Dear Editor,
Mutations in the β-globin gene, the essential component of adult hemoglobin (HbA; α2β2), results in either a production of aberrant sickle hemoglobin (HbS) leading to sickle cell disease (SCD) or an insufficient β-globin synthesis leading to β-thalassemia. These two major forms of β-hemoglobinopathies cause impaired erythropoiesis and life-threatening anemia. Clinical evidence has suggested that re-activation of fetal γ-globin (HBG) gene expression which is normally silenced after birth by certain genetic mutations can ameliorate the clinical course of β-hemoglobinopathies.1,2 In β-thalassemia, elevated levels of fetal γ-globin interact with α-globin to form fetal hemoglobin (HbF; α2γ2) restoring the α/β-like globin ratio and in SCD the γ-globin reduces HbS polymerization. There are two major strategies for re-activation of HBG expression: reducing the expression of critical trans-acting repression factors (such as BCL11A)3,4 or deletion of inhibitory cis-regulatory elements in the HBG1/2 promoter region. To develop a practical strategy for clinical implementation, we leveraged electroporation of a Cas9:sgRNA RNP to edit the HBG1/2 promoter in hematopoietic stem/progenitor cells (HSPCs). We successfully achieved an average editing efficiency of 85% in β-thalassemia patient-derived HSPCs, leading to increased γ-globin mRNA expression (up to 126% relative to α-globin) and an improved terminal erythroid differentiation rate. Importantly, we discovered that the BCL11A binding site (TGACCA: −114 to −119), which is critical for the repression of γ-globin,5 is an ideal target for base editor hA3A-BE3 induced mutation and elevation of HBG expression.
To mimic the effect of the naturally occurring Δ13 bp allele in the HBG1 promoter (−102 to −114) which was identified in patients with hereditary persistence of fetal hemoglobin (HPFH), we synthesized two sgRNAs (with or without 2′-O-methyl 3′phosphorothioate modifications at three terminal nucleotides at both the 5′ and 3′ ends) (sgRNA1, sgRNA2) targeting the HBG promoter region (Fig. 1a). After electroporation of either RNP complex into immortalized erythroid precursor HUDEP-2 cells,4 we found that chemical modification6 of sgRNA enhanced editing efficiency and that sgRNA1 was more efficient than sgRNA2 (Supplementary information, Fig. S1a). The enhancement effect of chemical modification on sgRNAs was also confirmed in T cell receptor (TCR) and beta-2-microglobulin (B2M) loci in human primary T cells (Supplementary information, Fig. S1b). Hence, unless specified, we used the chemically modified sgRNA1 in the following experiments. After titration of the optimal RNP concentration in the HUDEP-2 cell line and in human CD34+ HSPCs, we successfully achieved editing efficiency over 80% in both cells (Supplementary information, Fig. S1c, d). After erythroid differentiation, the γ-globin mRNA level in the edited HUDEP-2 cells reached a mean of 22.7% relative to the total β-like globin mRNA, which showed a 4-fold increase compared with controls (mean 5.6%) (Supplementary information, Fig. S1e), suggesting this strategy is able to efficiently reactivate HBG expression.
