Abstract
The present work explored the influence of individual and combinations of citral and linalool along with different Ostwald ripening inhibitors on the nanoemulsion stability and their antibacterial activity against Listeria monocytogenes. Nine different nanoemulsions (N1–N9) containing individual or combinations of citral, linalool with or without ripening inhibitors (medium chain triglycerides, coconut oil, sesame oil and castor oil) were formulated with 5% Tween-80 using ultrasonic emulsification. N1 formulation containing 5% citral without ripening inhibitors showed the least mean droplet diameter of 20.44 nm. Addition of linalool with the citral nanoemulsions was found to have deleterious effect on the thermodynamic and kinetic stabilities. Incorporation of ripening inhibitors controlled the increase of droplet size and polydispersity index in N6–N9 during the 90 days storage period, but decreased their antibacterial activity. N8 formulation containing sesame oil as ripening inhibitor was found to be the best in controlling Ostwald ripening. N1 formulation which showed the best antibacterial activity (MIC 0.312%) was found to disrupt the bacterial membrane integrity. N1 formulation also showed a promising biofilm inhibition of 83.51%. Therefore, N1 formulation containing 5% citral could be recommended as an efficient disinfectant against food-borne pathogen Listeria monoctyogenes in food industry. Moreover, addition of sesame oil in the nanoemulsion formulation of citral or linalool could increase their stability.
Keywords: Citral, Linalool, Nanoemulsions, Ripening inhibitors, Sesame oil, Listeria monocytogenes
Introduction
Essential oils (EOs) are complex mixture of volatile compounds which exhibit strong antimicrobial activity against several food-borne pathogens (Ryu et al. 2018) and can be used in maintaining food safety and quality (Moghimi et al. 2017). Citral, a monoterpene which occurs naturally in EOs of lemongrass is well documented to also possess potent antibacterial activity against food-borne pathogens (Katsukawa et al. 2010; Lu et al. 2018). However, as the application of citral may require high concentration in food system to achieve the same effects as those demonstrated in vitro because of the influence of the various intrinsic properties of food like water content, fat, protein, pH, salt etc. (Burt 2004), it would be judicious to use synergistic effect of citral with other EOs compounds. Herein linalool, a monoterpene alcohol, commonly found in EOs of plants like coriander which has been reported to possess strong antibacterial activity against food-borne pathogen (Prakash et al. 2019), could be explored along with citral for synergistic antibacterial activity.
Even though the citral and linalool exhibit considerable antibacterial activity, the low water solubility, stability and strong sensory characteristics restrict their application in food system (Ryu et al. 2018). Encapsulating these compounds into nanoemulsion could represent a viable remedy for such limitations. Nanoemulsions are colloidal dispersions with mean droplet size < 100 nm and possess distinct properties like optical transparency, tunable rheology and robust stability (Prakash et al. 2018). It can be produced using either high-energy (high pressure homogenizer, microfluidizer and ultrasonicator) and low energy methods (spontaneous emulsification and phase inversion) (McClements 2015).
The small particle size of nanoemulsion ensures its better stability against gravitational separation and aggregation than conventional emulsions (Karthik et al. 2017). Nevertheless, nanoemulsions are thermodynamically unstable and thus tend to breakdown over time through different physicochemical mechanisms like coalescence, flocculation, Ostwald ripening and chemical degradation (McClements 2015). Ostwald ripening is the main instability process of nanoemulsions, in which mean droplet increases over time due to the diffusion of oil molecules from small to large droplets through intervening fluid (McClements and Rao 2011). Recent studies have emphasized on the development of nanoemulsions that have long kinetic stability for commercial applications (Moghimi et al. 2017; Chang et al. 2012). In this context, Ostwald ripening can be prevented by the use of water insoluble carriers like soybean oil, corn oil and sunflower oil as ripening inhibitors which can dissolve the EOs and avert the transmission of polar components to the aqueous phase (Donsì and Ferrari 2016).
Keeping this in mind, the objective of the current research is to formulate synergistic nanoemulsions of citral and linalool and also to examine the impact of different Ostwald ripening inhibitors like medium chain triglycerides (MCT), coconut, sesame and castor oil on the nanoemulsions stability and the antibacterial activity against Listeria monocytogenes. The selected formulation was also studied for its anti-biofilm activity.
Materials and methods
Materials
Citral and linalool were purchased from Sigma Aldrich (St. Louis, MO, USA). Ripening inhibitors coconut oil, sesame oil and castor oil were provided as kind gift by from a local oil mill, Tiruchirappalli, Tamil Nadu. The ripening inhibitor MCT (Medium chain triglycerides) was provided as a gift by Adchems Specialities, Bengaluru, India. Tween-80 was purchased from Himedia, Mumbai, India. Listeria monocytogenes (ATCC 19111) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA).
