Abstract
The aim of this study was to evaluate the influence of adding copigment gallic acid (GA) on the stability of anthocyanin and color in blueberry juice, and assays were carried out with different anthocyanin:GA molar ratios (1:0, 1:1, 1:3, 1:5) in accelerated experiments (40 °C for 10 days). Results showed that the addition of GA made blueberry juice to appear more crimson color tonality, color saturation and anthocyanins stability. The most obvious hyperchromic effect appeared in juice with 1:5 of anthocyanin:GA molar ratios, and in this ratio, total anthocyanin content (137.67 mg/L) and main anthocyanin peonidin-3-glucoside content (51.68 mg/L) of the blueberry juice were higher than juice without copigment (total anthocyanin of 116.96 mg/L and peonidin-3-glucoside of 34.2 mg/L). Furthermore, anthocyanins in blueberry juice copigmented with molar ratios 1:5 of anthocyanin:GA were more stable at 4 °C than that at 25 °C and 40 °C. Thus, the addition of gallic acid at appropriate levels might be a promising juice process technology to obtain juices with high color quality and anthocyanin stability.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-04175-w) contains supplementary material, which is available to authorized users.
Keywords: Gallic acid, Blueberry juice, Color, Anthocyanin, Stability
Introduction
Blueberries (genus Vaccinium, family Ericaceae) are rich in polyphenols and appear dark purple on account of high amounts of anthocyanins (Prior et al. 1998). Due to an abundance of polyphenols, blueberry exhibits diverse biological properties such as antioxidant, cardioprotective, neuroprotective, antiinflammatory and anticarcinogenic properties (Benn et al. 2014; Signorelli et al. 2015). Blueberries are commonly processed into various forms including juice, wine, and jam. Blueberry juices are increasingly promoted and consumed due to nutritional and other health benefits (Michalska and Łysiak 2015; Diaconeasa et al. 2015).
Blueberry juice mainly contains 5 groups of anthocyanidins which include cyanidin (Cy), delphinidin (Dp), peonidin (Pn), malvidin (Mv), and petunidin (Pt) (You et al. 2011; Muller et al. 2012). Anthocyanins have relatively low stability and their degradation is influenced by factors such as pH, light, temperature, oxygen and non-covalent interactions with colorless compounds, coordination with metallic ions, and covalent reactions with other compounds present in the medium (Brenes et al. 2005; West and Mauer 2013). It is worth mentioning that the intermolecular copigmentation could better improve the stability of anthocyanins in berries (Markovic et al. 2000; Hernandez-Herrero and Frutos 2015; Fanzone et al. 2015; Chung et al. 2016).
Intermolecular copigmentation is a phenomenon in which pigments and copigments form complexes by weak hydrophobic and π–π interactions, and where the spectra of copigmented solutions usually display a shift of the visible λmax toward greater wavelengths (bathochromic effect) coupled with an increase of absorptivity (hyperchromic effect) (Castaneda-Ovando et al. 2009). A few copigments were successfully assayed in previous research, for example, the color and anthocyanin content of blueberry juice could be better retained through intermolecular copigmentation using the copigment vitexin, orientin and flavonoid C-glycoside (Pan et al. 2014). In addition, the intermolecular copigmentation of anthocyanins with sinapic acid, caffeic acid, ferulic acid, or rosemary polyphenolic extract could increase the stability of the anthocyanin (Sari et al. 2012; Kopjar and Pilizota 2009). Gallic acid (GA) is a low molecular weight polyphenol compound, which exhibits potent antioxidant properties (Abdelwahed et al. 2007). Previous research has stated that gallic acid facilitates the retention of anthocyanin in vitamin-C fortified cranberry juice (Roidoung et al. 2016).
There is some feasibility and benefits of using gallic acid in commercial juice production. The sources of food grade gallic acid are wide and it has a mildly sour taste (Srinivas et al. 2010). Moreover, in the human body, gallic acid could be absorbed better than other polyphenols (Daglia et al. 2014). In the present study, gallic acid as copigment was used in blueberry juice to enhance the stability of color and anthocyanins. The influence of different anthocyanin:GA molar ratios (1:0, 1:1, 1:3, 1:5) on the anthocyanins and chromatic parameters of juice was investigated, the stability of anthocyanins of blueberry juices was evaluated at different storage temperature and time, and copigmentation effect of GA on individual anthocyanins content in blueberry juice during storage has also been studied.
Materials and methods
Chemicals
The cyanidin-3-glucoside standard was purchased from Sigma-Aldrich (St. Louis, MO, USA). The food-grade gallic acid (99%) was obtained from Shanghai Kangting Bio-Technique Co. Ltd (Shanghai China). The Folin–Ciocalteu’s phenol reagent was purchased from Sinopharm Chemical Reagent (Shanghai, China). Other general chemicals with analytical grade were obtained from local suppliers.
Blueberry samples
Rabbiteye blueberry of cultivar ‘Baldwin’ was obtained from a commercial blueberry plantation located in Hefei (31° 52′ N, 117° 17′ E) in central-eastern China. The samples was frozen and stored at − 20 °C for subsequent chemical component analysis and making juice. Soluble solid content of the blueberry juice was 13.5°Brix, the pH was 3.3 and the concentration of titratable acid was 7.4 g/L.
Preparation of blueberry juice copigmented with gallic acid
The frozen blueberries were thawed at room temperature, blanched via steam for 3 min, and then cooled to room temperature. Steam blanching was performed in a food steamer at atmospheric pressure. Blanched berries were milled and treated with pectinase (Laffort, France) at a concentration of 0.05 g/kg fruit for 2 h at room temperature. To remove pomace, the blueberry puree by pectinase hydrolysis was strained through a piece of 200 mesh nylon mesh filter, and further centrifuged at 3100×g for 15 min by centrifugal machine (Xiangyi Laboratory Instrument Development Co., Ltd., Hunan, China). And at this moment, the blueberry juice containing 424.13 mg/L of total anthocyanin was prepared (the total anthocyanin was calculated with cyanidin-3-glucoside as standard).
In our previous experiment, the screening of the copigment, i.e., tea polyphenols, EGCG, P-coumaric acid and gallic acid (GA) were carried out, and gallic acid was confirmed as the copigment of blueberry juice to carry out the further research (Supplementary material, Fig. S1). Next, to obtain 1:0, 1:1, 1:3 and 1:5 ratio of anthocyanins to gallic acids in the copigmented juice samples, 0 mg, 16.06 mg, 48.19 mg and 80.31 mg GA was added to each 100 mL blueberry juice contained a total anthocyanin of 424.13 mg, respectively. Stir the juices until the gallic acid completely dissolved. The copigmented juices were pasteurized at 85 °C for 5 min for the next trials.
