Abstract
Carotenoids are group of colored terpenoids with antioxidant properties and widespread in nature including in microorganisms. Lactobacillus plantarum subsp. plantarum KCCP11226 was previously isolated from kimchi, while exhibiting the production of 4,4′-diaponeurosporene as a C30 carotenoid. In this study, full genome sequencing of the strain KCCP11226 was performed. Genome analysis revealed that the dehydrosqualene synthase (crtM) and dehydrosqualene desaturase (crtN) genes, which are major genes for biosynthesis of 4,4′-diaponeurosporene, were shown to act as an operon in most L. plantarum strains, but they were uncommon in other Lactobacillus species. In vitro experiments revealed that the production of 4,4′-diaponeurosporene was greatly increased by oxidative stress. In this situation, mRNA expressions of crtN and crtM were also significantly increased. In conclusion, genome analysis of L. plantarum subsp. plantarum KCCP11226 suggested the presence of a well-conserved C30 carotenoid biosynthetic pathway that includes the crtM–crtN operon. The genomic information on L. plantarum subsp. plantarum KCCP11226 could further elucidate the functions of genes involved in isoprenoid biosynthetic pathway, especially in C30 carotenoid biosynthesis.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2149-y) contains supplementary material, which is available to authorized users.
Keywords: Lactobacillus plantarum subsp. plantarum; 4,4′-Diaponeurosporene; Carotenoid; Genome
Introduction
Carotenoids are naturally occurring pigments in algae, plants, yeasts, fungi, and bacteria. In most bacteria, they are not essential compounds for growth, but have important biological roles, such as protection from oxidative stress by scavenging free radicals via their conjugated double bonds (Clauditz et al. 2006). Through these actions, carotenoids can produce many biological effects, such as immunomodulatory, anti-carcinogenic, and antioxidant on the human body when consumed in the diet. Therefore, they are considered important pigments and can be applied in food, cosmetic, and pharmaceutical industries (Chandi and Gill 2011). Carotenoids represent structurally diverse classes of lipophilic isoprenoids originating from the terpenoid biosynthetic pathway (Yatsunami et al. 2014). In general, carotenoid-producing bacteria utilize a farnesyl pyrophosphate (FPP), a product of the mevalonate pathway, to synthesize carotenoids. In order to synthesize triterpenoids (C30), a series of FPP desaturation steps by dehydrosqualene synthase (crtM) and dehydrosqualene desaturase (crtN) are required. In contrast, tetraterpenoids (C40) are synthesized from geranylgeranyl pyrophosphate, a converted form of FPP, while sequential desaturation steps were performed via phytoene synthase (crtB) and phytoene desaturase (crtI) (Maoka 2019).
Most carotenoids produced by bacteria are tetraterpenoids (C40); for example, astaxanthin, lycopene, and β-carotene (Phadwal 2005). However, triterpenoids (C30) have been reported only in some bacterial species, such as Lactobacillus plantarum, Staphylococcus aureus, Enterococcus faecium, Methylobacterium rhodinum, and helicobacteria species (Garrido-Fernández et al. 2010). L. plantarum species are easily detected in a wide variety of ecological environments such as fish, meat, dairy products, vegetables, and the human gut (Siezen and van Hylckama Vlieg 2011). L. plantarum, which may be present in a variety of products, is a very useful bacteria that can be used as a probiotic to provide various health beneficial effects on the human body (Zago et al. 2011).
Recently, some studies have focused on strains of L. plantarum that can synthesize the C30 carotenoid, 4,4′-diaponeurosporene. Garrido-Fernández et al. (2010) reported carotenoid production in L. plantarum strains and suggested that the functionality of a C30 carotenoid biosynthetic pathway in this species is regulated by an operon of crtM–crtN genes. Turpin et al. (2016) suggested that combining PCR analysis of crtM–crtN genes with analytical biochemical analyses is an efficient way to identify C30 carotenoid-producing lactic acid bacteria. They also revealed that the crtM and crtN genes were detected in 27 of the 29 L. plantarum strains examined.
Since the complete 3.3 Mb genome of L. plantarum that originated from human saliva, the first genome of a Lactobacillus species, was reported in 2003 (Kleerebezem et al. 2003), a large quantity of genome data on Lactobacillus has become available. Therefore, genome analysis across the extensive list of Lactobacillus species and strains may provide useful information related to the isoprenoid biosynthetic pathway for C30 carotenoids production in Lactobacillus.
Previously, we reported that L. plantarum subsp. plantarum KCCP11226, produced a notable amount of C30 carotenoid under oxidative stress conditions (Kim et al. 2019). In the present study, we report on the complete genome sequence of L. plantarum subsp. plantarum KCCP11226 and the bioinformatic analysis of its gene contents. Also, this study investigates the presence of all genes required for the C30 carotenoid biosynthetic pathway in the strain KCCP11226. Finally, the presence of a crtM–crtN operon as key enzymes for the biosynthesis of C30 carotenoids was investigated within a wide range of Lactobacillus species.