Preparation of nanoemulsions
The nanoemulsions were formulated by ultrasonication method described by Prakash et al. (2019), with slight modifications. Oil phase consisted of different ratios of citral and/or linalool mixed with 5% Tween 80 and 5% of different ripening inhibitors as shown in Table 1. Formulation N1 and N2 comprised of 5% citral and 5% linalool, respectively; N3, N4 and N5 comprised of different ratios of citral and linalool i.e., 25:75, 75:25, 50:50 v/v, respectively; N6, N7, N8 and N9 comprised of 50:50 v/v ratio of citral and linalool with 5% of different Ostwald ripening inhibitors i.e. MCT, coconut oil, sesame oil and castor oil, respectively. Initially, coarse emulsion was prepared by adding water to the oil phase, which was thereafter subjected to ultrasonication (PRO-250, Labman, Chennai, Tamilnadu, India) at 70 amplitude for 10 min with 30 s pulse on and 30 s off to reduce the droplet size. To reduce the heat generated during the ultrasonication, beaker containing emulsions was kept inside an ice bath.
Table 1.
Composition and characterization of citral and linalool nanoemulsions with or without different ripening inhibitors
| Sample code | Compound composition | Tween 80 | Ripening inhibitors | Zeta size | Polydispersity index (PDI) | Absorbance at 600 nm |
|---|---|---|---|---|---|---|
| N1 | 5% Citral | 5% Tween 80 | – | 20.44a ± 0.52 | 0.44a ± 0.03 | 1.48a ± 0.02 |
| N2 | 5% Linalool | 5% Tween 80 | – | 36.47b ± 1.28 | 0.43ac ± 0.04 | 2.64bj ± 0.01 |
| N3 | 1.25% Citral + 3.75% Linalool | 5% Tween 80 | – | 24.31ci ± 1.02 | 0.36bcd ± 0.41 | 0.80c ± 0.00 |
| N4 | 3.75% Citral + 1.25% Linalool | 5% Tween 80 | – | 25.05di ± 0.21 | 0.41ad ± 0.01 | 0.57d ± 0.00 |
| N5 | 2.5% Citral + 2.5% Linalool | 5% Tween 80 | – | 22.62ai ± 0.37 | 0.32d ± 0.02 | 0.89e ± 0.01 |
| N6 | 2.5% Citral + 2.5% Linalool | 5% Tween 80 | 5% Medium chain triglycerides | 105.05e ± 0.40 | 0.22e ± 0.01 | 2.91f ± 0.01 |
| N7 | 2.5% Citral + 2.5% Linalool | 5% Tween 80 | 5% Coconut oil | 88.80f ± 0.40 | 0.21e ± 0.00 | 2.67gj ± 0.01 |
| N8 | 2.5% Citral + 2.5% Linalool | 5% Tween 80 | 5% Sesame oil | 60.34g ± 0.70 | 0.18e ± 0.00 | 1.88h ± 0.04 |
| N9 | 2.5% Citral + 2.5% Linalool | 5% Tween 80 | 5% Castor oil | 126.53h ± 1.62 | 0.18e ± 0.01 | 1.74i ± 0.02 |
Values in the same column with different letter superscripts are significantly different (p < 0.05)
Characterization of nanoemulsions
The mean droplet size and polydispersity index (PDI) of the formulated nanoemulsions were measured using particle size analyzer (Zeta Sizer, Zeta NS, ZEN 3600, Malvern Instruments, Worcestershire, UK) at periodic intervals of 0th, 15th, 30th, 60th and 90th days. The samples were 100-folds diluted and analyzed in a disposable capillary cell (DTS1070, Malvern Instruments) at 25 °C with an equilibration time of 60 s. The turbidity of the formulated nanoemulsions was measured at 600 nm using an UV–visible spectrophotometer (Model Evolution 201, Thermo Scientific, Waltham, MA, USA).
The size and shape of selected nanoemulsion (N1) was also validated through transmission electron microscope (TEM), which was placed in a 200-mesh carbon coated copper grid (Electron Microscopy Science, Hatfield, PA, USA) and negatively stained with 2% (w/v) ammonium molybdate, dried and then visualized under TEM (JEOL-JEM 1011, Akishima, Tokyo, Japan).
Stability of nanoemulsions
Stability of the nanoemulsions was evaluated through the thermodynamic (Azeem et al. 2009) and kinetic stability studies (Ghosh et al. 2013). Thermodynamic studies involved three different thermo-mechanical stress conditions i.e. centrifugation, heating–cooling and freeze-thawing. For centrifugation, nanoemulsions were centrifuged for 800×g for 30 min at room temperature (Remi CM-12 Plus, Mumbai, Maharashtra, India). Heating–cooling involved storage of nanoemulsions at 45 °C and 4 °C and freeze–thawing involved storage at − 21 °C and 25 °C for 12 h each for three times. Any form of instability (phase separation or creaming) was visually observed at the end of stress conditions.