Storage stability of anthocyanins and color of blueberry juice
To evaluate the protection of anthocyanins and color by copigmentation of GA, blueberry juice with different anthocyanin/GA molar ratios (1:0, 1:1, 1:3, 1:5) were stored at 40 °C for 10 days to conduct an accelerated shelf life studies. The absorption spectra and individual anthocyanins content were determined on day 0 and day 10, respectively. The chromatic parameters [lightness (L*), chroma (C*) and hue angle (H*)] of blueberry juices were measured on day 0, 2, 5, 7 and 10. According to the degradation rates of anthocyanins in the blueberry juices, the changes of anthocyanins of juices at molar anthocyanins/GA ratios of 1:0 and 1:5 was further evaluated during storage at 4 °C for 50 days, 25 °C for 40 days and 40 °C for 10 days, respectively.
Measurements of hyperchromic effect and spectral shift
Anthocyanin copigmentation reactions can be detected by a hyperchromic effect or a bathochromic shift (Eiro and Heinonen 2002; Markovic et al. 2000). In this work, to evaluate the hyperchromic and bathochromic shift effect, the blueberry juice with different anthocyanin:GA molar ratios were diluted with deionized water according to their absorbance in advance, and then the absorption spectrum of juices were scanned in the visible range (between 400 and 700 nm) by a UV–visible light spectrophotometer with Lambda 35 UV–Vis systems (PerkinElmer, America).
Analysis of total anthocyanin content (TAC) and retention rate of TAC
The TAC and the retention rate of TAC of juices were estimated using the pH differential method as reported in our previous article (He et al. 2016). The samples of juice were diluted with pH 1.0 buffer and pH 4.5 buffer by the same multiple, respectively. The absorbance of diluted samples was measured at 510 nm and 700 nm. The TAC and the retention rate of TAC were calculated using the following formulas (1), (2) and (3):
| 1 |
| 2 |
| 3 |
where A510 and A700 is the absorbance detected at 510 nm and 700 nm, respectively; A is the difference in absorbance between pH 1.0 and 4.5, MW is the molecular weight of cyanidin-3-glucoside (449 g/mol); DF is the dilution factor; Ve is the extract volume, is the molar extinction coefficient of cyanidin-3-glucoside (29,600), and M is the mass of the blueberry extracted. Total anthocyanin content (TAC) was expressed as mg cyanidin-3-glucoside (C3G) equivalents per 1 L of juice (mg C3G/L). Further, TACt is the total anthocyanin content of storage for t day; TAC0 is the total anthocyanin content of storage for 0 day. TAC (%, of initial) expresses the retention rate of total anthocyanin content.
Polymeric color analysis
Polymeric color was determined using the method modified by Zou et al. (2016). For analysis, 0.3 mL of distilled water or 0.90 M (mol/L) potassium metabisulfite was added to 4.2 mL of diluted sample. After equilibrating for 30 min, samples were evaluated at λ = 700, 510, and 420 nm. Color density (CD) was calculated by data from the experimental group with distilled water using the following formula:
| 4 |
K was the dilution factor.
Polymeric color (PC) was determined from the experimental group with potassium metabisulfite using the following formula:
| 5 |
Percent polymeric color (PPC) was calculated using the following formula:
| 6 |
Quantification of individual anthocyanins by Ultra Performance Liquid Chromatograph
Anthocyanin profiles in blueberry juice were analyzed by Ultra Performance Liquid Chromatograph (UPLC) using Waters Acquity Ultra-Performance™ LC system (Waters, Millford, USA). The chromatographic system consisted of a pump, an Agilent C18 column (2.1 × 150 mm, 2.6 μm), and a Waters Tunable UV detector. Quantification method of individual anthocyanins was determined according to the method described previously (He et al. 2016) with slight modifications. Briefly, the sample was filtered through 0.45 μm microfiltration membrane prior to UPLC analysis. The mobile phase consisted of 6% formic acid aqueous (A) and acetonitrile containing 6% formic acid (B). The gradient elution program was set as follows: 5–50% B (0–20 min), 50–5% B (20–22 min), and 5% B (22–25 min). The injection volume, the flow rate and column temperature were 5 μL, 0.3 mL/min and 30 °C, respectively. Anthocyanins were detected by their absorbance at 520 nm. The levels of individual anthocyanins were quantified as cyanidin-3-glucoside equivalents.
Individual anthocyanin identification by UPLC-DAD-ESI/MS
Anthocyanin analysis of blueberry juices were carried out using a Waters Acquity UPLC System. (Waters, Millford, US) equipped with a Diode Array Detector (DAD) and a Triple Quadrupole Mass Spectrometer. Identification of individual anthocyanins, including delphinidin-3-galactoside, delphinidin-3-glucoside, cyanidin-3-galactoside, cyanidin-3-glucoside, petunidin-3-galactoside, petunidin-3-galactoside, peonidin-3-galactoside, peonidin-3-glucoside, malvidin-3-galactoside, malvidin-3-glucoside and malvidin-3-arabinoside, was performed according to the method in the literature (He et al. 2016) with a slight modification. Samples were separated using an Agilent C18 column packed with Zorbax (2.1 × 150 mm, 2.6 μm). The column temperature was maintained at 30 °C. The sample was filtered through 0.45 μm microfiltration membrane prior to UPLC analysis. The injection volume was 5 μL. The flow rate was 0.3 mL/min. The mobile phase consisted of aqueous 0.2% formic acid (A) and acetonitrile containing 0.2% formic acid (B). The gradient program was set as follows: 5–50% B (0–10 min), 50–5% B (10–11 min), and 5% B (11–14 min). Equilibrium time between runs was 10 min. Anthocyanins were detected at 520 nm. The conditions of MS analysis were as follows: electrospray ionization (ESI) interface, nitrogen for drying, nebulizing gas and nebulizer pressure at 35 psi, dry gas flowing at 10 mL/min, 350 °C dry gas temperature, capillary voltage at 2500 V, and spectra recording in the positive ion mode with scans from m/z 10 to 1000.
