Abstract
Objective:
We aimed to use diffusion tensor imaging (DTI) to detect alterations in white matter microstructure in older patients with breast cancer receiving chemotherapy.
Methods:
We recruited women aged ≥ 60 years with stage I–III breast cancer (chemotherapy [CT] group; n=19) to undergo two study assessments: at baseline and within one month after chemotherapy. Each assessment consisted of a brain magnetic resonance imaging scan with DTI and neuropsychological (NP) testing using the National Institutes of Health (NIH) Toolbox Cognition Battery. An age- and sex-matched group of healthy controls (HC, n=14) underwent the same assessments at matched intervals. Four DTI parameters (fractional anisotropy [FA], mean diffusivity [MD], axial diffusivity [AD], and radial diffusivity [RD]) were calculated and correlated with NP testing scores.
Results:
For CT group but not HCs, we detected statistically significant increases in MD and RD in the genu of the corpus callosum from time point 1 to time point 2 at p<0.01, effect size:0.3655 and 0.3173, and 95% confidence interval: from 0.1490 to 0.5821, and from 0.1554 to 0.4792, for MD and RD respectively. AD values increased for the CT group and decreased for the HC group over time, resulting in significant between-group differences (p=0.0056, effect size:1.0215, 95% confidence interval: from 0.2773 to 1.7657). There were no significant correlations between DTI parameters and NP scores (p>0.05).
Conclusions:
We identified alterations in white matter microstructures in older women with breast cancer undergoing chemotherapy. These findings may potentially serve as neuroimaging biomarkers for identifying cognitive impairment in older adults with cancer.
Keywords: chemotherapy; breast cancer; aging, white matter; diffusion tensor imaging
1. Introduction
Survival for breast cancer has improved, with a five-year relative survival rate of about 90%1. Unfortunately, many patients with cancer and cancer survivors suffer from cancer-related cognitive impairment (CRCI), which can affect their quality of life2–4. In addition, older adults with cancer and cancer survivors may also experience aging-related cognitive impairment5, 6. Despite these challenges and the critical need to address them, there are few studies focusing on older patients with cancer receiving adjuvant chemotherapy.
There have been several diffusion tensor imaging (DTI) studies in the scope of breast cancer. Among other findings, these neuroimaging studies have detected white matter microstructural alterations in patients with breast cancer who received chemotherapy7–13. DTI is a non-invasive imaging technique with the potential to study CRCI, as it provides a reliable assessment of white matter integrity14. The four DTI parameters most commonly used for clinical applications are fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD)15. FA measures the intravoxel coherence of white matter microstructures and reflects white matter integrity16. MD reflects the overall magnitude of water diffusion within the tissue, whereas RD and AD represent average diffusion perpendicular to or along the orientation of the first eigenvector, respectively14, 15.
Abraham et al. performed the first DTI study of CRCI in patients with breast cancer and they found lower FA values in the genu of the corpus callosum in the chemotherapy-treated patients compared to the healthy controls7. Deprez et al. showed that patients with breast cancer who received chemotherapy had lower FA and higher MD values in the frontal and temporal white matter than that of healthy and cancer controls10. Additional cross-sectional DTI studies have shown late effects of chemotherapy with lower FA and higher MD at ten or more8, 13 and 20 years after treatment11. White matter alterations due to chemotherapy have also been demonstrated in longitudinal DTI studies of CRCI in patients with breast cancer9, 12. Deprez et al. observed that patients with breast cancer had lower FA values and greater MD values in the frontal white matter months after receiving chemotherapy than what they observed before treatment began9. In addition, there were significant correlations between FA changes and performance changes in attention and verbal memory. A more recent longitudinal study by Menning et al. showed that patients with breast cancer who received chemotherapy had lower FA values in the right superior longitudinal fasciculus (SLF) than patients with breast cancer who did not receive chemotherapy12. The converging evidence from these prior studies indicates that patients with breast cancer who are exposed to chemotherapy exhibit alterations in white matter microstructure that potentially correlate with differences in their neurocognitive capabilities. However, although cancer is disease of aging, with more frequent occurrences in older populations17, no previously published DTI studies have focused on older patients with cancer receiving chemotherapy. Therefore, little is known about how chemotherapy- and aging-related changes in white matter microstructure and cognition may interact in this growing, potentially vulnerable older population.
Here, we present results from a longitudinal DTI study of older patients with breast cancer receiving adjuvant chemotherapy. We aimed to assess acute changes in the white matter integrity of these patients compared to that of similarly aged women without cancer. We hypothesized that patients receiving chemotherapy would exhibit alterations in DTI parameters and that these alterations may be correlated with their neuropsychological testing (NP) scores.
