Abstract
Benzeneperoxyseleninic acid has been proposed as the key intermediate in the widely used epoxidation of alkenes with benzeneseleninic acid and hydrogen peroxide. However, it reacts sluggishly with cyclooctene and instead rapidly decomposes in solution to a mixed selenonium-selenonate salt that was identified by x-ray absorption and 77Se NMR spectroscopy, as well as by single crystal x-ray diffraction. This process includes a selenoxide elimination of the peroxyseleninic acid with liberation of oxygen and additional redox steps. The salt is relatively stable in the solid state, but generates the corresponding selenonic acid in the presence of hydrogen peroxide. The selenonic acid is inert towards cyclooctene on its own; however, rapid epoxidation occurs when hydrogen peroxide is added. This shows that the selenonic acid must first be activated through further oxidation, presumably to the heretofore unknown benzeneperoxyselenonic acid. The latter is the principal oxidant in this epoxidation.
Keywords: benzeneperoxyseleninic acid, benzeneperoxyselenonic acid, epoxidation, oxidation, peroxides
Graphical Abstract
Peroxyseleninic or peroxyselenonic? What a difference a vowel makes! The main active intermediate in the epoxidation of cyclooctene with benzeneseleninic acid and hydrogen peroxide is not benzeneperoxyseleninic acid, but surprisingly, the corresponding postulated peroxyselenonic acid.

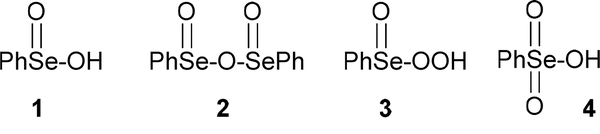
Benzeneseleninic acid (1) and anhydride (2), as well as their congeners, are widely utilized oxidants in a variety of synthetic transformations.[1,2] Of special interest are reactions that are conducted with stoichiometric or catalytic seleninic acids, or their precursor diselenides, in the presence of hydrogen peroxide. These include epoxidations[3] and dihydroxylations[3a,4] of alkenes, Baeyer-Villiger reactions,[5] dehydrogenations of alcohols and ketones[6] and numerous other processes.[1,2] It has been widely accepted that peroxyseleninic acids, such as 3, are the active oxidants, although there is little evidence to support this assumption or to exclude the possibility that selenonic acid 4 or other Se(VI) species are involved.
In 1987, Syper and Młochowski[7] reported that dissolution of benzeneseleninic acid 1 in 30% hydrogen peroxide resulted in the precipitation of the corresponding peroxyseleninic acid 3, which hydrolyzed back to the original seleninic acid when dissolved in water. When 3 was heated in acetonitrile solution at 80 °C, isomerization to 4 was reported, via the proposed selenadioxirane 5, which had been previously suggested as a potential alternative intermediate in the epoxidation of cyclooctene by Hori and Sharpless[3b] (Scheme 1). More recently, Orian et al.[8] employed NMR and computational methods to study the hydrogen peroxide oxidation of diphenyl diselenide to 1 and then to the peroxy acid 3, but did not address the possible formation of the selenonic acid 4. In contrast to seleninic acids, selenonic acids have been relatively little studied.[9] We now present evidence that, contrary to previous assumptions, 3 plays only a secondary role in a representative epoxidation of cyclooctene conducted with 1 and hydrogen peroxide, while a selenium (VI) species is the principal oxidant.
Scheme 1.
Syper and Mlochowski’s preparation and proposed isomerization of 3.
First, geometry optimizations (see the Supporting Information for details) indicated that the selenonic acid 4 is more stable than its peroxyseleninic acid isomer 3 by 28.8 kcal mol−1. Thus, we attribute the preparation of the less stable 3 from 1 and aqueous hydrogen peroxide to its equilibration and precipitation from the reaction medium. Attempts at geometry optimization of the highly strained intermediate 5 failed because it reverted to its valence bond isomer 4, but single point energy calculations on several trial conformations produced energies >100 kcal mol−1 higher than those of 4. We therefore propose the peroxyselenurane 6 as a possible alternative, dehydration of which could lead to either 3 or 4, thereby providing a means for their interconversion (Scheme 2), favouring the more stable 4 in solution.
Scheme 2.
