Abstract
Glycyrrhiza uralensis Fisch. is known as a common Chinese medicinal herb used to harmonize the effects of other ingredients in most Chinese herbal prescriptions. The rapid production of flavonoids in vitro remains unknown in G. uralensis Fisch. To investigate the in vitro adventitious root regeneration and flavonoid accumulation characteristics in G. uralensis for restrictions on collecting wild plants, suspension cultural and freezing microtomy with histochemical assays were carried out. We reported that multiple adventitious roots were initiated from hypocotyls and stems of G. uralensis. Indole-3-butyric acid (IBA) was more conducive than NAA (1-naphthaleneacetic acid) in inducing G. uralensis adventitious roots, but the addition of 6-BA (6-benzylaminopurine) and KT (kinetin) suppressed the formation of adventitious roots. While the concentration of IBA was 1.0 mg L−1, the flavonoid content and yield were the highest at 19.96 mg g−1 and 1.23 mg g−1, respectively. The optimum medium for adventitious root induction was 1/4-strength Murashige and Skoog’s medium containing 0.1 mg L−1 IBA. The content of flavonoids in adventitious roots and apicals cultured in vitro was higher than that in suspension callus, reaching 3.87 times the callus flavonoid content. The histochemical localization of flavonoids showed that G. uralensis flavonoids mainly distributed in the epidermal parenchyma cells of the callus outer layers and gradually accumulated in cell wall and cell gaps of the epidermis and endodermis of adventitious roots along with the primary growth of adventitious roots, indicating that there were no flavonoids in the roots at the early stage of adventitious roots formation. The results showed that calli inducing adventitious roots and apicals for 30 days obtained the highest yield of flavonoid, indicating effective production for flavonoids instead of wild culture. AlCl3 ethanol solution was better than NaOH aqueous solution in terms of chromogenic and localization effects. We concluded that the highest yield of flavonoid and effective production for flavonoid instead of wild culture could be obtained from calli inducing adventitious roots and apicals.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-04191-w) contains supplementary material, which is available to authorized users.
Keywords: Glycyrrhiza uralensis Fisch., Suspension culture, Adventitious root, Flavonoids, Histochemical localization
Introduction
With a 3000-year history of being used as a medicinal plant, Glycyrrhiza uralensis Fisch. is known as one of the most ancient herbal medicines (Zhang et al. 2009). G. uralensis inflata is a common Chinese medicinal herb used to harmonize the effects of the other ingredients in most Chinese herbal prescriptions (Yang et al. 2009). The main effective constituents are flavonoids and saponins (Yang et al. 2008). Many scholars have isolated and identified 61 kinds of triterpenoid saponins and more than 300 kinds of flavonoids from licorice (Fukai et al. 2002; Shul’ts et al. 2000). Furthermore, licorice extracts are used in cosmetics, food additives, tobacco flavors and confectionery foods (Hayashi and Sudo 2009). Many biological activities, such as anti-mutagenic activity, anti-ulcer effects, protective action against hepatotoxicity, antitumor-promoting activity and antimicrobial effects, have been reported (Li et al. 2000). Due to the decrease of wild Glycyrrhiza resources and restrictions on collecting wild Glycyrrhiza plants, researchers have attempted to enhance flavonoid accumulation in licorice cells and search for substitutes for licorice biotic resources by means of biotechnology (Yang et al. 2006). The flavanone liquiritigenin (F) and its 2’-hydroxychalcone isomer, isoliquiritigenin (C) compositions and proportions were found to be constant for all extracts from a Glycyrrhiza species. All G. uralensis extracts contained up to 2.5 more flavanone liquiritigenin than G. glabra extracts (Simmler et al. 2014).
In recent years, with the development of molecular biology, the use of genetic modification and genetic engineering to regulate the production of medicinal plant secondary metabolites has become a research hotspot, effectively alleviating the shortage of Chinese herbal medicine resources, and has become an effective way to improve secondary metabolites. For example, hairy root cultures of Saussurea involucrata have been transformed with the chalcone isomerase gene (CHI), producing more flavonoids than wild-type hairy roots (Li et al. 2006). In contrast, the silencing of the chalcone synthase gene (CHS) in transgenic hairy roots of Medicago truncatula decreased flavonoid production (Wasson et al. 2006). Zhang et al. (2009) significantly increased the licorice flavonoid yield in hairy roots through over-expression of CHI. They considered that different elicitors combined with the overexpression of the synthase gene can promote the accumulation of flavonoids in licorice more effectively. The overexpression of CHI promotes the yield of flavonoids in the hairy roots of Scutellaria baicalensis (Park et al. 2011). CHS and other synthetic genes have a synergistic expression effect (Tunen et al. 1988). Cinnamic acid-4-hydroxylase (C4H) is the second key enzyme of the phenylpropanoid pathway, affecting the metabolism of flavonoid synthesis. Studies showed the expression of C4H had a direct impact on the flavonoid content of Rubus triflorus and Camellia sinensis (Baek et al. 2008; Singh et al. 2009). The production of licorice chalcones and total flavonoids of hairy roots were improved by adding Tween-80, and increased flavonoids had a relationship with mRNA levels, the activities of phenylalanine ammonialyase, 4-coumarate-coenzyme a ligase and cinnamate-4-hydroxylase (Zhang et al. 2011). Jiang et al. (2010) used RNAi from Glycine max (L.) Merr. isoflavone synthase gene to promote the accumulation of isoflavones.
Plant in vitro culture is an alternative source for the production of valuable secondary metabolites. Hairy root cultures are valuable sources of medicinal compounds. The interest in hairy roots be due to their ability to grow fast without an external supply of any plant growth regulator. Many studies on in vitro culture of licorice, including callus, suspension, hairy roots and whole plant cultures, have been reported (Arias-Castro et al. 1993; Ayabe et al. 1990; Hayashi et al. 1988; Shabani et al. 2009; Wang et al. 2010; Wongwicha et al. 2008; Yang et al. 2007). Since Kamada et al. (1986) established the hairy root culture system using Agrobacterium to infect Atropa belladonna, culturing medicinal plant hairy roots to obtain secondary metabolites has become a new research hot spot; it is also considered to be an effective method to obtain secondary metabolites followed by cell culturing. Liquid culture systems with elicitors are increasingly being investigated to improve secondary metabolite production and to reduce processing costs in several plant cell/hairy root cultivation systems (Prakash and Srivastava 2008). A number of studies showed that the type and age of explant have a strong influence on hairy root induction since the age of the explant is a major factor that alters the physiological properties of the cell (Dupre et al. 2000). Hairy root cultures are characterized by high biosynthetic capacity and genetic as well as biochemical stability; therefore, they are considered to offer better prospects for the commercial production of secondary metabolites than undifferentiated cell cultures (Toivonen 1993). The effect of Tween 80 as an elicitor of licochalcone A from hairy root cultures of G. uralensis has been evaluated. After a 15-day treatment with 2% Tween 80, hairy roots grew well and produced higher levels of licochalcone A and total flavonoids than the control (Zhang et al. 2011). Wongwicha et al. (2011) induced hairy roots with licorice leaves, and stems grew fast in 1/2 MS medium.
