Abstract
Introduction
This study was aimed to compare ocular tissue distribution and systemic exposure of brimonidine and timolol after single topical administration to rabbits of fixed-combination ophthalmic solution of 0.1% brimonidine tartrate and 0.5% timolol and single drugs (0.1% brimonidine tartrate ophthalmic solution or 0.5% timolol ophthalmic solution) or concomitant administration of single drugs.
Methods
Rabbits were treated with a single topical administration of each ophthalmic solution or concomitant administration of single drugs. For concomitant administration, 0.1% brimonidine tartrate was administered after 0.5% timolol instillation successively within 10 s (without interval) or with 5-min intervals. Brimonidine and timolol concentrations in the aqueous humor, retina/choroid, vitreous body, and plasma were determined with liquid chromatography-tandem mass spectrometry.
Results
The area under the curve values of both drugs in the aqueous humor after fixed-combination administration were comparable to those after concomitant administration. The value of brimonidine was comparable to that after 0.1% brimonidine tartrate administration, whereas the value of timolol was 1.6-fold higher than that after 0.5% timolol administration. The plasma area under the curve value of brimonidine did not differ between fixed-combination and single-drug administrations, but that of timolol was higher after fixed-combination administration than after single-drug administration. Similar concentration-time curves of brimonidine were observed in the posterior ocular tissues in all groups. For concomitant administration, both drug concentrations in the aqueous humor without an administration interval were lower than those with 5-min intervals.
Conclusion
There was no difference in the effect of formulation compositions on ocular and systemic pharmacokinetics among the ophthalmic solutions, but brimonidine may alter the ocular and systemic absorption of timolol, which is possibly due to its pharmacologic action. We demonstrated the importance of an administration interval in the concomitant administration of these drugs. This concern could be avoided by using a fixed combination of brimonidine and timolol.
Keywords: Administration interval, Brimonidine, Fixed-combination ophthalmic solution, Ocular pharmacokinetics, Timolol
Key Summary Points
| Why carry out this study? |
| Fixed-combination therapies could simplify treatment regimens and additionally avoid diluting and/or washing each drug by non-interval administration of single drugs concomitantly. |
| Drug pharmacokinetics of fixed-combination ophthalmic solution would be affected by their formulation composition and drug-drug interaction, but the knowledge of these points is limited. |
| We tried to clarify and compare the pharmacokinetic profiles of the new fixed-combination ophthalmic solution of 0.1% brimonidine tartrate and 0.5% timolol with that of the respective single drugs alone and/or concomitantly. |
| What was learned from the study? |
| Ocular and systemic pharmacokinetics of brimonidine were comparable among fixed-combination, single drug and concomitant administration, but those of timolol were differ among these groups. |
| The present study suggested the difference of pharmacokinetics in timolol is caused by the pharmacologic action of brimonidine but not by formulation composition, providing an information in pharmacokinetics under fixed-combination therapies. |
Introduction
Glaucoma is a progressive neurodegenerative eye disease characterized by the degeneration and loss of retinal ganglion cells and their axons. As the intraocular pressure (IOP) is related to retinal ganglion cell death, the most common initial therapy for glaucoma is to decrease IOP through ophthalmic administration of one or more drugs [1].
Patients with glaucoma initially undergo monotherapy such as treatment with a prostaglandin analog or beta-blocker [2]. However, in 40–75% of patients with open-angle glaucoma, monotherapy fails to achieve sufficient IOP reduction after > 2 years of treatment, and combination therapy becomes necessary [3]. Administration of multiple medications could reduce patient compliance in terms of treatment schedule, such as increased number of instillations [4–7]. Furthermore, 22–59% of patients with multiple medications used eye drops without an appropriate administration interval, despite instructions to instill multiple medications with at least 5–10-min intervals to avoid diluting and/or washing each other from the cul-de-sac [8–10]. Fixed-combination therapies provide a number of ocular agents in a single formulation, thereby simplifying treatment regimens and reducing the number of daily instillations.
Brimonidine tartrate, a highly selective α2-adrenergic agonist, is an IOP-lowering drug. It decreases the IOP by reducing the production of aqueous humor and increasing its outflow via the uveoscleral pathway [11]. Timolol, a nonselective β-adrenergic receptor-blocking agent, acts by reducing the intracellular concentrations of cyclic adenosine monophosphate [12]. These two agents are used concomitantly in many patients to effectively lower the IOP because they have different mechanisms. A fixed-combination ophthalmic solution of 0.2% brimonidine tartrate and 0.5% timolol (Combigan®; Allergan Inc., Irvine, CA, USA) was first marketed in Canada and then in > 60 other countries including the USA. In Japan, a fixed-combination ophthalmic solution of 0.1% brimonidine tartrate and 0.5% timolol (Aibeta®; Senju Pharmaceutical Co., Ltd., Osaka, Japan) was approved recently. However, pharmacokinetic studies on the use of combination brimonidine and timolol ophthalmic solutions are limited [13].