Color measured
Color was determined with ColorQuest XE (Hunter Associates Laboratory Inc. Reston, VA, USA) (the 10° Standard Observer and Standard Illuminant D65). The chromatic parameters were assessed by using the method described previously (Zhang et al. 2016). The values a* and b* were used to calculate the hue angle and chroma value .
Statistical analysis
The experiments were conducted in triplicate and values were expressed as the means ± the standard deviation (SD). Data were subjected to one-way analysis of variance (ANOVA) followed by Duncan’s range test Duncan using Prism™ v6.0 software. A P < 0.05 was considered to be statistically significant and the differences are denoted by different letters.
Results and discussion
Copigmentation effect
The copigmentation phenomenon is usually assessed by looking at the changes in λmax of the visible absorption spectrum. Figure 1 illustrated the visible spectra of blueberry juices with different anthocyanins/GA molar ratios. It was found that the addition of copigments induced the increase of absorbance over all the visible range (400–700 nm), where the hyperchromic effect was elicited by copigments. These copigmented juices also induced bathochromic shift in λmax (bathochromic effect). As shown in Fig. 1, it could be seen that the hyperchromic effect was dependent on the magnitude of the added GA, and the most obvious hyperchromic effect was observed in the juice at a molar anthocyanins/GA ratio of 1:5. Hyperchromic effect or a bathochromic shift indicated that anthocyanins and copigments formed an overlapping arrangement through intermolecular association (Eiro and Heinonen 2002; Markovic et al. 2000). Fanzone et al. (2015) presented the experimental results of positive correlation between malvidin-3-O-glucoside:dihydroquercetin-3-O-glucoside molar ratio and hyperchromic effect. Petrova et al. (2017) found that Amax increases with the increase in the concentrations of strawberry anthocyanin and quercetin at 40 °C and 50 °C. The hyperchromic effect could enhance with the increase of copigment content (Fanzone et al. 2015; Lorenzo et al. 2005; Gonzalez-Manzano et al. 2008).
Fig. 1.
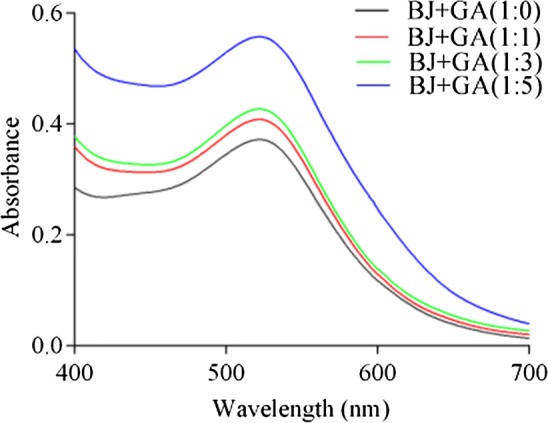
Absorption spectra of blueberry juices with different anthocyanins/gallic acid molar ratios. GA: gallic acid; BJ: blueberry juice; BJ + GA (1:0), BJ + GA (1:1), BJ + GA (1:3) and BJ + GA (1:5): blueberry juice with 1:0, 1:1, 1:3 and 1:5 anthocyanin/gallic acid molar ratio
Effects of copigmentation on anthocyanin stability and percent polymeric color
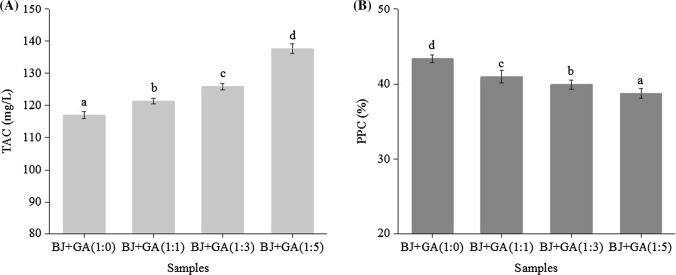
The retention rate of total anthocyanin content (TAC) and percent polymeric color (PPC) from blueberry juices of different anthocyanin/GA (GA) molar ratios at 40 °C for 10 days were respectively shown in Fig. 2A, B. As demonstrated by Fig. 2A, the addition of GA with higher molar ratios enhanced the retention rate of TAC in blueberry juice (P < 0.05). The improvement of anthocyanin stability in blueberry juice is due to the effect of the added polyphenolics (e.g. GA). Phenolic compound act as antioxidants can protect anthocyanins from oxidation (Roidoung et al. 2016; Juurlink et al. 2014). And phenolic compound as copigments can interact with anthocyanins by binding covalent or noncovalent bond with anthocyanins to form anthocyanin-cofactor complexes to resist the non-oxidative degradation of anthocyanins, such as hydration and further molecular division (Giustim and Wrolstad 2003; Trouillas et al. 2016; Zhang et al. 2018). Previous studies indicated that the addition of caffeic acid significantly increased (P < 0.05) the stability of anthocyanins in model systems of Cabernet Sauvignon grape extracts (Gris et al. 2007). The addition of chlorogenic acid increased the stability of anthocyanin in blackberry juice during storage (Kopjar et al. 2012). And quercetagetin was effective to stabilize the anthocyanins of grape skin (Xu et al. 2015). Gallic acid can improve the stability of anthocyanin in anthocyanin-rich foods (Navruz et al. 2016; Liu et al.2016; Zhang et al. 2018; Kopjar and Pilizota 2009; Srinivas et al. 2010).
Fig. 2.
The total anthocyanin content (a) and percent polymeric color (b) in blueberry juices with different anthocyanins/gallic acid molar ratios at 40 °C for 10 days. GA: gallic acid; BJ: blueberry juice; BJ + GA(1:0), BJ + GA(1:1), BJ + GA(1:3) and BJ + GA(1:5): blueberry juice with 1:0, 1:1, 1:3 and 1:5 anthocyanin/gallic acid molar ratio; TAC: total anthocyanin content; PPC: percent polymeric color. Different lowercase letters represent significant differences between samples (P < 0.05)
Furthermore, Retention rate of TAC in blueberry juice increased with the increasing of the anthocyanins/GA molar ratio. It had the highest retention rate of TAC in the blueberry juice with the anthocyanins/GA molar ratio of 1:5. The result showed that the copigmentation of high content GA was more beneficial to improving the stability of anthocyanin in blueberry juice. A large number of studies have found that the copigmentation effect become stronger with the increase of the concentration of copigments (Brouillard et al. 1989; Ghasemifar and Saeidian 2014). At the temperature of 20°C and pH 2.7–5.7, the higher the molar ratio of chlorogenic acid was, the stronger the copigmentation effect of anthocyanins in the aqueous solutions was (Mazza and Brouillard 1990). Sari (2016) also found that the anthocyanin retention rate increased with the increase of the molar ratio of GA in copigmented solution.
