Abstract
Purpose
The Xen® Gel Stent (Allergan, Irvine, CA, USA) is a minimally invasive glaucoma surgery device implanted to reduce intra-ocular pressure (IOP) in glaucoma. The stents can fail to drain post-operatively due to scarring of the conjunctiva around the stent opening. Data on the success rates of revision surgery in patients with Xen stent failure are scarce. We present the first detailed report of the steps, outcomes and complications of Xen revision surgery with 12 months of follow-up data.
Methods
We share our experiences on the circumstances in which to perform Xen revision surgery, the steps of the surgery and the results of a retrospective interventional case series of all Xen revisions performed at a single centre from 2013 to 2018.
Results
A total of 151 Xen implants were inserted into eyes at our tertiary centre during the study period, of which 21 eyes (patients) underwent revision surgery. Mean pre-operative IOP was 26.1 (standard deviation 8.3) mmHg with the patient on two drops of anti-glaucoma medication. Four patients were excluded from the analysis due to incomplete data (medical records were unavailable for 2 patients; 1 patient died shortly after surgery; and 1 patient moved to another area). A non-functioning Xen implant was identified in another patient during revision surgery, and the procedure was converted to a trabeculectomy. The remaining 16 patients were included in the analysis, all of whom had a minimum follow-up of 12 months, with the longest follow-up being 4 years following revision surgery. Of these 16 patients, four required needling and 5-fluorouracil injection in the first 12 months following revision surgery, three required further glaucoma drainage surgery due to the failure of the revision surgery to control IOP in the first year and one suffered bleb-related endophthalmitis at the site of previous trabeculectomy surgery. Mean IOP at 12 months following revision surgery was 16.3 (standard deviation 3.7) mmHg on 0.7 drops of anti-glaucoma medication, which equates to a 37.5% reduction in IOP and a 65% reduction in the amount of IOP-lowering drops required.
Conclusion
Our study shares experience on when to perform Xen revision surgery and the steps required. The results of our small cohort are the first in the literature and show that revisions can achieve promising IOP and medication reductions. Some patients still require needling in the post-operative period to optimise outcomes.
Keywords: Glaucoma, Minimally invasive glaucoma surgery, Revision surgery, Xen gel stent
Key Summary Points
| Why carry out this study? |
| To our knowledge this is the first detailed report of the steps, outcomes and complications of Xen revision surgery with 12 months of follow-up. |
| We explain the indications for Xen revision surgery and the steps of the procedure. |
| What was learned from the study? |
| Data from our small cohort show that Xen revision surgery can achieve intra-ocular pressure (IOP) outcomes similar to those achieved in primary Xen implantation. |
| A proportion of patients will still require needling following Xen implant surgery or adjunctive IOP-lowering drops in order to achieve their target IOP. |
Introduction
The Xen45 Gel Stent (Allergan, California, USA) is a minimally invasive glaucoma surgery device that can be implanted from inside the eye (ab-interno) or from outside the eye (ab-externo) to reduce intra-ocular pressure (IOP) in glaucoma. The 6-mm stent has an internal diameter of 45 μm, is composed of gelatin cross-linked with glutaraldehyde and comes in a 27-gauge pre-loaded injector. The stent creates a passage for aqueous to pass from the anterior chamber into the subconjunctival/subtenon’s space, enabling formation of a bleb similar to that seen in traditional trabeculectomy surgery.
Data published thus far support the Xen implant being a safe and effective approach to lowering IOP and reducing drop dependency in patients with glaucoma [1–14]. Although these reports show complications rates to be lower than those associated with traditional glaucoma surgery, it is standardly accepted that a proportion of eyes will require bleb needling in the post-operative period and that despite this some implants will fail due to progressive scarring of the conjunctiva around the stent opening that is unresponsive to slit lamp needling and antimetabolite injections [12]. In our tertiary glaucoma unit, Xen revision surgery has been performed for such cases. Here, we share our approach to managing the failing Xen implant, details of the surgical procedure to revise the Xen implant and the outcomes of the first 12 months of follow-up for our patient cohort undergoing Xen revision surgery.
Methods
Data were retrospectively collected from case notes and electronic records for all patients who underwent Xen revision surgery for failed Xen implant at our centre between 2013 and 2018. The original Xen implant procedures were all performed using the standard ab-interno method with a subconjunctival injection of mitomycin C (MMC) 0.2 mg/mL. The original Xen implants and revision procedures were all performed by a single surgeon (LA).