Adventitious root induction is influenced by various factors, including genotype, plant growth regulators, other medium components and culture conditions (Liu et al. 2010; Zhu et al. 2010; Reis et al. 2011). Awad et al. (2011) considered that MS medium with 3% sucrose was suitable for licorice apical in vitro culturing, and the licorice acid content was 1.32 mg g−1. In order to protect wild licorice resources, organ and tissue in vitro culture is a very effective way of obtaining licorice secondary metabolites (Karuppusamy 2009). Currently, studies on licorice products obtained using tissue culture are more concentrated on cell and hairy root culturing, while studies on culturing licorice adventitious root to effectively obtain secondary metabolites are limited. Cells and root tips of G. uralensis were used to induce adventitious roots to acquire secondary metabolites of licorice.
Materials and methods
Plant explants and treatments
Glycyrrhiza uralensis Fisch. seeds were collected from Province Neimenggu, China, in the middle of May 2017. The seeds were stripped and sterilized with 4% sodium hypochlorite solution for 10 min and then rinsed four times with sterile water. After sterilization, explants were placed in 100-mL flasks containing several media. They were incubated at a 22/20 °C thermoperiod (light/dark) under a 17-h photoperiod (40 μmol m−2 s−1, cool white fluorescent tubes).
Callus suspension culture
The hypocotyl of G. uralensis was inoculated on a medium of MS + 6-BA1.0 mg L−1 + NAA 2.0 mg L−1 for inducing callus. Then, 1.0 g callus was inoculated on a liquid medium of MS + 2,4-D 0.2 mg L−1 + 6-BA 0.5 mg L−1 + NAA 0.5 mg L−1 (pH 5.8), and the results for the remaining 15 liquid media are shown in supplementary Table S1 and Fig. S1. Callus were incubated at 25 °C on a rotary shaker (115 rpm) in darkness, subcultured 2–3 times in liquid medium every 10 days, and the dry weight, content and yield of flavonoids in callus were measured every 3 days. A 30-day growth curve of callus was drawn. Each treatment was repeated three times.
Adventitious root induction from callus
Callus (0.5 g) was inoculated on 1/2 MS culture solution; there was a total of seven media with different hormone combinations. Callus were incubated at 25 °C on a rotary shaker (115 rpm) in darkness. Each treatment was repeated three times. The induction of callus growth and adventitious roots were observed every day, and the fresh weight, dry weight and flavonoid accumulation of the callus were determined 30 days later.
Adventitious root induction from the stem
The stem of G. uralensis was inoculated on a medium of 1/2 MS + IBA (0.5 mg L−1) to induce adventitious roots. Seven days later, about 0.2 g of 1 cm-long adventitious root tips was clipped and inoculated on a total of seven media. They were incubated at 25 °C on a rotary shaker (115 rpm) in darkness for 3 weeks, and then the fresh weight, root length, number of lateral roots, content and yield of flavonoids were measured.
The standard sample
A 2.5 mg glycyrrhizin standard sample was dissolved in methanol as a control solution of 0.1 mg mL−1. Then, 0.2 mL control solution was added to methanol to a volume of 1 mL, and 1 mL of 10% KOH was added 5 min later, after which methanol was added to a volume of 5 mL. Methanol was used as a blank control for scanning under a 200–500 nm wavelength. Glycyrrhizin standard solution has a maximum absorption value at 336 nm (Fig. S2).
The method was as follows: place 0.0, 0.2, 0.4, 0.6, 0.8, 1.0 and 1.2 mL of the control solution into 10 mL centrifuge tubes; set the volume to 1.5 mL with methanol; add 1 mL 10% KOH solution. Shake centrifuge tubes and allow color development for 5 min; set the volume to 5 mL with methanol, and measure absorbance values at 336 nm. Standard curves were drawn using the reference concentration as the abscissa and the absorbance value as the vertical axis.
Glycyrrhizin standard sample concentration and absorbency
| liquiritin concentration (mg mL−1) | 0 | 0.004 | 0.008 | 0.012 | 0.016 | 0.02 | 0.024 |
|---|---|---|---|---|---|---|---|
| absorbance value (L g cm−1) | 0 | 0.333 | 0.663 | 1.011 | 1.366 | 1.709 | 2.069 |
Standard curve of liquiritin
The regression equation of glycyrrhizin: y = 86.268x − 0.0136 (R2 = 0.9998), where x is the glycyrrhizin concentration of the colored solution, and y is the absorbance of the colored solution at 336 nm.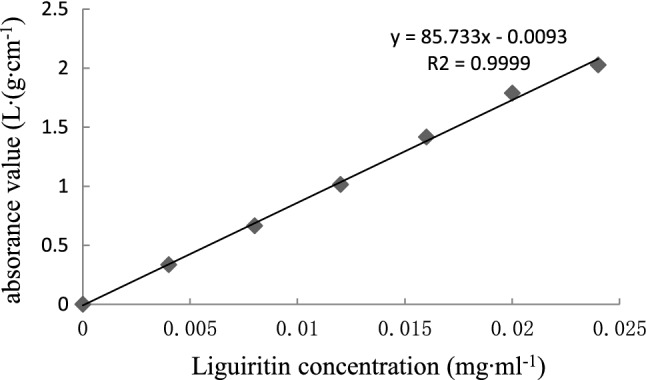
Extraction and measurement of flavonoids
Callus was put into Petri dishes for drying to a constant weight at 60 °C. Callus (0.1 g (± 0.002 g) was ground, sieved, and then put into centrifuge tubes. Ethanol (5 mL, 75%) was added to the centrifuge tubes, followed by shaking and soaking for 1 h. Ultrasonic wave were used to break the cell for 40 min, then centrifuged for 10 min (7104×g). The supernatant was used for the test sample solution: draw 0.2 mL of sample solution and add 0.8 mL of methanol and 1 mL of 10% KOH solution. Five minutes later, add methanol to 5 mL, and measure absorbance at 336 nm (See supplemental Fig. S2). Each sample was repeated three times. The total flavonoids of samples were calculated according to the standard curve. Flavonoid content formula:
The flavonoid content was calculated as glycyrrhizin. C is the concentration of flavonoids in the colored solution (mg mL−1), N is the dilution factor, V is the volume of extraction of flavonoids (5 mL), W is the weight of callus (0.1 g):
Histochemical localization of flavonoids
In order to identify the space distribution character of flavonoids in callus and adventitious roots, we observed freezing sections of fresh callus and adventitious roots. Samples were frozen, and 30-μm-thick sections were serially produced by a Leica CM1950 freezing microtome (Leica Instruments, Shanghai, China). The sections were stained with 10% NaOH and 1% AlCl3 ethanol solution for 20 min and were then mounted using glycerol / water (15:85). The sections were observed with a Leica DM 2500 light microscope (Germany) and a Leica DM 6000B fluorescence microscope (Germany) immediately. The location of flavonoids in G. uralensis was observed through two different staining methods.