The purpose of the present study was to compare ocular and systemic absorption in rabbits after topical administration of a fixed-combination ophthalmic solution of 0.1% brimonidine tartrate and 0.5% timolol (equivalent to 0.68% timolol maleate) with that after single and concomitant administration of the respective single drugs. We also investigated the distribution of brimonidine in the posterior ocular tissues such as the vitreous body and retina/choroid because recent studies demonstrated that brimonidine has a neuroprotective effect via the retinal α2-adrenergic receptor [14–16]. Additionally, the effect of an administration interval between brimonidine and timolol single drugs on ocular drug absorption was investigated when these single drugs were administered concomitantly to strengthen the relevance of fixed-combination drugs from the point of view of ocular pharmacokinetics.
Methods
Animals
Male Japanese white rabbits (Kbs:JW) weighing approximately 1.4–1.9 kg were obtained from Kitayama Labes Co., Ltd. (Nagano, Japan). The animals were maintained in conventional animal rooms and individually housed in plastic cages in an air-conditioned room with a temperature of 22 °C ± 3 °C, 55% ± 10% relative humidity, and a 12-h light/dark cycle. The animals were fed with a commercial diet (Lab R Stock; Nosan Corp., Tokyo, Japan) once daily and were given tap water ad libitum. Dumbbells (Bio-Serv; Flemington, NJ, USA) were provided in each animal cage for environmental enrichment. Standard procedures and housing conditions were applied in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International). All procedures were in accordance with the guidelines for animal experimentation at Senju Pharmaceutical Co., Ltd., and the protocol was reviewed by the Institutional Animal Care and Use Committee (IACUC).
Drugs and Chemicals
Fixed-combination ophthalmic solution of 0.1% brimonidine tartrate and 0.5% timolol (BMD/TIM) was prepared according to the formulation approved in Japan (Aibeta®). A 0.1% brimonidine tartrate ophthalmic solution (BMD; Aiphagan®; Senju Pharmaceutical Co., Ltd.) and a 0.5% timolol ophthalmic solution (TIM; Timoptol®; Santen Pharmaceutical Co., Ltd., Osaka, Japan) were obtained commercially. The reference standards used for quantitation were the same as those for the preparation of BMD/TIM. The internal standard, 5-chloro-6-(2-imidazolidinylideneamino) quinoxaline, was provided by Allergan Inc. (Irvine, CA, USA). All other reagents were special grade or higher and were obtained commercially.
Effect of Administration Interval on Ocular Absorption
TIM (35 µl) was concomitantly administered 5 min after BMD (35 µl) instillation to one eye of the rabbits (concomitant 5-min-interval group). For the other eye of each rabbit, TIM (35 µl) was concomitantly administered after BMD (35 µl) instillation successively within 10 s (concomitant non-interval group) to simulate a use of two eye drops without an administration interval. Aqueous humor was collected at 0.5, 1, and 2 h after the first drug administration (n = 3/time point).
Ocular Absorption After Topical Administration
Rabbits were single-dosed with BMD/TIM (combination group), BMD (brimonidine group), or TIM (timolol group) or were dosed concomitantly with BMD and TIM (concomitant group). A 35-µl drop of ophthalmic solution was topically administered to each eye of the rabbits (n = 3/time point). For the concomitant group, BMD was administered 5 min after TIM instillation. Aqueous humor was collected from the rabbits at 0.25, 0.5, 1, 2, and 4 h after the first drug administration.
Brimonidine Distribution in the Posterior Parts of the Eye
A single 35-µl drop of BMD/TIM (combination group), BMD (brimonidine group), or TIM (timolol group) was applied to one eye of each rabbit (n = 3/time point). In the concomitant group, a single 35-µl drop of BMD was applied to one eye of each rabbit followed 5 min later by a single 35-µl drop of TIM in the same eye. At three time points (0.5, 1, and 2 h) after the first administration, rabbits were euthanized by an intravenous overdose of pentobarbital sodium. The eyes were enucleated immediately after the rabbits had been euthanized. Enucleated eyes were frozen in liquid nitrogen and were divided at the equator. The vitreous body and retina/choroid were collected from each divided eye.
Systemic Absorption After Topical Administration
A single 35-µl drop of BMD/TIM (combination group), BMD (brimonidine group), or TIM (timolol group) was applied to one eye of each rabbit (n = 3). Blood samples were collected from each rabbit at 0.25, 0.5, 1, 2, or 4 h via the auricular vein. The blood samples were cooled in ice as soon as possible after blood sampling and plasma obtained by centrifugation (set at 4 °C, 2000 × g for 10 min).