IOP before and after revision surgery, measured with Goldmann applanation tonometry, best corrected visual acuity (BCVA) and number of glaucoma medications were documented. The number of interventions required after Xen implant surgery (bleb needling and antimetabolite injections) was recorded, as were any further surgeries undertaken or complications encountered. Bleb needling was performed at the slit lamp with 0.1 ml 5-fluorouracil (5-FU) (50 mg/ml) injected posterior to the bleb.
Data were entered into a pre-designed spreadsheet, and statistical analysis was performed using Apple Numbers version 2016 software (Apple Inc., Cupertino, CA, USA).
The primary outcome measures were IOP (mmHg) and number of drops of anti-glaucoma medication at 12 months following revision surgery. Similar to a previous study from our centre sharing the 1-year results from primary Xen surgery, success in the present study was determined in accordance with World Glaucoma Association guidance as IOP ≤ 21 mmHg and ≥ 20% reduction in IOP from baseline [13]. Outcomes were considered to be a ‘complete success’ if achieved without the use of adjunctive IOP-lowering drops (of anti-glaucoma medication) or as a ‘qualified success’ if IOP-lowering drops were required. Statistical significance of the results was determined using a paired t test, with P < 0.05 indicating statistical significance. Failure was defined as either the inability to meet these criteria or if a patient required alternative glaucoma surgery either at the time of revision or during the follow-up period.
Informed consent for revision surgery was obtained from all patients after a thorough explanation of the procedure and its risks. All procedures followed the tenets of the Declaration of Helsinki. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. As this study was not considered research by the NHS Health Research Authority, ethical approval was not required.
Indications for Xen Revision Surgery
The main indication for Xen revision surgery is a flat, non-draining bleb with raised IOP. In our experience, needling is unlikely to restore drainage, and revision surgery is our preferred option when the IOP cannot be controlled on medication. Patients with signs of a draining bleb but raised IOP are often needled first in the clinic combined with 5-FU injection, followed by recommencement of anti-glaucoma medications if the IOP is still not controlled. If despite previous needling and maximum tolerable drops the IOP remains uncontrolled, Xen revision surgery is performed.
Our Technique for Xen Revision Surgery
We place a 7/0 silk traction suture partial thickness through the cornea at the 12 o’clock position, and the eye is rotated to provide adequate access to the original Xen implant. A conjunctival peritomy is made at the limbus (Fig. 1), and blunt dissection with Spring scissors is used to expose the Xen implant, with care taken not to damage the implant (Fig. 2). We use a pushing motion with the scissors slightly open to dissect the tissue safely. The Xen implant is often found to be wrapped in a ‘sock’ of fibrotic tissue which requires very careful dissection.
Fig. 1.
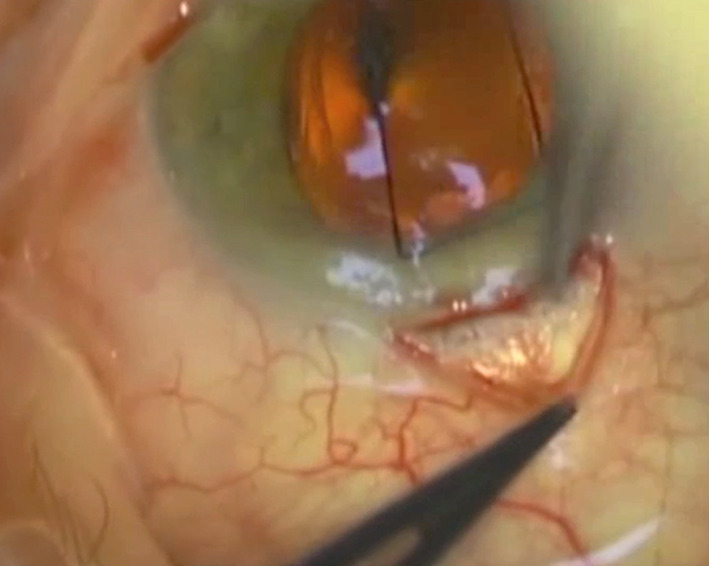
Colour photograph of Xen revision surgery with a silk traction suture at 12 o’clock, with rotation of the eye and a conjunctival peritomy made at the limbus
Fig. 2.