2. Methods
2.1. Study Participants
The study cohort consisted of patients with breast cancer receiving adjuvant chemotherapy (the “CT” group) and age- and sex-matched participants with no history of cancer as healthy controls (the “HC” group). Patients, recruited from our outpatient clinics, were eligible if the following criteria were met: female; aged ≥ 60 years; diagnosed with stage I–III breast cancer; no history of psychiatric disease, neurodegenerative disorders, or stroke; no other cancer diagnosis or treatment; and able to consent to this study. The eligible patients were enrolled in the CT group prior to receiving adjuvant chemotherapy. HCs, recruited via patients’ referrals, community health fairs, and advertisements in local newspapers, had to meet the same criteria, excluding the cancer diagnosis.
The participants’ characteristics, including age, race, and education, were obtained through a questionnaire administered upon enrollment. Cancer staging and treatment information were obtained through abstraction of patient electronic medical records.
Written informed consent was obtained from all study participants in accordance with institutional guidelines and the Declaration of Helsinki, as well as all local, state, and federal regulations. This study was approved by the Institutional Review Board of City of Hope National Medical Center.
2.2. Study Assessment
Each study assessment consisted of a questionnaire upon enrollment, administration of the NIH Toolbox Cognition Battery18, and a brain magnetic resonance imaging (MRI) scan with DTI. For patients in the CT group, the baseline assessment (at time point 1, TP1) was performed after surgery but before the start of adjuvant chemotherapy. The follow-up assessment (at time point 2, TP2) was conducted within one month after the last chemotherapy infusion. The HC group was tested using the same assessments at matched time points.
All brain MRI data was acquired on the same Verio 3T scanner (Siemens, Erlangen, Germany) with a 12-channel head coil. DTI was acquired in twenty directions using the following parameters: repetition time (TR)=7,800 millisecond (ms), echo time (TE)=87 ms, field of view (FOV)=224 × 224 mm2, voxel size=1.75 × 1.75 × 1.8 mm3, number of slices=35, and b-value=1,000 s/mm2.
The same scanner, software version, and parameters were used for acquiring all imaging data at both time points. All brain MRI scans included in this study were evaluated by a neuroradiologist with over 17 years of experience (BTC) for incidental brain pathology.
This research protocol also acquired additional neuroimaging data, including T1-weighted 3D, sagittal 3D fluid attenuated inversion recovery, susceptibility-weighted imaging, and resting-state functional MRI sequences, which were published previously19–22. However, data from DTI analysis of white matter microstructures has not yet been published.
All study participants were administered the computerized NIH Toolbox Cognition Battery at TP1 and TP2 outside the MRI scanner18. The NIH Toolbox Cognition Battery consisted of seven individual measures targeting the following subdomains: executive function, episodic memory, language, processing speed, working memory, and attention (Table 2). Higher scores indicated higher levels of cognitive functioning.
Table 2.
Summary of neuropsychological testing data obtained using the NIH Toolbox Cognition Battery (mean scores [SD]).
| NIH Toolbox Score | Time Point 1 | Time Point 2 | Change Over time | ||||
|---|---|---|---|---|---|---|---|
| Chemotherapy Group* | Healthy Control Group | Chemotherapy Group | Healthy Control Group | Chemotherapy Group | Healthy Control Group | pb | |
| Crystallized cognition composite | 111.04 (15.38) | 107.45 (15.61) | 111.64 (12.77) | 107.29 (18.61) | −0.12 (7.65) | −0.16 (6.38) | 0.9887 |
| Fluid cognition composite | 100.06 (13.6) | 99.79 (9.71) | 100.22 (13.54) | 105.58 (15.09) | 0.57 (10.97) | 5.79 (11.89) | 0.2083 |
| Total cognition composite | 105.39 (18.14) | 101.93 (14.82) | 105.09 (14.83) | 105.65 (20.01) | −0.71 (9.8) | 3.72 (8.7) | 0.1924 |
| Dimensional change card sort | 100.89 (11.24) | 102.68 (12.47) | 101.02 (6.96) | 108.27 (8.84) | 0.73 (8.06) | 5.59 (12.13) | 0.1847 |
| Flanker Inhibitory control and attention | 95.17 (10.62) | 98.32 (9.16) | 92.64 (8.66) | 100.23 (5.69) | −2.8 (11.67) | 1.91 (7.46) | 0.1992 |
| List sorting working memory | 102.21 (15.81) | 100.9 (15.83) | 106.94 (10.3) | 105.01 (17) | 5.25 (9.66)a | 4.11 (14.07) | 0.7869 |
| Oral reading recognition | 112.16 (11.38) | 103.09 (12.31) | 109.13 (12.1) | 103.84 (14.07) | −3.72 (6.23)a | 0.75 (6.27) | 0.0534 |
| Pattern comparison processing speed | 92.46 (14.6) | 97.32 (13.44) | 93.16 (16.86) | 95.4 (16.45) | 0.36 (14.14) | −1.92 (15.09) | 0.6637 |
| Picture sequence memory test | 111.23 (19.53) | 102.49 (16.32) | 108.44 (20.27) | 110.3 (25.05) | −2.25 (11.52) | 7.81 (20.83) | 0.092 |
| Picture vocabulary | 106.68 (14.94) | 109.7 (13.97) | 110.5 (10.9) | 107.71 (16.55) | 3.37 (9.6) | −1.99 (6.09) | 0.0784 |
p<0.05 comparing the scores from Time point 1 to Time point 2 in the chemotherapy group.
p-values comparing changes over time (Time Point 1 to Time Point 2) between the chemotherapy group and the healthy control group.