Kinetic stability of the formulations was evaluated by observing any form of phase separation or creaming at periodic intervals of 0th, 15th, 30th, 60th and 90th day in nanoemulsions stored at room temperature. Changes in droplet size and polydispersity index were also periodically investigated.
Antibacterial activity
The antibacterial activity of the nanoemulsion formulations against Listeria monocyotgenes (ATCC 19111) was determined according to the method described by Prakash et al. (2019). Pure citral and linalool were dissolved in 5% DMSO. Briefly, 50 μl of the serially diluted nanoemulsions (5–0.009%) were added to 50 μl of bacterial cell suspension and 100 μl of nutrient broth in a 96 well microtiter plate (Tarson, Kolkata, West Bengal, India). The plate was then incubated for 24 h at 37 °C and growth inhibition of the bacteria was visualized by addition of 20 µl of 5 mg/ml solution of MTT (methyl thiazolyldiphenyltetrazolium bromide; Sigma-Aldrich). The minimum inhibitory concentration (MIC) was determined as the lowest concentration of the samples which inhibited the bacterial growth. For determining the minimum bactericidal concentration (MBC), 100 μl of the aliquots from wells, where no visible growth was observed were plated on the nutrient agar. The plates were incubated for 24 h at 37 °C and observed for any viable colony. The lowest concentration that showed the absence of viable colony was the MBC.
Cell membrane integrity
The cell membrane integrity of Listeria monocytogenes treated with selected nanoemulsion formulation (N1) was studied by determining the release of cell contents as described by Sugumar et al. (2013) with slight modifications. For this, 10 ml of overnight grown bacterial culture at 37 °C was diluted 10 times and again incubated at 37 °C for 3 h. The culture was then centrifuged for 10 min at 4000×g, washed three times with sterile saline and finally re-suspended in 5 ml of sterile saline and the absorbance was adjusted to 0.2 at 600 nm to obtain a bacterial count of ~ 1 × 107 CFU/ml (0.5 McFarland turbidity). Thereafter, 0.5 ml of the bacterial suspension was added to 4.5 ml of the selected nanoemulsion at MIC and 2 × MIC. The sample mixture were then incubated at 37 °C with agitation for 1 h and then centrifuged at 3600×g for 10 min. The supernatant corresponding to the cytoplasmic contents released were measured at 260 and 280 nm.
To visualize the damage caused by the selected nanoemulsion (N1) on the cell membrane of Listeria monocytogenes, scanning electron microscopy (SEM) was performed as described by Prakash et al. (2019). Listeria monocytogenes cells (1 × 107 CFU/ml) treated with MIC of the selected nanoemulsion were fixed with 20% glutaraldehyde (Sigma-Aldrich) for 1 h and then washed with gradient alcohol concentrations of 30, 50, 70, 90 and 100%. The samples were thereafter fixed on a support, gold sputtered and visualized under SEM (Tescan Vega 3, Brno, Czech Republic).
Anti-biofilm activity
The anti-biofilm activity of the selected nanoemulsion (N1) was determined against Listeria monocytogenes using crystal violet assay (Banu et al. 2017). Briefly 100 μl of cell suspension (108 CFU/ml) was added to 1 ml of soyabean casein digest (SCD) broth (Himedia) containing 0.6% yeast extract and 50 μl of selected nanoemulsion (1/2 MIC) in a 24 well plate (NEST Biotechnology, Wuxi, Jiangsu, China) and incubated at 37 °C for 24 h. Following incubation, the spent media was discarded and stained with 0.4% crystal violet. After 30 min, the stain was discarded and washed with water to remove excess stain. The biofilms were then dissolved in 95% ethanol and quantified at 595 nm. The percent inhibition was calculated using the formula: [(Control OD − Test OD/Control OD) × 100].
The biofilm inhibition by selected nanoemulsion (N1) was also visualized under SEM. Listeria monocytogenes biofilms treated with or without 1/2 MIC of selected nanoemulsion were allowed to form on glass slides (1 × 1 cm) placed in a 24 well plate. Biofilms were then fixed with 20% glutaraldehyde, sequentially dehydrated in gradient percentage of ethanol (30, 50, 70, 90 and 100%), dried, gold spluttered and examined under SEM (Vega 3, Tescan, Brno, Czech Republic).
Statistical analysis
All the experiments were carried out in triplicates. Significant difference among means for each group was determined using ANOVA followed by Tukey post hoc test (p < 0.05) using GraphPad prism version 5.0 (GraphPad Software Inc., San Diego, CA, USA).