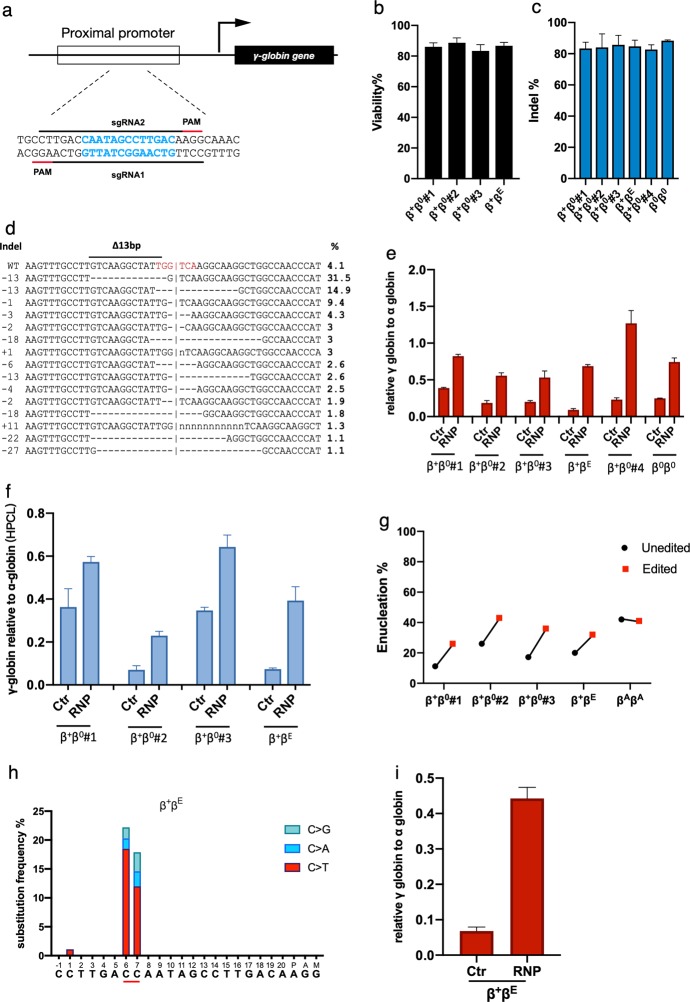
Fig. 1. Genome editing of HBG1/2 promoter reactivates the expression of γ-globin and ameliorates β-thalassemia.
a Schematic view of the targeted region in the HBG1/2 promoter. The 13-bp deletion in HPFH patient is labeled in blue. Spacers and protospacer adjacent motif (PAM) sequences are indicated with black and red lines, respectively. Black arrow indicates the direction of transcription. b Viability of the patient derived HSPCs 48 h after electroporation of the RNP complex. c Indel frequencies measured by TIDE analysis after RNP electroporation (data presented as mean ± SD, n = 3). d The most frequent indel patterns identified by TIDE analysis after sequencing the targeted site from the patient genomes. Frequency of each pattern is listed to the right. Red letters indicate the BCL11A-binding site. Δ13bp, 13-bp deletion site naturally occurred in HPFH patients. Horizontal dash lines indicate deletions and ‘n’ indicates substitutions. Vertical dash lines indicate the approximate cleavage site of Cas9. γ-globin level normalized to α-globin as measured by qPCR (e) or reverse HPLC (f) in patient HSPCs edited with Cas9/sg-HBG1 or Cas9 only (data presented as mean ± SD, n = 3). g The enucleation rate of edited or unedited patient HSPCs (data plotted as mean ± SD, n = 3). h C to D (A, T, or G) substitution frequencies induced by hA3A-BE3 in the targeted region in patient HSPCs. The −115C and −114C of the HBG promoter is underlined. i γ-globin level normalized to α-globin (qPCR) after targeting the HBG promoter by hA3A-BE3. β0β0 β-thalassemia major. β0β+ β-thalassemia intermedia. β+ βE β-thalassemia intermedia containing heterozygous E26K point on HBB gene. βA βA normal alleles.
Next, we used Cas9/sgRNA1 RNP to test this strategy in CD34+ HSPCs from a healthy donor. After RNP electroporation, the CD34+ cells were subjected to a three-phase erythroid differentiation regimen for further analysis. Tide analysis of the Sanger sequencing results suggested that the editing efficiency was ~74% (Supplementary information, Fig. S2a) and the majority (~44%) of mutant alleles were Δ13 bp which was identical to the desired HPFH deletion (−102 to −114) (Supplementary information, Fig. S2b). Importantly, almost all indel patterns carried at least 1 bp deletion within the BCL11A binding site (TGACCA; −114 to −200) (Supplementary information, Fig. S2b), suggesting that all mutant alleles would abolish or reduce BCL11A-mediated transcriptional repression.5,7 After differentiation, we found that the mRNA ratio of γ-globin to β-like globin increased by 7-fold (mean 46.7% vs 6.4%) in edited cells compared with the control (Supplementary information, Fig. S2c). It was further confirmed that the HbF protein level determined by HPLC analysis also increased in the edited group (mean 32.1%) compared to the control (mean 4.8%) (Supplementary information, Fig. S2d). Notably, clinical evidence showed that SCD patients with HbF level over 30% were almost asymptomatic, indicating a potential therapeutic effect of this strategy.2
To investigate whether editing of the HBG promoter would lead to clinically meaningful γ-globin induction, we electroporated the RNP into HSPCs derived from 6 β-thalassemia patients with different genotypes. Cell viability was over 85% at 48 h after electroporation, suggesting that the procedure was only marginally toxic (Fig. 1b). Tide analysis of the sequencing data demonstrated the disruption of the BCL11A binding motif and the procedure’s high editing efficiency in patient-derived HSPCs (mean 85%) (Fig. 1c, d), which was further confirmed by deep sequencing (see Supplementary Information). After differentiation, we detected a strong induction of γ-globin mRNA in each sample electroporated with the RNP compared with control groups electroporated with Cas9 only (range 53%–126% relative to α-globin in edited groups, compared with 15%–38% in unedited controls) (Fig. 1e). Promoter editing did not significantly affect β-globin