Abstract
Clear corneal incision (CCI) is a commonly used surgical approach in cataract surgery. In this prospective study, we evaluated the effect of CCI site on surgically induced astigmatism (SIA) and other postoperative astigmatic changes. CCIs were constructed based on the steep meridian of the total corneal refractive power in the 4.0-mm-zone (TCRP4.0), and patients were divided into four groups: temporal, superotemporal, superonasal, and superior according to the site of the incision. TCRP4.0 analysis demonstrated a statistically significant reduction of astigmatism with superior incisions (P < 0.001), and the combined mean polar values for SIA changed significantly in the temporal (Hotelling T2 = 1.977), superotemporal (Hotelling T2 = 0.544), superonsal (Hotelling T2 = 1.066), and superior incision groups (Hotelling T2 = 1.134) (all P < 0.001). The posterior axis alignment should be considered in cataract surgery with CCI, and the SIA is affected by axis rotation, and incision orientation.
Subject terms: Corneal diseases, Lens diseases, Refractive errors
Introduction
For an increasing number of younger patients diagnosed with cataract or presbyopia, refractive surgery is becoming an option to correct refractive errors. It not only improves visual acuity, but also vision quality. Since 1990, sutureless clear corneal incisions (CCI) have become the standard option in cataract surgery to minimize postoperative astigmatism and promote rapid visual rehabilitation1,2. However, corneal surgically-induced astigmatism (SIA) still occurs, and studies have reported that the severity of SIA depends on the length, width, and site of the incision3–6.
Today, precise estimation of corneal power and astigmatism is critical for anterior segment refractive surgery. Until now, corneal power and astigmatism have been measured using only the anterior corneal curvature, which is measured by methods such as manual keratometry, auto-keratometry, and simulated keratometry (Sim-K). All of these neglect the contribution of posterior corneal astigmatism. Therefore, only a few studies have described posterior corneal shape changes after cataract surgery6–10. The Pentacam is a diagnostic tool that uses a slit illumination system and works according to the Scheimpflug principle; it can directly measure both anterior and posterior corneal curvatures11.
Shajari et al.12 demonstrated that the parameters for differentiating keratoconus from healthy eyes included pachymetry, corneal curvature, and anterior and posterior corneal axis alignments. To date, the most accurate method for determining corneal astigmatism axis alignment remains unclear. However, posterior axis alignment is most likely vertical. Mean posterior corneal astigmatism values ranging from −0.26 to −0.78 D have been reported12–16. Despite numerous studies, there is still significant confusion regarding the best method to evaluate SIA of the cornea17–19. Abulafia et al. recently provided a new definition for SIA of the cornea (SIACornea)20.
The aim of the current prospective study was to determine the effects of the site of CCI on the total corneal refractive power (TCRP) at 4.0-mm zone (TCRP4.0) steep axis after cataract surgery.
Results
In post-hoc comparison analysis of deviation of means between the groups, SIACornea differed significantly with age (P = 0.001). There were no statistically significant differences in any preoperative demographic data between the four groups (Table 1).
Table 1.
Preoperative demographic data.
| Parameter | Incision Group | P value | |||
|---|---|---|---|---|---|
| Temporal | Superotemporal | Superonasal | Superior | ||
| Eyes (n) | 49 | 49 | 61 | 53 | — |
| Male/Female (n) | 8/38 | 12/36 | 22/34 | 15/33 | — |
| Mean age (y) ± SD | 70.14 ± 9.15 | 65.14 ± 12.17 | 67.49 ± 11.47 | 61.40 ± 11.18 | 0.001 |
| TCRP4.0 Kmean (D) | 43.42 ± 4.57 | 44.05 ± 2.04 | 44.04 ± 1.38 | 44.06 ± 1.49 | 0.687 |
| TCRP3.0 Kmean (D) | 43.73 ± 1.21 | 44.32 ± 2.09 | 44.23 ± 1.21 | 44.33 ± 1.41 | 0.614 |
| Sim-KAnt. Kmean (D) | 43.00 ± 4.56 | 43.63 ± 2.20 | 43.53 ± 1.16 | 42.94 ± 5.02 | 0.310 |
| SIACornea (D) | 0.27 ± 0.19 | 0.34 ± 0.35 | 0.38 ± 0.35 | 0.40 ± 0.37 | 0.310 |
| IOL Power (D) | 19.39 ± 4.38 | 19.32 ± 4.36 | 19.56 ± 3.88 | 18.58 ± 4.14 | 0.623 |
| ECD (mm2) | 2679.71 ± 283.60 | 2728.06 ± 280.74 | 2664.41 ± 315.80 | 2696.42 ± 273.07 | 0.705 |
y = year; SD = standard deviation; TCRP4 = total corneal refractive power at 4.0-mm-zone; TCRP3 = total corneal refractive power at 3.0-mm-zone; Sim-K = simulated karatometry; Ant. = anterior corneal curvature; Kmean = average of keratometry; D = diopters; IOL = intraocular lens; ECD = endothelium cell density.
There were statistically significant changes in TCRP4.0 polar values in each group (all P < 0.001; Hotelling T2 = 1.977 in the temporal; Hotelling T2 = 0.544 in the superotemporal; Hotelling T2 = 1.066 in the superonasal; Hotelling T2 = 1.134 in the superior) (Table 2). TCRP3.0 polar values were statistically significant changed in each group (all P < 0.001; Hotelling T2 = 1.777 in the temporal; Hotelling T2 = 0.698 in the superotemporal; Hotelling T2 = 1.203 in the superonasal; Hotelling T2 = 1.004 in the superior) (Table 3). However, Sim-KAnt. polar values were also statistically significant changed in all incision groups (all P < 0.001; Hotelling T2 = 1.713 in the temporal; Hotelling T2 = 0.680 in the superotemporal; Hotelling T2 = 1.464 in the superonasal; Hotelling T2 = 1.660 in the superior). In univariate analysis the AKP(+0) decreased significantly in all incision groups, and AKP(+45)—which is a representation of torsional force twisting in the astigmatic direction—was not significant after 2 months (Table 4).