Results and discussion
Callus suspension culture
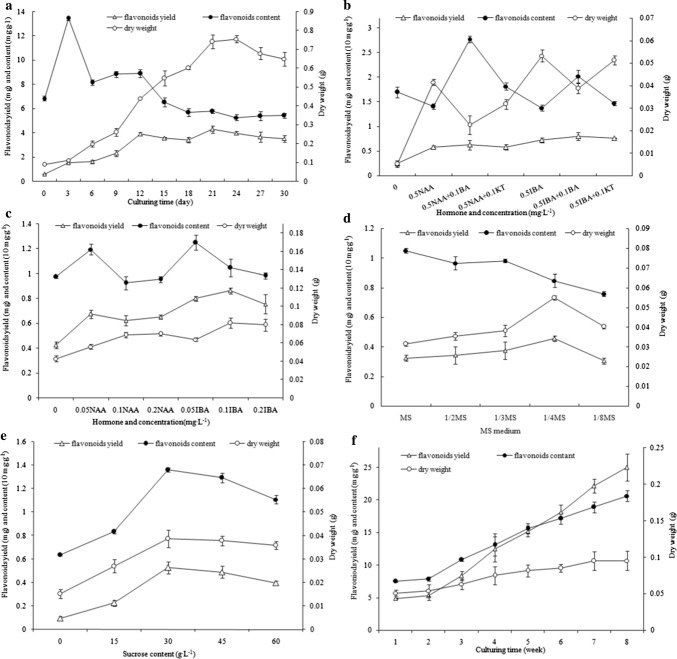
The growth curve of the suspension callus was S-shaped (Fig. 1a). The first 3 days of cell growth was a lag phase, and the cells grew very slowly, then quickly entered the logarithmic growth phase; the callus consisted of yellow grains. The culture solution became turbid on the 9th day, and cellular metabolic waste attached to the sides of the bottles gradually. The stable phase of cell growth began on the 21st day, and the cell dry weight almost stopped increasing, which was about 7.5 times of the initial dry weight. Twenty-four days later, the cell dry weight decreased significantly, and cell color changed from yellow brown to dark brown. Licorice callus cells in good condition on the 18th to 21st days were selected for subculturing.
Fig. 1.
Dry weight, flavonoid content and flavonoid yield curves of suspension culture cellus (a) and adventitious root (b, c, d, f) of G. uralensis.a Dry weight, flavonoid content and flavonoid yield curves of suspension culture cellus. b, c Effects of hormones impact on dry weight, flavonoid content and flavonoids yield of adventitious root which induced from callus (b) and from stem (c). d–f Effects of salt concentration (d), sucrose concentration (e) and culturing period (f) impact on the dry weight, flavonoid content and flavonoids yield of addvetitious roots which induced from stem
In the suspension culture process, the G. uralensis flavonoid content in callus cells increased first and then decreased (Fig. 1a). The initial content of flavonoids was 6.853 mg g−1. The highest content of 13.471 mg g−1 appeared on the third day, and it was 1.966 times higher than the initial content, probably resulting from the adaptation period in the new environment and the rapid generation of a stress response of flavonoids to prevent cell damage. The flavonoid content of the logarithmic growth phase cells decreased gradually and decreased rapidly on the sixth day, being slightly higher than the initial content of flavonoids, i.e., 8.166 mg g−1. Then, on the 15th day, there was a clear downward trend. Twenty-one days later the trend stabilized, but the content of flavonoids was 5.156 mg g−1 lower than the initial content. Therefore, the accumulation of flavonoids was associated with the cell growth rate. During suspension culture, flavonoid yield was proportional to the dry weight of cells (Fig. 1a). With culturing duration, the yield of flavonoids increased gradually. On the 21st day, the yield of flavonoids (3.818 mg g−1) increased to 6.332 times of the initial yield (0.603 mg g−1).
Adventitious root induction in callus
The effect of hormone combinations on the induction of adventitious roots
The effects of the NAA, IBA, 6-BA and KT combinations on the induction of adventitious roots in callus were studied. NAA and IBA either alone or with KT or 6-BA could induce suspension callus, forming adventitious roots. In all treatments, callus formed a white dot protuberance in the first 5 days of induction; adventitious roots formed from the 7th to 10th days.
The addition of auxin was conducive to the formation of adventitious roots, whether used alone or used in conjunction with the cytokinin, inducing callus to form adventitious roots, with a significant difference from the control. IBA is effective to induce adventitious root while used alone (Fig. 1b). The dry weight of the adventitious roots was 0.054 g in the medium with 0.5 mg L−1 IBA, and was significantly different from that of other groups. Adventitious root induction rates of IBA used alone or with 6-BA or KT were higher than those under the same level of NAA. The addition of cytokinin suppressed the formation of adventitious roots. The flavonoid content of adventitious roots in the medium with NAA 0.5 mg L−1 + 6-BA 0.1 mg L−1 was the highest, i.e., 27.79 mg g −1, then was the combination of IBA 0.5 mg L−1 and 6-BA 0.1 mg L−1 with a flavonoid content of 20.293 mg g−1, but the amount of adventitious roots was small, and the epidermis was easily broken. The flavonoid yield of IBA 0.5 mg L−1, IBA 0.5 mg L−1 + 6-BA 0.1 mg L−1 and IBA 0.5 mg L−1 + KT 0.1 mg L−1 treatment groups was significantly higher than that of the NAA treatment groups, i.e., 0.733 g, 0.802 g and 0.754 g, respectively (Fig. 1b). The adventitious roots in the medium with 6-BA and KT were short, grew slowly and were not conducive to secondary culture. In general, IBA was the most suitable auxin for G. uralensis callus to induce adventitious roots.
