Abstract
The desulfurization of fuel is currently enforced to meet environmental legislation and prevent pollution. The use of specific biodesulfurizing microbes with a unique 4S pathway allows the desulfurization without compromising the quality of fuel. These specific microbes can be screened by the detection of 2-hydroxybiphenol (2-HBP) in desulfurizing mixture of dibenzothiophene (DBT). At present, colorimetric Gibb’s assay is the most commonly employed screening method which requires a specific reagent, i.e., 2,6-dichloroquninone-4-chloramide. In the present study, a novel and simple spectrophotometric method was developed for the detection of 2-HBP for screening purpose based on dual wavelength method. The developed method facilitates the simultaneous analysis of DBT desulfurization and 2-HBP production in a sample by merely measuring the absorbance differences at two specified wavelengths, i.e., ΔA (λ320–λ247) for DBT and ΔA (λ286–λ324) for 2-HBP. The developed method was used to screen 57 microbes and two specific desulfurizing microbes Bacillus flexus MS-5 and Bacillus cereus BR-31 were selected based on 2-HBP production. The outcomes of developed method were validated by HPLC analysis. The strains MS-5 and BR-31 were employed in biodesulfurization and resulted in 54.88 ± 1.12% and 55.72 ± 1.32% desulfurization of 1.0 mM DBT, respectively. The developed method for screening of specific desulfurizing microbes does not require any specific reagent or sophisticated instrument in spite of being quick and reliable. The microbes selected by developed method exhibited excellent potential for biodesulfurization of fuel.
Keywords: Biodesulfurization, Screening, Bacillus, Dual wavelength, Spectrophotometer
Introduction
The sulfur dioxide discharge due to the combustion of sulfur-laden fuel is leading to various environmental and health problems (Martínez et al. 2016). The supply of ultra-low sulfur fuel from the refineries can diminish the effects of these harmful gases. However, the refineries must be backed up by the economical techniques such as biodesulfurization to furnish the fuel according to environment legislations at an affordable rate (Verma et al. 2016). Biodesulfurization is an imperative sulfur removal approach to degrade complex organosulfur compounds economically at mild temperature–pressure conditions using microbial potential (Sousa et al. 2012). The microbes are competent to remove sulfur from organosulfur compounds using different pathways. However, 4S pathway is the desired pathway which helps to retain the calorific value of fuels by particularly splitting of C–S bond rather C–C bonds of aromatic rings (Martínez et al. 2016).
Dibenzothiophene (DBT) is presented as a model compound in the crude oil and microbes competent to desulfurize DBT via 4S pathway are considered imminent candidates for biodesulfurization study (Bordoloi et al. 2016). The specific desulfurization (4S pathway) of DBT gives rise to 2-hydroxybiphenyl (2-HBP) as an end product. Therefore, the specific desulfurizing microbes can be screened by detecting 2-HBP in desulfurized medium (Verma et al. 2016). Gibb’s assay is the most common method to measure 2-HBP production which employs the use of a specific Gibb’s reagent, 2,6-dichloroquninone-4-chloramide (Rath et al. 2012). Though Gibb’s assay is a very useful colorimetric method for 2-HBP measurement, it requires a specific reagent and moreover, it is not competent for DBT desulfurization simultaneously in the same samples. Further, other analytical techniques available to study DBT and 2-HBP simultaneously such as high performance liquid chromatography (HPLC) and gas chromatography–mass spectrometer (GC–MS) are not suitable for the screening of a large number of samples easily (Nuhu 2013).
Hence, a novel simple spectrophotometric method was developed in the present study based on dual wavelength approach. The method facilitates the simultaneous detection of DBT and 2-HBP in a given sample by merely measuring the absorbance differences at two specified wavelengths selected for each component. The developed method presents the simple, quick and reliable way to screen the specific DBT desulfurizing microbes following 4S pathway. Two specific desulfurizing microbes Bacillus flexus MS-5 and Bacillus cereus BR-31 were selected and used for biodesulfurization study which present a very good potential for biodesulfurization of fuel.
Materials and methods
Reagents and instrumentation
The analytical grade chemicals were used throughout the study. Dibenzothiophene (DBT) and 2-hydroxybiphenyl (2-HBP) were purchased from Hi-Media, Mumbai. Ethyl acetate AR used as a solvent was procured from SDFCL, Mumbai. Fiber Optic Spectrophotometer, Maya 200 series (Ocean Optics) was used for spectrophotometric analysis.