18 chemotherapy patient: 1 patient without Time Point 1 neuropsychological testing data.
2.3. DTI Processing
The FMRIB Software Library (FSL) version 5.0 was used for DTI processing (Oxford, UK; http://www.fmrib.ox.ac.uk/fsl). Raw 4D diffusion data was first corrected for Eddy current and motion distortions using twelve-parameter affine transformation23. We then used the Brain Extraction Tool to generate brain masks24. A diffusion tensor model was fitted to every voxel in the brain to generate three eigenvectors and three eigenvalues. The eigenvalues were used to calculate FA, MD, AD, and RD25.
Voxel-wise analyses were performed for both study groups and time points, as previously described26. To minimize potential bias caused by within-subject differences over time, a longitudinal strategy for tract-based spatial statistics (TBSS: http://www.fmrib.ox.ac.uk/fsl/tbss/, part of FSL) was used27. For each subject, we registered the FA map from TP1 to the FA map from TP2 and from TP2 to TP1. A template was computed to capture the halfway point between TP1 and TP2, and the FA maps for both time points were registered to this template. The two registered FA maps were averaged to create a subject-specific FA template which was aligned to the templates for all other subjects. A study-specific target FA map with minimal mean and median deformation was then selected and was affine transformed into the standard space (Montreal Neurological Institute, MNI 152) at 1×1×1 mm328,29. Finally, each subject’s maps for both time points were transformed into the standard space by combining the non-linear transformation to the subject-specific template and then from the subject template to the standard space. A mean FA map was created by averaging the transformed FA maps from all subjects30. Using FA values larger than 0.2 as the threshold, the mean FA map was then thinned to produce an FA skeleton representing the centers of all tracts. Each subject’s aligned data was then projected onto the skeleton, and voxel-wise statistics were applied to the resulting data31.
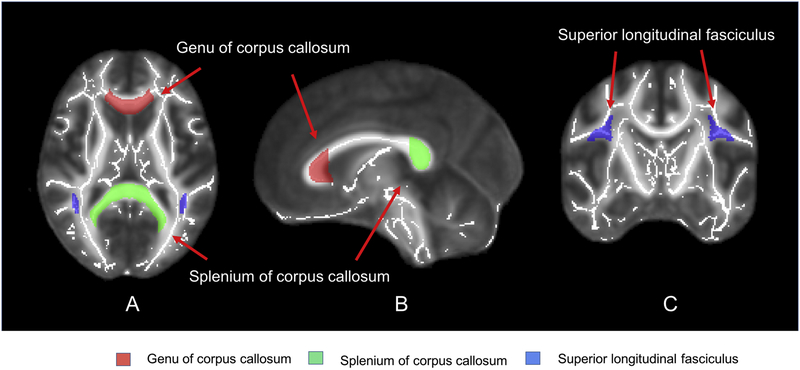
ROI analyses were obtained by calculating the mean values of the DTI parameters (FA, MD, RD, and AD) from the following four white matter tracts: left and right SLF, the genu and splenium of the corpus callosum (Fig. 1). We chose these four white matter tracts because they exhibited adverse chemotherapy-related effects in patients with breast cancer in previous DTI studies7, 9, 12. The ROI-based measurement was made semi-automatically according to the JHU White Matter template12, 32. First, the diffusivity maps were transformed into the standard space (spatial normalization). Then, we used the JHU white matter template to locate the four white matter tracts: left and right SLF, the genu and splenium of the corpus callosum. Each participant’s diffusivity map was carefully checked by a trained investigator (NY). Finally, the values of diffusivity parameters such as AD, RD, and MD of a given white matter tract of interest for each subject were computed by taking the arithmetic means of the values in all voxels within the specific tract.
Fig. 1.
(A) Axial, (B) sagittal, and (C) coronal images of the brain regions selected for region of interest (ROI) analysis.
2.4. Statistical Analysis
The HC group was matched to CT group by age distribution and gender. Race/ethnicity and education (categorical variables) distributions between the HC group and CT group were compared using Fisher’s exact tests. Linear mixed model taking into consideration of repeated measurements within each subject was used for longitudinal measurement of the NP data33. Within-subject correlations were accounted for by using a compound symmetry covariance structure. Time points (1 vs. 2) and groups (CT vs. HC) were both considered as categorical fixed effects in the model.