Percent polymeric color (PPC) can represent a measure of the pigment resistance to bleaching, and it can also show the polymerization of anthocyanin (Cao et al. 2011). As shown in Fig. 2B, copigment GA had a positive effect on PPC in the blueberry juices with different anthocyanin/GA molar ratios. PPC in the blueberry juices declined as the increase of the anthocyanin/GA molar ratio. Lowest PPC was found in the blueberry juice with 1:5 anthocyanin/GA molar ratio. With the increase of copigment concentration, the decrease of PPC values may be attributed to delay the gradual degradation of anthocyanins or condensation reactions of anthocyanins with protein and other phenolics (Zou et al. 2016).
Characterisation of anthocyanins in blueberry juice and effect of copigmentation on stability of individual anthocyanins
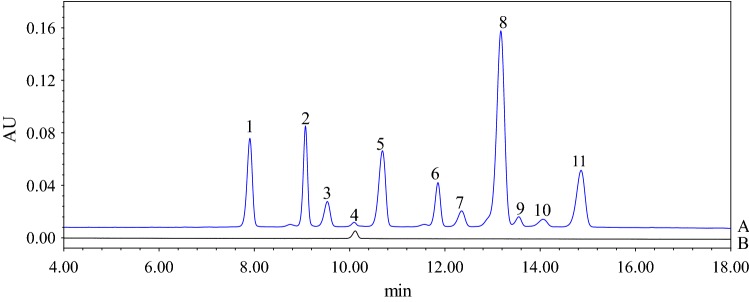
The anthocyanin profiles of blueberry juice and the cyanidin-3-glucoside standard were present in Fig. 3. Eleven anthocyanins were identified by chromatographic (Fig. 3). Individual anthocyanins were further detected by UPLC-DAD-ESI/MS (Table S1). According to the mass spectrum (Table S1) and previous report (Prior et al. 2001; You et al. 2011), eleven anthocyanins were identified and they belong to 5 main groups of anthocyanidins which include cyanidin (Cyd), delphinidin (Dpd), peonidin (Pnd), malvidin (Mvd), and petunidin (Ptd). The chromatograph analysis (Fig. 3) showed that delphinidin-3-glucoside and peonidin-3-glucoside were the major anthocyanins in the blueberry juice, and the result was in accord with Pan et al. (2014).
Fig. 3.
UPLC chromatograms of blueberry juice (A) and cyanidin-3-glucoside standard (B) at 520 nm. The chromatographic peak, 1: Delphinidin-3-galactoside; 2: Delphinidin-3-glucoside; 3: Cyanidin-3-galactoside; 4: Cyanidin-3-glucoside; 5: Petunidin-3-galactoside; 6: Petunidin-3-galactoside; 7: Peonidin-3-galactoside; 8: Peonidin-3-glucoside; 9: Malvidin-3-galactoside; 10: Malvidin-3-glucoside; 11: Malvidin-3- arabinoside
Copigmentation effect of GA on individual anthocyanins in blueberry juice storage for 10 days at 40 °C was investigated by UPLC (Table 1). Table 1 indicates that, as a main anthocyanin in all blueberry juices, the content of peonidin-3-glucoside from GA (1:0), GA (1:1), GA (1:3) and GA (1:5) juices were 34.2, 38.36, 42.08 and 51.68 mg/L, respectively.
Table 1.
Contents of individual anthocyanins of blueberry juices with different anthocyanins/gallic acid molar ratios after storage for 10 days at 40 °C
| Anthocyanin (mg/L) | RT (min) | Blueberry juices | |||
|---|---|---|---|---|---|
| BJ + GA(1:0) | BJ + GA(1:1) | BJ + GA(1:3) | BJ + GA(1:5) | ||
| Delphinidin-3-galactoside | 7.867 | 8.24 ± 0.67a | 10.97 ± 0.79b | 11.02 ± 0.93b | 15.86 ± 0.50c |
| Delphinidin-3-glucoside | 9.069 | 6.9 ± 0.45a | 7.04 ± 0.62a | 8.41 ± 0.91a | 10.25 ± 1.02b |
| Cyanidin-3-galactoside | 9.524 | 3.52 ± 0.12a | 3.66 ± 0.37a | 4.20 ± 0.34a | 6.51 ± 0.40b |
| Cyanidin-3-glucoside | 10.080 | 1.71 ± 0.06a | 1.32 ± 0.13a | 1.82 ± 0.40a | 1.69 ± 0.33a |
| Petunidin-3-galactoside | 10.667 | 10.59 ± 0.97a | 12.60 ± 1.02ab | 13.52 ± 1.88b | 18.28 ± 1.56c |
| Petunidin-3-glucoside | 11.835 | 3.99 ± 0.29a | 4.18 ± 0.48ab | 4.93 ± 0.56bc | 5.65 ± 0.51c |
| Peonidin-3-galactoside | 12.343 | 3.06 ± 0.18a | 3.60 ± 0.26ab | 3.88 ± 0.35b | 5.17 ± 0.39c |
| Peonidin-3-glucoside | 13.169 | 34.2 ± 2.88a | 38.36 ± 2.39ab | 42.08 ± 2.88b | 51.68 ± 2.99c |
| Malvidin-3-galactoside | 13.542 | 1.60 ± 0.11a | 1.75 ± 0.14a | 1.84 ± 0.23a | 2.50 ± 0.20b |
| Malvidin-3-glucoside | 14.083 | 3.04 ± 0.24a | 3.14 ± 0.26a | 3.51 ± 0.30a | 4.37 ± 0.31b |
| Malvidin-3- arabinoside | 14.879 | 9.22 ± 0.78a | 10.67 ± 0.83ab | 11.21 ± 0.91b | 15.86 ± 0.89c |
GA: gallic acid; BJ: blueberry juice; BJ + GA(1:0), BJ + GA(1:1), BJ + GA(1:3) and BJ + GA(1:5): blueberry juice with 1:0, 1:1, 1:3 and 1:5 anthocyanin/gallic acid molar ratio. Significance testing among the different samples was performed by one-way ANOVA followed by Duncan’s range test. Different lowercase letters represent significant differences between samples (P < 0.05)
Most of monomeric anthocyanin of juices (except Cyanidin-3-glucoside) at molar anthocyanins/ratio of 1:1, 1:3 and 1:5 increased than the juice without GA, while the addition of molar anthocyanins/GA ratio of 1:1 led to slight increase (Table 1). And with the increment of adding proportion on GA, the contents of monomeric anthocyanins of juice were gradually improved compared with the juice without GA (except Cyanidin-3-glucoside). GA exhibits potent antioxidant properties (Abdelwahed et al. 2007), a reason for higher antioxidant activities of juices protected anthocyanins from oxidative degradation with the increment of the addition proportion of anthocyanins/GA molar ratio. Gomez-Miguez et al. (2006) have observed a similar phenomenon that the presence of the caffeic acid at 1:1 copigment/pigment molar ratio does not impede the loss of the anthocyanin in model wine solutions. GA could improve the stabilities of cyanidin-3-rutinoside and cyanidin-3-glucosylrutinoside in sour cherry juice concentrates by protecting anthocyanins from oxidative degradation due to GA high antioxidant capacities (Navruz et al. 2016; Gil et al. 2000).