Preparation of standard stock and working solutions
The standard stock solutions (100 mM) of dibenzothiophene (DBT, mol. wt. 184.26 g/mol) and 2-hydroxybiphenyl (2-HBP, mol. wt. 170.21 g/mol) in ethyl acetate were prepared in 10 mL volumetric flasks by dissolving accurately weighed DBT (184 mg) and 2-HBP (170 mg). The stock solutions were further serially diluted to 10 mM and 1 mM before preparing working solutions (0.1–1.0 mM) to minimize errors due to manual handling.
Dual wavelength method for estimation of DBT and 2-HBP
A simple spectrophotometric method was developed in the present study based on dual wavelength approach for the simultaneous estimation of dibenzothiophene (DBT) and 2-hydroxybiphenyl (2-HBP) in a given sample. Dual wavelength approach facilitates the analysis of one component in the presence of an interfering component merely by measuring the absorbance difference at two specific wavelengths [A1(λ1)−A2(λ2)] at which no absorbance difference (ΔA = 0) is observed for interfering component (Jain et al. 2010). The absorbance difference at these selected wavelengths for the desired component is observed directly proportional to the concentration of that component irrespective of the presence of the interfering component in the same mixture. Hence, the key step for the method development was to select two wavelengths for each component showing significant absorbance difference for one component and none for other component.
Selection of two wavelengths from overlapped spectral study
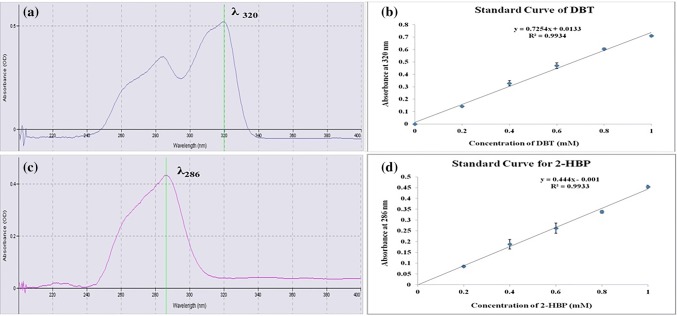
First of all, the λmax of DBT and 2-HBP (dissolved in ethyl acetate) were found out by scanning over the range of 200–800 nm using ‘Spectra Suite’ software in fiber optic spectrophotometer and were chosen as first analytical wavelength for estimation. The second specific wavelengths for DBT as well as 2-HBP were chosen according to a Dual-wavelength spectrophotometry method, first described by Shibata (1976).
As described in Fig. 1a, the second wavelength for component A was found out by drawing a vertical line from the peak at first analytical wavelength (λ1) to point of intersection on the overlapped spectra of two components. From this intersection point, a parallel horizontal line is drawn which meets at a point on the absorption spectrum of component B corresponding to second wavelength (λ2). At this moment, the absorbance difference (ΔA) at wavelengths λ1 and λ2 will be directly proportional to the concentration of component A since no absorbance difference is observed due to component B (same absorbance at λ1 and λ2). Similarly, the absorbance difference (ΔA) at wavelengths λ3 and λ4 will be directly proportional to the concentration of component B (Mabrouk et al. 2014). Using the same way, the two wavelengths selected for the estimation of DBT were 320 and 247 nm whereas 286 and 324 nm for 2-HBP from their overlapped spectra (Fig. 1b).
Fig. 1.
a Dual wavelength method for selection of second particular wavelength for each component (A and B) in mixture (modified from Mabrouk et al. 2014). b Overlapped spectra of DBT and 2-HBP and selection of wavelengths
Authentication of developed method
Different samples prepared by mixing DBT and 2-HBP (each 1.0 mM) in different ratios (5:0, 4:1, 3:2, 1:1, 2:3, 1:4 and 0:5) were analyzed by developed method. The measured absorption differences for two components were plotted against their actual concentration in the mixture to find out the regression coefficient (R2) of the graphs. The authenticity of developed method was estimated from regression coefficient of graph. The plotted graphs (absorbance difference vs concentration) were used to measure unknown concentration in the test samples for screening purpose. To make the preliminary screening of desulfurizing microbes rapid and smooth, the interference due to intermediate metabolites was not considered in this assay. However, the measurements on desulfurizing mixtures were done after 48 h of inoculation as explained in the next section. After 48 h, negligible amount of intermediates would be present which can hardly affect the estimation of DBT and 2-HBP (Bhatia and Sharma 2010; Derikvand and Etemedifar 2014).
Application of developed method for screening microbes
The developed method facilitates the screening of specific biodesulfurizing microbes as well as quantitative analysis of DBT desulfurization and 2-HBP production. Various desulfurizing microbes, isolated by standard method (Labana et al. 2005) using DBT as sole sulfur source and selection pressure whereas sodium succinate as carbon source in minimal salt media, (MSM) were screened for specific desulfurization.