mRNA expression in edited HSPCs (Supplementary information, Fig. S2e). To further confirm the increase of the γ-globin chain in the erythroid progeny, the level of single chain hemoglobin variants in differentiated cells was determined by reverse-phase HPLC. As shown in Fig. 1f, γ-globin chain was increased after gene editing in 4 tested samples (range 23%–64% in edited groups compared with 7%–34% in unedited controls). These data strongly suggest that the elevated γ-globin could form HbF tetramers to ameliorate the terminal erythroid maturation defects in β-thalassemia patients. Indeed, we observed elevated enucleation frequency, increased cell size, and more circular terminal erythroid cells in each of the edited patient samples (Fig. 1g; Supplementary information, Figs. S2f and S3a, b). Moreover, through deep-sequencing of the top 12 predicted off-target sites (web-based tool Cas-off Finder), no off-target editing events were detected (Supplementary information, Table S1).
Since two identical sgRNA target sites presented in the promoter of HBG1 and HBG2 gene respectively, we also considered the possibility that a 5.2 kb deletion of the intergenic DNA fragment could have been generated. Through a quantitative PCR (qPCR) assay based on two edited patient samples, we found that Cas9 editing caused a decrease of the HBG copy number by ~32% and ~39%, respectively (Supplementary information, Fig. S3c). Although the long-term clinical effect of the 5.2 kb deletion has not been determined, studies in the HUDEP-2 Δ-G γ cell line demonstrated that the deletion of the entire HBG2 and the intergenic region would not interfere with the induction of HBG1 expression.8 During the process of revising this manuscript, a study in monkeys with similar strategy suggested that the proportion of HSPCs carrying the intergenic deletion is significantly reduced in the population of cells observed after transplantation although the underlying mechanism remains unknown.9 These results suggested that targeting the HBG1/2 promoter by Cas9:sgRNA RNP electroporation is a promising gene therapy strategy toward β-thalassemia for clinical translation.
Base editing (BE) is a promising genome editing technology which efficiently catalyzes base conversion without inducing double-strand DNA breaks (DSBs) and is thus considered to be preferable to using Cas9. To test whether base editing can reactivate HBG and reduce the Cas9-induced deletion mentioned above, RNP electroporation of CBE/sgRNA strategy was utilized. A recent study demonstrated that single nucleotide substitution at −115C or −114C was enough to disrupt the BCL11A binding and increase HBG expression.5 The hAPOBEC3A-Cas9n (hA3A-BE3) recombinant protein and sgRNA2 (targeting the −115C and −114C in HBG promoter, Supplementary information, Fig. S3d) were electroporated into CD34+ cells of healthy or patient donor. Deep sequencing result showed that the overall C-T mutation frequency of both sites was around 12%–18%, and the C>D (D equals to A, T, G) frequency was about 22% in both donor cells (Fig. 1h and Supplementary information, Fig. S3e). We observed a low indel frequency (<2%) induced by hA3A-BE3 (Supplementary information, Table S2). Importantly, the HBG copy number was not affected compared with controls (Supplementary information, Fig. S3c). Interestingly, the γ-globin level increased significantly from ~13.7% to ~58.1% in the healthy HSPCs (Supplementary information, Fig. S3f) or from ~6.8% to ~44.2% in the patient-derived CD34+ cells (Fig. 1i) after differentiation, reaching a level that has potential clinical benefits for patients with β-hemoglobinopathies. Next we investigated sgRNA-dependent off-target event by deep sequencing of the top 18 off-target sites. No significant off-target event was detected (Supplementary information, Table S3). Since BEs generate fewer double-strand DNA breaks, this strategy would induce fewer DNA damage responses and has the potential to edit multiple targets for precision therapy.