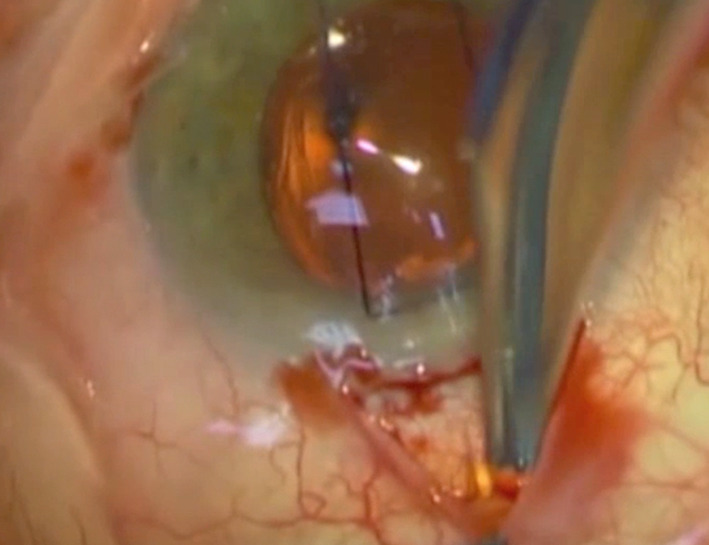
Colour photograph demonstrating blunt dissection to expose the Xen implant (yellow)
After the Xen implant is freed, we demonstrate the patency of the device by using a dry spear swab to dab the opening of the stent (Fig. 3). If good flow is witnessed, then the surgeon can proceed to the final steps, otherwise alternative surgery needs to be considered at this stage. Flushing of the Xen implant is difficult due to the flexible nature of the stent as well as the small lumen. If the Xen implant is blocked, truncating the very end of the external portion might re-establish flow if the blockage is due to in-growing fibrotic tissue. If the Xen implant still fails to drain despite these measures, we then insert a second Xen implant ab-externally or we proceed to a trabeculectomy, depending on the clinical circumstances.
Fig. 3.
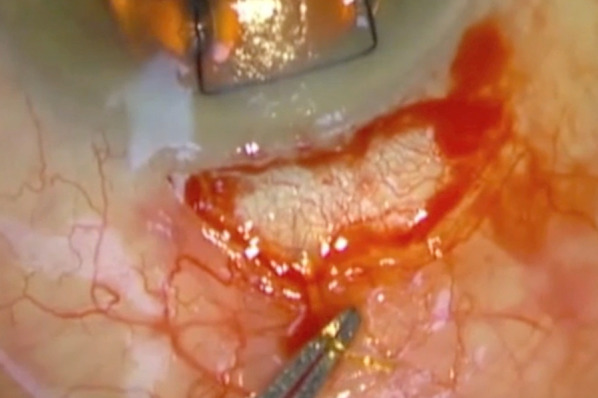
Colour photograph demonstrating the freed Xen implant after dissection (within tip of forceps)
We place small sponges soaked in 0.2 mg/ml MMC in the posterior aspect of the conjunctival pocket for 3 min and then remove them (Fig. 4), following which the surgical field is thoroughly irrigated with saline solution. We close the conjunctiva using interrupted 10-0 nylon sutures, ensuring that the Xen implant is lying flat on the scleral surface free from any tenon tissue. All patients receive subconjunctival steroid and antibiotic medications at the end of the procedure.
Fig. 4.
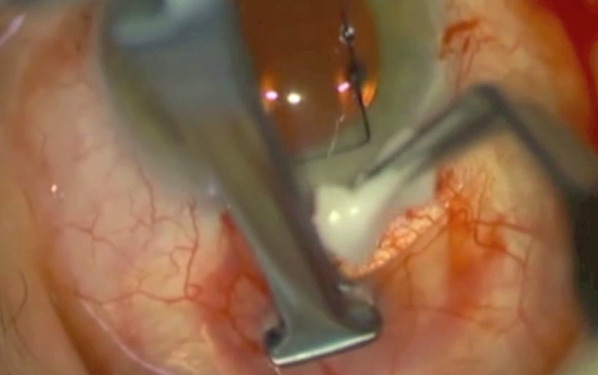
Colour photo showing insertion of sponges soaked in mitomycin C being placed in the posterior aspect of the conjunctival pocket
Glaucoma drops are discontinued until the IOP is checked the following day. Patients are given chloramphenicol eye drops four times per day for 2 weeks, as well as prednisolone 1% eye drops six times per day which is subsequently tapered on an individual basis dependent on conjunctival appearance at the follow-up assessments. Treatment with topical steroid is continued for a minimum period of 2 months following revision surgery.