Results and discussion
Characterization of nanoemulsions
The characterization results of nanoemulsion formulations are shown in Table 1 and Fig. 1. Among the investigated formulations, N1 had the least mean average droplet diameter of 20.44 nm (Fig. 1a). The mean droplet diameter of nanoemulsions (N6–N9) was found to significantly increase upon addition of any of the ripening inhibitor. This could have been due to the relative difference in ultrasonication efficacy to reduce particle size upon differential interfacial interaction of oils with ripening inhibitors. In fact, the droplet size and distribution are dependent on the solubility and concentration of the components in the nanoemulsions (McClements and Rao 2011).
Fig. 1.
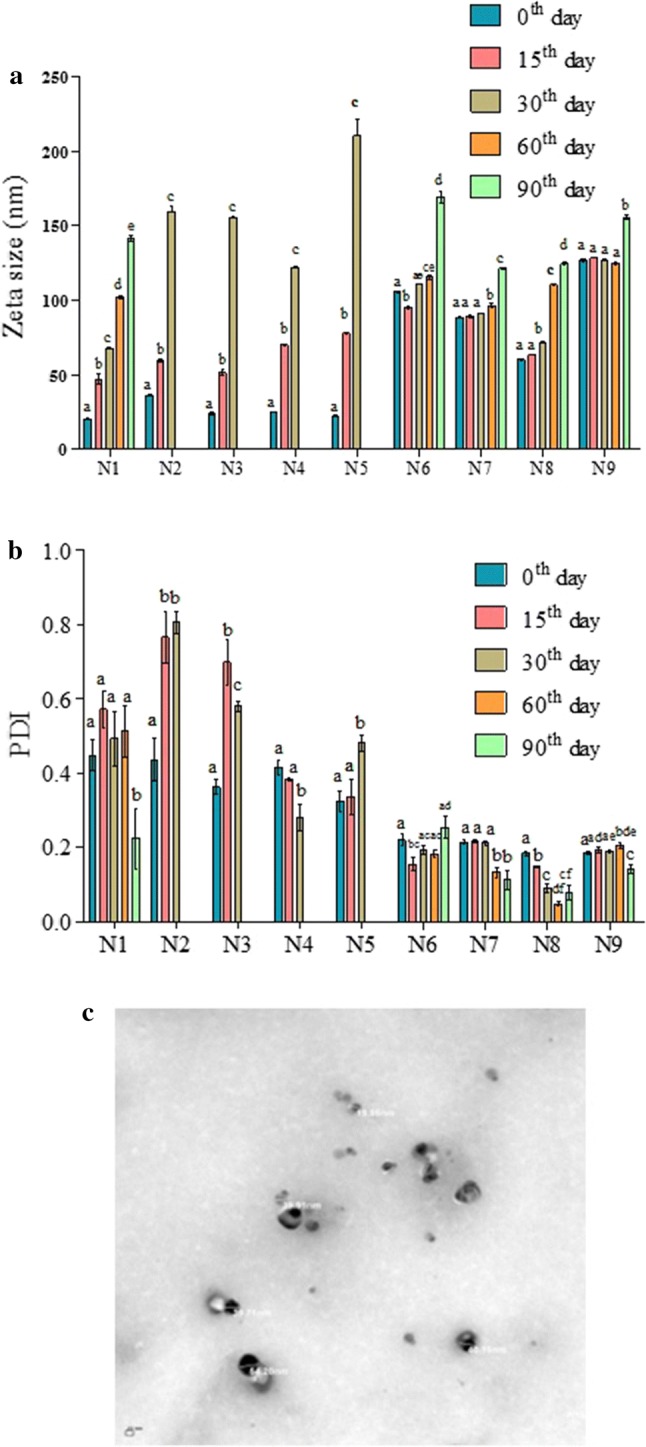
Changes in the mean droplet size (a) and polydispersity index (b) of nanoemulsion formulations during 90 days of storage. TEM image of the N1 formulation (c). Bars with asterisk indicate significant difference (p < 0.05)
Droplet size distribution in the nanoemulsions was evaluated through the polydispersity index (PDI). Small PDI values below 0.2 indicate uniformity among oil droplet sizes with monomodal distributions and better stability, whereas values close to 1 designate a heterogeneous distribution (Guerra-Rosas et al. 2017). In the present study, nanoemulsions containing ripening inhibitors i.e. N6, N7, N8 and N9 were monodispersed as they had the PDI values either below or close to 0.2 (Table 1), while all the other formulations were polydispersed. The polydispersive nature observed in such nanemulsions could be due to the large difference in refractive index between dispersed and continuous phases (McClements 2015).
The turbidity of the nanoemulsions was expressed based on their absorbance at 600 nm. Nanoemulsions containing combination of citral and linalool without ripening inhibitors (N3, N4 and N5) showed a lower absorbance from 0.57 to 0.89, in comparison with nanoemulsion prepared from only citral or linalool (N1 and N2) and nanoemulsions containing ripening inhibitors N6–N9 (1.74–2.91) (Table 1). The increased turbidity in N1 and N2 could be due to their high PDI values, whereas the decreased turbidity of nanoemulsions in N3, N4 and N5 could be due to their small droplet diameter, which scatters light weakly making emulsion optically more clear (Ghosh et al. 2014).