Effect of different concentrations of NAA and IBA on Glycyrrhiza uralensis root tips
For inducing G. uralensis root, using NAA plus IBA or NAA alone was better than that of using IBA alone. In suspension culture medium with different concentrations of NAA and IBA, adventitious roots began to expand 3 days after induction. The epidermis of adventitious roots cultured in the medium containing NAA broke and gradually formed. With the NAA and IBA concentration increasing, adventitious roots began to form, and the broken epidermis dissolved into the medium, such that the culture solution was yellow and turbid.
The growth of adventitious roots was different in the media with IBA and NAA. The adventitious roots in the medium with IBA grew longer with many lateral roots, while those in the medium with NAA were stubby (Fig. 1c). The results showed that the adventitious root dry weight increased with increasing concentration of IBA and NAA. The dry weight of adventitious roots was the highest (0.0824 g) in the medium with IBA 0.1 mg L−1, but when the concentration was higher than 0.1 mg L−1, in either IBA or NAA treatment, the growth of adventitious root was suppressed (Fig. 1c).
The effect of auxin concentration on flavonoid synthesis in G. uralensis adventitious roots was also highly significant. The flavonoid content in adventitious roots decreased with increasing IBA and NAA concentrations in the medium. The flavonoid content (12.5484 mg g−1) was highest in the medium with IBA 0.05 mg L−1 and differed significantly from other treatments with the IBA concentration ranging from 0.05 to 0.2 mg L−1, indicating that a low concentration of IBA was conducive to the synthesis of flavonoids in adventitious roots (Fig. 1c). The yield of flavonoids of adventitious roots (0.8641 mg) was highest in the medium with IBA 0.1 mg L−1 and was significantly higher than that in other treatments (Fig. 1c). Therefore, 1/4-strength MS medium with IBA 0.1 mg L−1 is suitable for G. uralensis adventitious root culture.
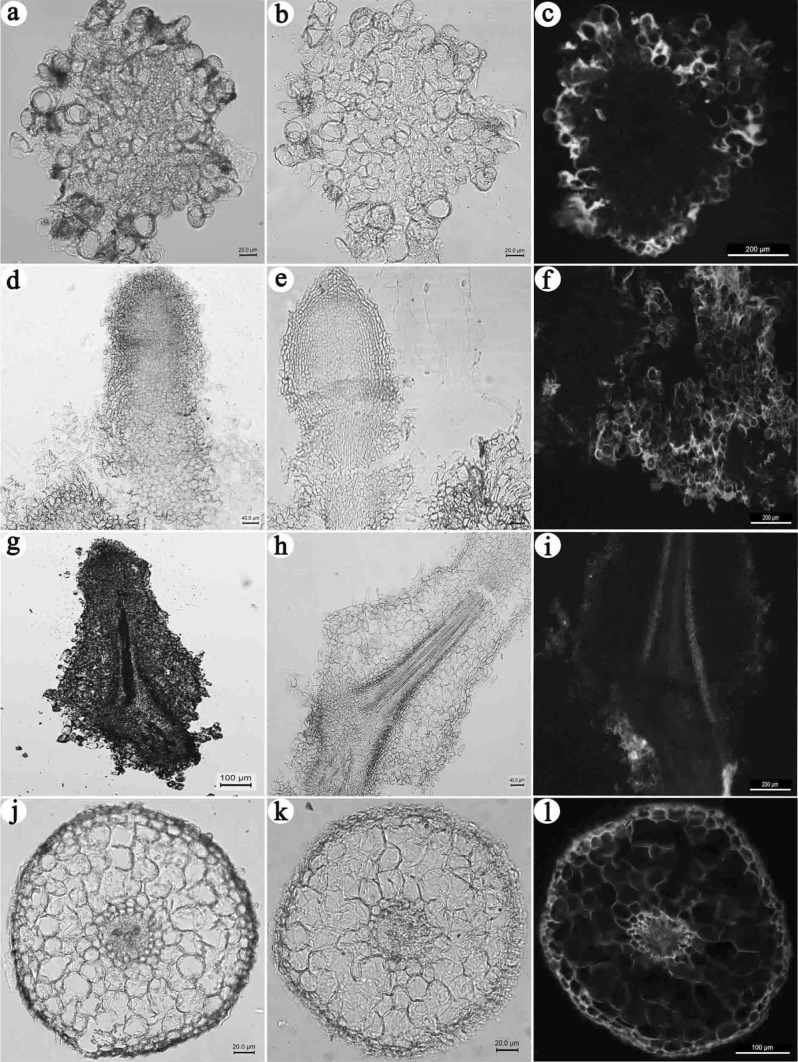
Compared with control root tips that were brown, slender and single (Fig. 2a), roots induced by NAA were mostly single per block of callus, and their tips were yellow, shorter and intumescent (Fig. 2b). In response to NAA 0.5 mg L−1 + 6-BA 0.1 mg L−1, adventitious roots were brown and short, and the epidermis was almost entirely broken (Fig. 2c). The adventitious roots under NAA 0.5 mg L−1 and KT 0.1 mg L−1 were yellow, most of which were in segments (Fig. 2d), whereas the adventitious roots induced by IBA 0.25 mg L−1 were yellow-white, numerous and slender, about 1–2 cm; grew rapidly; and formed as a single root or stellate-shaped (Fig. 2e, h). The adventitious roots in medium with IBA 0.5 mg L−1 plus 6-BA 0.1 mg L−1 or IBA 0.5 mg L−1 plus KT 0.1 mg L−1 were short and thick (Fig. 2f, g). The adventitious roots induced by IBA 1.0 mg L−1 were yellow-white, short and stellate-shaped (Fig. 2i), and the adventitious roots induced by IBA 2.0 mg L−1 were yellow and stellate-shaped, with a broken epidermis (Fig. 2j). The adventitious roots induced by IBA 4.0 mg L−1 were yellow, with root tip protuberance (Fig. 2k). Some adventitious roots in IBA 0.5 mg L−1 formed lateral roots (Fig. 2l).