Materials and methods
Genome sequencing and assembly
Lactobacillus plantarum subsp. plantarum KCCP11226 was previously isolated from kimchi, a Korean fermented vegetable (Kim et al. 2019). For this study, the strain KCCP11226 was aerobically cultivated in De Man, Rogosa and Sharpe (MRS) broth (Difco, Detroit, MI, USA). The genomic DNA of the strain KCCP11226 was extracted by using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). The extracted genomic DNA was quantified using a NanoDrop 2000 UV-Vis spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and a Qubit 2.0 fluorometer (Thermo Fisher Scientific). The PacBio RS II (Pacific Biosciences, Menlo Park, CA, USA) sequencing platform was used to analyze the whole genome sequence of the strain KCCP11226. The raw data for the sequenced reads were assembled using HGAP 3.0 with a 3-Mb expected genome size (Chin et al. 2013). The genome map of the chromosome and plasmids was visualized using the Circlator tool (Hunt et al. 2015). The prediction of open reading frames and the annotation of the coding genes were carried out using the NCBI prokaryotic genome annotation pipeline (PGAP) (Tatusova et al. 2016).
Functional classification of coding sequences in L. plantarum subsp. plantarum strains
The functional classification of protein-coding genes was classified by the assignment of a clusters of orthologous group (COG) code to each gene (Tatusov et al. 1997). Classification was performed by COGnitor in the COGsoft (Kristensen et al. 2010) with default options. Each gene was classified into a best-hit COG function, and unassigned genes were described as ‘Not assigned’. The protein data for eight L. plantarum subsp. plantarum strains (ST-III, P-8, SRCM100434, LB1-2, TS12, nF1-FD, CGMCC 1.557, and BNH17) were obtained from the NCBI RefSeq assembly data. The average nucleotide identity (ANI) values were computed using the pyani module with the default option (https://github.com/widdowquinn/pyani). The nine complete genomes of L. plantarum subsp. plantarum strains were obtained from the NCBI RefSeq assembly data (accession numbers are listed in Table S1). Also, the complete genomes of nine L. plantarum subsp. plantarum strains were aligned using the Progressive Mauve genome alignment tool (Darling et al. 2010).
Culture conditions with external stress
All culture experiments involving L. plantarum subsp. plantarum KCCP11226 were performed in 100 mL of MRS broth at 30 °C for 24 h. The air condition was a static growth condition in which more than two-thirds of the flask was filled with air. To analyze carotenoid levels under external stress conditions, glycerol (1.8 or 2.0 M), sodium chloride (4–9%), high temperature (40 °C or 45 °C), and oxygen conditions (anaerobic, static, and aerobic) were applied. In order to maintain anaerobic and aerobic conditions, an AnaeroPack system (Mitsubishi Gas Chemical, Tokyo, Japan) and shaking condition (110 rpm) were applied, respectively. Bacterial growth was monitored at 600 nm using a spectrophotometer (Shimadzu, Kyoto, Japan).
Extraction and analysis of carotenoids
After incubation as described above, yellow pigments of L. plantarum subsp. plantarum KCCP11226 were extracted. In brief, 100 mL of cultured cells were harvested by centrifugation at 7000 × g for 10 min, followed by overnight extraction with 5 mL of methanol. Subsequently, 5 mL of hexane and 2.5 mL of distilled water were added to the methanol extract. After centrifugation at 2000 × g for 10 min, the carotenoid-containing organic phase was transferred to a 15 mL tube. The organic phase underwent evaporation and was then re-suspended in 1 mL petroleum ether. The pigmentation levels in the extract were measured at an absorbance of 470 nm using a spectrophotometer (Shimadzu).
Real-time quantitative reverse transcription-polymerase chain reaction
Total RNA of L. plantarum subsp. plantarum KCCP11226 was extracted using an RNeasy Protect Bacteria Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNA concentrations were measured using a Nanodrop (Thermo Scientific) spectrophotometer at 260 nm. Purity was confirmed by determining the 260/280 nm ratio. To synthesize cDNA, 0.1 μg of total RNA was used. The reverse transcription-PCR was performed using a Diastar RT Kit (Solgent, Seoul, Korea) and random hexamers (Neoprobe, Daejeon, South Korea). The qRT-PCR was carried out using a SYBR Green real-time PCR Master Mix (TOYOBO, Osaka, Japan) and a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Amplification was performed using primer sets of crtN; 179-bp amplicon (forward: 5′-ACC GAA GCA TTA CAC ACG ATC C-3′ and reverse: 5′-TCA GGA ACT GGT ACT AAA ACA T-3′) and crtM; 247-bp amplicon (forward: 5′-GCA TTG CGC CAA TCA TTG AC-3 and reverse: 5′-GCG GGT TAG TTG CTA GCA TT-3′). Also, the partial region of the 16S rRNA gene of the strain KCCP11226 was amplified with the universal primers; 194-bp amplicon (341F: 5′-CCT ACG GGA GGC AGC AG-3′ and 518R: 5′-ATT ACC GCG GCT GCT GG-3′). The PCR conditions were as follows: initial denaturation at 95 °C for 15 min; followed by 30 cycles of 20 s at 95 °C, 40 s at 55 °C, and 30 s at 72 °C. Upon completion, amplification was immediately followed by melting program which was 95 °C for 15 s, 55 °C for 1 min, and 95 °C for 15 s.