The size and morphology of the nanoemulsion formulation, which exhibited least droplet size (N1) was also validated through TEM (Fig. 1c). The spherical droplet with an average size of 38.78 nm was observed which was in accordance with the results obtained using particle size analyzer (20.44 nm). Similarly, Lu et al. (2018) who prepared citral nanoemulsions also observed a spherical shaped droplet.
Thermodynamic stability of nanoemulsions
For the commercial applications, it is important that nanoemulsions should remain physically stable for a long period. When compared to coarse emulsion, the nanoemulsion shows enhanced stability due to the reduction in the droplet size which results in increased strength of repulsive forces than the attractive forces (Ghosh et al. 2014). In the present study, the thermodynamic and long term stability of the nanoemulsions depended on the composition of the formulations. A non-ionic surfactant, Tween-80 was used uniformly in all the nanoemulsions, as it can stabilize the emulsions by generating a steric barrier via the bulky molecular groups that are directed towards the continuous medium (Saranya et al. 2012). Among three thermodynamic stability studies, in the freeze-thaw cycle all the nanoemulsions were unstable (Table 2). N2 formulation containing 5% linalool was unstable in all the three thermodynamic stress conditions. Probably, addition of linalool could have deleterious effect on the stability of all the nanoemulsions containing citral (N3–N5). This might be due to the relatively higher water solubility of linalool (1590 mg/L) in comparison to citral (1.34 mg/L) (Wan et al. 2019), which could result in Ostwald ripening.
Table 2.
Thermodynamic stability of different citral and linalool nanoemulsions
| Nanoemulsion formulations | Thermodynamic stability | ||
|---|---|---|---|
| Centrifuge | Heat cool | Freeze thaw | |
| N1 | Stable | Stable | Unstable |
| N2 | Unstable | Unstable | Unstable |
| N3 | Stable | Stable | Unstable |
| N4 | Stable | Stable | Unstable |
| N5 | Stable | Stable | Unstable |
| N6 | Stable | Stable | Unstable |
| N7 | Stable | Stable | Unstable |
| N8 | Stable | Stable | Unstable |
| N9 | Stable | Stable | Unstable |
Kinetic stability of nanoemulsions
The kinetic stability of nanoemulsions was evaluated by measuring their droplet diameter and their distribution at periodic intervals (Fig. 1a, b) and observed for any phase separation during the storage period of 90 days (Fig. 2). A linear increase in the droplet size was observed during the storage period in nanoemulsions which did not contain ripening inhibitors (N1–N5). For instance, the droplet size of N1 increased from an initial size of 20.44 to 141.28 nm at 90th day (Fig. 1a). The increase in size of the droplets could be due to the Ostwald ripening (McClements and Rao 2011). Except N1 formulation which had only citral, creaming was observed in the N2–N5 formulations (Fig. 2). Creaming started in N2 and N3 formulations on 15 and 30 days of storage, respectively and in N4 and N5 formulation on 60 days of storage. Creaming is a phenomenon wherein due to increase in the droplet size to few microns, the droplets rise due to buoyancy which finally leads to phase separation (Gupta et al. 2016). Eventually, by 90th day phase separation was seen in linalool nanoemulsion (N2) or formulations containing different ratios of citral and linalool i.e. N3, N4 and N5. Herein, nanoemulsions containing linalool were found to be specifically more prone to Ostwald ripening because of high water solubility of linalool (Wan et al. 2019). Moreover, addition of ripening inhibitors in the nanoemulsion was able to control the increase in droplet size of the formulations during storage (Fig. 1a). No phase separation was also observed in nanoemulsions containing any of the ripening inhibitor (N6–N9). These ripening inhibitors which are hydrophobic in nature can counteract the Ostwald ripening by generating an entropy of mixing effect (McClements and Rao 2011). Nevertheless, irrespective of the ripening inhibitor type, the initial droplet size of the nanoemulsions N6–N9 (60.34–126.53 nm) were found to be more than the nanoemulsions which were not containing ripening inhibitors (20.44–36.47 nm). Among the different ripening inhibitors, N8 formulation (containing sesame oil) showed least initial droplet size of 60.34 nm which remained relatively constant till 30th day with size of 71.48 nm and then increased to 110.46 and 124.46 nm at the 60th and 90th day, respectively. Similar to our results, Chang et al. (2012) also observed that the addition of corn oil as ripening inhibitor improved the stability of thyme oil nanoemulsion with insignificant changes in particle size distribution till 30 days of storage.