The Randomize function in FSL (http://www.fmrib.ox.ac.uk/fsl/randomize/) was used to assess voxel-wise changes in the four DTI parameters (FA, MD, RD and AD) from TP1 to TP2 in the CT and HC groups, as well as the group differences in these changes. This function implemented permutation-based inference (5,000 permutations) within a general linear model to test group differences and within-group longitudinal differences34. Age was included as a covariate in all DTI analyses. In addition, multiple comparisons were corrected for family wise error (FWE) using threshold-free cluster enhancement35. Cluster level p-corrected < 0.05 was considered statistically significant.
A paired t-test was used for assessing the statistical significance of the change in the diffusivity parameters from TP1 to TP2 for each group, and two sample t-test was used to assess the statistical significance of the between-group differences of the diffusivity parameters at TP1 and the between-group differences in the changes of the diffusivity parameters. The effect sizes and 95% confidence intervals of the within-group change over time in the diffusivity parameters were assessed using Cohen’s d, and the effect sizes and the 95% confidence intervals of between-group difference in the changes of diffusivity parameters were assessed using Hedges’ g. The analysis was performed using R (https://CRAN.R-project.org/package=effsize).
ROI-based correlative analysis was performed by computing pair-wise Pearson correlation coefficients and the associated p-values between the ten NP scores and the mean values of the DTI parameters within the genu of the corpus callosum and left SLF. We focused on the genu of the corpus callosum and the left SLF because these were the only two ROI areas that showed significant changes over time in the CT group. Specifically, the following correlative analyses were performed for both groups: between DTI values and NP scores at TP1, between DTI values at TP1 and changes in NP scores, and between changes in DTI values and changes in NP scores.
3. Results
3.1. Demographic Data
In this cohort, there were nineteen older female patients with breast cancer receiving adjuvant chemotherapy (mean age 66.6, SD= 5.24 years, range 60–82 years) and fourteen age- and sex-matched healthy women serving as controls (mean age 68.1, SD= 5.69 years, range 60–78 years) as shown in Table 1. No significant differences between the CT and HC groups were detected in age (p=0.32) or overall education (p=0.79).
Table 1.
Demographic information for all participants and disease stage and treatment regimen for patients with breast cancer
| Chemotherapy Group (n=19) | Healthy Control Group (n=14) | |
|---|---|---|
| Age, years | ||
| Mean (SD) | 66.6 (5.24) | 68.1 (5.69) |
| Range | 60–82 | 60–78 |
| Race, N (%) | ||
| White | 13 (68.4%) | 14 (100%) |
| Black | 6 (31.6%) | 0 (0%) |
| Ethnicity, N (%) | ||
| Hispanic or Latina | 2 (10.5%) | 2 (14.3%) |
| Non-Hispanic | 17 (89.5%) | 12 (85.7%) |
| Education, N (%) | ||
| High School | 4 (21.1%) | 1 (7.1%) |
| Some College or junior college | 6 + 4 (52.6%) | 4 + 4 (57.1%) |
| College degree | 3 (15.8%) | 3 (21.4%) |
| Advance degree | 2 (10.5%) | 2 (14.3%) |
| Stage, N (%) | ||
| I | 7 (36.8%) | |
| II | 9 (47.4%) | |
| III | 3 (15.8%) | |
| Regimen, N (%) | ||
| TCa | 10 (52.6%) | |
| TCPHb | 2 (10.5%) | |
| Paclitaxel/trastuzumab | 4 (21.1%) | |
| Carboplatin/paclitaxel | 1 (5.3%) | |
| ddAC-Tc | 1 (5.3%) | |
| TACd | 1 (5.3%) |
TC: docetaxel (Taxotere) and cyclophosphamide
TCPH: docetaxel (Taxotere), carboplatin, and trastuzumab (Herceptin)
ddAC-T: dose dense doxorubicin (Adriamycin) and cyclophosphamide, followed by paclitaxel (Taxol)
TAC: docetaxel (Taxotere), doxorubicin (Adriamycin), and cyclophosphamide
3.2. DTI Data
Using voxel-wise whole brain analysis with TBSS, we did not observe any significant differences between the CT and HC group in FA, MD, RD, or AD at TP1 (cluster level p-corrected>0.05). When comparing the between-group differences (the CT group vs the HC group) in the changes of DTI parameters from TP1 to TP2, voxel-wise analysis with TBSS showed no significant difference (cluster level p-corrected>0.05).