Results
A total of 151 Xen implant procedures were performed during the study period at our centre, of which 21 patients (eyes) required revision surgery. Four patients were excluded from the analysis due to incomplete data (medical records were unavailable for 2 patients; 1 patient died shortly after surgery; 1 patient moved to another area). One patient’s Xen implant was not draining when revision surgery was performed, and therefore the procedure was converted to a trabeculectomy. This left a cohort of 16 patients for analysis. All 16 patients completed 1 year of follow-up, with four, one, and one patients reaching 2, 3 and 4 years of post-revision surgery follow-up, respectively. The pre-revision demographic characteristics of these patients are given in Table 1.
Table 1.
Pre-revision demographics of the patient cohort
| Pre-revision demographics | Values |
|---|---|
| Mean age (years) | 71 (SD 12.6) |
| Female:male ratio | 3:1 |
| Primary diagnosis (no. of patients) | |
| Primary open-angle glaucoma | 13 |
| Uveitic glaucoma | 2 |
| Pseudoexfoliation | 1 |
| Mean time from Xen to revision surgery (months) | 9.9 (range 1–38) |
| Mean intra-ocular pressure (mmHg) | 26.1 (SD 8.3) |
| Mean number of medications | 2 (range 1–3) |
| Mean cup:disc ratio | 0.6 (range 0.3–0.9) |
| Mean visual field mean deviation | − 7.23 (SD 5.8) |
SD Standard deviation
Change in IOP and Medication
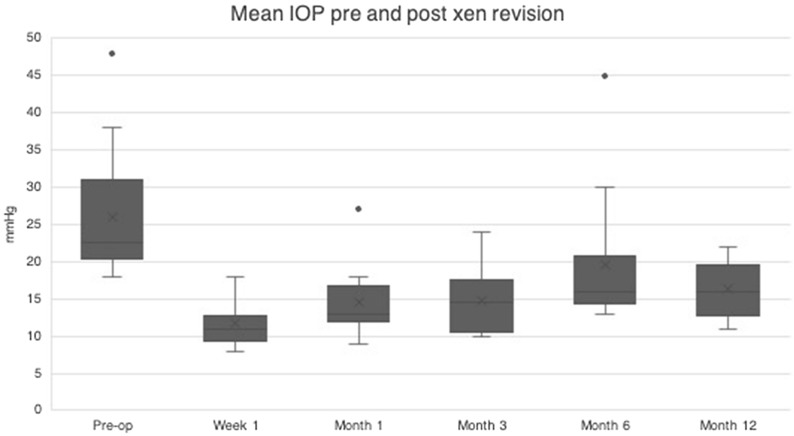
The mean IOP pre-revision surgery was 26.1 mmHg (SD 8.3) on two drops (range 1–3 drops) of anti-glaucoma medication, with four patients taking additional acetazolamide tablets. Post-operative change in IOP and number of drops is shown in Figs. 5 and 6. The average reduction in IOP was 37.5%, and there was a 65% reduction in number of drops needed at 1 year. Four patients in this cohort are currently at the 2-year post revision surgery time point, with a mean IOP of 17.0 mmHg (standard devation 1.4) on 1.25 drops; one patient is at the 3-year follow-up, with an IOP of 10 mmHg without drops; and one patient is at the 4-year follow-up with an IOP of 17 mmHg on one drop.
Fig. 5.
Change in intra-ocular pressure (IOP; mmHg) pre- and post-Xen revision surgery
Fig. 6.
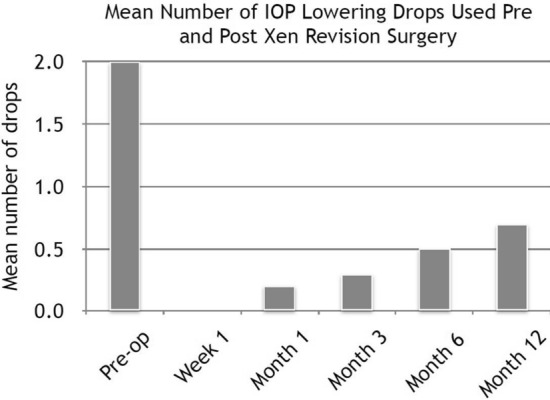
Number of IOP-lowering drops (of anti-glaucoma medication) required pre- and post-Xen revision surgery
Complete and Qualified Success
Based on our pre-defined success criteria, nine of the 16 patients (56%) achieved at least qualified success, of whom seven (44%) achieved complete success with no medication. Xen surgery was considered to have failed in the remaining seven patients for the reasons explained below.