Fig. 2.
Visual appearance of nanoemulsion formulations at 0th day (a), 15th day (b), 30th day (c), 60th day (d) and 90th days of storage (e). Creaming started in N2 and N3 on 15th and 30th day, respectively and in N4 and N5 on 60 days of storage (indicated by arrow mark). Phase separation was observed in N2–N5 formulations an 90th day of storage (indicated by arrow mark)
Stability of the nanoemulsion is dependent on droplet size and it’s PDI (Klang et al. 2012). N1 formulation containing only citral had a PDI value ranging from 0.224 to 0.572 during the 90 days of storage (Fig. 1b). Nanoemulsion formulations containing only linalool (N2) or its combination with citral not having ripening inhibitors (N2–N5) were found to be polydispersed with PDI values from 0.279 to 0.806 on the 30th day of storage. The high PDI values observed in N2–N5 corresponding to their low stability is in accordance to the creaming phenomenon observed in them. Nevertheless, nanoemulsion formulations containing ripening inhibitors (N6–N9) were monodispersed till the end of storage period. N8 formulation (containing sesame oil ripening inhibitor) which was most effective in controlling particle size also showed the least PDI value of 0.184 initially and 0.078 at the end of 90th day storage period (Fig. 1b).
Antibacterial activity of nanoemulsions
Among the food-borne pathogens, Listeria monocytogenes is a major pathogen which causes listeriosis, a disease having a very high mortality rate of 20–30% (Choi et al. 2018). Listeria monocytogenes is of particular concern in food sector, because it is able to multiply even at the low temperature where produce are generally stored (Ajayeoba et al. 2016). The antibacterial activity of the nanoemulsion formulations against Listeria monocytogens is shown in Fig. 3a. Pure citral and linalool had MIC of 0.625 and 1.25%, respectively, while their respective nanoemulsions (N1 and N2) showed twofolds enhanced antibacterial activity with MIC of 0.312 and 0.625%, respectively. Enhanced antibacterial activity of nanoemulsions in comparison to pure compounds could have been due to the better interaction of the small sized droplets of nanoemulsion with the cell membrane (Prakash et al. 2019). Addition of different ratios of linalool into the citral nanoemulsion (N3, N4 and N5) did not increase the antibacterial activity. Herein, the addition of linalool into the citral nanoemulsion might have affected the interfacial tension among the oils phase and therein prevented it’s interaction with the bacterial membrane. In fact, the antibacterial efficacy of nanoemulsions is dependent on various variables like droplet size, oil phase composition and the surfactant concentration (Prakash et al. 2018). Previous studies have shown that citral and linalool kill bacterial cell by disrupting its cell membrane integrity (Leonard et al. 2010; Prakash et al. 2019).
Fig. 3.
Antibacterial activity of different nanoemulsions against Listeria monocytogenes (a); The effect of citral and N1 formulation on the cell constituents release of L. monocytogenes at 260 and 280 nm (b); SEM images of control, citral and N1 treated L. monocytogenes (c). Arrows indicate damage seen in the cell membrane. Bars with asterisk indicate significant difference (p < 0.0001)
It was also observed that addition of any of the ripening inhibitor into the nanoemulsion caused a two-fold reduction in the antibacterial activity in comparison to their respective nanoemulsions (Fig. 3a). In accordance to our results, Ryu et al. (2018) also observed a decrease in antibacterial activity of nanoemulsions with ripening inhibitors. This might be due to the fact that the ripening inhibitors limit the diffusion of active compounds to the bacterial cell membrane. N1 formulation showed the best antibacterial activity among all the formulations and it was also stable in long term storage study, therefore its effect on bacterial cell membrane integrity and anti-biofilm activity were further investigated.
Cell membrane integrity
Components of EOs are known to lyse cells by fusing and destabilizing the integrity of cell membranes (McClements and Rao 2011). The leakage of cytoplasmic contents released upon cell lysis can be measured at 260 and 280 nm, which correspond to the nucleic acid and proteins, respectively. The influence of nanoemulsions on the leakage of cytoplasmic contents from the Listeria monocytogens was expressed in terms of absorbance at 260 and 280 nm (Fig. 3b). Treatment with N1 at MIC showed a significant increase in the absorbance at 260 and 280 nm in comparison to the control. A small increase in the efficacy of N1 was observed at 2 × MIC when compared to MIC. Hence, these results suggest the considerable damage caused by N1 to bacterial membrane, which led to the release of cytoplasmic constituents. The alteration in surface morphology of the bacterial cells upon treatment with citral and N1 formulation was also validated through SEM (Fig. 3c). Significantly distorted cell membrane morphology was observed in N1 treated bacterial cells in comparison to the intact cells observed in control samples.