Meantime, each gene was amplified and sub-cloned into pGEM-T-easy vector (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Escherichia coli DH5α (Takara, Shiga, Japan) was introduced as a host for vector propagation. For calibration, the cloned plasmids were extracted and used as a standard template. The mRNA levels were normalized to 16S rRNA gene transcript levels as described previously (Tasara and Stephan 2007; Chen et al. 2010). The copy numbers were determined using the following equation: DNA (copy) = [6.0221 × 1023 (copies/mol) × DNA amount (g)]/[DNA length (bp) × 660 (g/mol/bp)]. Target gene expression levels were calculated using StepOne software v2.3 (Applied BioSystems). Experiments were repeated in triplicate.
Statistical analysis
The results of the experiments were expressed as means ± standard deviations of three independent measurements. Results were analyzed by applying ANOVA with the Tukey–Kramer multiple comparisons test or with Bonferroni’s multiple comparisons test.
Results and discussion
The complete genome of L. plantarum subsp. plantarum KCCP11226
The genome of L. plantarum subsp. plantarum KCCP11226 is composed of one chromosome and three plasmids. The chromosome is 3,382,104 bps with a 44.4% GC content; plasmids 1, 2, and 3 are 80,744 bps with 40.96% GC, 54,114 bps with 39.87% GC, and 40,982 bps with 40.32 GC content, respectively (Fig. 1; Table 1). The 3247 genes, including 3152 protein-coding genes, 93 pseudogenes, and 95 RNA genes, were annotated in the strain KCCP11226. Five or six full-length 5S, 16S, and 23S rRNA genes are located in the genome, as well, 75 tRNA genes and four non-coding RNA were identified. So far, only nine complete genome sequences of L. plantarum subsp. plantarum strains have been reported to the NCBI genome database, including that for the strain KCCP11226. Comparison of complete genome sequences of nine L. plantarum subsp. plantarum strains including the strain KCCP11226 showed that genome size and the number of coding genes were significantly different, ranging from 3,195,678 to 3,541,869 bps and 3097 to 3499, respectively (Table S1). Interestingly, most of the L. plantarum subsp. plantarum strains had plasmids and, typically, a fairly large number of plasmids were present (up to seven). However, those strains with a large number of plasmids did not necessarily have a large genome. On the other hand, GC content among the strains was very similar (approximately 44%). Although there were differences in the number of tRNAs, the number of rRNAs (5S, 16S, and 23S) were similar, 6, 5, and 5, respectively. A single CRISPR array was placed in the genomes of strains TS12 and CGMCC 1.557, whereas no CRISPR array was detected in the genomes of the other strains.
Fig. 1.
Visualized circular map of the chromosome and plasmids in L. plantarum subsp. plantarum KCCP11226. a Chromosome, b–d plasmids. From the outermost; track 1 (deep blue): forward-strand coding genes; track 2 (blue): reverse-strand coding genes; track 3 (light blue): tRNAs; track 4 (orange): rRNAs; track 5 (black): GC content; and track 6 (green and purple): G + C skew
Table 1.