In the current study, with the increment of the addition proportion of anthocyanins/GA molar ratio, the content change of Cyanidin-3-glucoside showed the small fluctuations. Many factors have influenced copigmentation of monomeric anthocyanin, such as anthocyanin structure, concentration of anthocyanin relative to copigment and copigment structure (Eiro and Heinonen 2002). Besides, GA has a different protective effect on different monomeric anthocyanin, which may be related to the complexity of juice system during storage. Gallic acid, approximately from 300 to 928 mg/kg, has been explored in the production of sour cherry juice and Cabernet Sauvignon wine, and it has been found to retain more anthocyanins in juices and wines (Navruz et al. 2016; Liu et al.2016; Zhang et al. 2018). GA as a good copigment (Kopjar and Pilizota 2009; Srinivas et al. 2010), it played an important role in enhancing the stability of total individual anthocyanins in blueberry juice.
Stability of anthocyanin in blueberry juices storage at 4 °C, 25 °C and 40 °C
The retention rate of total anthocyanin in different blueberry juices during storage at 4 °C, 25 °C and 40 °C were given in Fig. 4A–C. The results showed that retention rate of total anthocyanin content (TAC) in blueberry juices presented decrease tendency with increasing of storage period at different storage temperature. Among them, the blueberry juice storaged at 4 °C had the highest retention rate of TAC (62.23–71.69%, 40 days), followed by 25 °C (12.83–23.04%, 40 days) and 40 °C (27.57–32.35%, 10 days). Anthocyanin, as a heat-sensitive substance, is highly susceptible to degradation under high temperature, and anthocyanins degradation could be accelerated with increase of storage temperatures, so low temperature is an effective method to delay anthocyanins degradation of blueberry juice (Syamaladevi et al. 2012; Kechinski et al. 2010; Buckow et al. 2010).
Fig. 4.
Changes in retention rate of total anthocyanin content of blueberry juices at a molar anthocyanins/gallic acid ratio of 1:5 during storage at 4 °C (A), 25 °C (B) and 40 °C (C). GA: gallic acid; BJ: blueberry juice; BJ + GA(1:0) and BJ + GA(1:5): blueberry juice with 1:0 and 1:5 anthocyanin/gallic acid molar ratio; TAC: total anthocyanin content. Different lowercase letters represent significant differences between samples (P < 0.05)
During storage period at different temperature, the anthocyanin degradation of blueberry juices with GA was significantly slower than the juice without GA (P < 0.05). The blueberry juice copigmented with GA had higher retention rate (4.23–13.65%) of TAC than untreated juice. In addition to higher antioxidant activities of GA protected anthocyanins from oxidative degradation (Abdelwahed et al. 2007; Navruz et al. 2016; Gil et al. 2000), GA and anthocyanin pigments in blueberry juice could form stable pigment-cofactor complexes through molecule association, which inhibits the hydration of anthocyanins and improves the retention rate of anthocyanins (Chen and Hrazdina 1981; Galli and Clemente 2013). Pan et al. (2014) also reported that anthocyanins in juices with copigment showed gentle downward trend, while control showed a faster downward trend during storage.
Effect of copigmentation on color stability
Color is considered the basis for assessing food quality. Moreover, color as a key sensory feature is thought to influence consumers’ preference, acceptability and ultimate choice of food (Zhang and Wang 2017; Bridle and Timberlake 1997). With the aim of evaluating the effect of copigmentation on the color expression of blueberry juice, chromatic analyses in the CIELab space were performed. The chromaticity parameters (L*, C*, and H*) of all juices were shown in Table 2. During storage, the addition of the copigment to the blueberry juice induced a decrease in lightness (L*) and a rise in chroma (C*) compared to juice without GA, indicating that the color of copigmented juice samples was more intense and saturated (Pan et al. 2014). After seventh days, juices with GA showed significantly (P < 0.05) lower value of hue angle (H*) and lightness (L*) compared with juice without GA, reflecting a more reddish color tonality of copigmented solutions. These changes were more marked with increasing copigment concentration. As reported by Shikov et al. (2008), the color stability was increased due to copigmented anthocyanins. The more color saturation are also connected with hyperchromic effect produced by copigmentation (Gomez-Miguez et al. 2006). The interaction between the anthocyanin and cofactors leads to hyperchromic and bathochromic effects, enhancing color intensity and stability (Marković et al. 2005; Zhang et al. 2015). Previous studies have shown that copigmentation can stabilize the color of natural anthocyanins (Gras et al. 2016; Chitgar et al. 2018; Fan et al. 2019).
Table 2.