Anti-biofilm activity
The formation of biofilms by Listeria monocytogenes on food surface is a serious challenge in food industry as biofilms are more resistant to the disinfectants in comparison to planktonic cells (Srey et al. 2013). The anti-biofilm activity of the citral and N1 formulation are shown in Fig. 4a, which indicated 81.54% and 83.51% biofilm inhibitions, respectively. In accordance to our results, Leonard et al. (2010) also observed a high anti-biofilm activity of citral against Listeria monocytogenes. A higher anti-biofilm activity of the N1 nanoemulsion could have been due its ability to inhibit the attachment of bacteria on the surfaces, which is the initial step of biofilm formation (Lou et al. 2017). The anti-biofilm activity of the citral and N1 was also confirmed using SEM (Fig. 4b). Control samples showed dense aggregates of bacterial cells, which is a typical characteristic of biofilms. Treatment with citral and N1 formulation was found to remarkably reduce the biofilm formation in comparison to the control. As the formation of Listeria monoctyogens biofilms is major problem in the food sector, N1 could be tapped as a natural disinfectant in food industry.
Fig. 4.
Biofilm inhibitory effect of citral and N1 against Listeria monocytogenes (a) and SEM visualization of anti-biofilm activity of citral and N1 (b). Bars with asterisk indicate significant difference (p < 0.0001)
Conclusion
Present study investigated the influence of individual and combinations of citral and linalool along with different ripening inhibitors on the nanoemulsion stability and antibacterial activity against Listeria monocytogenes. Incorporation of linalool with the citral nanoemulsions was found to have deleterious effect on it’s the stability and antibacterial activity. Incorporating ripening inhibitors like sesame oil in linalool combined citral nanoemulsions, controlled the increase in size and distribution of droplets during the 90 days storage period, but decreased their antibacterial activity. N1 formulation (containing 5% citral nanoemulsion) which was kinetically stable also showed the best antibacterial activity by disrupting bacterial membrane integrity. N1 formulation also showed a promising biofilm inhibition of 83.51% and therefore, it could be potentially explored as an efficient disinfectant against Listeria monoctyogenes in food industry. Moreover, information obtained from the present study could provide leads for formulating stable and efficient antibacterial nanoemulsion systems.
Acknowledgements
Financial support provided by CSIR in the form of Junior Research Fellowship (09/1095/0020/2017-EMR-I) to A.P. is acknowledged. Authors are grateful to the management of SASTRA Deemed University for their encouragement and support.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ajayeoba TA, Atanda OO, Obadina AO, Bankole MO, Adelowo OO. The incidence and distribution of Listeria monocytogenes in ready-to-eat vegetables in South-Western Nigeria. Food Food Sci Nutr. 2016;4(1):59–66. doi: 10.1002/fsn3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeem A, Rizwan M, Ahmad FJ, Iqbal Z, Khar RK, Aqil M, Talegaonkar S. Nanoemulsion components screening and selection: a technical note. AAPS Pharm Sci Tech. 2009;10(1):69–76. doi: 10.1208/s12249-008-9178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu FS, Rubini D, Rakshitaa S, Chandrasekar K, Murugan R, Wilson A, Gowrishankar S, Pandian SK, Nithyanand P. Antivirulent properties of underexplored Cinnamomum tamala essential oil and its synergistic effects with DNase against Pseudomonas aeruginosa biofilms—an in vitro study. Front Microbiol. 2017;8:1144. doi: 10.3389/fmicb.2017.01144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Chang Y, McLandsborough L, McClements DJ. Physical properties and antimicrobial efficacy of thyme oil nanoemulsions: influence of ripening inhibitors. J Agric Food Chem. 2012;60(48):12056–12063. doi: 10.1021/jf304045a. [DOI] [PubMed] [Google Scholar]
- Choi MH, Park YJ, Kim M, Seo YH, Kim YA, Choi JY, Yong D, Jeong SH, Lee K. Increasing incidence of listeriosis and infection-associated clinical outcomes. Ann Lab Med. 2018;38:102–109. doi: 10.3343/alm.2018.38.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsì F, Ferrari G. Essential oil nanoemulsions as antimicrobial agents in food. J Biotechnol. 2016;233:106–120. doi: 10.1016/j.jbiotec.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Ghosh V, Mukherjee A, Chandrasekaran N. Formulation and characterization of plant essential oil based nanoemulsion: evaluation of its larvicidal activity against Aedes aegypti. Asian J Chem. 2013;25:S321–S323. doi: 10.14233/ajchem.2013.13557. [DOI] [Google Scholar]