Genome features of L. plantarum subsp. plantarum KCCP11226
| Attributes | Total | Chromosome | Plasmid 1 | Plasmid 2 | Plasmid 3 |
|---|---|---|---|---|---|
| Sequence accession | CP046262.1 | CP046263.1 | CP046264.1 | CP046265.1 | |
| Genome size (bp) | 3,382,104 | 3,206,264 | 80,744 | 54,114 | 40,982 |
| GC content (%) | 44.40 | 44.61 | 40.96 | 39.87 | 40.32 |
| Total genes | 3247 | 3062 | 85 | 56 | 44 |
| Total CDS | 3152 | 2967 | 85 | 56 | 44 |
| Coding genes | 3059 | 2909 | 78 | 35 | 37 |
| Pseudo genes | 93 | 58 | 7 | 21 | 7 |
| RNAs | 95 | 95 | – | – | – |
| rRNAs (5S, 16S, 23S) | 6, 5, 5 | 6, 5, 5 | – | – | – |
| tRNAs | 75 | 75 | – | – | – |
| ncRNAs | 4 | 4 | – | – | – |
| Repeat region | – | – | – | – | – |
| CRISPRs | – | – | – | – | – |
| Regulatory | 8 | 8 | – | – | – |
Genomic features of L. plantarum subsp. plantarum KCCP11226
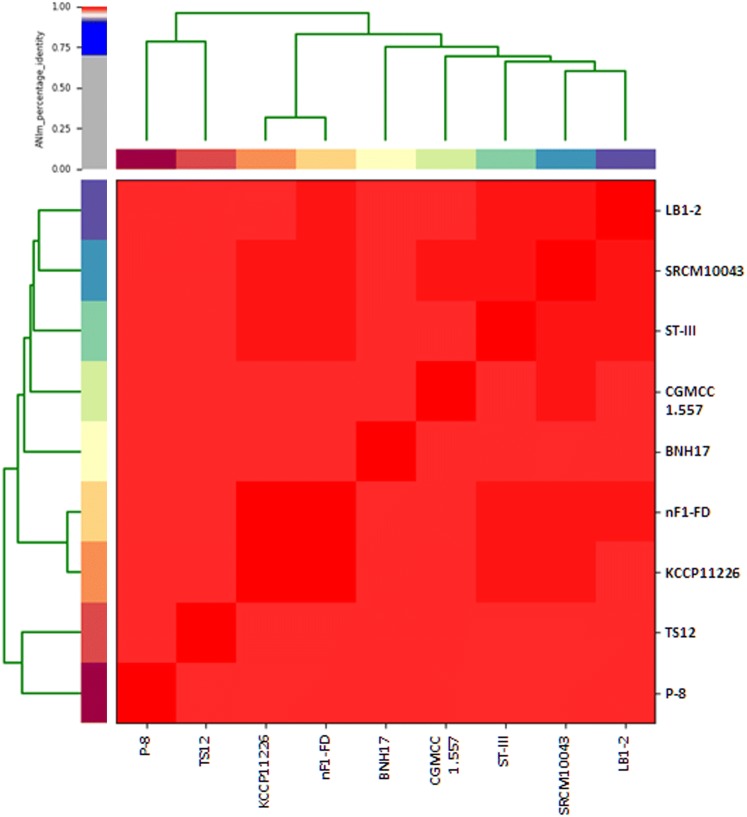
The protein COGs were generated based on the functional features of the protein-coding genes of L. plantarum subsp. plantarum KCCP11226, and the COG categories were classified (Table S2). Among the 3059 protein-coding genes, 2670 proteins were classified and there were 389 unassigned proteins. As nine strains belonged to the same species, the assigned code’s proportions were not significantly different. Only the ‘Mobilome: prophages, transposons (X)’ showed a 30% difference in the number of genes among the compared strains, ranging from a minimum of 25 to a maximum of 79. The proportions of unknown function proteins, including the ‘general function prediction only (R)’, ‘function unknown (S)’, and ‘not assigned (–)’, were greater than 27% in all the strains. As each of those strains belongs to the same sub-species, the ANI values were quite similar, ranging from a minimum of 98.6% to a maximum of 99.8% (Fig. 2). As there was no significant difference among the ANI values, genome alignment results showed that each conserved region was distinctly collinear and homologous (Fig. S1). However, in the case of strain TS12, it was observed that the middle part of the genome (ca 1,050,000–2,300,000 bps) was in present in the opposite direction; regardless, the region was well conserved.
Fig. 2.