Recently, a proof-of-concept study by Traxler et al. also demonstrated dramatic reactivation of γ-globin via CRISPR/Cas9-mediated disruption of HBG promoter in SCD patient-derived HSPCs.10 The RNP electroporation strategy we utilized may have advantages over the lentiviral-mediated delivery system they used, due to its high editing efficiency and the fact that no additional DNA component is introduced into the cells.3 Moreover, RNP has a much shorter half-life,11 which would significantly reduce Cas9- or CBE-induced off-target events at both the DNA and RNA level. A recent study has also shown that edited HSPCs are able to reconstitute the hematopoietic system in human, further suggesting the delivery system is suitable for clinical application.12 In conclusion, we demonstrated highly efficient HBG promoter editing and γ-globin re-activation in β-thalassemia patient-derived HSPCs via Cas9:sgRNA and hA3A-BE3:sgRNA RNP electroporation, suggesting a clinically applicable selection-free approach for the therapy of β-hemoglobinopathies. Theoretically, editing of the HBG1/2 promoter will not influence other genes and will have advantages in safety especially in long-term adult stem cells.
Supplementary information
Acknowledgements
We thank Dr Stefan Siwko for proofreading of this manuscript and the support of ECNU Public Platform for innovation (011). We thank Dr Merlin Crossley for his advice on the editing of the manuscript. This work was partially supported by grants from the National Key R&D Program of China (2019YFA0110802, 2019YFA0802800), Major Science and Technology Program of Hainan Province (ZDKJ2017007), the National Natural Science Foundation of China (81670470, 81873685), grants from the Shanghai Municipal Commission for Science and Technology (18411953500) and the Innovation program of Shanghai Municipal Education Commission (2019-01-07-00-05-E00054), and the Fundamental Research Funds for the Central Universities.
Author contributions
D.L., L.W., Y.L., M.L., designed the experiments, analyzed the data and wrote the manuscript; L.W.,Y.M., H.H., L.L, Q.L., performed the experiments and analyzed the data; Y.Y., B.F., maintained HSPCs and HUDEP-2. W.L., S.Y., purified recombinant proteins. S.Y., helped to perform the HPLC assay. R.K. and Y.N. provided the HUDEP-2 cell and analyzed the data.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Liren Wang, Linxi Li, Yanlin Ma, Handan Hu
Contributor Information
Mingyao Liu, Email: myliu@bio.ecnu.edu.cn.
Yongrong Lai, Email: laiyongrong@hotmail.com.
Dali Li, Email: dlli@bio.ecnu.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41422-019-0267-z.
References
- 1.Liu D, et al. Blood. 2014;124:803–811. doi: 10.1182/blood-2014-03-561779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lettre G, Bauer DE. Lancet. 2016;387:2554–2564. doi: 10.1016/S0140-6736(15)01341-0. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, et al. Nat. Med. 2019;25:776–783. doi: 10.1038/s41591-019-0401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canver MC, et al. Nature. 2015;527:192–197. doi: 10.1038/nature15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martyn GE, et al. Nat. Genet. 2018;50:498–503. doi: 10.1038/s41588-018-0085-0. [DOI] [PubMed] [Google Scholar]
- 6.Hendel A, et al. Nat. Biotechnol. 2015;33:985–989. doi: 10.1038/nbt.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martyn GE, et al. Blood. 2019;133:852–856. doi: 10.1182/blood-2018-07-863951. [DOI] [PubMed] [Google Scholar]
- 8.Wienert B, et al. Blood. 2017;130:803–807. doi: 10.1182/blood-2017-02-767400. [DOI] [PubMed] [Google Scholar]
- 9.Humbert O, et al. Sci. Transl. Med. 2019;11:eaaw3768. doi: 10.1126/scitranslmed.aaw3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traxler EA, et al. Nat. Med. 2016;22:987–990. doi: 10.1038/nm.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Kim D, Cho SW, Kim J, Kim JS. Genome Res. 2014;24:1012–1019. doi: 10.1101/gr.171322.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L, et al. N. Engl. J. Med. 2019;381:1240–1247. doi: 10.1056/NEJMoa1817426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.