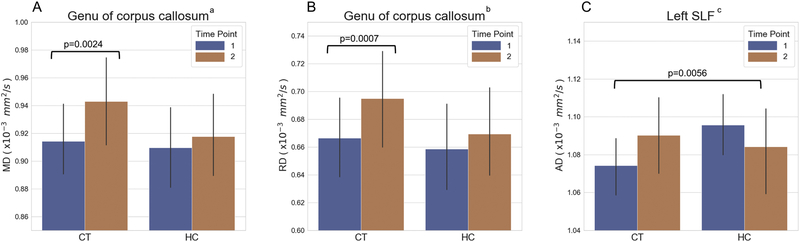
Using ROI-based analysis, we also did not observe any significant differences between CT and HC groups at TP1 (p>0.05). However, from TP1 to TP2, the mean MD and RD values in the genu of the corpus callosum increased significantly in the CT group but not in the HC group (p=0.0024, effect size: 0.3655, 95% confidence interval: from 0.1490 to 0.5821 for MD, and p=0.0007, effect size: 0.3173, 95% confidence interval: from to 0.4792, for RD; Fig. 2A–B). Although not statistically significant, the AD values increased in the CT group and decreased in the HC group over time (p >0.05). However, the different trajectories of AD values from TP1 to TP2 between the two groups were significant for group by time interaction (p=0.0056, effect size: 1.0215, 95% confidence interval: from 0.2773 to 1.7657, Fig. 2C).
Fig. 2.
(A) Mean diffusivity (MD) and (B) radial diffusivity (RD) values for the genu of corpus callosum and (C) axial diffusivity (AD) values in the left superior longitudinal fasciculus (SLF) of the chemotherapy (CT) group and the healthy control (HC) group. Error bars represent the standard error of the mean. a: comparing MD from time point 1 (TP1) to time point 2 (TP2) within the CT group. b: comparing RD from TP1 to TP2 within the CT group. c: comparing the changes in AD from TP1 to TP2 between the CT group and the HC group.
3.3. NP Data
We reported NP testing scores obtained using the NIH Toolbox Cognition Battery for both the CT and HC groups in our previous publications19–22. However, since our previous studies, we added three patients to the CT group (n=19). In addition, we added data for a HC participant who was not included in the prior studies, but also removed one HC participant who did not have DTI data (n=14). We therefore re-analyzed the NP data, which was presented in Table 2.
There were no statistically significant differences between the CT and HC groups in any of the ten NP testing scores at TP1 (p>0.05). From TP1 to TP2, there were statistically significant within-group increase in the List-Sorting Working Memory score in the CT group and similar but non-significant increase in the HC group, possibly due to practice effect for both groups. The Oral Reading Recognition score was decreased from TP1 to TP2 (p<0.05) in the CT group. However, these changes over time were not different between the CT group and HC group (p>0.05).
3.4. Correlations Between DTI Parameters and NP Scores
ROI-based correlative analysis showed no significant correlation between any of the four DTI parameters including FA, MD, RD and AD and the ten NP scores at TP1, between DTI values at TP1 and changes in NP scores, or between changes in DTI values and changes in NP scores with p>0.05 for all correlations after corrected for multiple comparisons.
4. Discussion
To the best of our knowledge, this is the first longitudinal study of aging white matter microstructure in older patients with cancer undergoing adjuvant chemotherapy. We observed significant increases in MD and RD values in the genu of the corpus callosum in the CT group over time after receiving chemotherapy. We also observed changes in AD values in the left SLF that were significantly different between the CT and HC groups.
Similar to the findings reported by Menning et al.12, our voxel-based whole brain analysis revealed no significant between-group differences in the magnitude of the DTI alterations between the CT group and the HC group. However, our findings differed from those of Deprez et al.9 whose voxel-wise analysis found significantly decreased FA values over time in the corpus callosum, SLF, and forceps major in patients with breast cancer who received chemotherapy but not in their healthy controls or the patients with breast cancer who did not receive chemotherapy. We found increased MD and RD values in the older patients receiving chemotherapy in our ROI-based analysis. Our DTI study did not include an untreated patient group to make similar comparisons to the Deprez et al. study9.
There are several possible explanations for the discrepancies between our results and those of others. First, by design, our participants were older than those in other studies. Our CT cohort may have been more vulnerable to the detrimental effects of aging, manifested as decreased FA and increased diffusivity values in the longitudinal DTI analysis36–38. In addition, we observed a decrease in diffusivity values in our HC participants, who were older (mean 68.1 ± 5.69 years) than the healthy controls included in the Deprez et al. study (mean 43.8 ± 4.9 years)9 who did not exhibit this effect. This is supported by findings from Menning et al. who also observed a decrease in FA values in an intermediately aged cohort of healthy controls (mean 50.5 ± 8.0 years)12. Our results were also in line with previous reports that alterations in DTI parameters accelerate with advancing age38. Second, we recognize that our baseline and follow-up assessments were obtained within a shorter time frame (one month after chemotherapy) than that of Deprez et al. (three to four months after chemotherapy) and Menning et al. (six months after chemotherapy)9, 12. We speculate that our shorter interval might not have been long enough to reveal all the white matter alterations observed in the studies by Menning et al. and Deprez et al. Third, the chemotherapy regimens administered to the patients in our cohort were different from the regimens administered to patients in the studies. Prior reports have shown that different chemotherapy regimens exert distinct neurotoxic effects39. For example, Menning et al. observed milder neurotoxicity than Deprez et al., which they attributed to the use of newer anthracycline-based chemotherapeutic regimens that are less toxic than the 5-fluorouracil-containing regimen used in the older study by Deprez et al9, 12. The patients in our study received several different chemotherapy regimens, with most (ten out of nineteen) on the TC regimen consisting of docetaxel and cyclophosphamide (Table 1). This adjuvant TC regimen has been recommended for older patients with breast cancer because of its favorable cardiotoxicity profile as reported in a position paper from International Society of Geriatric Oncology Task Force40. However, there is limited information about the effects of this regimen on white matter integrity in older patients.