Bioanalysis of Drug Concentration
All bioanalysis samples were stored at − 80 °C until sample processing. Brimonidine and timolol concentrations in ocular tissues and plasma samples were determined by liquid chromatography-tandem mass spectrometry. Ocular tissues excluding aqueous humor and plasma samples were pretreated with solid-phase extract methods before analysis using OASIS HLB (Waters, Milford, MA, USA).
Pharmacokinetics Analysis
Pharmacokinetics analysis was performed by noncompartmental analysis using Phoenix® WinNonlin® version 6.3 (Certara LP, Princeton, NJ, USA). The time to the maximum concentration (Tmax), maximum concentration (Cmax), and area under the curve (AUC) in the aqueous humor and plasma were determined.
Results
Administration Interval
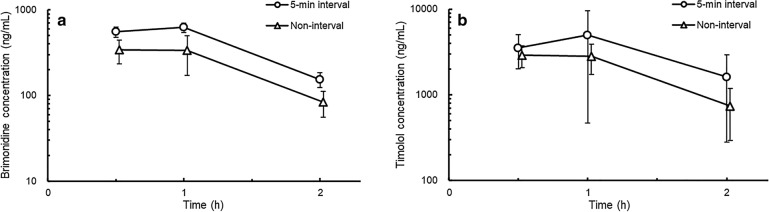
In both groups, the aqueous humor concentrations of brimonidine and timolol attained their peak values within 1 h after administration (Fig. 1). The brimonidine concentration of the aqueous humor in the concomitant non-interval group was lower than that in the concomitant 5-min-interval group. The Cmax and AUC0-2 values of brimonidine in the concomitant non-interval group were 0.5- and 0.6-fold lower, respectively, than that in the concomitant 5-min-interval group (Table 1). In addition, the timolol concentration in the aqueous humor in the concomitant non-interval group was also lower than that in the concomitant 5-min-interval group.
Fig. 1.
Brimonidine (a) and timolol (b) concentrations in the aqueous humor after concomitant administration of 0.1% brimonidine tartrate ophthalmic solution (BMD) and 0.5% timolol ophthalmic solution (TIM) in rabbits. TIM (35 µl) was administered 5 min after BMD (35 µl) instillation to one eye of each rabbit. For the other eye of each rabbit, TIM was administered after BMD (35 µl) instillation in succession within 10 s in the non-interval group. Data are presented as mean ± standard deviation (n = 3)
Table 1.
Pharmacokinetic parameters of brimonidine and timolol in the aqueous humor after concomitant administration of 0.1% brimonidine ophthalmic solution and 0.5% timolol ophthalmic solution with or without administration interval in rabbits
| Group | Brimonidine | Timolol | ||||
|---|---|---|---|---|---|---|
| Tmax (h) | Cmax (ng/ml) | AUC0–2 (ng·h/ml) | Tmax (h) | Cmax (ng/ml) | AUC0–2 (ng·h/ml) | |
| Concomitant | ||||||
| 5-min interval | 1 | 622 ± 78 | 821 ± 41 | 1 | 4990 ± 4520 | 6300 ± 2040 |
| Non-interval | 0.5 | 340 ± 105 | 463 ± 77 | 0.5 | 2900 ± 840 | 3920 ± 540 |
Cmax is presented as mean ± standard deviation (n = 3). AUC0–2 is presented as mean ± standard error (n = 3)
Tmax time to maximum concentration, Cmax maximum concentration, AUC0–2 area under the curve from time 0–2 h
Ocular Absorption
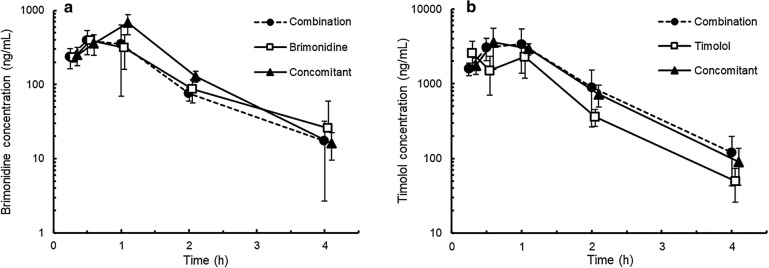
The aqueous humor concentration of brimonidine in the combination group attained peak values at 0.5 h after administration and thereafter decreased rapidly (Fig. 2). In the other groups, the ocular absorption of brimonidine was also rapid after ocular administration, and maximum concentrations peaked at 0.5 or 1 h after administration. No differences in Cmax or AUC0–4 values were observed between the combination and brimonidine groups, which were 0.6- and 0.7-fold lower, respectively, than those in the concomitant group (Table 2).