Alternative formation and isomerization of 4 from 3.
In further investigations, seleninic acid 1 was dissolved in CDCl3:CD3OD (95:5) and treated with a slight excess (1.15 equiv) of 30% hydrogen peroxide at room temperature. A rapid reaction accompanied by gentle gas evolution occurred and the 77Se NMR spectrum (recorded over ca. 40 min) revealed a new signal at 1026 ppm, compared to 1173 ppm (CDCl3)[10] for 1. Furthermore, when the NMR experiment was repeated at higher concentration with the UDEFT protocol,[11] an additional weaker peak appeared at 1218 ppm. Thus, while a new product(s) was clearly produced, the paucity of existing 77Se NMR data for peroxyseleninic and selenonic acids precluded unequivocal assignment of these peaks to 3, 4 or other oxidized species.
Since the isomeric peroxyseleninic and selenonic acids 3 and 4, respectively, have different oxidation states, a sample of the product was prepared from the treatment of benzeneseleninic acid (1) with hydrogen peroxide. It was evaporated under vacuum and the residue was subjected to x-ray absorption spectroscopy (XAS) at the Stanford Synchrotron Radiation Lightsource (SSRL) in order to obtain additional structural information (for details and an illustrative Figure, see the Supporting Information). Thus, the selenium K-edge x-ray absorption near-edge spectrum of the above sample was compared with the spectrum of unreacted 1. The latter displayed a peak energy of 12663.4 eV which is characteristic of Se (IV), while two peaks were observed in the sample that was reacted with hydrogen peroxide. The lower energy peak was seen at 12662.7 eV, while the higher energy peak appeared at 12666.4 eV. The 12662.7 eV peak is consistent with an Se (IV) species like peroxyseleninic acid 3, while the 12666.4 eV peak indicates an Se(VI) species such as selenonic acid 4.[12] Thus, unexpectedly, these results indicated that both Se(IV) and Se(VI) oxidation states were present in the oxidized sample subjected to XAS analysis.
Crystals of the product obtained by the similar oxidation of (1) with hydrogen peroxide proved suitable for x-ray diffraction (see Figure 2 and the Supporting Information). To our surprise, the x-ray structure[13] showed that the product was neither 3 nor 4, but instead the selenonium-selenonate salt 7, formed by protonation of 1 by an equimolar amount of the selenonic acid 4. The presence of two selenium atoms in different oxidation states was clearly evident and consistent with the 77Se NMR and XAS results. Furthermore, 7 was isolated in 74% yield when only 0.5 equiv of hydrogen peroxide was employed in the oxidation of 1. When the peroxyseleninic acid 3 was heated in acetonitrile in the absence of hydrogen peroxide, gas evolution was again observed and product 7 was obtained in 63% yield (Scheme 3). The same product was obtained within 40 min at room temperature. Moreover, the melting point attributed to the selenonic acid 4 (144 °C) reported by Syper and Młochowski,[7] is almost identical to that of 7 (142–144 °C). These experiments indicate that the peroxyseleninic acid 3 is relatively stable in the solid state, but labile in solution, rapidly affording salt 7[14] and not the selenonic acid 4 as previously reported.[7]
Figure 2.
ORTEP diagram of 7. The disorder in the selenonate anion portion is due to rotational freedom of the aryl ring, resulting in nonparallel alignment of the aryl rings in alternating molecules in the unit cell.
Scheme 3.
Formation and further oxidation of selenonium-selenonate salt 7.
In order to assign the 77Se NMR signals at 1026 and 1217 ppm of 7 unequivocally to its two respective selenium atoms, 1 was treated with trifluoroacetic acid and the 77Se NMR spectrum of the resulting selenonium ion 8 produced a signal at 1215 ppm. This closely correlates with the signal at 1218 ppm in 7 and permits its assignment to the selenonium moiety, while the peak at 1026 ppm is therefore attributed to the selenonate anion (Figure 3).
Figure 3.