The changes in the diffusivity parameters (MD, RD, and AD) but not the directional parameter (FA) for the CT group in our ROI-based analysis may be related to axonal injury and white matter demyelination9, 41. It has been suggested that MD might be more sensitive than FA to short-term changes due to aging and brain injuries, including those related to chemotherapy and radiation27, 41. The post-mortem examination of a patient with breast cancer who received chemotherapy identified demyelination in the white matter regions42, and previous animal studies have shown that demyelination and RD are related43. Therefore, it is possible that chemotherapy causes demyelination that can be detected by RD. Based on these previous studies, we were not surprised by our findings of increased MD and RD in the CT group over time and of greater increases in AD over time in the CT group than in the HC group. Nevertheless, the underlying neurobiological changes responsible for the altered white matter diffusivity in older patients undergoing chemotherapy are not clear.
We observed microstructural alterations in the anterior white matter, specifically the genu of corpus callosum in the chemotherapy-treated group. The corpus callosum is the largest white matter bundle in the brain and has been widely used as an index of changes of cerebral white matter. This finding is consistent with prior studies showing that DTI changes due to chemotherapy or aging preferentially occur in the anterior white matter7, 9, 27. Prior studies by Abraham et al. and Deprez et al. showed lower FA values in the genu of the corpus callosum in the patients treated with chemotherapy7, 9. White matter alterations follow an anterior-to-posterior gradient, with greater susceptibility to aging and other insults in the anterior regions27. In a similarly designed longitudinal study20, we found that gray matter density decreased significantly over time in older patients with breast cancer receiving chemotherapy. Taken together, these studies indicate that frontal brain regions including both gray matter and white matter are altered in older patients with breast cancer undergoing chemotherapy.
There were several limitations to this study. First, our small sample size and the heterogeneity of the chemotherapy regimens administered to our patient group may have limited our ability to identify DTI alterations related to any one regimen. Second, the small sample size and short-term follow-up may have limited our ability to detect significant changes in NP scores between the CT group and the HC group, or possible correlation between the DTI parameters and the NP scores. Our short-term follow-up also limited our ability to assess the potential recovery of white matter microstructural changes after chemotherapy44. Third, the lack of a control group of patients with breast cancer who did not receive chemotherapy limited our ability to identify DTI changes specifically related to the cancer and not the chemotherapy. Fourth, white matter hyperintensities (WMHs), which are commonly seen on brain MRI scans of older adults45, may represent cerebral small vessel disease and can potentially influence DTI parameters46. We did not analyze WMHs in our DTI analysis because our current study was focused on microstructural rather than macrostructural white matter changes. Nevertheless, we recognize the value of a future investigation regarding any possible differences in WMHs in our cohort, as these may have potentially contributed to the observed differences in DTI parameters, but that would not diminish the strength of our findings. Lastly, it should be mentioned that variation in DTI data analyses may influence the results. For example, FA is reduced in voxels with multiple crossing fibers. Thus, resolving individual fibers in fiber-crossing areas might help to reveal subtle alterations in white matter microstructure47. Nevertheless, this was the first longitudinal DTI study on the effects of chemotherapy focusing on older patients with cancer and has provided promising pilot data from which to generate hypotheses for larger studies of CRCI in older cancer survivors.
5. Conclusion
We observed microstructural alterations in white matter including corpus callosum and SLF in older women with breast cancer undergoing chemotherapy. These DTI alterations may potentially serve as neuroimaging biomarkers for identifying neural correlates of cognitive functioning, thus improving our ability to diagnose cognitive impairment and to restore the quality of life for older patients with cancer.
Table 3.
Diffusion tensor imaging parameters for the white matter skeleton and selected regions for region of interest analysis. Values indicate the mean change in each parameter from time point 1 to time point 2.