Table 2.
Polar value analysis of TCRP4.0.
| Group | Mean Keratometry (D) ± SD | P Valuea | P Valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative | Change | |||||||
| AKP(+0) | AKP(+45) | AKP(+0) | AKP(+45) | △AKP(+0) | △AKP(+45) | △AKP(+0) | △AKP(+45) | ||
| Temporal | 0.87 ± 0.45 | 0 | −0.09 ± 0.62 | −0.04 ± 0.64 | −0.96 ± 0.76 | −0.04 ± 0.64 | <0.001 | <0.001 | 0.629 |
| Superotemporal | 0.99 ± 1.02 | 0 | −0.07 ± 1.05 | −0.02 ± 0.74 | −1.06 ± 1.79 | −0.02 ± 0.74 | <0.001 | <0.001 | 0.887 |
| Superonasal | 0.90 ± 0.62 | 0 | 0.16 ± 0.81 | −0.10 ± 0.67 | −0.73 ± 0.96 | −0.10 ± 0.67 | <0.001 | <0.001 | 0.234 |
| Superior | 1.12 ± 0.75 | 0 | 0.24 ± 0.88 | −0.03 ± 0.74 | −0.87 ± 1.05 | −0.03 ± 0.74 | <0.001 | <0.001 | 0.774 |
D = diopters; SD = standard deviation; △ = change; AKP = astigmatic polar value.
aHotelling trace between preoperative and postoperative keratometry.
bUnivariate analysis for △AKP(+0) and △AKP(+45).
Table 3.
Polar value analysis of TCRP3.0.
| Group | Mean Keratometry (D) ± SD | P Valuea | P Valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative | Change | |||||||
| AKP(+0) | AKP(+45) | AKP(+0) | AKP(+45) | △AKP(+0) | △AKP(+45) | △AKP(+0) | △AKP(+45) | ||
| Temporal | 0.88 ± 0.48 | 0 | −0.50 ± 0.57 | 0.04 ± 0.73 | −1.37 ± 0.79 | 0.04 ± 0.73 | <0.001 | <0.001 | 0.697 |
| Superotemporal | 0.73 ± 0.63 | 0 | −0.29 ± 0.58 | −0.04 ± 0.70 | −1.02 ± 1.01 | −0.04 ± 0.70 | <0.001 | <0.001 | 0.671 |
| Superonasal | 0.80 ± 0.53 | 0 | −0.36 ± 0.69 | 0.06 ± 0.77 | −1.16 ± 0.87 | 0.06 ± 0.77 | <0.001 | <0.001 | 0.553 |
| Superior | 0.89 ± 0.63 | 0 | −0.31 ± 0.69 | −0.08 ± 0.72 | −1.20 ± 1.10 | −0.08 ± 0.72 | <0.001 | <0.001 | 0.449 |
D = diopters; SD = standard deviation; △ = change; AKP = astigmatic polar value.
aHotelling trace between preoperative and postoperative keratometry.
bUnivariate analysis for △AKP(+0) and △AKP(+45).
Table 4.
Polar value analysis of Sim-KAnt..
| Group | Mean Keratometry (D) ± SD | P Valuea | P Valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative | Change | |||||||
| AKP(+0) | AKP(+45) | AKP(+0) | AKP(+45) | △AKP(+0) | △AKP(+45) | △AKP(+0) | △AKP(+45) | ||
| Temporal | 0.76 ± 0.41 | 0 | −0.04 ± 0.47 | −0.03 ± 0.57 | −0.80 ± 0.61 | −0.03 ± 0.57 | <0.001 | <0.001 | 0.698 |
| Superotemporal | 0.94 ± 0.83 | 0 | 0.04 ± 0.59 | 0.10 ± 0.83 | −0.90 ± 1.05 | 0.10 ± 0.83 | <0.001 | <0.001 | 0.382 |
| Superonasal | 0.81 ± 0.48 | 0 | 0.05 ± 0.63 | 0.03 ± 0.66 | −0.76 ± 0.79 | 0.03 ± 0.66 | <0.001 | <0.001 | 0.697 |
| Superior | 1.10 ± 0.62 | 0 | −0.01 ± 0.95 | −0.13 ± 0.69 | −1.11 ± 1.08 | −0.13 ± 0.69 | <0.001 | <0.001 | 0.159 |
D = diopters; SD = standard deviation; △ = change; AKP = astigmatic polar value.
aHotelling trace between preoperative and postoperative keratometry.
bUnivariate analysis for △AKP(+0) and △AKP(+45).
The changes of keratometric astigmatism in TCRP4.0, TCRP3.0, and Sim-KAnt. were shown in Table 5. There was statistically significant reduction of astigmatism in TCRP4.0 on the site of superior direction (P < 0.001), and significant reduction of astigmatism in Sim-KAnt. on the site of superotemporal, superonasal, and superior directions. There were no statistically significant differences in Kmean between preoperative and postoperative in TCRP4.0, TCRP3.0, and Sim-KAnt. (all P > 0.05, Table 6).
Table 5.