Fig. 2.
Brimonidine (a) and timolol (b) concentrations in the aqueous humor after the administration of fixed-combination ophthalmic solution of 0.1% brimonidine tartrate and 0.5% timolol (combination group), 0.1% brimonidine tartrate ophthalmic solution (BMD) (brimonidine group), or 0.5% timolol ophthalmic solution (TIM) (timolol group) or concomitant administration of these single drugs (concomitant group) in rabbits. A 35-µl drop of ophthalmic solution was topically applied to each eye of the rabbits. In the concomitant group, BMD was administered 5 min after TIM instillation. Data are presented as mean (n = 2) or mean ± standard deviation (n = 3–6)
Table 2.
Pharmacokinetic parameters of brimonidine and timolol in the aqueous humor after topical administration to rabbits
| Group | Brimonidine | Timolol | ||||
|---|---|---|---|---|---|---|
| Tmax (h) | Cmax (ng/ml) | AUC0–4 (ng·h/ml) | Tmax (h) | Cmax (ng/ml) | AUC0–4 (ng·h/ml) | |
| Combination | 0.5 | 393 ± 140 | 599 ± 91 | 1 | 3410 ± 2020 | 5550 ± 750 |
| Brimonidine | 0.5 | 395a | 595 ± 93 | – | – | – |
| Timolol | – | – | – | 0.25 | 2580 ± 1080 | 3520 ± 540 |
| Concomitant | 1 | 673 ± 201 | 911 ± 93 | 0.5 | 3560 ± 2020 | 5160 ± 530 |
Cmax is presented as mean ± standard deviation (n = 3–6). AUC0–4 is presented as mean ± standard error (n = 2–6)
Tmax time to maximum concentration, Cmax maximum concentration, AUC0–4 area under the curve from time 0–4 h
aThis value is presented as mean (n = 2)
The aqueous humor concentration of timolol in the combination group attained peak values 1 h after administration and thereafter decreased rapidly (Fig. 2). In the other groups, topically administered timolol was also rapidly absorbed and eliminated in the aqueous humor. The Cmax and AUC0–4 values in the combination group were comparable to those in the concomitant group and were 1.3- and 1.6-fold higher, respectively, than those in the timolol group (Table 2).
Ocular Tissue Distribution of Brimonidine in the Posterior Parts of the Eye
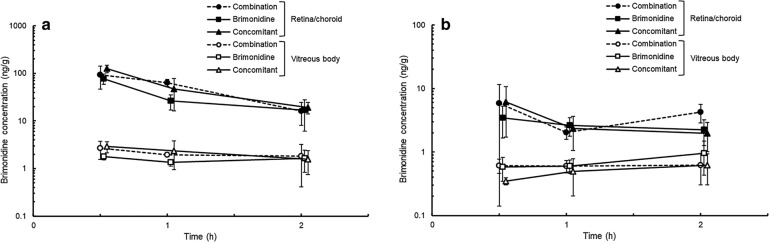
Topically applied brimonidine was distributed rapidly in the vitreous body and retina/choroid in all groups (Fig. 3). In all posterior ocular tissues, similar brimonidine concentration-time curves were observed among these groups. The posterior vitreous body showed the lowest brimonidine concentration in posterior ocular tissues. The Cmax values in the posterior vitreous body were 0.622 ± 0.318, 0.954 ± 0.520, and 0.618 ± 0.316 ng/g in the combination, brimonidine, and concomitant groups, respectively.
Fig. 3.
Brimonidine concentrations in the anterior (a) and posterior (b) parts of the retina/choroid and vitreous body after the administration of fixed-combination ophthalmic solution of 0.1% brimonidine tartrate and 0.5% timolol (combination group) or 0.1% brimonidine tartrate ophthalmic solution (BMD) (brimonidine group) or concomitant administration of BMD and 0.5% timolol ophthalmic solution (TIM) (concomitant group) in rabbits. A 35-µl drop of ophthalmic solution was topically applied to each eye of the rabbits. In the concomitant group, TIM was administered 5 min after BMD instillation. Data are presented as mean ± standard deviation (n = 3–4)
Systemic Absorption
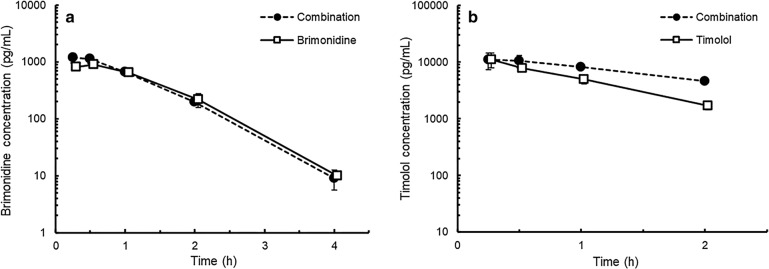
The brimonidine Tmax values in the plasma were similar between the combination and brimonidine groups, which were 0.33 and 0.43 h after administration, respectively (Fig. 4). After the Tmax, brimonidine concentrations rapidly decreased in both groups. No differences in Cmax or AUC0–4 values were observed between these two groups (Table 3).