2% (v/v) of freshly grown isolated cultures (A600 1.0–1.2) were inoculated in MSM supplemented with filter sterilized DBT (1.0 mM) and incubated at 30 °C. The aliquots (1 mL) withdrawn at 48 h were acidified to pH 2 with 6 N HCl followed by the addition of equal volume of ethyl acetate (Derikvand et al. 2015). Following vigorous mixing, the mixture was centrifuged (3000 rpm, 5 min) to remove biomass. The upper layer of ethyl acetate with organosulfur compounds was extracted and used to analyze DBT and 2-HBP with developed method. DBT desulfurization % and 2-HBP production % was estimated as follow:
Confirmation of screening results
2-HBP production on DBT desulfurization by selected desulfurizing microbes was further confirmed from other screening method Gibb’s assay as well as with HPLC analysis.
Gibb’s assay
The aliquots withdrawn from DBT desulfurizing mixture were assayed by Gibb’s assay. The pH of aliquots was adjusted to 8 with 10% (w/v) sodium carbonate. 20 µL of Gibb’s reagent (2,6-dichloroquninone-4-chloramide in ethanol) was added in the samples. The samples were analyzed for color development after incubation at 30 °C for 30 min (Ansari et al. 2007).
High performance liquid chromatography (HPLC) analysis
The ethyl acetate extracted biodesulfurizing mixtures (as described in “Application of developed method for screening microbes”) of the selected microbes were analyzed by HPLC and compared with that of procured specific desulfurizing microbe Rhodococcus rhodochrous MTCC 3552. The analysis was performed on an advanced scientific HPLC instrument (Shimadzu) equipped with C-18 column (5 μm to 250 mm) and UV detector (Smartline 2600) set at 280 nm. The mobile phase was acetonitrile–water (80:20, v/v) with a flow rate of 1.0 mL/min (Bhatia and Sharma 2010). The retention times for DBT and 2-HBP were determined by passing their standard solutions. The presence of 2-HBP was confirmed from the presence of peak at its retention time.
Biodesulfurization study with selected microbes
The selected microbes (MS-5 and BR31) were employed in DBT biodesulfurization study. The desulfurization of DBT (1.0 mM) in minimal salt media was studied with 2% (4.0–4.5 × 107 cells/mL) fresh inoculum of each Isolate MS-5 and BR-31 at 30 °C in an orbital shaker at 150 rpm for 72 h. A small aliquot was withdrawn at the interval of 6 h to estimate the amount of DBT desulfurization (Rath et al. 2012).
Identification of selected microbes
The selected microbial isolates were identified by 16S rRNA gene sequencing by Yaazh’s Xenomics, Mumbai (Maharashtra, India). The amplification of 16S rRNA gene was done with universal primers 27F and 1492R using MJ Research Peltier Thermal Cycler. The sequences of forward and reverse primers were AGAGTTTGATCMTGGCTCAG and TACGGYTACCTTGTTACGACTT, respectively. NCBI BLAST search tool was used to compare 16S rRNA gene sequences. The isolates were identified based on the highest sequence similarity with closely related microorganisms (Garcha et al. 2016).
Results and discussion
Dual wavelength spectrophotometric method
In the standard solutions of dibenzothiophene (DBT) and 2-hydroxybiphenyl (2-HBP), the absorbance at their λmax was found to be linear with their concentrations (0.1–1.0 mM). The regression coefficients (R2) of the standard curves of DBT and 2-HBP were 0.9934 and 0.9933, respectively, at their λmax’s, i.e., 320 nm for DBT and 286 nm for 2-HBP (Fig. 2). However, the linear relationship gets disturbed when both components are present in the same sample due to interference with each other. The overlapped spectrum of DBT and 2-HBP (Fig. 1b) clearly demonstrated that 2-HBP displayed some absorption at 320 nm (λmax for DBT) and similarly, DBT presented strong absorption at 286 nm (λmax for 2-HBP) interfering the analysis by simple spectrophotometric method. Hence, the additional absorbance at λmax due to the interfering component was nullified by subtracting the absorbance measured at second wavelength which was selected according to dual wavelength method (Jain et al. 2010). The second wavelength selected were 247 and 324 nm for DBT and 2-HBP, respectively as described in “Selection of two wavelengths from overlapped spectral study”. Therefore, the concentration of DBT and 2-HBP in the mixed spectrum was estimated from the absorbance differences, i.e., ΔA (λ320–λ247) for DBT and ΔA (λ286–λ324) for 2-HBP.