UDEFT 77Se NMR spectra of the salt 7 and the selenonium trifluoroacetate 8 in CDCl3
The formation of the stable salt 7 from 1 and hydrogen peroxide, as shown in path a of Scheme 3, is easily rationalized. As selenonic acid 4 is produced via isomerization of the initially formed peroxyseleninic acid 3, as shown in Scheme 2, it protonates the remaining amphoteric[15] starting material 1, resulting in the formation of salt 7. This reaction is complete when 50% of 1 is oxidized, thus requiring only 0.5 equiv of hydrogen peroxide. On the other hand, the transformation of 3 directly to 7 in the absence of hydrogen peroxide (path b of Scheme 3), is less obvious, as both the selenonic acid 4 and seleninic acid 1 are required for the formation of 7. We propose that the observed instability of 3 in solution, accompanied by observable gas evolution, is due to its facile formal selenoxide elimination that produces benzeneselenenic acid (9) and dioxygen. The oxidation of 9 by a second molecule of 3 then generates the seleninic acid 1 (Scheme 4). If this process proceeds at a comparable rate to the isomerization of 3 to 4, then both components required for the formation of 7 are produced simultaneously.
Scheme 4.
Selenoxide elimination of 3 leading to 7 in the absence of hydrogen peroxide.
Upon further oxidation of 7 with 2.2 equiv of hydrogen peroxide in acetonitrile at 60 °C, it afforded selenonic acid 4 quantitatively (Scheme 3), identical to an authentic sample (see Supporting Information) with a 77Se NMR signal (CD3CN) at 1025 ppm.
Finally, we examined the reactions of stoichiometric amounts of independently prepared 1, 4 and 7 with cyclooctene (10) in the absence of hydrogen peroxide at room temperature in dichloromethane in separate control experiments. GC analysis revealed no detectable conversion to the corresponding epoxide 11, even after several hours. When the reaction was similarly repeated with 3, epoxidation reached only 19% conversion in 1 h and did not proceed further. Salt 7 was produced simultaneously in 62% isolated yield and was identified by comparison of its m.p. and 77Se NMR spectrum with those of an authentic sample. The relative amounts of the epoxide and the salt indicate that the pathway to 7 proceeds at least 3.3 times faster than the epoxidation and explains why direct epoxidation with 3 is relatively ineffective. Furthermore, when the epoxidation was repeated with seleninic acid 1 in the presence of one equiv of hydrogen peroxide, a more rapid reaction ensued, but afforded only 50% of the epoxide. When 7 was employed under similar conditions, epoxide formation was rapid, requiring 3.5 h to reach ca. 82% conversion, after which no further reaction was observed (Scheme 5 and Figure 4a). The even more striking behaviour of 4 is illustrated in Figure 4b, where no reaction was observed after 2 h, but upon addition of 1 equiv of hydrogen peroxide, the epoxide 11 was formed in 86% yield after only one additional hour.
Scheme 5.
Epoxidation of cyclooctene
Figure 4.
A) Yields of cyclooctene epoxide were obtained by GC analysis with an internal standard. Reactions were performed in dichloromethane with equimolar amounts of cyclooctene, the selenium compound and, where indicated, hydrogen peroxide. B) Epoxidation was conducted similarly with selenonic acid 4 alone, followed by addition of one equiv of hydrogen peroxide after 2 h. Rate constants are provided in the Supporting Information.
Although it is possible that seleninic acid 1 can produce 4 via selenurane 6 (Scheme 2) without the intermediacy of peroxyseleninic acid 3, we have shown that any 3 that might form would be rapidly converted to the salt 7. The same salt would also be produced by the reaction of 1 and 4 (Scheme 4). Thus, the formation of 7 competes effectively with the relatively slow and limited epoxidation of cyclooctene with 3 in the absence of hydrogen peroxide (Scheme 6). In addition, the faster epoxidations observed with 7, and especially with 4, in the presence of hydrogen peroxide, together with their inertness in its absence, indicate that downstream intermediates are the principal oxidants of the alkene. We therefore propose that the selenonium ion moiety of 7 reacts further with hydrogen peroxide to regenerate the peroxyseleninic acid, which is then recycled back to 7, while the simultaneous liberation of 4 from 7 accompanies each cycle of this process (Scheme 6). Moreover, the observation that the selenonic acid 4 can only effect epoxidation in the presence of hydrogen peroxide indicates that a new species such as the peroxyselenonic acid 13, probably produced via 12, must serve as the principal active oxidant. In order to confirm the formation of 13, a sample of 4 was treated with 0.5 equiv of hydrogen peroxide and subjected to UDEFT 77Se NMR spectroscopy. The spectrum showed two peaks of roughly equal intensity, one at 1026 ppm from unreacted 4 and a second signal at 1024 ppm attributed to 13.16 When excess hydrogen peroxide was added, only the upfield signal was observed. This appears to be the first observation, albeit a tentative one, of a peroxyselenonic acid. Unfortunately, 13 proved too unstable for isolation and further characterization.