Xen surgery was considered to have failed in three of these seven patients due to failure of these patients to meet the IOP success criteria at 12 months of follow-up. Of these three patients, one did achieve an IOP reduction (IOP < 21 mmHg) relative to the starting IOP and was on fewer drops than at the pre-operative stage but did not achieve a 20% reduction from the starting IOP of 18 mmHg. This patient had undergone one needling procedure with 5-FU during the follow-up. Xen surgery in the second patient was considered to have failed because the pre-operative IOP and IOP at the 12-month follow-up was the same, on the same number of drops. This patient had not had any needling of the Xen bleb and has subsequently been listed for another glaucoma drainage surgery combined with cataract surgery. In the third patient, the pre-operative IOP and the IOP at the 12-month follow-up were the same, and the patient was taking more medications post surgery than pre-surgery. This patient did not have any needling, and no further surgery is planned at present. All three patients had an underlying diagnosis of primary open angle glaucoma.
Of the four other Xen surgeries that were considered to have failed, the IOP of three patients was not controlled during the first 12-months of follow-up, and these three patients had to undergo further glaucoma surgery (see below); the fourth patient suffered bleb-related endophthalmitis, as detailed below.
Needling and Further Surgery
Overall four of the 16 patients in our cohort required bleb needling with 5-FU injection in the first 12 months following revision surgery. One of these four patients had two needling and 5-FU procedures, but unfortunately the IOP stayed at 30 mmHg and the patient was listed for a trabeculectomy 6 months after the revision surgery. Each of the other three patients required one bleb needling with 5-FU injection procedure: one patient met the criteria for complete success, one met the criteria for qualified success and the third patient was the one who did not achieve a 20% reduction in IOP despite having an IOP of < 21 mmHg and being on fewer medications.
Xen revision surgery failed to control IOP in three of the 16 patients, and these proceeded to further surgery during the 12-month follow-up period. One of these patients, as mentioned in the previous section, had two needlings but the IOP remained high and therefore a trabeculectomy was performed. One patient with an underlying diagnosis of uveitis had an IOP of 42 mmHg at 2 months of follow-up and underwent Baerveldt tube surgery. The third patient had an underlying diagnosis of primary open-angle glaucoma and had an IOP of 45 mmHg at 6 months of follow-up; this patient proceeded to have a Cypass stent (Alcon, Fort Worth, TX, USA).
Complications
The complication rate in our cohort undergoing revision surgery was low. One eye suffered hypotonous maculopathy even though the IOP never fell below 10 mmHg when measured. This condition self-resolved without treatment within 1 month and final visual acuity was not compromised. One eye suffered bleb-related endophthalmitis at the site of a previous trabeculectomy surgery 3 months after revision; this patient had a history of nasolacrimal duct obstruction and recurrent conjunctivitis.
One patient was found to have a Xen implant that was not functioning during the revision procedure; the procedure was therefore converted to a trabeculectomy. It is worth mentioning that if the Xen implant is non-functional, a second Xen implant can be inserted either ab-internally or ab-externally prior to conjunctival closure. However, in this case, the patient had uveitic glaucoma and high IOP; she was also very anxious and required intra-operative sedation. Hence, the decision was made to convert to trabeculectomy which has an overall higher success rate and lower chance of repeat surgery. This patient was excluded from our cohort as she did not strictly have a Xen revision; had we inserted a second Xen implant (instead of converting to trabeculectomy), we would have included this patient in our analysis.
We had no cases of the iris blocking the stent, hyphaema, bleb leak or implant exposure. However, our cohort was small, and these conditions have been reported in larger series of patients with the original Xen implant.
Discussion
It is important to consider how to approach Xen implants following the initial surgery to understand when it may be appropriate to perform revision surgery and in which patients it is indicated. In their article, Vera et al. [15] provide an excellent flow chart on the management of high IOP post Xen implantation which is largely based on bleb morphology. We agree that in situations where there is no or minimal bleb it is vital to perform gonioscopy to identify lumen occlusion, which can be then treated with YAG laser. If gonioscopy reveals no occlusion, then ocular massage may help to establish flow and overcome some fibrosis at the outflow site. At this stage, if the bleb remains flat we would proceed to surgical revision to ensure the correct position and function of the implant. Vera et al. also [15] describe the option of revision at this stage, but they do not document the details of the surgical technique and the likelihood of success.