Fig. 2.
Adventitious roots and callus in different media. a Control (× 10): brown, slender, single; b NAA0.5 mg L−1 (× 12.5): yellow, root tip intumescent, shorter; c NAA0.5 mg L−1 + 6-BA0.1 mg L−1 (× 12.5): brown, short, epidermis broken; d NAA0.5 mg L−1 + KT0.1 mg L−1 (× 10): yellow, in segments; e IBA0.5 mg L−1 (× 7.3): yellow-white, slender; f IBA0.5 mg L−1 + 6-BA0.1 mg L−1 (× 7.3): yellow-brown, short and thick, epidermis broken; g IBA0.5 mg L−1 + KT0.1 mg L−1 (× 12.5): yellow, short and thick; h IBA0.25 mg L−1 (× 7.3): brown, slender, single; i IBA1.0 mg L−1 (× 10): yellow-white, short, stellate-shaped; j IBA2.0 mg L−1 (× 10): yellow, stellate-shaped, epidermis broken; k IBA4.0 mg L−1 (× 10): yellow, root tip protuberance; l IBA0.5 mg L−1 (× 10): Adventitious root with lateral roots (color figure online)
The auxins commonly used in G. uralensis rooting are NAA and IBA. NAA is better in inducing roots than IBA. In different concentrations of NAA and IBA suspension culture medium, adventitious roots began to expand from the 3rd day. The epidermis of adventitious roots cultured in the medium containing NAA broke and gradually formed callus. As the concentration increased, the adventitious roots began to form, and the broken epidermis dissolved into the medium, such that the culture solution became yellow and turbid.
Adventitious root suspension culture
The effect of inorganic salt concentration on adventitious root growth
The results showed that the concentration of inorganic salts in the medium had a strong impact on adventitious root growth and the accumulation of flavonoids. 1/4-strength MS medium was the most conducive to the growth of adventitious roots; a very low (1/8 MS) or high (MS) concentration of inorganic salt could inhibit adventitious root growth. The roots were white initially; lateral roots formed from the 2nd and 3rd day; apicals began to elongate 3–5 days later; 10 days later the roots gradually became yellow-white and intertwined with each other; and the roots were yellow-brown 3 weeks later. Adventitious root apicals elongated slowly; the number of lateral roots was lower and the lengths of lateral roots was shorter in MS medium. With a decrease in the major element in the medium and the number of lateral roots, the length of lateral roots was significantly longer than that in the MS medium. The adventitious root apicals in the 1/4-strength MS medium grew obviously strong. The difference in fresh weight, dry weight and the length reached a significant level compared with other treatments (Table 1, Fig. 1d). The number of lateral roots was significantly increased, and fresh weight and dry weight increased rapidly. Taproots were strong. Therefore, the 1/4-strength MS medium was more suitable for the growth of adventitious roots.
Table 1.
Effects of salt in medium on the growth of Licorice adventitious roots
| Medium | The length of taproot (mm) | The number of lateral roots | The length of lateral root (mm) | Fresh weight (g) | Dry weight (g) |
|---|---|---|---|---|---|
| MS | 36.24d | 6.406b | 2.118d | 0.322 ± 0.01b | 0.0314 ± 0.001c |
| 1/2MS | 56.13ab | 6.958b | 7.017ab | 0.338 ± 0.02b | 0.0356 ± 0.002bc |
| 1/3MS | 51.92b | 7.125b | 7.934b | 0.376 ± 0.03b | 0.0382 ± 0.003bc |
| 1/4MS | 58.87a | 10.958a | 8.608a | 0.472 ± 0.01a | 0.055 ± 0.001a |
| 1/8MS | 42.84c | 6.417b | 4.316c | 0.368 ± 0.02b | 0.0402 ± 0.001b |
Duncan multiple comparison, the same letter means the difference is not significant (P < 0.05)
With the decreasing concentration of inorganic salts in the medium, the content of flavonoids in adventitious roots showed a decreasing trend. The flavonoid content was the highest (10.4317 mg g−1) in MS medium. The yield of flavonoids in adventitious roots was highest, i.e., 0.4638 mg g−1, in 1/4-strength MS medium.
Sucrose concentration
Roots were hardly grown without sucrose in medium. Whereas a lower sucrose concentration of 15 g L−1 was added, and fewer yellowish-white roots were growing and the dry matter was 9.48%. With increasing sucrose concentration up to 30 g L−1, both fresh and dry weights of roots reached the highest, 0.3759 g and 0.0387 g, respectively, and reached a significant level compared with other sucrose concentration treatments. With increasing sucrose concentration, the nutrient solution gradually turned deep yellowish-brown, and the fresh weight decreased gradually, which showed that excessive sucrose concentration restrained root growth. Similar to the root growth trend, the effect of sucrose concentration on flavonoid synthesis increased first and then decreased. When the sucrose concentration was 30 g L−1, the content and production reached the highest values of 13.6215 mg g−1 and 0.5267 mg g−1, respectively, which means that a sucrose concentration of 30 g L−1 is suitable for G. uralensis growth (Fig. 1e).
Period culture
The roots grew slowly in vitro 1–2 weeks after inoculation (Fig. 1f), but grew rapidly during the 3rd–7th weeks, and the increase in roots remained at a stable level during the 7th–8th weeks. Whereas the flavonoid yield exhibited different trends from those of root growth during the 1–8-week period, both the G. uralensis flavonoid content and flavonoid yield increase diminished with prolonged culture time. In conclusion, the 7th day was the optimum period for harvesting G. uralensis roots in vitro.
Comparison of the content and yield of flavonoids in different cultures
The experimental results proved that the best harvest time of the suspension callus was the 21st day, and that of the suspension apical was the 49th day. In this study, we compared the content and yield of G. uralensis flavonoids under different cultures. The flavonoid content in apicals was 19.354 mg g−1, which was higher than that in suspension callus, but the growth of roots was so slow that the flavonoid yield was significantly lower than that in suspension callus. While the adventitious roots were induced from suspension callus about 30 days later, the flavonoid content was significantly higher, 19.918 mg g−1 (Table 2), about 4-fold that of the initial suspension callus. Therefore, the generation of adventitious roots promoted the accumulation of flavonoids.
Table 2.