Scheme 6.
Proposed mechanism of epoxidation of cyclooctene with 1 and hydrogen peroxide.
In summary, these experiments indicate that the peroxyselenonic acid 13, and not the peroxyseleninic acid 3, is the principal oxidant in the epoxidation of cyclooctene. Further work will be required to determine if this is also true for other oxidations conducted with seleninic acid 1 or its congeners in the presence of hydrogen peroxide.17
Supplementary Material
Figure 1.
Structures of benzeneseleninic acid (1) and several of its congeners.
Acknowledgements
We thank the Natural Sciences and Engineering Research Council of Canada for financial support. K.N.S. thanks Walpole Island First Nation and the Province of Alberta for postgraduate scholarships. G.N.G. and I.J.P. are Canada Research Chairs. E.M.R. acknowledges a Dean’s Scholarship. Use of SSRL, SLAC National Accelerator Laboratory, is supported by the U.S. DOE, Office of Science, OBES under Contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE OBER, and by the NIH, NIGMS (P41GM103393). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
Supporting information for this article is given via a link at the end of the document.
References
- [1].a) T. G. Back in Organoselenium Chemistry – A Practical Approach, (Ed.: Back TG), Oxford University Press, Oxford, 1999, Chapter 5; [Google Scholar]; b) Singh FV, Wirth T in Organoselenium Chemistry–Synthesis and Reactions, (Ed.: Wirth T), Wiley-VCH, Weinheim, 2012, Chapter 8.2.12; [Google Scholar]; c) Nomoto A, Ogawa A in The Chemistry of Organic Selenium and Tellurium Compounds, Vol. 3, Part 1 (Ed.: Rappoport Z), J. Wiley and Sons, Chichester, 2012, Chapter 11. [Google Scholar]; d) Braga AL, Schwab RS, Rodrigues OED in Organoselenium Chemistry: Between Synthesis and Biochemistry, (Ed.: Santi C), Bentham, Sharjah, 2014, Chapter 8. [Google Scholar]; e) Lenardão EJ, Santi C, Sancineto L, New Frontiers in Organoselenium Compounds, Springer, Heidelberg, 2018, pp. 1–97. [Google Scholar]
- [2].For reviews of seleninic acids and related compounds as catalysts for oxidations, see:; a) Młochowski J, Brzaszcz M, Giurg M, Palus J, Wójtowicz H, Eur. J. Org. Chem 2003, 4329–4339; [Google Scholar]; b) Młochowski J, Wójtowicz-Młochowska H, Molecules 2015, 20, 10205–10243; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Back TG, Curr. Green Chem 2016, 3, 76–91; [Google Scholar]; (d) Freudendahl DM, Santoro S, Shahzad SA, Santi C, Wirth T, Angew. Chem. Int. Ed 2009, 48, 8409–8411; Angew. Chem. 2009, 121, 8559–8562. [DOI] [PubMed] [Google Scholar]
- [3].a) Grieco PA, Yokoyama Y, Gilman S, Nishizawa M, J. Org. Chem 1977, 42, 2034–2036; [Google Scholar]; b) Hori T, Sharpless KB, J. Org. Chem 1978, 43, 1689–1696; [Google Scholar]; c) Reich HJ, Chow F, Peake SL, Synthesis 1978, 299–301; [Google Scholar]; d) Kametani T, Nemoto H, Fukumoto K, Bioorg. Chem 1978, 7, 215–220; [Google Scholar]; e) Betzemeier B, Lhermitte F, Knochel PA, Synlett 1999, 489–491; [Google Scholar]; (f) ten Brink G-J, Fernandes BCM, van Vliet MCA, Arends IWCE, Sheldon RA, J. Chem. Soc., Perkin Trans 1 2001, 224–228. [Google Scholar]