Change of chromatic parameters lightness (L*), chroma (C*) and hue angle (H*) of blueberry juices with different anthocyanins/gallic acid molar ratios during storage at 40 °C
| Chromatic parameters | Samples | Storage (days) | ||||
|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 7 | 10 | ||
| L* | BJ + GA(1:0) | 1.58 ± 0.02Aa | 6.11 ± 0.01Bc | 8.61 ± 0.02Cd | 10.58 ± 0.01Dd | 11.11 ± 0.01Ed |
| BJ + GA(1:1) | 1.56 ± 0.02Aa | 6.07 ± 0.02Bb | 8.18 ± 0.01Cc | 9.88 ± 0.01Dc | 10.49 ± 0.02Ec | |
| BJ + GA(1:3) | 1.55 ± 0.02Aa | 6.06 ± 0.01Bb | 8.07 ± 0.03Cb | 9.78 ± 0.02Db | 10.41 ± 0.01Eb | |
| BJ + GA(1:5) | 1.55 ± 0.02Aa | 5.48 ± 0.01Ba | 7.49 ± 0.06Ca | 9.67 ± 0.02 Da | 10.26 ± 0.01Ea | |
| C* | BJ + GA(1:0) | 11.54 ± 0.13Aa | 33.92 ± 0.10Ba | 39.12 ± 0.08Ca | 42.56 ± 0.12 Da | 43.99 ± 0.11Ea |
| BJ + GA(1:1) | 11.56 ± 0.09Aa | 35.90 ± 0.13Bb | 41.27 ± 0.10Cc | 42.76 ± 0.09Db | 44.51 ± 0.13Eb | |
| BJ + GA(1:3) | 11.58 ± 0.08Aa | 35.79 ± 0.07Bb | 40.43 ± 0.09Cb | 42.89 ± 0.06Db | 44.47 ± 0.14Eb | |
| BJ + GA(1:5) | 11.57 ± 0.09Aa | 35.81 ± 0.08Bb | 40.26 ± 0.14Cb | 43.16 ± 0.10Dc | 45.83 ± 0.11Ec | |
| H* | BJ + GA(1:0) | 13.58 ± 0.04Aa | 17.06 ± 0.03Bb | 21.09 ± 0.04Ca | 23.58 ± 0.04Dd | 24.72 ± 0.03Ed |
| BJ + GA(1:1) | 13.56 ± 0.05Aa | 17.00 ± 0.06Bb | 20.41 ± 0.03Ca | 22.78 ± 0.05Dc | 23.98 ± 0.04Ec | |
| BJ + GA(1:3) | 13.58 ± 0.03Aa | 17.00 ± 0.06Bb | 20.22 ± 0.05Ca | 22.58 ± 0.08Db | 23.81 ± 0.03Eb | |
| BJ + GA(1:5) | 13.55 ± 0.04Aa | 16.17 ± 0.04Ba | 19.29 ± 2.04Ca | 21.58 ± 0.04 Da | 23.72 ± 0.04Ea | |
GA: gallic acid; BJ: blueberry juice; BJ + GA(1:0), BJ + GA(1:1), BJ + GA(1:3) and BJ + GA(1:5): blueberry juice with 1:0, 1:1, 1:3 and 1:5 anthocyanin/gallic acid molar ratio. Significance testing among the different samples was performed by one-way ANOVA followed by Duncan’s range test. Different uppercase letters in the same row represent significant differences within sample and different lowercase letters represent significant differences between samples (P < 0.05)
Conclusion
According to the results acquired in this work, the high amount of gallic acid (GA) added into blueberry juice did indeed delay the degradation of main anthocyanins and prolong the color deterioration during the storage period. GA led to the hyperchromic effect and the increase in main anthocyanin content indicating the existence of copigmentation in blueberry juice. GA treatment do not only influence the color characteristics and anthocyanin compounds of juice, but also have a longer effect on the evolution of color and anthocyanins in juice during storage. In addition, the GA as a phenolic acid can bring juices appropriate taste and good functionality, improving the quality of blueberry juice. Therefore, the application of GA could be as a feasible and promising novel technology to produce a stabile blueberry juice.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Anhui Province Natural Science Foundation (1508085SMC217) and Anhui Province District Key Project (15czz03101).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lingli Zhang and Wenbo Wang have contributed equally to this work.
References
- Abdelwahed A, Bouhlel I, Skandrani I, Valenti K, Kadri M, Guiraud P, Steiman R, Mariotte AM, Ghedira K, Laporte F, Dijoux-Franca MG, Chekir-Ghedira L. Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus confirmation by microarray expression profiling. Chem Biol Interact. 2007;165(1):1–13. doi: 10.1016/j.cbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Benn T, Kim B, Park YK, Wegner CJ, Harness E, Nam TG, Kim DO, Lee JS, Lee JY. Polyphenol-rich blackcurrant extract prevents inflammation in diet-induced obese mice. J Nutr Biochem. 2014;25(10):1019–1025. doi: 10.1016/j.jnutbio.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Brenes CH, Del Pozo-Insfran D, Talcott ST. Stability of copigmented anthocyanins and ascorbic acid in a grape juice model system. J Agric Food Chem. 2005;53(1):49–56. doi: 10.1021/jf049857w. [DOI] [PubMed] [Google Scholar]
- Bridle P, Timberlake CF. Anthocyanins as natural food colours—selected aspects. Food Chem. 1997;58(1):103–109. [Google Scholar]
- Brouillard R, Mazza G, Saad Z, Albrecht-Gary AM, Cheminat A. The co-pigmentation reaction of anthocyanins: a microprobe for the structural study of aqueous solutions. J Am Chem Soc. 1989;111(7):2604–2610. [Google Scholar]
- Buckow R, Kastell A, Terefe NS, Versteeg C. Pressure and temperature effects on degradation kinetics and storage stability of total anthocyanins in blueberry juice. J Agric Food Chem. 2010;58(18):10076–10084. doi: 10.1021/jf1015347. [DOI] [PubMed] [Google Scholar]
- Cao XM, Zhang Y, Zhang FS, Wang YT, Yi JY, Liao XJ. Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J Sci Food Agric. 2011;91(5):877–885. doi: 10.1002/jsfa.4260. [DOI] [PubMed] [Google Scholar]
- Castaneda-Ovando A, Pacheco-Hernández ML, Páez-Hernández ME, Rodríguez JA, Galán-Vidal CA. Chemical studies of anthocyanins: a review. Food Chem. 2009;113(4):859–871. [Google Scholar]
- Chen LJ, Hrazdina G. Structural aspects of anthocyanin-flavonoid complex formation and its role in plant color. Phytochemistry. 1981;20(2):297–303. [Google Scholar]
- Chitgar MF, Aalami M, Kadkhodaee R, Maghsoudlou Y, Milani E. Effect of thermosonication and thermal treatments on phytochemical stability of barberry juice copigmented with ferulic acid and licorice extract. Innov Food Sci Emerg Technol. 2018;50:102–111. [Google Scholar]
- Chung C, Rojanasasithara T, Mutilangi W, McClements DJ. Stabilization of natural colors and nutraceuticals: inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chem. 2016;212:596–603. doi: 10.1016/j.foodchem.2016.06.025. [DOI] [PubMed] [Google Scholar]