Preoperative and postoperative keratometric astigmatism changes.
| Parameter | Incision Group | P Value* | |||
|---|---|---|---|---|---|
| Temporal | Superotemporal | Superonasal | Superior | ||
| TCRP4.0 | |||||
| Pre-op Ast. (D) | 0.87 ± 0.45 | 0.99 ± 1.02 | 0.90 ± 0.62 | 1.12 ± 0.75 | 0.177 |
| Post-op Ast. (D) | 0.79 ± 0.47 | 0.94 ± 1.00 | 0.89 ± 0.59 | 0.86 ± 0.73 | 0.145 |
| P value | 0.055 | 0.332 | 0.621 | <0.001 | |
| TCRP3.0 | |||||
| Pre-op Ast. (D) | 0.88 ± 0.48 | 0.73 ± 0.63 | 0.80 ± 0.53 | 0.89 ± 0.63 | 0.265 |
| Post-op Ast. (D) | 0.79 ± 0.49 | 0.74 ± 0.48 | 0.91 ± 0.57 | 0.80 ± 0.77 | 0.871 |
| P value | 0.281 | 0.801 | 0.212 | 0.110 | |
| Sim-KAnt. | |||||
| Pre-op Ast. (D) | 0.76 ± 0.41 | 0.94 ± 0.83 | 0.81 ± 0.48 | 1.10 ± 0.62 | 0.004 |
| Post-op Ast. (D) | 0.60 ± 0.41 | 0.79 ± 0.68 | 0.71 ± 0.48 | 0.92 ± 0.71 | 0.073 |
| P value | 0.095 | 0.004 | 0.042 | <0.001 | |
TCRP4.0 = total corneal refractive power at 4.0-mm-zone; TCRP3.0 = total corneal refractive power at 3.0-mm-zone; Pre-op Ast. = Preoperative astigmatism; Post-op Ast. = Postoperative astigmatism; D = diopters; Sim-K = simulated keratometry; Ant. = anterior corneal curvature.
Table 6.
Preoperative and postoperative Kmean changes in TCRP4.0, TCRP3.0, Sim-KAnt., and Sim-KPost..
| Parameter | Incision Group | P Value | |||
|---|---|---|---|---|---|
| Temporal | Superotemporal | Superonasal | Superior | ||
| TCRP4.0 | |||||
| Pre-op Kmean (D) | 43.63 ± 1.42 | 44.05 ± 2.04 | 44.04 ± 1.38 | 44.06 ± 1.49 | 0.472 |
| Post-op Kmean (D) | 43.63 ± 1.40 | 43.99 ± 2.08 | 44.07 ± 1.38 | 44.03 ± 1.44 | 0.511 |
| TCRP3.0 | |||||
| Pre-op Kmean (D) | 43.21 ± 1.43 | 43.63 ± 2.20 | 43.53 ± 1.16 | 43.64 ± 1.45 | 0.482 |
| Post-op Kmean (D) | 43.10 ± 1.36 | 43.59 ± 2.29 | 43.52 ± 1.25 | 43.62 ± 1.39 | 0.339 |
| Sim-KAnt. | |||||
| Pre-op Kmean (D) | 43.99 ± 1.33 | 44.32 ± 2.09 | 44.23 ± 1.21 | 44.33 ± 1.41 | 0.663 |
| Post-op Kmean (D) | 43.93 ± 1.33 | 44.31 ± 2.22 | 44.22 ± 1.25 | 44.33 ± 1.36 | 0.549 |
| Sim-KPost. | |||||
| Pre-op Kmean (D) | −6.41 ± 0.23 | −6.46 ± 0.43 | −6.42 ± 0.21 | −6.41 ± 0.24 | 0.746 |
| Post-op Kmean (D) | −6.43 ± 0.24 | −6.49 ± 0.44 | −6.43 ± 0.24 | −6.44 ± 0.25 | 0.730 |
TCRP4.0 = total corneal refractive power at 4.0-mm-zone; TCRP3.0 = total corneal refractive power at 3.0-mm-zone; Sim-K = simulated keratometry; Ant. = Anterior corneal curvature; Post. = Posterior corneal curvature; D = diopters; Pre-op Kmean = preoperative average of keratometry; Post-op Kmean = postoperative average of keratometry.
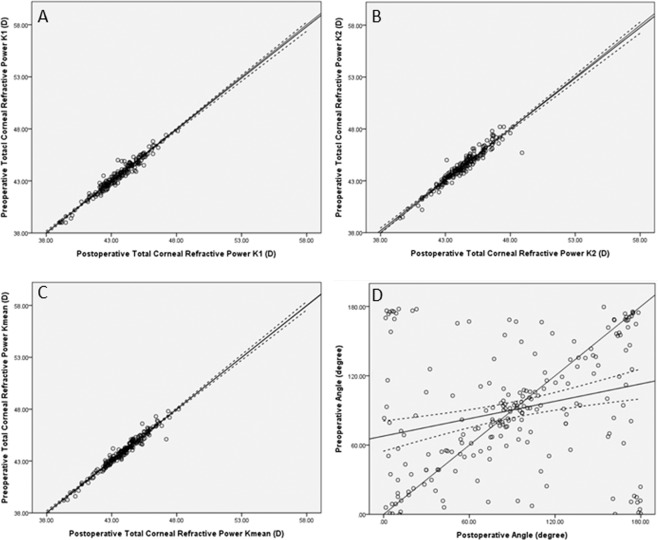
Overall, 95.8% of the cases were within the 95% confidence intervals of different values of flattest keratometry (K1), with R-square (R2) = 0.95, adjusted R2 = 0.95, and standard error = 0.34 D. For steepest keratometry (K2), 97.6% of the cases were within the 95% confidence intervals of different values of K2, with R2 = 0.93, adjusted R2 = 0.93, and standard error = 0.44 D. For the average of keratometry (Kmean), 95.3% of the cases were within the 95% confidence intervals of different values of Kmean, with R2 = 0.96, adjusted R2 = 0.96 and standard error = 0.30 D. For the angle of astigmatism, 91.5% of the cases were within the 95% confidence intervals of different values, with R2 = 0.05, adjusted R2 = 0.05, and standard error = 50.6 degrees (Fig. 1).