Dendrogram of the average nucleotide identity of L. plantarum subsp. plantarum strains. Color bars representing correlation coefficients (0–1.0) are shown with a color scale based on their respective percentage identity
Isoprenoid synthetic pathway in L. plantarum subsp. plantarum KCCP11226
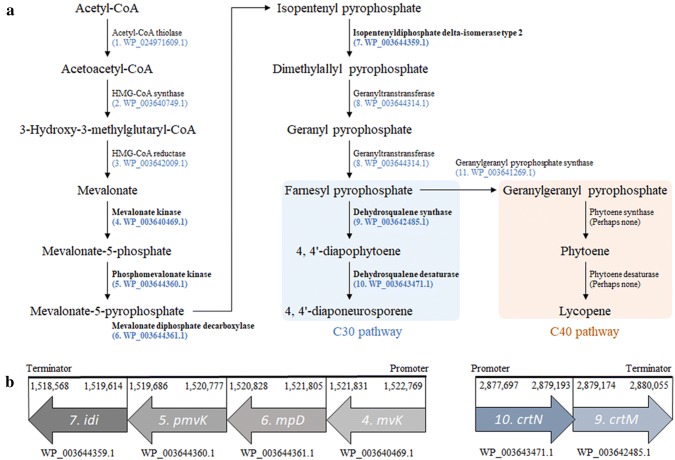
The yellow pigment produced by L. plantarum subsp. plantarum KCCP11226 was previously identified as a C30 carotenoid 4,4′-diaponeurosporene (Kim et al. 2019). The complete genome of the strain KCCP11226 revealed that all enzymes involved in the biosynthetic pathways of C30 carotenoid are present in its chromosome (Table S3 and Fig. 3a). Acetyl-CoA thiolase was not detected in the genome. However, WP_024971609.1, assigned as a hypothetical protein in PGAP, showed 99% amino acid (AA) identity in the alignment results with the β-ketoadipyl-CoA thiolase of L. plantarum (OUT06548.1). That thiolase is a synonym of acetyl-CoA thiolase and, thus, is expected to function equivalently. FPP may be successfully biosynthesized via the mevalonate pathway starting from acetyl-CoA (Fig. 3a). Of particular note, four genes (nos. 4, 5, 6, and 7) required for bioconversion from mevalonate to dimethylallyl pyrophosphate (DPP) were present as a cluster in the chromosome (Fig. 3b). The four genes shared one promoter and terminator like polycistronic mRNA. Although there were approximately 25–70 bps of extra nucleotides between genes, promoters or terminators corresponding to each gene were not found except for those of the start and end of the genes. Two proteins, WP_003644314.1 and WP_003641269.1, classed as polyprenyl synthetase family proteins, showed 99% AA identity with geranlytranstransferase (AHN69035.1) and geranylgeranyl pyrophosphate synthase (QDJ19411.1) of L. plantarum, respectively. Also, there were genes that have the roles of dehydrosqualene synthase (crtM) and dehydrosqualene desaturase (crtN), which are essential genes for synthesis of 4,4′-diaponeurosporene from FPP. They were assigned as a phytoene/squalene synthase family protein and as phytoene desaturase in PGAP, respectively, but their AA sequences were identical to those of dehydrosqualene synthase (crtM) and dehydrosqualene desaturase (crtN), respectively (Turpin et al. 2016). In addition, these two genes shared one promoter and terminator. Meanwhile, a geranylgeranyl pyrophosphate (GPP) synthase (no. 11) is involved in the biosynthesis of GPP, a precursor for producing C40 carotenoid. Based on amino acid alignment results, the observed phytoene synthase (crtB) and phytoene desaturase (crtI) corresponded as best matches to crtM (WP_003642485.1) and crtN (WP_003643471.1) in the strain KCCP11226, respectively (Table 2). In addition, although queries of crtB and crtI cover up to 94%, most of the identity was only about 30%. Therefore, it was predicted that crtB and crtI are absent in the strain KCCP11226. In fact, it is unclear whether crtM and crtN can have roles equivalent to those of the intermediate enzymes (crtB and crtI) in the synthesis of lycopene. However, to the best of our knowledge, C40 production in L. plantarum strain has not yet been reported. Perhaps, the intermediate enzymes (crtB and crtI) for lycopene synthesis are absent from L. plantarum subsp. plantarum KCCP11226.
Fig. 3.
Isoprenoid synthetic pathway in L. plantarum subsp. plantarum KCCP11226. a Carotenoid production-related genes and corresponding enzymes in the strain KCCP11226. b Clustered genes in the C30 carotenoid production pathway. Promoter prediction was performed by BPROM (https://softberry.com) and terminator prediction via ARNold-finding terminators (Gautheret and Lambert 2001; Macke et al. 2001)
Table 2.