- Daglia M, Di Lorenzo A, Nabavi S, Talas Z, Nabavi S. Polyphenols: well beyond the antioxidant capacity: gallic acid and related compounds as neuroprotective agents: you are what you eat! Curr Pharm Biotechnol. 2014;15(4):362–372. doi: 10.2174/138920101504140825120737. [DOI] [PubMed] [Google Scholar]
- Diaconeasa Z, Leopold L, Rugina D, Ayvaz H, Socaciu C. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int J Mol Sci. 2015;16(2):2352–2365. doi: 10.3390/ijms16022352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiro MJ, Heinonen M. Anthocyanin color behavior and stability during storage: effect of intermolecular copigmentation. J Agric Food Chem. 2002;50(25):7461–7466. doi: 10.1021/jf0258306. [DOI] [PubMed] [Google Scholar]
- Fan LL, Wang Y, Xie PJ, Zhang LX, Li YH, Zhou JZ. Copigmentation effects of phenolics on color enhancement and stability of blackberry wine residue anthocyanins: chromaticity, kinetics structural simulation. Food Chem. 2019;275:299–308. doi: 10.1016/j.foodchem.2018.09.103. [DOI] [PubMed] [Google Scholar]
- Fanzone M, González-Manzano S, Pérez-Alonso J, Escribano-Bailón MT, Jofré V, Assof M, Santos-Buelga C. Evaluation of dihydroquercetin-3-O-glucoside from Malbec grapes as copigment of malvidin-3-O-glucoside. Food Chem. 2015;175:166–173. doi: 10.1016/j.foodchem.2014.11.123. [DOI] [PubMed] [Google Scholar]
- Galli D, Clemente E. Influence of organic acids on the stability of anthocyanins extracted from residues of grape processing. J Food Agric Environ. 2013;11(1):36–39. [Google Scholar]
- Ghasemifar E, Saeidian S. The effects catechin on stability of grape anthocyanin-copigment complex. Int J Sci Res Environ Sci. 2014;2(4):150–155. [Google Scholar]
- Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48(10):4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Giustim M, Wrolstad RE. Acylated anthocyanins from edible sources and their application food systems. Biochem Eng J. 2003;14(3):217–255. [Google Scholar]
- Gomez-Miguez M, Gonzalez-Manzano S, Escribano-Bailon MT, Heredia FJ, Santos-Buelga C. Influence of different phenolic copigments on the color of malvidin 3-glucoside. J Agric Food Chem. 2006;54(15):5422–5429. doi: 10.1021/jf0604586. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Manzano S, Mateus N, Freitas VD, Santos-Buelga C. Influence of the degree of polymerisation in the ability of catechins to act as anthocyanin copigments. Eur Food Res Technol. 2008;227:83–92. [Google Scholar]
- Gras CC, Bogner H, Carle R, Schweiggert RM. Effect of genuine non-anthocyanin phenolics and chlorogenic acid on color and stability of black carrot (Daucus carota ssp sativus var. atrorubens Alef.) anthocyanins. Food Res Int. 2016;85:291–300. doi: 10.1016/j.foodres.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Gris EF, Ferreira EA, Falcao LD, Bordignon-Luiz MT. Caffeic acid copigmentation of anthocyanins from Cabernet Sauvignon grape extracts in model systems. Food Chem. 2007;100(3):1289–1296. [Google Scholar]
- He B, Zhang LL, Yue XY, Liang J, Jiang J, Gao XL, Yue PX. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016;204:70–76. doi: 10.1016/j.foodchem.2016.02.094. [DOI] [PubMed] [Google Scholar]
- Hernandez-Herrero JA, Frutos MJ. Influence of rutin and ascorbic acid in colour, plum anthocyanins and antioxidant capacity stability in model juices. Food Chem. 2015;173:495–500. doi: 10.1016/j.foodchem.2014.10.059. [DOI] [PubMed] [Google Scholar]
- Juurlink BH, Azouz HJ, Aldalati AM, AlTinawi BM, Ganguly P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr J. 2014;13:1475–2891. doi: 10.1186/1475-2891-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kechinski CP, Guimaraes PVR, Norena CPZ, Tessaro IC, Marczak LDF. Degradation kinetics of anthocyanin in blueberry juice during thermal treatment. J Food Sci. 2010;75(2):C173–C176. doi: 10.1111/j.1750-3841.2009.01479.x. [DOI] [PubMed] [Google Scholar]
- Kopjar M, Pilizota V. Copigmentation effect of phenolic compounds on red currant juice anthocyanins during storage. Croat J Food Sci Technol. 2009;1(2):16–20. [Google Scholar]
- Kopjar M, Jaksic K, Pilizota V. Influence of sugars and chlorogenic acid addition on anthocyanin content, antioxidant activity and color of blackberry juice during storage. J Food Process Preserv. 2012;36(6):545–552. [Google Scholar]
- Liu Y, Zhang B, He F, Duan CQ, Shi Y. The influence of prefermentative addition of gallic acid on the phenolic composition and chromatic characteristics of Cabernet Sauvignon wines. J Food Sci. 2016;81(7):C1669–C1678. doi: 10.1111/1750-3841.13340. [DOI] [PubMed] [Google Scholar]
- Lorenzo C, Pardo F, Zalacain A, Alonso GL, Salinas MR. Effect of red grapes co-winemaking in polyphenols and color of wines. J Agric Food Chem. 2005;53(19):7609–7616. doi: 10.1021/jf050848c. [DOI] [PubMed] [Google Scholar]
- Marković D, Petranović NA, Baranac JM. A spectrophotometric study of the copigmentation of malvin with caffeic and ferulic acids. J Agric Food Chem. 2000;48(11):5530–5536. doi: 10.1021/jf000038v. [DOI] [PubMed] [Google Scholar]
- Marković JMD, Petranović NA, Baranac JM. The copigmentation effect of sinapic acid on malvin: a spectroscopic investigation on colour enhancement. J Photochem Photobiol B. 2005;78(3):223–228. doi: 10.1016/j.jphotobiol.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Mazza G, Brouillard R. The mechanism of copigmentation of anthocyanins in aqueous solutions. Phytochemistry. 1990;29(4):1097–1102. [Google Scholar]