Figure 1.
Equivalency plot of the preoperative keratometry value and angle of astigmatism on the y-axis versus the postoperative keratometry value and angle of astigmatism on the x-axis: (A). K1 (flattest keratometry); (B). K2 (steepest keratometry); (C). Kmean (average of keratometry); and (D). Angle of axis.
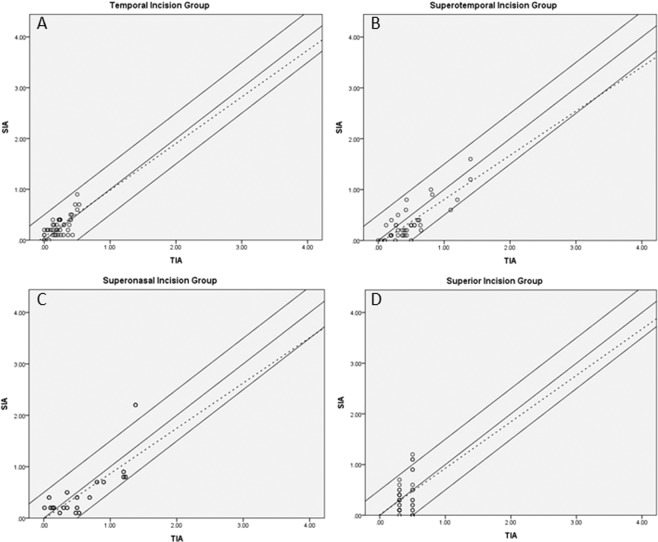
The difference of correction index between the magnitude of Predicted SIACornea and SIACornea was less than 0.50 D in 43 of 49 eyes (87.8%) in the temporal group, 39 of 49 eyes (79.6%) in the superotemporal group, 46 of 61 eyes (75.4%) in the superonasal group, and 40 of 53 eyes (75.5%) in the superior group (Fig. 2).
Figure 2.
Effect of TCRP4.0 (total corneal refractive power at 4 mm) steep meridian incision. Individual corneal astigmatism correction (Alpins) in: (A). Temporal group; (B). Superotemporal group; (C). Superonasal group; and (D). Superior group.
Discussion
Multiple studies have investigated the effects of posterior surface astigmatism and curvature in refractive surgery, especially in cases of keratoconus. Implantation of premium IOLs, particularly multifocal and toric IOLs in cataract surgery, requires reliable prediction of the refractive results. Given the controllable nature of postoperative corneal astigmatism and visual quality after operations21–25, the present study aimed to analyze the effects of the site of CCI on TCRP4.0 steep meridians in cataractous eyes.
Keratometry has traditionally been the favored method for evaluating astigmatic changes; however, it cannot be used to evaluate the peripheral cornea or irregularities of the central cornea26,27. In order to conduct a thorough, detailed, and objective evaluation of corneal tomography and topography, computer-assisted video keratography (CVK) is widely used in optometry and ophthalmology5. The Pentacam biometric device provides precise values for corneal power and topometric indices, both in normal and in keratoconic eyes28. In the current study, Scheimpflug tomography was used to analyze anterior and posterior curvatures based on a ray tracing technique combined with a rotating Scheimpflug camera.
According to a previous study, the percentage of eyes with vertical alignment of the steep meridian decreased with increasing age (from 64% in patients aged 60–69 years to 48.1% in those aged 70–79 years), and the percentage of patients with horizontal alignment increased from 17.5% to 31.8% for the anterior corneal surfaces; however, almost cases of eyes in vertical alignment on the posterior corneal surface11. In our study, patients in the 60–69 years age group were more likely to undergo superior incision, while patients in the 70–79 years age group were more likely to undergo temporal incision. In principle, when the steep meridian on the anterior surface is aligned vertically, it creates with-the-rule (WTR) astigmatism; in contrast, vertical alignment on the posterior surface creates against-the-rule (ATR) astigmatism, which has negative power and partially compensates for anterior astigmatism.
In 2012, Rho et al.4 reported that steep meridian incision in CCI surgery was associated with significantly reduced keratometric astigmatism at the temporal, superotemporal, and superior locations. Astigmatic polar value of net astigmatism (AKP)19 analysis was used in that study; the changes in AKP(+0) were −0.26 ± 0.53 D in a temporal group, −0.39 ± 0.45 D in a superotemporal group, and −0.46 ± 0.63 D in a superior group. Moreover, there were significant decreases in astigmatism values in AKP(+0), but no significant changes in AKP(+45). In the analysis of the effects of CCI in each steep meridian axis group in the current study, superior incisions were more effective than the others (P < 0.001). In the present study, the changes in AKP(+0) were −0.96 ± 0.76 D in the temporal group, −1.06 ± 1.79 D in the superotemporal group, −0.73 ± 0.96 D in the superonasal group, and −0.87 ± 1.05 D in the superior group, and all of these changes were statistically significant (TCRP4.0). Moreover, the changes of TCRP3.0 in AKP(+0) were −1.37 ± 0.79 D, −1.02 ± 1.01 D, −1.16 ± 0.87 D, and −1.20 ± 1.10 D, respectively; the changes of Sim-KAnt. in AKP(+0) were −0.80 ± 0.61 D, −0.90 ± 1.05 D, −0.76 ± 0.79 D, and −1.11 ± 1.08 D, respectively. Notably, however, torsional force twisting in the astigmatic direction, represented by AKP(+45) values was not significant after 2 months. These results were similar to the results of previously reported studies involving similar groups4, but the changes in SIACornea AKP(+0) polar values were greater in our study. Moreover, analysis the reduction of astigmatism, there were statistically significant in Sim-KAnt. on the incision site of superotemporal, superonasal, and superior directions, and significant reduction of astigmatism in TCRP4.0 was investigated on the incision site of superior direction (all P < 0.05).