Fig. 4.
Brimonidine (a) and timolol (b) concentrations in the plasma after single administration of fixed-combination ophthalmic solution of 0.1% brimonidine tartrate and 0.5% timolol (combination group), 0.1% brimonidine tartrate ophthalmic solution (BMD) (brimonidine group), or 0.5% timolol ophthalmic solution (TIM) (timolol group) in rabbits. A 35-µl drop of ophthalmic solution was topically applied to each eye of the rabbits. Data are presented as mean ± standard deviation (n = 3)
Table 3.
Pharmacokinetic parameters of brimonidine and timolol in the plasma after topical administration to rabbits
| Group | Brimonidine | Timolol | ||||
|---|---|---|---|---|---|---|
| Tmax (h) | Cmax (pg/ml) | AUC0–4 (pg·h/ml) | Tmax (h) | Cmax (pg/ml) | AUC0–2 (pg·h/ml) | |
| Combination | 0.33 | 1190 ± 230 | 1540 ± 100 | 0.33 | 11,300 ± 3500 | 15,700 ± 2200 |
| Brimonidine | 0.43 | 947 ± 70 | 1380 ± 100 | – | – | – |
| Timolol | – | – | – | 0.33 | 11,400 ± 2900 | 10,300 ± 1200 |
Tmax is presented as mean (n = 3). Cmax, AUC0–4, and AUC0–2 are presented as mean ± standard deviation (n = 3)
Tmax time to maximum concentration, Cmax maximum concentration, AUC0–4 or AUC0–2 area under the curve from time 0 to 4 or 2 h
In both the combination and TIM groups, the plasma concentrations of timolol attained their peak values at 0.33 h after administration and decreased thereafter (Fig. 4). The Cmax values were similar between these two groups, whereas the AUC0–2 value in the combination group was 1.5-fold higher than that in the timolol group (Table 3).
Discussion
Several reports have shown that an insufficient administration interval between two drugs in the concomitant administration lowers the pre-corneal drug concentration by diluting and/or by washing each other and reducing the penetration of drugs to the aqueous humor [17, 18]. In the present study, brimonidine concentrations in the aqueous humor after concomitant administration of BMD and TIM without administration interval were approximately two times lower than those with a 5-min interval. In addition, the concentrations of timolol, which was the second administered drug, in the aqueous humor after concomitant administration without administration interval tended to be lower than those with 5-min interval. These results strongly support the findings of previous reports [17, 18] as well as the general recommendation of instilling with an appropriate interval when successive administration of eye-drop drugs from the viewpoint of ocular pharmacokinetics. Fixed-combination ophthalmic solutions could avoid this issue by instilling each eye-drop drug concomitantly. In this study, the ocular tissue distribution of brimonidine or timolol after administration of a fixed-combination ophthalmic solution of these drugs, BMD/TIM, was investigated by comparing it with that after single or concomitant administration of these single drugs, BMD and TIM.
The formulation composition influences corneal drug penetration [19–22]. BMD/TIM contains benzalkonium chloride (BAK) and ethylenediaminetetraacetic acid (EDTA), which are typical agents that influence ocular drug pharmacokinetics. BMD does not contain BAK, but TIM does at the concentration of 0.005%. Neither BMD nor TIM contains EDTA. In addition, the bioavailability of brimonidine and timolol in the aqueous humor following ophthalmic administration is enhanced by increasing the solution pH, in accordance with the pH partition hypothesis [23–25]. For a fixed-combination drug, these differences in formulation raised a concern about whether corneal drug penetrations of brimonidine and timolol were changed or not. However, many researchers reported that the enhancing effect of BAK on corneal permeability of ophthalmic drugs appears to be concentration-dependent in the range of 0.005–0.05% [22, 26, 27], and the BAK concentration in BMD/TIM is < 0.005%. The concentration of EDTA in both BMD/TIM and TIM is lower than that reported to affect the ocular absorption of timolol [28]. Moreover, BMD/TIM was formulated at a target pH similar to that of BMD and TIM. Thus, it was expected that BMD/TIM would show comparable drug concentrations of brimonidine and timolol in the aqueous humor with BMD and TIM. The present result showed that the brimonidine Cmax and AUC values in the aqueous humor were comparable between BMD/TIM and BMD. Regarding systemic pharmacokinetics, a similar trend with the aqueous humor was seen in the plasma concentrations of brimonidine.