Protein identities of L. plantarum subsp. plantarum KCCP11226 corresponding to previously identified phytoene synthase and phytoene desaturase
| Organism | Protein ID | Best match* | Query cover (%) | Identity (%) | |
|---|---|---|---|---|---|
| Phytoene synthase (crtB) | Paracoccus sp. | BAA09595.2 | WP_003642485.1 | 87 | 28.41 |
| Synechocystis sp. | CAA48922.1 | WP_003642485.1 | 81 | 30.74 | |
| Neurospora crassa | AAA19428.1 | WP_003642485.1 | 46 | 24.64 | |
| Arabidopsis thaliana | AAA32836.1 | WP_003642485.1 | 63 | 32.25 | |
| Phytoene desaturase (crtI) | Rhodobacter capsulatus | CAA77540.1 | WP_003643471.1 | 94 | 33.40 |
| Erwinia uredevora | BAA14127.1 | WP_003643471.1 | 99 | 32.93 | |
| Neurospora crassa | AAA33555.1 | WP_003643471.1 | 84 | 29.46 |
*The second matches are not represented because all query covers of them did not exceed 10%
Analysis of crtM–crtN operon in Lactobacillus species
The crtM–crtN operon is not popular in Lactobacillus species. The BlastP results suggested that crtM and crtN could be detected only in L. plantarum (43 and 50 results, respectively), L. paraplantarum (3 results), L. paracasei (1 result), L. herbarum (1 result), L. mudanjiangensis (3 results), and L. florum (2 results). Other than those, most of the other similar identities were found in Enterococcus species. Among all 100 complete genomes selected from the 468 reported genomes of L. plantarum strains, most strains (87/100) had intact crtM–crtN operons. All 12 reported genomes of L. paraplantarum contained the crtM–crtN operon (12/12 genomes). A total 178 genomes have been reported for L. paracasei, but, only one result was shown in the BlastP results. In addition, the crtM–crtN operon were not detected in all of the 29 reported complete genomes. The crtM–crtN operon was found in L. herbarum, L. mudanjiangensis, and L. florum, but, very few genomes of those species (1, 4, and 4 genomes, respectively) have been reported. A PCR-based experiment revealed that some L. fermentum strains isolated from African fermented foods contain crtM–crtN operon (16 of 64 strains) (Turpin et al. 2016). However, considering the redundant isolations, it is unclear whether they are different strains. Seventy genomes of L. fermentum have been reported; however, there was no result of crtM–crtN operon presences in the BlastP results. At least, 20 of the reported complete genomes of L. fermentum do not have crtM–crtN operon. Genome-based analysis suggests that the crtM–crtN operon was uncommon in Lactobacillus species. As a supplement to this suggestion, only L. plantarum and L. fermentum have been reported to date as C30 carotenoid producing Lactobacillus species (Turpin et al. 2016). In addition, although the crtM–crtN operon has been detected in some Lactobacillus species, it is most common in of L. plantarum strains and is well conserved.
Stress condition affecting C30 carotenoid production of L. plantarum subsp. plantarum KCCP11226
In general, biosynthesis of carotenoids is associated with various stress conditions (Clauditz et al. 2006), and L. plantarum subsp. plantarum KCCP11226 was cultivated to examine the effects of high temperature, glycerol, sodium chloride, and oxygen condition on C30 carotenoid production. The results indicated that high temperature and glycerol did not affect the production of carotenoids (data not shown). However, the sodium chloride and oxygen condition stressors resulted in a significant increase in carotenoid production. The strain KCCP11226 was grown with a range of concentrations of sodium chloride (4–9%) at 30 °C under static growth conditions (Fig. 4a). Inhibition of cell growth with increasing sodium chloride concentration of up to 5% could be because C30 carotenoids produced at up to 5% salt concentration did not sufficiently sustain the cell growth by osmotic pressure. However, the protection against the inhibition of cell growth by osmotic pressure exhibited at 7% salt concentration by the increase of C30 carotenoid production which could prevent the release of water molecules within the cells (Britton 1995). In addition, values of A470/OD600 gradually increased as the sodium chloride concentration increased. However, cell growth was not possible at or above 8% sodium chloride; hence, the optimum A470/OD600 ratio (0.25) was obtained at a sodium chloride concentration of 7%. In order to examine the effects on carotenoid production of oxygen conditions, the strain KCCP11226 was cultured under anaerobic, static, and aerobic conditions (Fig. 4b). Cells could be fully grown under all conditions, resulting in the optical densities of 5.0, 5.0 and 6.0, respectively. Cell growth was slightly increased under the aerobic condition, but the carotenoid production showed a significant increase. The carotenoid productions (A470) under the anaerobic, static, and aerobic conditions were 0.11, 0.55, and 1.49, respectively. Values of A470/OD600 were significantly higher under the aerobic condition (0.02, 0.11, and 0.25 under anaerobic, static, and aerobic conditions, respectively) (Fig. 4b). It has been reported that the induction of carotenoid production upon oxygen exposure is one of the stress tolerance mechanisms in lactic acid bacteria (LAB) (Hagi et al. 2014). In addition, the production of some antioxidants by LAB has been reported to occur under aerobic conditions (Miyoshi et al. 2003). However, the specific relationship between salt-resistance and carotenoid production in bacteria has not yet been clarified, although the effects of various salt concentrations on carotenoid production have been reported (Ben-Amotz and Avron 1983; Fong et al. 2001; Liu and Lee 2000; Orosa et al. 2001).
Fig. 4.