In the present study, the Alpins method29 was used to investigate the effect of each incision site on SIACornea. Scatterplots of the astigmatic correction, SIACornea/Predicted SIACornea, depicted a greater bias toward unity and zero in each group. The values were widely and more evenly distributed, suggesting a lower potential for intended induced astigmatism. With regard to corneal anatomy, the distance from the cornea to the limbus is shorter in the horizontal direction than the vertical direction30. Moreover, as the strength of corneal fibers is much stronger for short distances from the central cornea, it is reasonable to suggest that there are changes in the morphology of the cornea and astigmatism after surgery31–33. Hence, the SIACornea is different depending on the location of CCI. In current study, we investigated the mean and standard deviation values of SIACornea in each location of CCI were 0.27 ± 0.19 D, 0.34 ± 0.35 D, 0.38 ± 0.35 D, and 0.40 ± 0.37 D, respectively (P = 0.310). Slade et al.34 reported that the image-guided surgical planning system was effective for accurate correction of preexisting astigmatism in cataract surgery, especially when combined with femtosecond laser-assisted cataract surgery. Byun et al.35 studied similar groups and suggested that anterior keratometry does not accurately reflect total corneal astigmatism. They also reported that the correction index was higher with ATR astigmatism than with WTR astigmatism. Accordingly, the refractive cylinder was higher than the keratometric astigmatism in the ATR group and the oblique group, and lower in eyes with WTR astigmatism. However, the similar correction index was investigated in each group in the present study, and superior outcomes were found in the ATR and WTR groups.
Alió et al.36 reported that SIA was influenced by the size and location of the incision, and Denoyer et al.37 proposed that corneal biomechanical properties should be taken into account when making the incision in refractive surgery. In the current study, SIACornea was influenced by the steep meridian axis location, irrespective of the incision width. Furthermore, there was a statistically significant difference between preoperative and postoperative Kmean (TCRP4.0) values (a difference of 0.16 ± 0.30 D), but there were no statistically significant differences in TCRP3.0 and Sim-KAnt. (a difference of 0.02 ± 0.32 D and 0.04 ± 0.49 D, respectively).
As recommended by Alpins et al.38, the flattening effect at the incision meridian should be used for toric IOL calculation. In a previous study, Ferreira et al.39 compared flattening effects after CCI in a femtosecond laser group and a manual group, which involves making the side port at 90 to 110 degrees from the main incision. They reported flattening effects of −0.11 D in the femtosecond laser group and −0.13 D in the manual group, and additionally, with regard to superior oblique incisions, the values were −0.21 D in the femtosecond laser group and −0.34 D in the manual group. Interestingly, in the present study, the flattening effects were −0.04 ± 0.26 D in the temporal group, −0.06 ± 0.27 D in the superotemporal group, 0.03 ± 0.38 D in the superonasal group, and −0.03 ± 0.33 D in the superior group.
Park and Kim8 recently reported the effects of CCI on the TCRP3.0 steep meridian axis. The procedure they utilized involved the use of a 1-mm side port, 70 degrees to the left of the main incision, including angles of incisions that were 40 degrees larger than those in the groups in the current study, at each location. According to their results, total corneal astigmatism (TCA – 3 mm apex/zone data from the Scheimpflug) was associated with significant changes in AKP(+0) and AKP(+45) 2 months postoperatively. The AKP(+0) means and standard deviations were −0.22 ± 0.20 D in the WTR microcoaxial group and −0.20 ± 0.21 D in the ATR microcoaxial group, and the respective AKP(+45) values were 0.12 ± 0.10 D and 0.11 ± 0.10 D. However, there were no statistically significant changes in keratometric astigmatism. Nevertheless, their study indicated that steep meridian incisions may have a significant torsional effect and that TCA is an effective tool for analysis of corneal astigmatic changes after cataract surgery. However, in our study, the postoperative AKP(+0) of Sim-KAnt. were −0.04 ± 0.47 D, 0.04 ± 0.59 D, 0.05 ± 0.63 D, and −0.01 ± 0.95 D, respectively, and it was much lower than the results of the previous study.
The present study has several limitations. It has been reported that incision width and corneal hysteresis influence SIACornea37. Dry eye syndrome remains one of the postoperative symptoms experienced by some patients40–42, and is associated with ocular soreness, pain, and a burning sensation, among other things. In an analysis reported by Kasetsuwan et al.40, only 9.8% of eyes had dry eye symptoms after 3 months following cataract surgery; in the present study, patients with severe dry eyes were excluded. In addition, only Pentacam measurements labeled with the quality specification “OK” were accepted as valid in the current study. These factors could not affect the postoperative evaluation of SIACornea. In the context of study limitations, notably optimized corneal incision sites would improve ocular aberration results25–27. Further studies are required for comparative evaluation of their effects on SIACornea and corneal higher-order aberrations (HOAs).
In conclusion, unsutured CCI on the TCRP4.0 steep meridian is effective in reducing SIACornea changes, and has a slight flattening effect after surgery. The VERION Image-Guided System is effective in guiding the surgeon to achieve accurate CCI during cataract surgery. The current study is potentially meaningful in the context of clinical research because all phacoemulsification procedures were performed by one experienced surgeon. The above-described surgical option may be used more frequently in cataract surgery.