Meanwhile, our data showed that the timolol Cmax and AUC values of BMD/TIM in the aqueous humor were higher than those of TIM, whereas these values of BMD/TIM were comparable to that after concomitant administration of the single drugs. These findings indicate that the ocular absorption of timolol might be affected by brimonidine, but not by differences in formulation compositions, such as preservatives, for the above-described reason. Transporters in the corneal epithelium have been reported to module the ocular bioavailability of their substrates administered topically [29]. However, enhanced timolol concentration in the aqueous humor may not be caused by the transporter effect because Sakanaka et al. [30] reported that timolol permeation through the corneal epithelium was predominantly contributed by passive diffusion. Urtti and Kyyrönen [31] have shown that epinephrine and phenylephrine, which induce vasoconstriction, slowed the absorption of timolol from the conjunctiva and nasal mucosa to systemic circulation by reducing the blood flow in the main sites for the systemic absorption of topically applied timolol. Brimonidine was reported to induce ocular vasoconstriction primarily via the α2-adrenergic receptor [32]. Therefore, the brimonidine-induced vasoconstriction in the conjunctiva and nasal mucosa could cause the retention of timolol in the conjunctiva, probably resulting in an increase in the timolol transition into ocular tissues.
This consideration in ocular pharmacokinetics would be supported by the results on the systemic pharmacokinetics of timolol. Elimination of timolol in the plasma seemed to be slower in BMD/TIM administration than that in TIM administration. This implies a delay of systemic absorption that is probably caused by the brimonidine-induced vasoconstriction in the conjunctiva and nasal mucosa. Taken together, these results suggest that systemic exposure of timolol is not affected by the difference of formulation composition, but by the pharmacologic action of brimonidine.
Since the target tissue of the neuroprotective effect of brimonidine is the retina, it is important to investigate the drug distribution in the posterior parts of the eye after BMD/TIM administration. A previous study showed that, in most patients, topical multiple administration of BMD twice daily reached a brimonidine vitreous concentration > 2 nM [33], which is the concentration necessary to activate the α2-adrenergic receptor [34]. In addition, Shinno et al. [35] suggest that the brimonidine concentration in the vitreous body can be a surrogate indicator of the free concentration in the posterior retina/choroid, which is regarded as an available fraction for receptor binding. In this study, topical administration of BMD/TIM showed similar concentration-time curves of brimonidine to BMD administration and concomitant administration of BMD and TIM in the posterior parts of the eye, including the vitreous body, and produced a brimonidine concentration in the vitreous body and retina/choroid > 2 nM (0.584 ng/g). Therefore, topical administration of BMD/TIM could achieve a brimonidine concentration that can sufficiently exert a neuroprotective effect on target tissues.
The present study has a few limitations. This study of BMD/TIM focused on pH and preservatives; hence, changing other formulation factors such as viscosity, ion pair formation, and others can possibly alter ocular absorption. Further studies with other fixed-combination drugs would be necessary to clarify not only the above formulation factors but also drug-drug interaction of ocular pharmacokinetics and pharmacodynamics in fixed-combination ophthalmic solution.
Conclusions
We demonstrated that the ocular pharmacokinetics of brimonidine and timolol after ophthalmic administration of a fixed-combination ophthalmic solution (BMD/TIM) was comparable to that of the single drugs (BMD or TIM) alone and/or their concomitant administration. A trend similar to that of ocular absorption was observed in systemic absorption. These results indicate that the effect of the formulation composition of BMD/TIM on drug pharmacokinetics is comparable to that of the respective single drugs after ophthalmic administration. Meanwhile, the present results demonstrated that brimonidine somewhat altered the ocular and systemic absorption of timolol, which is possibly due to local vasoconstriction induced by brimonidine. This study also clearly showed that concomitant administration of BMD and TIM without an administration interval lowered ocular absorption of both drugs. BMD/TIM could be a new therapeutic alternative for the treatment of glaucoma that eliminates concerns about administration interval, making it a convenient treatment for patients.
Acknowledgements
The authors thank their coworkers in the DMPK team of Senju Pharmaceutical Co., Ltd., for their technical assistance in the distribution study.
Funding
This research and publication processing costs were supported by Senju Pharmaceutical Co., Ltd. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
All authors of this paper, Gen Suzuki, Eriko Kunikane, Keisuke Shinno, Seiko Kozai, Masaaki Kurata, and Akio Kawamura, are employees of Senju Pharmaceutical Co., Ltd.