- Ghosh V, Mukherjee A, Chandrasekaran N. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids Surf B Biointerface. 2014;114:392–397. doi: 10.1016/j.colsurfb.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Guerra-Rosas M, Morales-Castro J, Cubero-Márquez M, Salvia-Trujillo L, Martín-Belloso O. Antimicrobial activity of nanoemulsions containing essential oils and high methoxyl pectin during long-term storage. Food Control. 2017;77:131–138. doi: 10.1016/j.foodcont.2017.02.008. [DOI] [Google Scholar]
- Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12:2826–2841. doi: 10.1039/C5SM02958A. [DOI] [PubMed] [Google Scholar]
- Karthik P, Ezhilarasi P, Anandharamakrishnan C. Challenges associated in stability of food grade nanoemulsions. Crit Rev Food Sci Nutr. 2017;57(7):1435–1450. doi: 10.1080/10408398.2015.1006767. [DOI] [PubMed] [Google Scholar]
- Katsukawa M, Nakata R, Takizawa Y, Hori K, Takahashi S, Inoue H. Citral, a component of lemongrass oil, activates PPARα and γ and suppresses COX-2 expression. BBA Mol Cell Biol Lipid. 2010;1801(11):1214–1220. doi: 10.1016/j.bbalip.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Klang V, Matsko NB, Valenta C, Hofer F. Electron microscopy of nanoemulsions: an essential tool for characterisation and stability assessment. Micron. 2012;43(2–3):85–103. doi: 10.1016/j.micron.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Leonard C, Virijevic S, Regnier T, Combrinck S. Bioactivity of selected essential oils and some components on Listeria monocytogenes biofilms. S Afr J Bot. 2010;76(4):676–680. doi: 10.1016/j.sajb.2010.07.002. [DOI] [Google Scholar]
- Lou Z, Chen J, Yu F, Wang H, Kou X, Ma C, Zhu S. The antioxidant, antibacterial, antibiofilm activity of essential oil from Citrus medica L. var. sarcodactylis and its nanoemulsion. LWT Food Sci Technol. 2017;80:371–377. doi: 10.1016/j.lwt.2017.02.037. [DOI] [Google Scholar]
- Lu W-C, Huang D-W, Wang C-C, Yeh C-H, Tsai J-C, Huang Y-T, Li P-H. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J Food Drug Anal. 2018;26(1):82–89. doi: 10.1016/j.jfda.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements DJ. Food emulsions: principles, practices, and techniques. 3. Boca Raton: CRC Press; 2015. [Google Scholar]
- McClements DJ, Rao J. Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit Rev Food Sci Nutr. 2011;51(4):285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- Moghimi R, Aliahmadi A, Rafati H. Ultrasonic nanoemulsification of food grade trans-cinnamaldehyde: 1, 8-Cineol and investigation of the mechanism of antibacterial activity. Ultrason Sonochem. 2017;35:415–421. doi: 10.1016/j.ultsonch.2016.10.020. [DOI] [PubMed] [Google Scholar]
- Prakash A, Baskaran R, Paramasivam N, Vadivel V. Essential oil based nanoemulsions to improve the microbial quality of minimally processed fruits and vegetables: a review. Food Res Int. 2018;111:509–523. doi: 10.1016/j.foodres.2018.05.066. [DOI] [PubMed] [Google Scholar]
- Prakash A, Vadivel V, Rubini D, Nithyanand P. Antibacterial and antibiofilm activities of linalool nanoemulsions against Salmonella Typhimurium. Food Biosci. 2019;28:57–65. doi: 10.1016/j.fbio.2019.01.018. [DOI] [Google Scholar]
- Ryu V, McClements DJ, Corradini MG, McLandsborough L. Effect of ripening inhibitor type on formation, stability, and antimicrobial activity of thyme oil nanoemulsion. Food Chem. 2018;245:104–111. doi: 10.1016/j.foodchem.2017.10.084. [DOI] [PubMed] [Google Scholar]
- Saranya S, Chandrasekaran N, Mukherjee A. Antibacterial activity of eucalyptus oil nanoemulsion against Proteus mirabilis. Int J Pharm Pharm Sci. 2012;4(3):668–671. [Google Scholar]
- Srey S, Jahid IK, Ha SD. Biofilm formation in food industries: a food safety concern. Food Control. 2013;31(2):572–585. doi: 10.1016/j.foodcont.2012.12.001. [DOI] [Google Scholar]
- Sugumar S, Nirmala J, Ghosh V, Anjali H, Mukherjee A, Chandrasekaran N. Bio-based nanoemulsion formulation, characterization and antibacterial activity against food-borne pathogens. J Basic Microbiol. 2013;53(8):677–685. doi: 10.1002/jobm.201200060. [DOI] [PubMed] [Google Scholar]
- Wan J, Zhong S, Schwarz P, Chen B, Rao J. Physical properties, antifungal and mycotoxin inhibitory activities of five essential oil nanoemulsions: impact of oil compositions and processing parameters. Food Chem. 2019;291:119–206. doi: 10.1016/j.foodchem.2019.04.032. [DOI] [PubMed] [Google Scholar]