Effect of external stress conditions on C30 carotenoid production in L. plantarum subsp. plantarum KCCP11226. a Effect of sodium chloride stress. Cells were grown for 24 h at 30 °C under static conditions. b Effect of aerobic condition stress. Cells were grown for 24 h at 30 °C under anaerobic, static, and aerobic conditions. The numbers shown on the graph represent the A470/OD600 values
In the previous section, we mentioned that the crtM–crtN cassette is uncommon in Lactobacillus species. Therefore, the expression levels of the key enzymes (crtM–crtN genes) in the biosynthesis of 4,4′-diaponeurosporene under stress (sodium chloride and oxygen) were investigated (Fig. 5). The mRNA expression of crtM was increased by almost twofold under 7% sodium chloride condition (1.91 × 10–3 ± 1.82 × 10–4 to 3.82 × 10–3 ± 8.7 × 10–4) (Fig. 5a). In addition, significant differences in mRNA expressions of crtN and crtM were showed under oxidative stress conditions (Fig. 5b). Under aerobic conditions, mRNA expressions of crtN and crtM (1.64 × 10–1 ± 6.69 × 10–3 and 2.15 × 10–1 ± 1.1 × 10–2, respectively) were significantly higher than those of anaerobic (2.28 × 10–2 ± 4.04 × 10–3 and 3.25 × 10–2 ± 7.73 × 10–3, respectively) and static conditions (2.46 × 10–2 ± 1.59 × 10–3 and 3.45 × 10–2 ± 4.89 × 10–3, respectively), under which the mRNA expression of crtN and crtM increased by almost 7.1 and 6.6 times, respectively. These results correspond to the results showing an increase in the 4,4′-diaponeurosporene under the aerobic condition, suggesting that oxidative stress is a very important factor in inducing mRNA expressions of crtN and crtM in L. plantarum subsp. plantarum KCCP11226.
Fig. 5.
The mRNA expression levels of crtN and crtM under external stress conditions. a Sodium chloride and b oxidative stress conditions. Cells were cultivated under the same conditions as indicated in Fig. 4
In conclusion, this study shows that the C30 carotenoid biosynthetic pathway is conserved in L. plantarum subsp. plantarum KCCP11226. Among the identified genes, the crtM–crtN operon is uncommon in other Lactobacillus species, but it is present in most of L. plantarum strains. The expression of mRNA of crtN and crtM can be induced by external stresses, particularly, oxidative stress. In short, the crtM–crtN genes form a well-conserved operon in L. plantarum, and are highly involved in the biosynthesis of 4,4′-diaponeurosporene production under oxidative stress.
Nucleotide sequence accession number
The complete genome sequence of L. plantarum subsp. plantarum KCCP11226 was deposited in the NCBI genome database under accession numbers CP046262 for the chromosome and CP046263 to CP046265 for the three plasmids.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by an Incheon National University Research Grant in 2017.
Author contributions
M-JS and W-HC designed and coordinated all the experiments; MK performed in vitro experiments into C30 carotenoid production and wrote the manuscript; D-HJ analyzed the genome data; D-HS performed the genome sequencing and sequence assembly. All authors have read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Mibang Kim and Dong-Hyun Jung contributed equally to this work.
Won-Hyong Chung and Myung-Ji Seo contributed equally to this work.
Contributor Information
Won-Hyong Chung, Email: whchung@kfri.re.kr.
Myung-Ji Seo, Email: mjseo@inu.ac.kr.
References
- Ben-Amotz A, Avron M. On the factors which determine massive β-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol. 1983;72:593–597. doi: 10.1104/pp.72.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton G. History: 175 years of carotenoid chemistry. In: Britton G, Pfander H, Liaaen-Jensen S, editors. Carotenoids. Basel: Birkhauser; 1995. pp. 13–26. [Google Scholar]
- Chandi GK, Gill BS. Production and characterization of microbial carotenoids as an alternative to synthetic colors: a review. Int J Food Prop. 2011;14:503–513. doi: 10.1080/10942910903256956. [DOI] [Google Scholar]
- Chen B, Zhang A, Li R, Mu X, He H, Chen H, Jin M. Evaluation of the protective efficacy of a newly identified immunogenic protein, HP0272, of Streptococcus suis. FEMS Microbiol Lett. 2010;307:12–18. doi: 10.1111/j.1574-6968.2010.01944.x. [DOI] [PubMed] [Google Scholar]
- Chin C-S, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- Clauditz A, Resch A, Wieland K-P, Peschel A, Götz FJI. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun. 2006;74:4950–4953. doi: 10.1128/IAI.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One. 2010;5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong N, Burgess M, Barrow K, Glenn D. Carotenoid accumulation in the psychrotrophic bacterium Arthrobacter agilis in response to thermal and salt stress. Appl Microbiol Biotechnol. 2001;56:750–756. doi: 10.1007/s002530100739. [DOI] [PubMed] [Google Scholar]