The efficacy of primary Xen surgeries has been well described. The mean IOP reduction at 1 year reported in the literature ranges from 22.7 to 60.4% (mean 35.1%) [1–14]. Our 12-month post-revision mean IOP reduction was 37.5%, demonstrating a success rate similar to that reported in the literature and indicating that revision surgery for failed Xen implants can successfully restore the function of the Xen implant at least within the first year. Additionally, as revision surgery has a shorter surgery time and faster recovery time, it is a favourable option over traditional filtration procedures when considering how to approach a failed Xen implant [12]. All of our Xen revision surgeries that required further drainage procedures failed within the first year of follow-up. A number of our revision cases have completed 4 years of follow-up following revision surgery and have not required any further interventions.
Needling rates at 12 months after the initial Xen implant surgery reported in the literature range from 0 to 51.3% (mean 29.8%) [1–14]. In our study, 25% of the cohort required needling and antimetabolite injection; in the majority of cases, this treatment was successful in preventing the patient from needing further glaucoma surgery. One patient was needled twice but still had elevated IOP and therefore went on to have a trabeculectomy at 6 months after revision surgery.
Complications detailed in the literature due to primary Xen implants include hypotony (range 0.7–37.5%), endophthalmitis (range 0–4.2%), failure requiring further surgery (range 0–16.7%), iris blocking the stent (range 0.5–7.7%), hyphaema (range 0.7–10.3%), wound and bleb leaks (range 1.5–9.2%), implant exposure (range 0.5–8.3%) and anecdotal reports of stent migration into the anterior chamber [1–14, 16, 17]. In our case series of Xen revisions, complications were uncommon. One patient had hypotonous maculopathy that self-resolved within 1 month of revision surgery and which had no long-term effects on vision. Another patient with recurrent conjunctivitis secondary to nasolacrimal pathology suffered bleb-related endophthalmitis at the site of previous trabeculectomy surgery. Finally, one patient was found to have a non-functioning Xen during revision surgery and required conversion to trabeculectomy. Despite the low number of complications, we acknowledge that our cohort is small, and caution is still required when recommending revision surgery, especially with the additional application of antimetabolite injections.
Although Xen revision surgery is a short and relatively easy procedure, one must be careful not to damage the stent while dissecting the scar tissue surrounding it. We do not report any instances of stent damage during revision surgery, but Mansouri et al. [7] reported a 2% rate of damage to the stent during post-operative needling at the slit lamp. However, with revision surgery performed by opening up the conjunctiva at the limbus, there is better exposure than needling at the slit lamp and therefore we expect the incidence of this to be infrequent in revision surgery.
Our study is limited by the small sample size, but revision surgery of any kind, by nature, is uncommon. Additionally, there was a range of patients in the cohort with different underlying glaucoma diagnoses and varying severity of disease at the outset. However, as data on Xen revision surgery in the literature are extremely rare, we thought it important to share the outcomes from our centre so that other surgeons may consider this relatively simple and effective approach to tackling failed Xen implant due to conjunctival scarring.
Conclusion
The is the first detailed report of the surgical steps of Xen revision surgery and the post-operative outcome. We believe that Xen revision surgery should be considered first before any other filtration surgery when IOP is uncontrolled by medication. Overall, our study shows that Xen revision surgery can achieve IOP reductions similar to those achieved by primary Xen implantation. However, a proportion of the patients undergoing Xen revision surgery will still require further needling at the slit lamp during the post-operative period to achieve target IOP, and some may need adjunctive IOP-lowering drops, albeit to a lesser degree than prior to Xen surgery. Larger studies with a prolonged follow-up are needed to establish the safety and efficacy of revision surgery over the long term, and the success or risk of subsequent glaucoma surgery in failed cases must also be fathomed.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosures
Emma Linton declares that she has no conflict of interest. Leon Au has received research support, honorarium and travel reimbursement from Glaukos, Allergan, Ivantis, Santen, Thea, EyeDPharma and Alcon Pharmaceuticals. Leon Au is a member of the journal’s Editorial Board.