| Chemotherapy Group (n=19) | Healthy Control Group (n=14) | ||
|---|---|---|---|
| Mean change (SD) | Mean change (SD) | ||
| Skeleton | FA | −0.007 (0.012) | −0.016 (0.010) |
| AD (×10−3 mm2/s) | 0.013 (0.023) | −0.010 (0.017) | |
| MD (×10−3 mm2/s) | 0.013 (0.014) | 0.003 (0.013) | |
| RD (×10−3 mm2/s) | 0.014 (0.012) | 0.010 (0.013) | |
| Genu of corpus callosum | FA | −0.006 (0.015) | −0.008 (0.016) |
| AD (×10−3 mm2/s) | 0.029 (0.052) | 0.002 (0.021) | |
| MD (×10−3 mm2/s) | 0.029 (0.035)a | 0.008 (0.014) | |
| RD (×10−3 mm2/s) | 0.028 (0.030)b | 0.011 (0.017) | |
| Splenium of corpus callosum | FA | −0.002 (0.011) | −0.009 (0.010) |
| AD (×10−3 mm2/s) | 0.028 (0.043) | 0.004 (0.015) | |
| MD (×10−3 mm2/s) | 0.017 (0.024) | 0.010 (0.010) | |
| RD (×10−3 mm2/s) | 0.011 (0.018) | 0.011 (0.013) | |
| Superior longitudinal fasciculus (Left) | FA | 0.003 (0.015) | −0.006 (0.009) |
| AD (×10−3 mm2/s)c | 0.016 (0.027) | −0.011 (0.025) | |
| MD (×10−3 mm2/s) | 0.008 (0.013) | −0.005 (0.016) | |
| RD (×10−3 mm2/s) | 0.004 (0.011) | −0.002 (0.014) | |
| Superior longitudinal fasciculus (Right) | FA | 0.001 (0.018) | −0.013 (0.017) |
| AD (×10−3 mm2/s) | 0.015 (0.028) | −0.007 (0.014) | |
| MD (×10−3 mm2/s) | 0.009 (0.017) | 0.004 (0.007) | |
| RD (×10−3 mm2/s) | 0.007 (0.017) | 0.009 (0.011) |
FA: fractional anisotropy, MD: mean diffusivity, RD: radial diffusivity, AD: axial diffusivity
indicates significant within-group changes from time point 1 to time point 2 in the chemotherapy group (p<0.01 after Bonferroni correction).
indicates significant between-group difference when comparing the changes from time point 1 to time point 2 in the chemotherapy group to the changes in the healthy control group (p<0.05).
Acknowledgements
Kerin Higa, PhD, provided editing assistance.
Funding
This study was funded by National Institutes of Health/National Institute on Aging grants R03 AG045090-02 (BTC) and K24 AG055693-01 (Dr. Arti Hurria, this award has been transitioned to Dr. William Dale). BTC also received pilot funding support from the City of Hope Center for Cancer and Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest and Disclosures
The authors declare no competing interests.
Ethical Approval
All procedures involving human participants were performed in accordance with the ethical standards of the Institutional Review Board of City of Hope and with the 1964 Helsinki Declaration and its later amendments, as well as with all local, state, and federal regulations. This study is registered on ClinicalTrials.gov ().
We dedicate this manuscript to the memory of Dr. Arti Hurria whose vision and support made this work possible.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Lyon DE, Cohen R, Chen H, Kelly DL, Starkweather A, Ahn HC et al. The relationship of cognitive performance to concurrent symptoms, cancer- and cancer-treatment-related variables in women with early-stage breast cancer: a 2-year longitudinal study. J Cancer Res Clin Oncol 2016;142:1461–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calvio L, Peugeot M, Bruns GL, Todd BL, Feuerstein M. Measures of cognitive function and work in occupationally active breast cancer survivors. J Occup Environ Med 2010;52:219–27. [DOI] [PubMed] [Google Scholar]
- 4.Mandelblatt JS, Jacobsen PB, Ahles T. Cognitive effects of cancer systemic therapy: implications for the care of older patients and survivors. J Clin Oncol 2014;32:2617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahles TA, Root JC. Cognitive Effects of Cancer and Cancer Treatments. Annu Rev Clin Psychol 2018;14:425–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandelblatt JS, Small BJ, Luta G, Hurria A, Jim H, McDonald BC et al. Cancer-Related Cognitive Outcomes Among Older Breast Cancer Survivors in the Thinking and Living With Cancer Study. J Clin Oncol 2018;Jco1800140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abraham J, Haut MW, Moran MT, Filburn S, Lemiuex S, Kuwabara H. Adjuvant chemotherapy for breast cancer: effects on cerebral white matter seen in diffusion tensor imaging. Clin Breast Cancer 2008;8:88–91. [DOI] [PubMed] [Google Scholar]
- 8.de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp 2012;33:2971–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van Hecke W et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol 2012;30:274–81. [DOI] [PubMed] [Google Scholar]
- 10.Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Van den Stock J et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp 2011;32:480–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koppelmans V, de Groot M, de Ruiter MB, Boogerd W, Seynaeve C, Vernooij MW et al. Global and focal white matter integrity in breast cancer survivors 20 years after adjuvant chemotherapy. Hum Brain Mapp 2014;35:889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menning S, de Ruiter MB, Veltman DJ, Boogerd W, Oldenburg HSA, Reneman L et al. Changes in brain white matter integrity after systemic treatment for breast cancer: a prospective longitudinal study. Brain Imaging Behav 2018;12:324–334. [DOI] [PubMed] [Google Scholar]
- 13.Stouten-Kemperman MM, de Ruiter MB, Koppelmans V, Boogerd W, Reneman L, Schagen SB. Neurotoxicity in breast cancer survivors >/=10 years post-treatment is dependent on treatment type. Brain Imaging Behav 2015;9:275–84. [DOI] [PubMed] [Google Scholar]