- [4].a) Santi C, Di Lorenzo R, Tidei C, Bagnoli L, Wirth T, Tetrahedron 2012, 68, 10530–10535; [Google Scholar]; b) Yu L, Wang J, Chen T, Wang Y, Xu Q, Appl. Organomet. Chem 2014, 28, 652–656. [Google Scholar]
- [5].Grieco PA, Yokoyama Y, Gilman S, Ohfune Y, J. Chem. Soc. Chem. Commun 1977, 870–871. [Google Scholar]
- [6].In related work, the authors employed benzeneseleninic anhydride (2) as the catalyst and iodoxybenzene as the cooxidant: Barton DHR, Godfrey CRA, Morzycki JW, Motherwell WB, Ley SV, J. Chem. Soc., Perkin Trans 1 1982, 1947–1952. [Google Scholar]
- [7].Syper L, Młochowski J, Tetrahedron, 1987, 43, 207–213. [Google Scholar]
- [8].Ribaudo G, Bellanda M, Menegazzo I, Wolters LP, Bortoli M, Ferrer-Sueta G, Zagotto G, Orian L, Chem. Eur. J 2017, 23, 2405–2422. [DOI] [PubMed] [Google Scholar]
- [9].a) D. L. Klayman in Organic Selenium Compounds: Their Chemistry and Biology, (Eds.: Klayman DL, Günther WHH), Wiley, New York, 1973, Chapter 4; [Google Scholar]; b) Drabowicz J, Midura WH, Krasowska D in The Chemistry of Organic Selenium and Tellurium Compounds, Vol. 3, Part 2 (Ed.: Rappoport Z), J. Wiley and Sons, Chichester, 2012, Chapter 17; [Google Scholar]; c) Boese R, Haas A, Herkt S Pryka M, Chem. Ber 1995, 128, 423–428; [Google Scholar]; d) Abdo M, Knapp S, J. Am. Chem. Soc 2008, 130, 9234–9235; [DOI] [PubMed] [Google Scholar]; e) Satheeshkumar K, Raju S, Singh HB, Butcher RJ, Chem. Eur. J 2018, 24, 17513–17522. [DOI] [PubMed] [Google Scholar]
- [10].The 77Se NMR chemical shift of seleninic acid 1 was also reported as δ 1152 ppm in an aqueous buffer at pH 8.0: House KL, Dunlap RB, Odom JD, Wu ZP, Hilvert D, J. Am. Chem. Soc 1992, 114, 8573–8579. [Google Scholar]
- [11].Piotto M, Bourdonneau M, Elbayed K, Wieruszeski J-M, Lippens G, Magn. Reson. Chem. 2006, 44, 943–947. [DOI] [PubMed] [Google Scholar]
- [12].For a review of XAS of selenium compounds, see: Dolgova NV, Nehzati S, Choudhury S, MacDonald TC, Regnier NR, Crawford AM, Ponomarenko O, George GN, I. J. Pickering Biochim. Biophys. Acta 2018, 1862, 2383–2392. [DOI] [PubMed] [Google Scholar]
- [13].Deposition number 1958185 contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures Service (www.ccdc.cam.ac.uk/structures).
- [14].More than 100 years ago, Doughty reported the preparation of benzeneselenonic acid (4) by heating H2SeO4 in benzene in a sealed tube for 100 h at 110 °C. In 1964, Paetzold and Lienig repeated the experiment and used a Bunsen test to show that both Se(IV) and Se(VI) were present in the product. On that basis, they proposed the selenonium-selenonate salt 7 as a revised structure for Doughty’s product.; a) Doughty HW, Am. Chem. J 1909, 41, 326–337; [Google Scholar]; b) Paetzold R, Lienig D, Z. Chem 1964, 4, 186. [Google Scholar]
- [15].Ayrey G, Barnard D, Woodbridge DT, J. Chem. Soc 1962, 2089–2099. [Google Scholar]
- [16].The broadened shape of the singlet at 1024 ppm suggests that the peroxyselenonic acid 13 may be in rapid equilibrium with its hydrate, the selenurane 12.
- [17]. Additional information, including charge densities of 3 and 13, and pKa values of 1 and 4, is provided in the Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.