The flavonoids yield in callus and adventitious roots
| Cultures | Culturing time (days) | Mediums (mg L−1) | Flavonoid content (mg g−1) | Flavonoid yield (mg g−1) | Production efficiency (mg g−1 day−1) |
|---|---|---|---|---|---|
| Suspension callus | 21 | MS + 2,4-D0.2 + NAA0.5 + 6-BA 0.5 | 5.783 ± 0.142b | 4.286 ± 0.315b | 0.204 |
| Adventitious roots inducing in stem | 49 | 1/4MS + IBA 0.1 | 19.354 ± 0.975a | 0.927 ± 0.054c | 0.095 |
| Adventitious roots inducing in callus | 30 | 1/2MS + IBA 1.0 | 19.918 ± 0.109a | 8.255 ± 0.214a | 0.550 |
Means followed by the same letter with in each column are not significantly different at the P < 0. 05 indicated by a Duncan s multiple range test. Culture for 30 days
The flavonoid contents of apicals cultured in vitro were similar to the flavonoid contents of adventitious roots induced from the suspension callus, but because of the slow growth of roots, the flavonoid yield of apicals cultured in vitro was the lowest. Therefore, the expanding culture of apicals cannot accumulate numerous flavonoids. In the current experimental results, the accumulation of flavonoids in different cultures in descending order was adventitious roots, suspended callus, root and tips cultured in vitro.
The flavonoid content did not gradually increase with the extension of the incubation time during the first week, probably because the callus cells synthesized flavonoids in a suitable growth environment. If there are no external stimuli, then fewer flavonoids are synthesized. Many medicinal plants accumulate secondary metabolites from apical meristem culture gradual accumulation of flavonoids, although the roots grow more slowly, but the final flavonoid yield obtained from culture alone or adventitious root tips due to the higher flavonoid content root itself. In the adventitious callus induction process, flavonoids gradually increased, the same as the gradual accumulation of adventitious roots, so adventitious co-culture with callus material accumulated the maximum amount of flavonoids.
In Wang’s (2013) study, the contents in different G. uralensis materials were analyzed using cluster analysis. They concluded that adventitious roots had a greater capability of flavonoid production compared to seedlings, callus and cells, but another study showed that the number of flavanones was lower in cultured Glycyrhiza cells (6.31 mg g−1) than in native cells (9.82 mg g−1) (Man et al. 2013). In our study, the content of flavonoid in callus was lower than that in native cells, and adventitious roots showed a fairly high capacity to produce flavonoids. Li et al. (2012) studied the efficient genetic transformation of licorice (Glycyrrhiza inflata Batalin) cells in suspension culture using Agrobacterium tumefaciens-mediated T-DNA delivery. The results showed that the introduced genes had no discernable effect on cell growth or the accumulation of total licorice flavonoids in the transgenic cell lines. It seems that the technique of molecular biology is also likely to be a method to improve flavonoid synthesis, but not the most effective one. We conclude that callus induction of adventitious roots was the most suitable method for obtaining flavonoids effectively.
Flavonoid histochemical localization
NaOH can result in a color reaction with flavanone compounds to make chalcone, showing a yellow color. The suspended secondary culture callus epidermal cells were yellow, but the internal region was not stained (Fig. 3b). In the early days of rooting (10 days), only the callus epidermis was yellow, with less staining of the adventitious roots (Fig. 3e), suggesting low flavonoid accumulation in the early induction of adventitious roots. On the 20th day, the adventitious root endodermis was light yellow, and the color of the epidermis was not obvious (Fig. 3h), whereas the callus was stained deeper than the epidermis of adventitious root. On the 30th day, adventitious root epidermal cells were yellow, but other cells were not stained (Fig. 3k).
Fig. 3.
Accumulation of flavonoids during adventitious root formation process. a callus-unstained (× 200); b callus-NaOH staining (× 200); c callus-AlCl3 ethanol staining (× 100); d the 10th day of adventitious root induction-unstained (× 200); e the 10th day of adventitious root induction-NaOH staining (× 200); f the 10th day of adventitious root induction-AlCl3 ethanol staining (× 100); g the 20th day of adventitious root induction-unstained (× 40); h the 20th day of adventitious root induction-NaOH staining a less (× 200); i the 20th day of adventitious root induction-AlCl3 ethanol staining (× 100); j the 30th day of adventitious root induction-unstained (× 100); k the 30th day of adventitious root induction-NaOH staining (× 100); l the 30th day of adventitious root induction-AlCl3 ethanol staining (× 100)
The AlCl3 ethanol solution can react with flavonoids to produce a yellow complex, which presented blue-white, yellow, or yellow-green fluorescence under a fluorescence microscope. Results showed that suspended callus parenchyma cells presented clear yellow-green and were mainly distributed in the cell wall and intercellular space. In the early days of rooting (10 days), both callus (Fig. 3c) and adventitious roots (Fig. 3f) presented yellow-green fluorescence, obviously brighter than the similar tissues or organs stained by NaOH. On the 20th day, adventitious root endodermal cells presented yellow-green fluorescence; epidermal cells also had considerable light green fluorescence (Fig. 3i). On the 30th day, adventitious root epidermal cells presented orange-yellow fluorescence; the epidermal cells presented yellow-green fluorescence; xylem parenchyma cells also had a small amount of blue-green fluorescence (Fig. 3l). The results were consistent with the results of NaOH histochemical localization, but the tissues or organs stained by AlCl3 ethanol solution is more obvious than NaOH staining. Therefore, AlCl3 ethanol solution was better than NaOH aqueous solution in terms of chromogenic and localization effects. During the primary growth of adventitious roots, flavonoids may first be transported from callus epidermal cells to the endodermis of adventitious roots and eventually accumulate in the epidermal cells.
The cycle of suspension callus culture was 18–21 days. IBA was more conducive than NAA in inducing G. uralensis adventitious roots, but the addition of 6-BA and KT suppressed the formation of adventitious roots. The flavonoid content and yield reached the highest values of 19.962 mg g−1 and 1.232 mg g−1, respectively, when the IBA concentration was 1.0 mg L−1. The study of histochemical localization of flavonoids suggested that G. uralensis flavonoids were mainly distributed in the epidermal cells of the callus outer layers. There was no accumulation of flavonoids in the roots in the early stage of the induction of adventitious roots. With the primary growth of adventitious roots, flavonoids gradually distributed in the cell wall and cell gaps of the epidermis and endodermis of adventitious roots. The best medium for the induction and growth of adventitious roots was 1/4-strength MS + 0.1 mg L−1 IBA. In this medium, apicals grew faster, and the yield of flavonoids was the highest. The content of flavonoids in adventitious roots and apicals cultured in vitro was higher than that in suspension callus and reached 3.87 times the callus flavonoid content.