Compliance with Ethics Guidelines
All institutional and national guidelines for the care and use of laboratory animals were followed. All procedures were in accordance with the guidelines for animal experimentation at Senju Pharmaceutical Co., Ltd., and the protocol was reviewed by the Institutional Animal Care and Use Committee (IACUC).
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.11537694.
Change history
12/22/2021
The license text was incorrectly structured. The article has been corrected.
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Japan Glaucoma Society Guidelines for Glaucoma (4th edition). Nihon Ganka Gakkai Zasshi. 2019;122:5–53.
- 3.Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 4.Barnebey HS, Robin AL. Adherence to Fixed-combination versus unfixed travoprost 0.004%/timolol 0.5% for glaucoma or ocular hypertension: a randomized trial. Am J Ophthalmol. 2017;176:61–69. doi: 10.1016/j.ajo.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Konstas AG, Maskaleris G, Gratsonidis S, Sardelli C. Compliance and viewpoint of glaucoma patients in Greece. Eye. 2000;14:752–756. doi: 10.1038/eye.2000.197. [DOI] [PubMed] [Google Scholar]
- 6.Olthoff CM, Schouten JS, van de Borne BW, Webers CA. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112(6):953–961. doi: 10.1016/j.ophtha.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 7.Robin AL, Covert D. Does adjunctive glaucoma therapy affect adherence to the initial primary therapy? Ophthalmology. 2005;112(5):863–868. doi: 10.1016/j.ophtha.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Abe H. Improving compliance with medical treatment of glaucoma. J Eye. 1999;16(7):907–912. [Google Scholar]
- 9.Ikeda H, Sato M, Tsukamoto H, et al. Evaluation and multivariate statistical analysis of factors influencing patient adherence to ophthalmic solutions. Yakugaku Zasshi. 2001;121(11):799–806. doi: 10.1248/yakushi.121.799. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman TJ, Zalta AH. Facilitating patient compliance in glaucoma therapy. Surv Ophthalmol. 1983;28(Suppl):252–258. doi: 10.1016/0039-6257(83)90142-X. [DOI] [PubMed] [Google Scholar]
- 11.Adkins JC, Balfour JA. Brimonidine. A review of its pharmacological properties and clinical potential in the management of open-angle glaucoma and ocular hypertension. Drugs Aging. 1998;12(3):225–241. doi: 10.2165/00002512-199812030-00005. [DOI] [PubMed] [Google Scholar]
- 12.Mittag TW. Adrenergic and dopaminergic drugs in glaucoma. In: Ritch R, Schields MB, Krupin T, editors. The glaucomas. Maryland Heights: Mosby; 1996. pp. 1409–1424. [Google Scholar]
- 13.Lee AJ, McCluskey P. Fixed combination of topical brimonidine 0.2% and timolol 0.5% for glaucoma and uncontrolled intraocular pressure. Clin Ophthalmol. 2008;2(3):545–555. doi: 10.2147/OPTH.S3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S. A randomized trial of brimonidine versus timolol in preserving visual function: results from the low-pressure glaucoma treatment study. Am J Ophthalmol. 2011;151(4):671–681. doi: 10.1016/j.ajo.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama Y, Kawasaki R, Takahashi H, et al. Effects of brimonidine and timolol on the progression of visual field defects in open-angle glaucoma: a single-center randomized trial. J Glaucoma. 2019;28(7):575–583. doi: 10.1097/IJG.0000000000001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saylor M, McLoon LK, Harrison AR, Lee MS. Experimental and clinical evidence for brimonidine as an optic nerve and retinal neuroprotective agent: an evidence-based review. Arch Ophthalmol. 2009;127(4):402–406. doi: 10.1001/archophthalmol.2009.9. [DOI] [PubMed] [Google Scholar]
- 17.Chrai SS, Makoid MC, Eriksen SP, Robinson JR. Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J Pharm Sci. 1974;63(3):333–338. doi: 10.1002/jps.2600630304. [DOI] [PubMed] [Google Scholar]
- 18.Sieg JW, Robinson JR. Mechanistic studies on transcorneal permeation of pilocarpine. J Pharm Sci. 1976;65(12):1816–1822. doi: 10.1002/jps.2600651230. [DOI] [PubMed] [Google Scholar]
- 19.Grass GM, Wood RW, Robinson JR. Effects of calcium chelating agents on corneal permeability. Invest Ophthalmol Vis Sci. 1985;26(1):110–113. [PubMed] [Google Scholar]
