la Tuberculosis Norma Técnica para al Prevención y Control de la Tuberculosis en el Perú, 2013. Lima, Peru: Ministerio de Salud Perú; 2013. [Google Scholar]
- 8.Otero L, Shah L, Verdonck K, Battaglioli T, Brewer T, Gotuzzo E, et al. A prospective longitudinal study of tuberculosis among household contacts of smear-positive tuberculosis cases in Lima, Peru. BMC Infect Dis. 2016. June 8;16:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Triasih R, Robertson C, Duke T, Graham SM. Risk of infection and disease with Mycobacterium tuberculosis among children identified through prospective community-based contact screening in Indonesia. Trop Med Int Health TM IH. 2015. June;20(6):737–43. [DOI] [PubMed] [Google Scholar]
- 10.Triasih R, Robertson CF, Duke T, Graham SM. A prospective evaluation of the symptom-based screening approach to the management of children who are contacts of tuberculosis cases. Clin Infect Dis Off Publ Infect Dis Soc Am. 2015. January 1;60(1):12–8. [DOI] [PubMed] [Google Scholar]
- 11.Egere U, Sillah A, Togun T, Kandeh S, Cole F, Jallow A, et al. Isoniazid preventive treatment among child contacts of adults with smear-positive tuberculosis in The Gambia. Public Health Action. 2016. December 21;6(4):226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marais BJ, van Zyl S, Schaaf HS, van Aardt M, Gie RP, Beyers N. Adherence to isoniazid preventive chemotherapy: a prospective community based study. Arch Dis Child. 2006. September;91(9):762–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins HE, Yuen CM, Rodriguez CA, Nathavitharana RR, McLaughlin MM, Donald P, et al. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(3):285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blount RJ, Tran B, Jarlsberg LG, Phan H, Thanh Hoang V, Nguyen NV, et al. Childhood tuberculosis in northern Viet Nam: a review of 103 cases. PloS One. 2014;9(5):e97267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zawedde-Muyanja S, Nakanwagi A, Dongo JP, Sekadde MP, Nyinoburyo R, Ssentongo G, et al. Decentralisation of child tuberculosis services increases case finding and uptake of preventive therapy in Uganda. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2018. 01;22(11):1314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutherford ME, Hill PC, Triasih R, Sinfield R, van Crevel R, Graham SM. Preventive therapy in children exposed to Mycobacterium tuberculosis: problems and solutions: TB preventive therapy in exposed children. Trop Med Int Health. 2012. October;17(10):1264–73. [DOI] [PubMed] [Google Scholar]
- 17.Islam Z, Sanin KI, Ahmed T. Improving case detection of tuberculosis among children in Bangladesh: lessons learned through an implementation research. BMC Public Health. 2017. 28;17(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayakaka I, Ackerman S, Ggita JM, Kajubi P, Dowdy D, Haberer JE, et al. Identifying barriers to and facilitators of tuberculosis contact investigation in Kampala, Uganda: a behavioral approach. Implement Sci [Internet]. 2017. December [cited 2019 May 25];12(1). Available from: http://implementationscience.biomedcentral.com/articles/10.1186/s13012-017-0561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tadesse Y, Gebre N, Daba S, Gashu Z, Habte D, Hiruy N, et al. Uptake of Isoniazid Preventive Therapy among Under-Five Children: TB Contact Investigation as an Entry Point. PloS One. 2016;11(5):e0155525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pothukuchi M, Nagaraja SB, Kelamane S, Satyanarayana S, Shashidhar, Babu S, et al. Tuberculosis contact screening and isoniazid preventive therapy in a South Indian district: operational issues for programmatic consideration. PloS One. 2011;6(7):e22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banu Rekha VV, Jagarajamma K, Wares F, Chandrasekaran V, Swaminathan S. Contact screening and chemoprophylaxis in India’s Revised Tuberculosis Control Programme: a situational analysis. Int J Tuberc Lung Dis. 2009. December;13(12):1507–12. [PubMed] [Google Scholar]
- 22.Arguello Perez E, Seo SK, Schneider WJ, Eisenstein C, Brown AE. Management of Latent Tuberculosis Infection Among Healthcare Workers: 10-Year Experience at a Single Center. Clin Infect Dis Off Publ Infect Dis Soc Am. 2017. November 29;65(12):2105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichler MR, Reves R, Bur S, Ford J, Thompson V, Mangura B, et al. Treatment of latent tuberculosis infection in contacts of new tuberculosis cases in the United States. South Med J. 2002. April;95(4):414–20. [PubMed] [Google Scholar]
- 24.Gomes VF, Wejse C, Oliveira I, Andersen A, Vieira FJ, Carlos LJ, et al. Adherence to isoniazid preventive therapy in children exposed to tuberculosis: a prospective study from Guinea-Bissau. Int J Tuberc Lung Dis. 2011. December 1;15(12):1637–43. [DOI] [PubMed] [Google Scholar]
- 25.Lackey B, Seas C, Van der Stuyft P, Otero L. Patient Characteristics Associated with Tuberculosis Treatment Default: A Cohort Study in a High-Incidence Area of Lima, Peru. PloS One. 2015;10(6):e0128541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Zyl S, Marais BJ, Hesseling AC, Gie RP, Beyers N, Schaaf HS. Adherence to anti-tuberculosis chemoprophylaxis and treatment in children. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2006. January;10(1):13–8. [PubMed] [Google Scholar]
- 27.Villarino ME, Scott NA, Weis SE, Weiner M, Conde MB, Jones B, et al. Treatment for Preventing Tuberculosis in Children and Adolescents: A Randomized Clinical Trial of a 3-Month, 12-Dose Regimen of a Combination of Rifapentine and Isoniazid. JAMA Pediatr. 2015. March 1;169(3):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spyridis NP, Spyridis PG, Gelesme A, Sypsa V, Valianatou M, Metsou F, et al. The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11-year randomized study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2007. September 15;45(6):715–22. [DOI] [PubMed] [Google Scholar]
- 29.Global Tuberculosis Programme. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. [Internet]. 2018. [cited 2019 May 25]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK531235/ [PubMed]
- 30.Marais BJ, Graham SM, Maeurer M, Zumla A. Progress and challenges in childhood tuberculosis. Lancet Infect Dis. 2013. April;13(4):287–9. [DOI] [PubMed] [Google Scholar]
- 31.Fox GJ, Dobler CC, Marais BJ, Denholm JT. Preventive therapy for latent tuberculosis infection—the promise and the challenges. Int J Infect Dis. 2017. March;56:68–76. [DOI] [PubMed] [Google Scholar]
- 32.Hill PC, Rutherford ME, Audas R, van Crevel R, Graham SM. Closing the policy-practice gap in the management of child contacts of tuberculosis cases in developing countries. PLoS Med. 2011. October;8(10):e1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brent AJ, Nyundo C, Langat J, Mulunda C, Wambua J, Bauni E, et al. Prospective Observational Study of Incidence and Preventable Burden of Childhood Tuberculosis, Kenya. Emerg Infect Dis. 2018. March;24(3):514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dodd PJ, Yuen CM, Becerra MC, Revill P, Jenkins HE, Seddon JA. Potential effect of household contact management on childhood tuberculosis: a mathematical modelling study. Lancet Glob Health. 2018. December;6(12):e1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]