Conclusion
We concluded that calli inducing adventitious roots and apicals for 30 days obtained the highest yield of flavonoid. The best medium for induction and growth of adventitious roots was 1/4-strength MS + 0.1 mg L−1 IBA. In this medium, apicals grew faster, and the yield of flavonoids was the highest. AlCl3 ethanol solution was better than NaOH aqueous solution in terms of the chromogenic and localization effects on flavonoid. Using in vitro culture instead of wild culture is an effective method for producing flavonoids.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Project No. 31870573), Science and technology project of Beijing Municipal Administration Center of Parks (Project Nos. ZX2018014, ZX2020023), the open project of Beijing key Laboratory of Greening Plants Breeding in China (Beijing Institute of Landscape Architecture) in 2019 (Project No. YZ201904), Beijing Natural Science Foundation of China (project No. 6202012), Beijing Key Laboratory of Greening Plants Breeding in China, the National Engineering Laboratory for Tree Breeding, the Key Laboratory of Genetics and Breeding in Forest Trees and Ornamental Plants of the Ministry of Education, and the Tree and Ornamental Plant Breeding and Biotechnology Laboratory of the State Forestry Administration in China.
Abbreviations
- IBA
Indole-3-butyric acid
- NAA
1-Naphthlcetic acid
- 6-BA
6-Benzylaminopurine
- KT
Kinetin
- MS
Murashige and Skoog
- F
Flavanone liquiritigenin
- C
2’-Hydroxychalcone isomer, isoliquiritigenin
- CHI
Chalcone isomerase gene
- CHS
Chalcone synthase gene
- C4H
Cinnamic acid-4-hydroxylase
Author contributions
SG and YZ designed the study and conceived the experiments. YZ and YJ carried out the experiments, and analyzed data, drafted the manuscript. YS partly participated writing manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arias-Castro C, Scragg A, Stafford A, Rodriguez- Mendiola M. Growth characteristic of Glycyrrhiza glabra cell suspension cultures. Plant Cell Tissue Organ Cult. 1993;34:77–82. doi: 10.1007/BF00048466. [DOI] [Google Scholar]
- Awad V, Shirke R, Mukherjee S, Khadke S, Pawar P, Meti N, Harsulkar A. Somatic embryo genesis, regeneration and in vitro production of glycyrrhizic acid from root cultures of Taverniera cuneifolia (Roth) Arn. In Vitro Cell Dev Biol Plant. 2011;47:525–535. doi: 10.1007/s11627-011-9356-5. [DOI] [Google Scholar]
- Ayabe S, Takano H, Fujita T, Furuya T, Hirota H, Takahashi T. Triterpenoid biosynthesis in tissue cultures of Glycyrrhiza glabra var glandulifera. Plant Cell Rep. 1990;9:181–184. doi: 10.1007/BF00232175. [DOI] [PubMed] [Google Scholar]
- Baek MH, Chung BY, Kim JH, Kim JS, Lee SS, An BC, Lee IJ, Kim TH. cDNA cloning and expression patternof cinnamate-4-hydroxylase in the Korean black raspberry. Biochem Mol Biol Rep. 2008;41:529–536. doi: 10.5483/bmbrep.2008.41.7.529. [DOI] [PubMed] [Google Scholar]
- Dupre P, Lacoux J, Neutelings G, Mattar-Laurain D, Fliniaux MA, David A, Jacquin-Dubreuil A. Genetic transformation of Ginkgo biloba by A. tumefaciens. Physiol Plant. 2000;108:413–419. doi: 10.1034/j.1399-3054.2000.t01-1-100411.x. [DOI] [Google Scholar]
- Fukai T, Marumo A, Kaitou K, Kanda T, Terada S, Nomura T. Antimicrobial activity of licorice flavonoids against methicillin-resistant Staphylococcus aureus. Fitoterapia. 2002;73:536–539. doi: 10.1016/S0367-326X(02)00168-5. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Sudo H. Economic importance of licorice. Plant Biotechnol. 2009;26:101–104. doi: 10.5511/plantbiotechnology.26.101. [DOI] [Google Scholar]
- Hayashi H, Fukui H, Tabata M. Examination of triterpenoids produced by callus and cell suspension cultures of Glycyrrhiza glabra. Plant Cell Rep. 1988;7:508–511. doi: 10.1007/BF00272743. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Wang B, Li H, Yao L, Wu T. Flavonoid production is effectively regulated by RNAi interference of two flavone synthase genes from Glycine max. J Plant Biol. 2010;53:425–432. doi: 10.1007/s12374-010-9132-9. [DOI] [Google Scholar]
- Kamada H, Okamura N, Satake M, Harada H, Shimomura K. Alkaloid production by hairy root cultures in Atropa belladonna. Plant Cell Rep. 1986;5:239–242. doi: 10.1007/BF00269811. [DOI] [PubMed] [Google Scholar]
- Karuppusamy S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res. 2009;3:1222–1239. [Google Scholar]
- Li W, Asada Y, Yoshikawa T. Flavonoid constituents from Glycyrrhiza glabra hairy root cultures. Phytochemistry. 2000;55:447–456. doi: 10.1016/S0031-9422(00)00337-X. [DOI] [PubMed] [Google Scholar]
- Li F, Jin Z, Qu W, Zhao D, Ma F. Cloning of cDNA encoding the Saussurea medusa chalcone isomerase and its expression in transgenic tobacco. Plant Physiol Biochem. 2006;44:455–461. doi: 10.1016/j.plaphy.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Dong Y, Zhang Y, Fu C, Yu L. Stable transformation of suspension-cultured Glycyrrhiza inflata batalin cells with agrobacterium tumefaciens. Z Naturforschung Sect CA J Biosci. 2012;67:603–610. doi: 10.1515/znc-2012-11-1211. [DOI] [PubMed] [Google Scholar]
- Liu C, Callow P, Rowland LJ, Hancock JF, Song GQ. Adventitious shoot regeneration from leaf explants of southern highbush blueberry cultivars. Plant Cell Tissue Organ Cult. 2010;103:137–144. doi: 10.1007/s11240-010-9755-z. [DOI] [Google Scholar]