- Michalska A, Łysiak G. Bioactive compounds of blueberries: post-harvest factors Influencing the nutritional value of products. Int J Mol Sci. 2015;16(8):18642–18663. doi: 10.3390/ijms160818642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Schantz M, Richling E. High performance liquid chromatography analysis of anthocyanins in bilberries (Vaccinium myrtillus L.), blueberries (Vaccinium corymbosum L.), and corresponding juices. J Food Sci. 2012;77(4):C340–C345. doi: 10.1111/j.1750-3841.2011.02605.x. [DOI] [PubMed] [Google Scholar]
- Navruz A, Türkyılmaz M, Özkan M. Colour stabilities of sour cherry juice concentrates enhanced with gallic acid and various plant extracts during storage. Food Chem. 2016;197(Part A):150–160. doi: 10.1016/j.foodchem.2015.10.098. [DOI] [PubMed] [Google Scholar]
- Pan YZ, Guan Y, Wei ZF, Peng X, Li TT, Qi XL, Zu YG, Fu YJ. Flavonoid C-glycosides from pigeon pea leaves as color and anthocyanin stabilizing agent in blueberry juice. Ind Crops Prod. 2014;58:142–147. [Google Scholar]
- Petrova I, Shikov V, Gandova V, Mihalev K, Dimitrov DI. Spectrophotometric and thermodynamic study on the co-pigmentation interaction between strawberry anthocyanins and quercetin in model systems. Bulg Chem Commun. 2017;49(1):115–120. [Google Scholar]
- Prior RL, Cao GH, Martin A, Sofic E, McEwen J, O’Brien C, Lischner N, Ehlenfeldt M, Kalt W, Krewer G. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of vaccinium species. J Agric Food Chem. 1998;46(7):2686–2693. [Google Scholar]
- Prior RL, Lazarus SA, Cao G, Muccitelli H, Hammerstone JF. Identification of procyanidins and anthocyanins in blueberries and cranberries (Vaccinium spp.) using high performance liquid chromatography/mass spectrometry. J Agric Food Chem. 2001;49(3):1270–1276. doi: 10.1021/jf001211q. [DOI] [PubMed] [Google Scholar]
- Roidoung S, Dolan KD, Siddiq M. Gallic acid as a protective antioxidant against anthocyanin degradation and color loss in vitamin-C fortified cranberry juice. Food Chem. 2016;210:422–427. doi: 10.1016/j.foodchem.2016.04.133. [DOI] [PubMed] [Google Scholar]
- Sari F. The copigmentation effect of different phenolic acids on Berberis crataegina anthocyanins. J Food Process Preserv. 2016;40(3):422–430. [Google Scholar]
- Sari P, Wijaya CH, Sajuthi D, Supratman U. Colour properties, stability, and free radical scavenging activity of jambolan (Syzygium cumini) fruit anthocyanins in a beverage model system: natural and copigmented anthocyanins. Food Chem. 2012;132(4):1908–1914. [Google Scholar]
- Shikov V, Kammerer DR, Mihalev K, Mollov P, Carle R. Heat stability of strawberry anthocyanins in model solutions containing natural copigments extracted from rose (Rosa damascena Mill.) petals. J Agric Food Chem. 2008;56(18):8521–8526. doi: 10.1021/jf801946g. [DOI] [PubMed] [Google Scholar]
- Signorelli P, Fabiani C, Brizzolari A, Paroni R, Casas J, Fabrias G, Caretti A. Natural grape extracts regulate colon cancer cells malignancy. Nutr Cancer Int J. 2015;67(3):494–503. doi: 10.1080/01635581.2015.1004591. [DOI] [PubMed] [Google Scholar]
- Srinivas K, King JW, Howard LR, Monrad JK. Solubility of gallic acid, catechin, and protocatechuic acid in subcritical water from 298.75 K to 415.85 K. J Chem Eng Data. 2010;55(9):3101–3108. [Google Scholar]
- Syamaladevi RM, Andrew PK, Davies NM, Walters T, Sablani SS. Storage effects on anthocyanins, phenolics and antioxidant activity of thermally processed conventional and organic blueberries. J Sci Food Agric. 2012;92(4):916–924. doi: 10.1002/jsfa.4670. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Sancho-Garcia JC, de Freitas VA, Gierschner J, Otyepka M, Dangles O. Stabilizing and modulating color by copigmentation: insights from theory and experiment. Chem Rev. 2016;116(9):4937–4982. doi: 10.1021/acs.chemrev.5b00507. [DOI] [PubMed] [Google Scholar]
- West ME, Mauer LJ. Color and chemical stability of a variety of anthocyanins and ascorbic acid in solution and powder forms. J Agric Food Chem. 2013;61(17):4169–4179. doi: 10.1021/jf400608b. [DOI] [PubMed] [Google Scholar]
- Xu HG, Liu X, Yan Q, Yuan F, Gao YX. A novel copigment of quercetagetin for stabilization of grape skin anthocyanins. Food Chem. 2015;166:50–55. doi: 10.1016/j.foodchem.2014.05.125. [DOI] [PubMed] [Google Scholar]
- You Q, Wang BW, Chen F, Huang ZL, Wang X, Luo PJG. Comparison of anthocyanins and phenolics in organically and conventionally grown blueberries in selected cultivars. Food Chem. 2011;125(1):201–208. [Google Scholar]
- Zhang QA, Wang TT. Effect of ultrasound irradiation on the evolution of color properties and major phenolic compounds in wine during storage. Food Chem. 2017;234:372–380. doi: 10.1016/j.foodchem.2017.05.022. [DOI] [PubMed] [Google Scholar]
- Zhang B, Liu R, He F, Zhou PP, Duan CQ. Copigmentation of malvidin-3-O-glucoside with five hydroxybenzoic acids in red wine model solutions: experimental and theoretical investigations. Food Chem. 2015;170:226–233. doi: 10.1016/j.foodchem.2014.08.026. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Li N, Gao XL. Phenolic compounds and antioxidant activity of wines fermented using ten blueberry varieties. Am J Food Technol. 2016;11(6):291–297. [Google Scholar]
- Zhang XK, He F, Zhang B, Reeves MJ, Liu Y, Zhao X, Duan CQ. The effect of prefermentative addition of gallic acid and ellagic acid on the red wine color, copigmentation and phenolic profiles during wine aging. Food Res Int. 2018;106:568–579. doi: 10.1016/j.foodres.2017.12.054. [DOI] [PubMed] [Google Scholar]
- Zou H, Lin TT, Bi XF, Zhao L, Wang YT, Liao XJ. Comparison of high hydrostatic pressure, high-pressure carbon dioxide and high-temperature short-time processing on quality of mulberry juice. Food Bioprocess Technol. 2016;9(2):217–231. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.