Compliance with Ethics Guidelines
Informed consent for revision surgery was obtained from all patients after a thorough explanation of the procedure and its risks. All procedures followed the tenets of the Declaration of Helsinki. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. As this study was not considered research by the NHS Health Research Authority, ethical approval wasn’t required.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.11771391.
Change history
12/22/2021
The license text was incorrectly structured. The article has been corrected.
References
- 1.De Gregorio A, Pedrotti E, Stevan G, et al. XEN glaucoma treatment system in the management of refractory glaucomas: a short review on trial date and potential role in clinical practice. Clin Ophthalmol. 2018;12:773–782. doi: 10.2147/OPTH.S146919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galal A, Bilgic A, Eltanamly R, et al. XEN glaucoma implant with mitomycin C 1-year follow-up: results and complications. J Ophthalmol. 2017; article ID 5457246. 10.1155/2017/5457246. [DOI] [PMC free article] [PubMed]
- 3.Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25–36. doi: 10.1016/j.ajo.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Heidinger A, Schwab C, Lindner E, et al. A retrospective study of 199 Xen45 stent implantations from 2014 to 2016. J Glaucoma. 2019;28:75–79. doi: 10.1097/IJG.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 5.Ibáñez-Muñoz A, Soto-Biforcos VS, Rodríguez-Vicente L, et al. XEN implant in primary and secondary open-angle glaucoma: a 12-month retrospective study. Eur J Ophthalmol. 2019 doi: 10.1177/1120672119845226. [DOI] [PubMed] [Google Scholar]
- 6.Karimi A, Lindfield D, Turnbull A, et al. A multi-centre interventional case series of 259 ab-interno Xen gel implants for glaucoma, with and without combined cataract surgery. Eye. 2019;33:469–477. doi: 10.1038/s41433-018-0243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansouri K, Guidotti J, Rao HL, et al. Prospective evaluation of standalone XEN gel implant and combined phacoemulsification–XEN gel implant surgery: 1-year results. J Glaucoma. 2018;27:140–147. doi: 10.1097/IJG.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 8.Reitsamer H, Sng C, Vera V, et al. Two-year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2019;257:983–996. doi: 10.1007/s00417-019-04251-z. [DOI] [PubMed] [Google Scholar]
- 9.Schlenker MB, Gulamhusein H, Conrad-Hengerer I, et al. Efficacy, safety, and risk factors for failure of standalone Ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017;124:1579–1588. doi: 10.1016/j.ophtha.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Smith M, Charles R, Abdel-Hay A, et al. 1-year outcomes of the Xen45 glaucoma implant. Eye. 2019;33:761–766. doi: 10.1038/s41433-018-0310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sng CCA, Wang J, Hau S, et al. XEN-45 collagen implant for the treatment of uveitis glaucoma. Clin Exp Ophthalmol. 2018;46:339–345. doi: 10.1111/ceo.13087. [DOI] [PubMed] [Google Scholar]
- 12.Szigiato A, Sandhu S, Ratnarajan G, et al. Surgeon perspectives on learning ab-interno gelatin microstent implantation. Can J Ophthalmol. 2018;53:246–251. doi: 10.1016/j.jcjo.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Tan SZ, Walkden A, Au L. One-year result of XEN45 implant for glaucoma: efficacy, safety, and post-operative management. Eye. 2018;32:324–332. doi: 10.1038/eye.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widder RA, Dietlein TS, Dinslage S, et al. The XEN45 gel stent as a minimally invasive procedure in glaucoma surgery: success rates, risk profile, and rates of re-surgery after 261 surgeries. Graefe’s Arch Clin Exp Ophthalmol. 2017;256:765–771. doi: 10.1007/s00417-018-3899-7. [DOI] [PubMed] [Google Scholar]
- 15.Vera V, Ahmed I, Stalmans I, et al. Gel stent implantation—recommendations for preoperative assessment, surgical technique, and postoperative management. US Ophthalmic Rev. 2018;11(1):38–46. doi: 10.17925/USOR.2018.11.1.38. [DOI] [Google Scholar]
- 16.Dervenis N, Mikropoulou AM, Dervenis P, et al. Dislocation of a previously successful XEN glaucoma implant into the anterior chamber: a case report. BMC Ophthalmol. 2017;17:148. doi: 10.1186/s12886-017-0540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salinas L, Chaudhary A, Guidotti J, et al. Revision of a leaking bleb with XEN gel stent replacement. J Glaucoma. 2018;27(1):e11–e13. doi: 10.1097/IJG.0000000000000811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.