- 20.Chrai SS, Patton TF, Mehta A, Robinson JR. Lacrimal and instilled fluid dynamics in rabbit eyes. J Pharm Sci. 1973;62(7):1112–1121. doi: 10.1002/jps.2600620712. [DOI] [PubMed] [Google Scholar]
- 21.Lee VH, Chien DS, Sasaki H. Ocular ketone reductase distribution and its role in the metabolism of ocularly applied levobunolol in the pigmented rabbit. J Pharmacol Exp Ther. 1988;246(3):871–878. [PubMed] [Google Scholar]
- 22.Podder SK, Moy KC, Lee VH. Improving the safety of topically applied timolol in the pigmented rabbit through manipulation of formulation composition. Exp Eye Res. 1992;54(5):747–757. doi: 10.1016/0014-4835(92)90030-V. [DOI] [PubMed] [Google Scholar]
- 23.Acheampong AA, Small D, Baumgarten V, Welty D, Tang-Liu D. Formulation effects on ocular absorption of brimonidine in rabbit eyes. J Ocul Pharmacol Ther. 2002;18(4):325–337. doi: 10.1089/10807680260218498. [DOI] [PubMed] [Google Scholar]
- 24.Dong JQ, Babusis DM, Welty DF, Acheampong AA, Tang-Liu D, Whitcup SM. Effects of the preservative purite on the bioavailability of brimonidine in the aqueous humor of rabbits. J Ocul Pharmacol Ther. 2004;20(4):285–292. doi: 10.1089/1080768041725326. [DOI] [PubMed] [Google Scholar]
- 25.Cantor LB, WuDunn D, Catoira-Boyle Y, Yung CW. Absorption of brimonidine 0.1% and 0.15% ophthalmic solutions in the aqueous humor of cataract patients. J Glaucoma. 2008;17(7):529–534. doi: 10.1097/IJG.0b013e318162257f. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki H, Nagano T, Yamamura K, Nishida K, Nakamura J. Ophthalmic preservatives as absorption promoters for ocular drug delivery. J Pharm Pharmacol. 1995;47(9):703–707. doi: 10.1111/j.2042-7158.1995.tb06726.x. [DOI] [PubMed] [Google Scholar]
- 27.Saettone MF, Chetoni P, Cerbai R, Mazzanti G, Braghiroli L. Evaluation of ocular permeation enhancers: in vitro effects on corneal transport of four β-blockers, and in vitro/in vivo toxic activity. Int J Pharm. 1996;142(1):103–113. doi: 10.1016/0378-5173(96)04663-7. [DOI] [Google Scholar]
- 28.Ashton P, Podder SK, Lee VH. Formulation influence on conjunctival penetration of four beta blockers in the pigmented rabbit: a comparison with corneal penetration. Pharm Res. 1991;8(9):1166–1174. doi: 10.1023/A:1015810619869. [DOI] [PubMed] [Google Scholar]
- 29.Nirmal J, Singh SB, Biswas NR, et al. Potential pharmacokinetic role of organic cation transporters in modulating the transcorneal penetration of its substrates administered topically. Eye. 2013;27(10):1196–1203. doi: 10.1038/eye.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakanaka K, Kawazu K, Nishida K, et al. Transport of timolol and tilisolol in rabbit corneal epithelium. Biol Pharm Bull. 2006;29(10):2143–2147. doi: 10.1248/bpb.29.2143. [DOI] [PubMed] [Google Scholar]
- 31.Urtti A, Kyyrönen K. Ophthalmic epinephrine, phenylephrine, and pilocarpine affect the systemic absorption of ocularly applied timolol. J Ocul Pharmacol. 1989;5(2):127–132. doi: 10.1089/jop.1989.5.127. [DOI] [PubMed] [Google Scholar]
- 32.McLaurin E, Cavet ME, Gomes PJ, Ciolino JB. Brimonidine ophthalmic solution 0.025% for reduction of ocular redness: a randomized clinical trial. Optom Vis Sci. 2018;95(3):264–271. doi: 10.1097/OPX.0000000000001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takamura Y, Tomomatsu T, Matsumura T, et al. Vitreous and aqueous concentrations of brimonidine following topical application of brimonidine tartrate 0.1% ophthalmic solution in humans. J Ocul Pharmacol Ther. 2015;31(5):282–285. doi: 10.1089/jop.2015.0003. [DOI] [PubMed] [Google Scholar]
- 34.Burke J, Schwartz M. Preclinical evaluation of brimonidine. Surv Ophthalmol. 1996;41(Suppl 1):9–18. doi: 10.1016/S0039-6257(96)82027-3. [DOI] [PubMed] [Google Scholar]
- 35.Shinno K, Kurokawa K, Kozai S, Kawamura A, Inada K, Tokushige H. The relationship of brimonidine concentration in vitreous body to the free concentration in retina/choroid following topical administration in pigmented rabbits. Curr Eye Res. 2017;42(5):748–753. doi: 10.1080/02713683.2016.1238941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.