Methods
Patients
This prospective study included 212 eyes from 200 patients who underwent phacoemulsification. A single, experienced surgeon (C. K. J.) performed all the procedures at the Seoul St. Mary’s Hospital Eye Center between January 2016 and August 2017. Informed consent was obtained from all of the patients before the commencement of the study. The protocol adhered to the tenets of the Declaration of Helsinki, and St. Mary’s Hospital’s institutional ethics committee approved the study.
All patients underwent a routine comprehensive ophthalmic examination preoperatively. Refractive power and corneal curvature were measured using an auto-keratometer (KR-1, Topcon Medical Systems Inc, New Jersey, USA). Ophthalmoscopy was performed to evaluate the fundus under a dilated pupil. Cataract nuclear density was graded according to the Lens Opacities Classification System III43. TCRP, Sim-K and its steep meridian were measured using the Scheimpflug analysis system, Pentacam HR (Oculus Optikgeräte GmbH, Wetzlar, Germany). To ensure sufficient quality, only measurements assigned the quality specification “OK” were accepted as valid. The site of the CCI just in the quadrant of the steep meridian was determined according to patients’ TCRP4.0 steep meridian axes.
The inclusion criteria were a diagnosis of cataract with regular corneal astigmatism ranging from 0.50–3.50 D (as determined via an auto-keratometer), a postoperative follow-up period of at least 2 months, a corneal endothelial cell count >2000 per mm2, and no viral or bacterial infection. Exclusion criteria were a history of previous ocular surgery, surgical complications, or any corneal or macular pathology, and poor fixation. Cases involving severe dry eye were also excluded.
Surgical technique
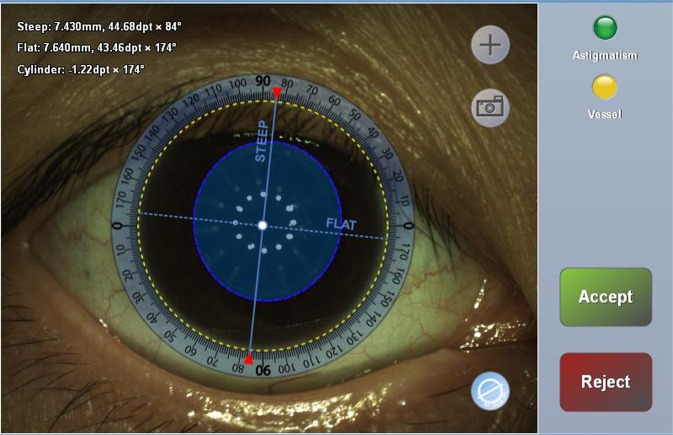
All phacoemulsification procedures were performed under topical anesthesia (proparacaine 0.5%). The VERION Image-Guided System (Alcon Laboratories Inc., Fort Worth, Texas, USA) was used to determine the steep meridian axis orientation and digital markers for the microscopy (Fig. 3). A 2.2-mm, single-step CCI was made in the pre-limbal region of the steep meridian using a dual-beveled, angled, slit knife (Alcon Laboratories Inc.), and a side port incision (full-thickness puncture) was made using a 1.0-mm knife, 60 degrees left of the main incision site. Lastly, the CCI size was enlarged from 2.3 mm to 3.2 mm for correcting corneal astigmatism and intraocular lens (IOL) insertion. The corneal wounds were left unsutured (sutureless). Preoperative and 2-month postoperative keratometric data were compared. Polar value analysis was used to assess SIACornea. Patients were divided into four groups based on the incision site: temporal, superotemporal, superonasal (opposite site of incision: inferiortemporal), and superior4.
Figure 3.
The VERION Image-Guided System was used to determine the steep meridian axis orientation and digital markers for the microscopy.
Methods of calculation
The formula for calculating SIACornea19 is the same as that used for calculating prediction error by Holladay et al.44. In general, the goal of refractive surgery is to neutralize the refractive error such that the final postoperative refraction is plano. When this is achieved, the Predicted SIACornea and the actual SIACornea are equal. When the actual SIACornea does not match the Predicted SIACornea, a postoperative refractive error is induced. In cataract surgery with CCI, the intended outcome is postoperative astigmatism of 0.50 D. The differences between preoperative and postoperative polar values were calculated and compared. For example, the preoperative and postoperative astigmatic polar values (AKP) for the preoperative net cylinder A@a and the postoperative net cylinder B@b were defined as follows19:
Statistical analysis
Descriptive and theoretical analyses were performed using SPSS for Windows, version 18.0 (SPSS Inc, Chicago IL, USA). Data normality was confirmed using the Shapiro-Wilk test. The Wilcoxon Rank-Sum test was used for nonparametric analyses. Comparative evaluation of preoperative and 2-month postoperative keratometric parameters was performed. The Kruskal-Wallis H test was used for multiple comparisons among the four groups. Scatterplots, the R2 correlation coefficient, and correlation coefficients (Pearson or Spearman, depending on the data; i.e., whether normality could be assumed) were used to assess the associations between pairs of variables. Hotelling trace test was used for comparison of intraindividual changes, and univariate analysis was used to examine changes in respective polar values in each group. P values < 0.05 were considered statistically significant.