- Man S, Guo S, Gao W, Wang G, Zhang L, Li X. Identification of metabolic profiling of cell culture of licorice compared with its native one. Anal Bioanal Chem. 2013;405:3321–3329. doi: 10.1007/s00216-013-6776-6. [DOI] [PubMed] [Google Scholar]
- Park NI, Xu H, Li XH, Kim SJ, Park SU. Enhancement of flavone levels through overexpression of chalcone isomerase in hairy root cultures of Scutellaria baicalensis. Funct Integr Genomics. 2011;11:3491–3496. doi: 10.1007/s10142-011-0229-0. [DOI] [PubMed] [Google Scholar]
- Prakash G, Srivastava AK. Statistical elicitor optimization studies for the enhancement of azadirachtin production in bioreactor Azadirachta indica cell cultivation. Biochem Eng J. 2008;40:218–226. doi: 10.1016/j.bej.2007.12.017. [DOI] [Google Scholar]
- Reis RV, Borges APPL, Chierrito TPC, Souto ER, Souza LM, Iacomini M, Oliveira AJB, Gonçalves RAC. Establishment of adventitious root culture of Stevia rebaudiana Bertoni in a roller bottle system. Plant Cell Tissue Organ Cult. 2011;106:329–335. doi: 10.1007/s11240-011-9925-7. [DOI] [Google Scholar]
- Shabani L, Ehsanpour AA, Asghari G, Emami J. Glycyrrhizin production by in vitro cultured Glycyrrhiza glabra elicited by methyl jasmonate and salicylic acid. Russ J Plant Physiol. 2009;56:621–626. doi: 10.1134/S1021443709050069. [DOI] [Google Scholar]
- Shul’ts EE, Petrova TN, Shakirov MM, Chernyak EI, Tolstikov GA. Flavonoids of roots of Glycyrrhiza uralensis growing in Siberia. Chem Nat Compd. 2000;36:362–368. doi: 10.1023/A:1002836729067. [DOI] [Google Scholar]
- Simmler C, Jones T, Anderson JR, Nikolić DC, van Breemen RB, Soejarto DD, Chen SN, Pauli GF. Species-specific standardisation of licorice by metabolomic profiling of flavanones and chalcones. Phytochem Anal. 2014;25:378–388. doi: 10.1002/pca.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Kumar S, Rani A, Gulati A, Ahuja PS. Phenylalanine ammonia-lyase (PAL) and cinnamate 4-hydroxylase (C4H) and catechins (flavan-3-ols) accumulationintea. Funct Integr Genomics. 2009;9:125–134. doi: 10.1007/s10142-008-0092-9. [DOI] [PubMed] [Google Scholar]
- Toivonen L. Utilization of hairy root cultures for production of secondary metabolites. Biotechnol Prog. 1993;9:12–20. doi: 10.1021/bp00019a002. [DOI] [Google Scholar]
- Tunen AJV, Koes RE, Spelt CE, Krol ARVD, Stuitje AR, Mol JN. Cloning of the two chalcone flavanone isomerase genes from Petunia hybrida: coordinate, light-regulated and differential expression of flavonoid genes. EMBO J. 1988;7:1257–1263. doi: 10.1002/j.1460-2075.1988.tb02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Qi N, Wang Z. Application of a stir-tank bioreactor for perfusion culture and continuous harvest of Glycyrrhiza infl ata suspension cells. Afr J Biotechnol. 2010;9:347–351. [Google Scholar]
- Wang J, Gao W, Zhang L, Huang L. Establishment and quality assessment of tissue cultures in Glycyrrhiza uralensis Fisch. Appl Biochem Biotechnol. 2013;169:588–594. doi: 10.1007/s12010-012-0012-2. [DOI] [PubMed] [Google Scholar]
- Wasson AP, Pellerone FI, Mathesius U. Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell. 2006;18:1617–1629. doi: 10.1105/tpc.105.038232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongwicha W, Tanaka H, Shoyama Y, Tuvshintokh I, Putalun W. Production of glycyrrhizin in callus cultures of licorice. Z Naturforsch. 2008;63:413–417. doi: 10.1515/znc-2008-5-617. [DOI] [PubMed] [Google Scholar]
- Wongwicha W, Tanaka H, Shoyama Y, Putalun W. Methyl jasmonate elicitation enhances glycyrrhizin production in Glycyrrhiza inflata hairy roots cultures. Z Naturforsch. 2011;66:423–428. doi: 10.1515/znc-2011-7-815. [DOI] [PubMed] [Google Scholar]
- Yang S, Liu X, Shen X, Zheng J. Ri plasmid transformation of Glycyrrhiza uralensis and effects of some factors on growth of hairy roots. Zhongguo Zhong Yao Za Zhi. 2006;31:875–878. [PubMed] [Google Scholar]
- Yang Y, He F, Yu L, Chen X, Lei J, Ji J. Influence of drought on oxidative stress and flavonoid production in cell suspension culture of Glycyrrhiza inflata Batal. Z Naturforsch. 2007;62:410–416. doi: 10.1515/znc-2007-5-615. [DOI] [PubMed] [Google Scholar]
- Yang Y, He F, Yu L. Dynamics analyses of nutrients consumption and flavonoids accumulation in cell suspension culture of Glycyrrhiza inflate. Biol Plant. 2008;52:732–734. doi: 10.1007/s10535-008-0141-1. [DOI] [Google Scholar]
- Yang Y, He F, Yu L, Ji J, Wang Y. Flavonoid accumulation in cell suspension cultures of Glycyrrhiza inflata Batal under optimizing conditions. Z Naturforsch. 2009;64:68–72. doi: 10.1515/znc-2009-1-212. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu J, Lu H, Gao S. Enhanced flavonoid production in hairy root cultures of Glycyrrhiza uralensis Fisch by combining the over-expression of chalcone isomerase gene with the elicitation treatment. Plant Cell Rep. 2009;28:1205–1213. doi: 10.1007/s00299-009-0721-3. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu J, Chen H, Gao C, Lu H, Zhou H, Li Y, Gao S. Up-regulation of licochalcone A biosynthesis and secretion by Tween 80 in hairy root cultures of Glycyrrhiza uralensis Fisch. Mol Biotechnol. 2011;47:50–56. doi: 10.1007/s12033-010-9311-4. [DOI] [PubMed] [Google Scholar]
- Zhu X, Chai S, Chen L, Zhang M, Yu J. Induction and origin of adventitious roots from chimeras of Brassica juncea and Brassica oleracea. Plant Cell Tissue Organ Cult. 2010;101:287–294. doi: 10.1007/s11240-010-9686-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.