- 14.Deprez S, Billiet T, Sunaert S, Leemans A. Diffusion tensor MRI of chemotherapy-induced cognitive impairment in non-CNS cancer patients: a review. Brain Imaging Behav 2013;7:409–35. [DOI] [PubMed] [Google Scholar]
- 15.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 1995;8:333–44. [DOI] [PubMed] [Google Scholar]
- 16.Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev 2010;20:209–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252–71. [DOI] [PubMed] [Google Scholar]
- 18.Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ et al. Cognition assessment using the NIH Toolbox. Neurology 2013;80:S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen BT, Sethi SK, Jin T, Patel SK, Ye N, Sun CL et al. Assessing brain volume changes in older women with breast cancer receiving adjuvant chemotherapy: a brain magnetic resonance imaging pilot study. Breast Cancer Res 2018;20:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen BT, Jin T, Patel SK, Ye N, Sun CL, Ma H et al. Gray matter density reduction associated with adjuvant chemotherapy in older women with breast cancer. Breast Cancer Res Treat 2018;172:363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen BT, Ghassaban K, Jin T, Patel SK, Ye N, Sun CL et al. Subcortical brain iron deposition and cognitive performance in older women with breast cancer receiving adjuvant chemotherapy: A pilot MRI study. Magn Reson Imaging 2018;54:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen BT, Jin T, Patel SK, Ye N, Ma H, Wong CW et al. Intrinsic brain activity changes associated with adjuvant chemotherapy in older women with breast cancer: a pilot longitudinal study. Breast Cancer Res Treat 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016;125:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 1994;103:247–54. [DOI] [PubMed] [Google Scholar]
- 26.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–505. [DOI] [PubMed] [Google Scholar]
- 27.Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth O, Larsen VA et al. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum Brain Mapp 2012;33:2390–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonov VS, Evans AC, McKinstry RC, Almli C, Collins D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage 2009;S102. [Google Scholar]
- 29.Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL et al. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 2011;54:313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersson JL, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford 2007;2:1–21. [Google Scholar]
- 31.Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med 2009;61:1255–60. [DOI] [PubMed] [Google Scholar]
- 32.Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 2008;39:336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38:963–74. [PubMed] [Google Scholar]
- 34.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014;92:381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44:83–98. [DOI] [PubMed] [Google Scholar]
- 36.Pelletier A, Periot O, Dilharreguy B, Hiba B, Bordessoules M, Chanraud S et al. Age-Related Modifications of Diffusion Tensor Imaging Parameters and White Matter Hyperintensities as Inter-Dependent Processes. Front Aging Neurosci 2015;7:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage 2005;26:891–9. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev 2006;30:749–61. [DOI] [PubMed] [Google Scholar]
- 39.Kesler SR, Blayney DW. Neurotoxic Effects of Anthracycline- vs Nonanthracycline-Based Chemotherapy on Cognition in Breast Cancer Survivors. JAMA Oncol 2016;2:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biganzoli L, Aapro M, Loibl S, Wildiers H, Brain E. Taxanes in the treatment of breast cancer: Have we better defined their role in older patients? A position paper from a SIOG Task Force. Cancer Treat Rev 2016;43:19–26. [DOI] [PubMed] [Google Scholar]
- 41.Chapman CH, Nazem-Zadeh M, Lee OE, Schipper MJ, Tsien CI, Lawrence TS et al. Regional variation in brain white matter diffusion index changes following chemoradiotherapy: a prospective study using tract-based spatial statistics. PLoS One 2013;8:e57768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore-Maxwell CA, Datto MB, Hulette CM. Chemotherapy-induced toxic leukoencephalopathy causes a wide range of symptoms: a series of four autopsies. Mod Pathol 2004;17:241–7. [DOI] [PubMed] [Google Scholar]
- 43.Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 2005;26:132–40. [DOI] [PubMed] [Google Scholar]
- 44.Billiet T, Emsell L, Vandenbulcke M, Peeters R, Christiaens D, Leemans A et al. Recovery from chemotherapy-induced white matter changes in young breast cancer survivors? Brain Imaging Behav 2018;12:64–77. [DOI] [PubMed] [Google Scholar]
- 45.Habes M, Erus G, Toledo JB, Zhang T, Bryan N, Launer LJ et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 2016;139:1164–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frey BM, Petersen M, Mayer C, Schulz M, Cheng B, Thomalla G. Characterization of White Matter Hyperintensities in Large-Scale MRI-Studies. Front Neurol 2019;10:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh M, Wong CW. Independent component analysis-based multifiber streamline tractography of the human brain. Magn Reson Med 2010;64:1676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]