Author contributions
J.P. and C.-K.J. were involved in the study’s conception, design, and funding; C.-K.J. conducted the study; J.P. collected the data; J.P. and C.-K.J. analyzed the data; J.P. wrote the manuscript, reviewed the manuscript, and gave final approval of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sinskey RM, Stoppel JO. Induced astigmatism in a 6.0 mm no-stitch frown incision. J. Cataract Refract. Surg. 1994;20:406–409. doi: 10.1016/S0886-3350(13)80175-7. [DOI] [PubMed] [Google Scholar]
- 2.Ernest PH. Corneal lip tunnel incision. J. Cataract Refract. Surg. 1994;20:154–157. doi: 10.1016/S0886-3350(13)80156-3. [DOI] [PubMed] [Google Scholar]
- 3.Masket S, Wang L, Belani S. Induced astigmatism with 2.2- and 3.0-mm coaxial phacoemulsification incisions. J. Refract. Surg. 2009;25:21–24. doi: 10.3928/1081597X-20090101-04. [DOI] [PubMed] [Google Scholar]
- 4.Rho CR, Joo CK. Effects of steep meridian incision on corneal astigmatism in phacoemulsification cataract surgery. J. Cataract Refract. Surg. 2012;38:666–671. doi: 10.1016/j.jcrs.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Joo CK, Han HK, Kim JH. Computer-assisted videokeratography to measure changes in astigmatism induced by sutureless cataract surgery. J. Cataract Refract. Surg. 1997;23:555–561. doi: 10.1016/S0886-3350(97)80213-1. [DOI] [PubMed] [Google Scholar]
- 6.Savini G, Barboni P, Carbonelli M, Hoffer KJ. Comparison of methods to measure corneal power for intraocular lens power calculation using a rotating Scheimpflug camera. J. Cataract Refract. Surg. 2013;39:598–604. doi: 10.1016/j.jcrs.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Koch DD, Jenkins RB, Weikert MP, Yeu E, Wang L. Correcting astigmatism with toric intraocular lenses: effect of posterior corneal astigmatism. J. Cataract Refract. Surg. 2013;39:1803–1809. doi: 10.1016/j.jcrs.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Park Y, Kim HS. Torsional and flattening effect on corneal astigmatism after cataract surgery: a retrospective analysis. BMC Ophthalmol. 2017;17:10. doi: 10.1186/s12886-017-0399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi K, Sato T, Yoshida M, Yoshimura K. Corneal shape changes of the total and posterior cornea after temporal versus nasal clear corneal incision cataract surgery. Br J Ophthalmol. 2019;103:181–185. doi: 10.1136/bjophthalmol-2017-311710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi K, Yoshida M, Hirata A, Yoshimura K. Changes in shape and astigmatism of total, anterior, and posterior cornea after long versus short clear corneal incision cataract surgery. J. Cataract Refract. Surg. 2018;44:39–49. doi: 10.1016/j.jcrs.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 11.Tonn B, Klaproth OK, Kohnen T. Anterior surface-based keratometry compared with Scheimpflug tomography-based total corneal astigmatism. Invest. Ophthalmol. Vis. Sci. 2014;56:291–298. doi: 10.1167/iovs.14-15659. [DOI] [PubMed] [Google Scholar]
- 12.Shajari M, Friderich S, Pour Sadeghian M, Schmack I, Kohnen T. Characteristics of corneal astigmatism of anterior and posterior surface in a normal control group and patients with keratoconus. Cornea. 2017;36:457–462. doi: 10.1097/ICO.0000000000001143. [DOI] [PubMed] [Google Scholar]
- 13.Dunne MC, Royston JM, Barnes DA. Posterior corneal surface toricity and total corneal astigmatism. Optom. Vis. Sci. 1991;68:708–710. doi: 10.1097/00006324-199109000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Dubbelman M, Sicam VA, Van der Heijde GL. The shape of the anterior and posterior surface of the aging human cornea. Vision Res. 2006;46:993–1001. doi: 10.1016/j.visres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Módis L, Jr, Langenbucher A, Seitz B. Evaluation of normal corneas using the scanning-slit topography/pachymetry system. Cornea. 2004;23:689–694. doi: 10.1097/01.ico.0000126315.05519.0b. [DOI] [PubMed] [Google Scholar]
- 16.Ho JD, Tsai CY, Liou SW. Accuracy of corneal astigmatism estimation by neglecting the posterior corneal surface measurement. Am. J. Ophthalmol. 2009;147:788–795. doi: 10.1016/j.ajo.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Naesar K. Popperian falsification of methods of assessing surgically induced astigmatism. J. Cataract Refract. Surg. 2001;27:25–30. doi: 10.1016/S0886-3350(00)00605-2. [DOI] [PubMed] [Google Scholar]
- 18.Koch DD. Reporting astigmatism data. J. Cataract Refract. Surg. 1998;24:1545. doi: 10.1016/S0886-3350(98)80335-0. [DOI] [PubMed] [Google Scholar]
- 19.Naeser K, Hjortdal J. Polar value analysis of refractive data. J. Cataract Refract. Surg. 2001;27:86–94. doi: 10.1016/S0886-3350(00)00799-9. [DOI] [PubMed] [Google Scholar]
- 20.Abulafia A, Koch DD, Holladay JT, Wang L, Hill W. Pursuing perfection in intraocular lens calculations: IV. Rethinking astigmatism analysis for intraocular lens-based surgery: Suggested terminology, analysis, and standards for outcome reports. J. Cataract Refract. Surg. 2018;44:1169–1174. doi: 10.1016/j.jcrs.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 21.Koch DD, et al. Contribution of posterior corneal astigmatism to total corneal astigmatism. J. Cataract Refract. Surg. 2012;38:2080–2087. doi: 10.1016/j.jcrs.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 22.Garzón N, et al. Comparing surgically induced astigmatism calculated by means of simulated keratometry versus total corneal refractive power. Eur. J. Ophthalmol. 2018;28:573–581. doi: 10.1177/1120672118757666. [DOI] [PubMed] [Google Scholar]