- Garrido-Fernández J, Maldonado-Barragán A, Caballero-Guerrero B, Hornero-Méndez D, Ruiz-Barba JL. Carotenoid production in Lactobacillus plantarum. Int J Food Microbiol. 2010;140:34–39. doi: 10.1016/j.ijfoodmicro.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Gautheret D, Lambert A. Direct RNA motif definition and identification from multiple sequence alignments using secondary structure profiles. J Mol Biol. 2001;313:1003–1011. doi: 10.1006/jmbi.2001.5102. [DOI] [PubMed] [Google Scholar]
- Hagi T, Kobayashi M, Nomura M. Aerobic condition increases carotenoid production associated with oxidative stress tolerance in Enterococcus gilvus. FEMS Microbiol Lett. 2014;350:223–230. doi: 10.1111/1574-6968.12341. [DOI] [PubMed] [Google Scholar]
- Hunt M, De Silva N, Otto TD, Parkhill J, Keane JA, Harris SR. Circlator: automated circularization of genome assemblies using long sequencing reads. Genome Biol. 2015;16:294. doi: 10.1186/s13059-015-0849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Seo D, Park Y, Cha I, Seo M. Isolation of Lactobacillus plantarum subsp. plantarum producing C30 carotenoid 4,4'-diaponeurosporene and the assessment of its antioxidant activity. J Microbiol Biotechnol. 2019;29:1925–1930. doi: 10.4014/jmb.1909.09007. [DOI] [PubMed] [Google Scholar]
- Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MW. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci USA. 2003;100:1990–1995. doi: 10.1073/pnas.0337704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen DM, Kannan L, Coleman MK, Wolf YI, Sorokin A, Koonin EV, Mushegian A. A low-polynomial algorithm for assembling clusters of orthologous groups from intergenomic symmetric best matches. Bioinformatics. 2010;26:1481–1487. doi: 10.1093/bioinformatics/btq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B-H, Lee Y-K. Secondary carotenoids formation by the green alga Chlorococcum sp. J Appl Phycol. 2000;12:301–307. doi: 10.1023/A:1008185212724. [DOI] [Google Scholar]
- Macke TJ, Ecker DJ, Gutell RR, Gautheret D, Case DA, Sampath R. RNAMotif, an RNA secondary structure definition and search algorithm. Nucleic Acids Res. 2001;29:4724–4735. doi: 10.1093/nar/29.22.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maoka T. Carotenoids as natural functional pigments. J Nat Med. 2019;74:1–16. doi: 10.1007/s11418-019-01364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi A, Rochat T, Gratadoux J-J, Le Loir Y, Oliveira SC, Langella P, Azevedo V. Oxidative stress in Lactococcus lactis. Genet Mol Res. 2003;2:348–359. [PubMed] [Google Scholar]
- Orosa M, Valero J, Herrero C, Abalde J. Comparison of the accumulation of astaxanthin in Haematococcus pluvialis and other green microalgae under N-starvation and high light conditions. Biotechnol Lett. 2001;23:1079–1085. doi: 10.1023/A:1010510508384. [DOI] [Google Scholar]
- Phadwal K. Carotenoid biosynthetic pathway: molecular phylogenies and evolutionary behavior of crt genes in eubacteria. Gene. 2005;345:35–43. doi: 10.1016/j.gene.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Siezen RJ, van Hylckama Vlieg JE. Genomic diversity and versatility of Lactobacillus plantarum, a natural metabolic engineer. Microb Cell Fact. 2011;10:S3. doi: 10.1186/1475-2859-10-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasara T, Stephan R. Evaluation of housekeeping genes in Listeria monocytogenes as potential internal control references for normalizing mRNA expression levels in stress adaptation models using real-time PCR. FEMS Microbiol Lett. 2007;269:265–272. doi: 10.1111/j.1574-6968.2007.00633.x. [DOI] [PubMed] [Google Scholar]
- Tatusov RL, Koonin EV, Lipman DJ. A genomic perspective on protein families. Science. 1997;278:631–637. doi: 10.1126/science.278.5338.631. [DOI] [PubMed] [Google Scholar]
- Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin W, Renaud C, Avallone S, Hammoumi A, Guyot J-P, Humblot C. PCR of crtNM combined with analytical biochemistry: an efficient way to identify carotenoid producing lactic acid bacteria. Syst Appl Microbiol. 2016;39:115–121. doi: 10.1016/j.syapm.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Yatsunami R, Ando A, Yang Y, Takaichi S, Kohno M, Matsumura Y, Ikeda H, Fukui T, Nakasone K, Fujita N. Identification of carotenoids from the extremely halophilic archaeon Haloarcula japonica. Front Microbiol. 2014;5:100. doi: 10.3389/fmicb.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago M, Fornasari ME, Carminati D, Burns P, Suàrez V, Vinderola G, Reinheimer J, Giraffa G. Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol. 2011;28:1033–1040. doi: 10.1016/j.fm.2011.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.