- 23.Hidaka Y, et al. Changes in corneal aberrations after cataract surgery. Jpn. J. Ophthalmol. 2016;60:135–141. doi: 10.1007/s10384-016-0431-7. [DOI] [PubMed] [Google Scholar]
- 24.Chu L, et al. Optimal incision sites to reduce corneal aberration variations after small incision phacoemulsification cataract surgery. Int. J. Ophthalmol. 2016;9:540–545. doi: 10.18240/ijo.2016.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He W, et al. Clinical efficacy of implantation of toric intraocular lenses with different incision positions: a comparative study of steep-axis incision and non-steep-axis incision. BMC Ophthalmol. 2017;17:132. doi: 10.1186/s12886-017-0528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders DR, Gills JP, Martin RG. When keratometric measurements do not accurately reflect corneal topography. J. Cataract Refract. Surg. 1993;19(Suppl):131–135. doi: 10.1016/S0886-3350(13)80396-3. [DOI] [PubMed] [Google Scholar]
- 27.Martin RG, Sanders DR, Miller JD, Cox CC, III, Ballew C. Effect of cataract wound incision size on acute changes in corneal topography. J. Cataract Refract. Surg. 1993;19(Suppl):170–177. doi: 10.1016/S0886-3350(13)80402-6. [DOI] [PubMed] [Google Scholar]
- 28.Kosekahya P, et al. Repeatability and reliability of ectasia display and topometric indices with the Scheimpflug system in normal and keratoconic eyes. J. Cataract Refract. Surg. 2018;44:63–70. doi: 10.1016/j.jcrs.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 29.Alpins N. Astigmatism analysis by the Alpins method. J. Cataract Refract. Surg. 2001;27:31–49. doi: 10.1016/S0886-3350(00)00798-7. [DOI] [PubMed] [Google Scholar]
- 30.Borasio E, Mehta JS, Maurino V. Torque and flattening effects of clear corneal temporal and on-axis incisions for phacoemulsification. J. Cataract Refract. Surg. 2006;32:2030–2038. doi: 10.1016/j.jcrs.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Boote C, Dennis S, Meek K. Spatial mapping of collagen fibril organization in primate cornea-an X-ray diffraction investigation. J. Struct. Biol. 2004;146:359–367. doi: 10.1016/j.jsb.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Boote C, Dennis S, Newton RH, Puri H, Meek KM. Collagen fibrils appear more closely packed in the prepupillary cornea: optical and biomechanical implications. Invest. Ophthalmol. Vis. Sci. 2003;44:2941–2948. doi: 10.1167/iovs.03-0131. [DOI] [PubMed] [Google Scholar]
- 33.Boote C, Dennis S, Huang Y, Quantock AJ, Meek KM. Lamellar orientation in human cornea in relation to mechanical properties. J. Struct. Biol. 2005;149:1–6. doi: 10.1016/j.jsb.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Slade S, Lane S, Solomon K. Clinical outcomes using a novel image-guided planning system in patients with cataract and IOL implantation. J. Refract. Surg. 2018;34:824–831. doi: 10.3928/1081597X-20181115-01. [DOI] [PubMed] [Google Scholar]
- 35.Byun YS, et al. Astigmatic correction by intrastromal astigmatic keratotomy during femtosecond laser-assisted cataract surgery: factors in outcomes. J. Cataract Refract. Surg. 2018;44:202–208. doi: 10.1016/j.jcrs.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 36.Alió J, Rodríguez-Prats JL, Galal A, Ramzy M. Outcomes of microincision cataract surgery versus coaxial phacoemulsification. Ophthalmology. 2005;112:1997–2003. doi: 10.1016/j.ophtha.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Denoyer A, Ricaud X, Van Went C, Labbé A, Baudouin C. Influence of corneal biomechanical properties on surgically induced astigmatism in cataract surgery. J. Cataract Refract. Surg. 2013;39:1204–1210. doi: 10.1016/j.jcrs.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 38.Alpins N, Ong JKY, Stamatelatos G. Asymmetric corneal flattening effect after small incision cataract surgery. J. Refract. Surg. 2016;32:598–603. doi: 10.3928/1081597X-20160608-01. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira TB, Ribeiro FJ, Pinheiro J, Ribeiro P, O’Neill JG. Comparison of surgically induced astigmatism and morphologic features resulting from femtosecond laser and manual clear corneal incisions for cataract surgery. J. Refract. Surg. 2018;34:322–329. doi: 10.3928/1081597X-20180301-01. [DOI] [PubMed] [Google Scholar]
- 40.Kasetsuwan N, Satitpitakul V, Changul T, Jariyakosol S. Incidence and pattern of dry eye after cataract surgery. PLoS One. 2013;8:e78657. doi: 10.1371/journal.pone.0078657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh T, Jung Y, Chang D, Kim J, Kim H. Changes in the tear film and ocular surface after cataract surgery. Jpn. J. Ophthalmol. 2012;56:113–118. doi: 10.1007/s10384-012-0117-8. [DOI] [PubMed] [Google Scholar]
- 42.Hwang HB, Kim HS. Phototoxic effects of an operating microscope on the ocular surface and tear film. Cornea. 2014;33:82–90. doi: 10.1097/ICO.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 43.Chylack LT, Jr, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch. Ophthalmol. 1993;111:831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 44.Holladay JT, Moran JR, Kezirian GM. Analysis of aggregate surgically induced refractive change, prediction error, and intraocular astigmatism. J. Cataract Refract. Surg. 2001;27:61–79. doi: 10.1016/S0886-3350(00)00796-3. [DOI] [PubMed] [Google Scholar]