Fig. 2.
a Selection of λmax for DBT, b standard curve of DBT in ethyl acetate, cλmax of 2-HBP, d standard curve for 2-HBP in ethyl acetate
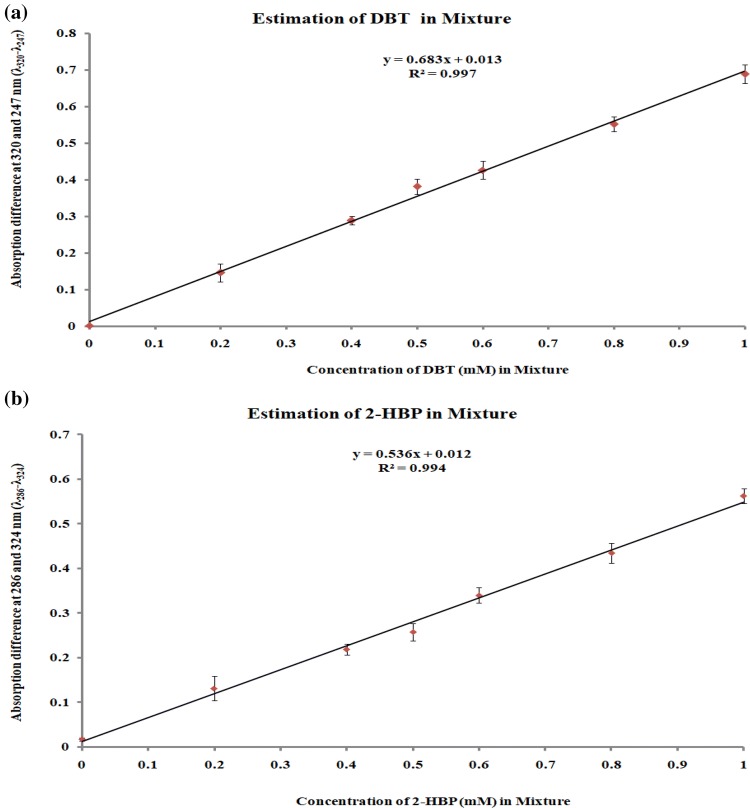
Table 1 presents the absorbance differences measured from the mixture of DBT and 2-HBP taken in different ratios. The graphs plotting between actual concentrations of DBT and 2-HBP in mixture with respect to their corresponding absorbance difference values were linear in the range of 0.1–1.0 mM (Fig. 3). The developed method appeared highly reliable based on the regression coefficient (R2) values closer to one. The regression equations of standard curves were Y = 0.683X + 0.013, R2 = 0.997 for DBT and Y = 0.536X + 0.012, R2 = 0.994 for 2-HBP. These standard graphs were used to measure unknown concentration in the test samples for the screening of specific desulfurizing microbes.
Table 1.
Analysis of mixed samples of DBT and 2-HBP with developed spectrophotometric method
| The ratio of DBT (1 mM) and HBP (1 mM) in mixed samples | Actual conc. of DBT (mM) in mixture | Actual conc. of 2-HBP (mM) in mixture | Absorbance difference | |
|---|---|---|---|---|
| ΔA (λ320–λ247) | ΔA (λ286–λ324) | |||
| 5:0 | 1.0 | 0.0 | 0.688 ± 0.025 | 0.018 ± 0.003 |
| 4:1 | 0.8 | 0.2 | 0.552 ± 0.020 | 0.132 ± 0.028 |
| 3:2 | 0.6 | 0.4 | 0.426 ± 0.024 | 0.219 ± 0.012 |
| 1:1 | 0.5 | 0.5 | 0.381 ± 0.021 | 0.257 ± 0.019 |
| 2:3 | 0.4 | 0.6 | 0.289 ± 0.012 | 0.340 ± 0.018 |
| 1:4 | 0.2 | 0.8 | 0.146 ± 0.024 | 0.437 ± 0.022 |
| 0:5 | 0.0 | 1.0 | 0.002 ± 0.002 | 0.562 ± 0.017 |
Fig. 3.
The linear relationship between the absorbance difference (λ320–λ247 for DBT and λ286–λ324 for 2-HBP) with respect to the actual concentration of, a DBT and b 2-HBP in the mixed solutions, data derived from Table 1
The developed method possesses several advantages over the existing screening methods. Instead of being quite simple and reliable, the developed method does not need a specific chemical like 2,6-dichloroquinone-4-chloramide (Gibb’s reagent) for Gibb’s assay, the most commonly used screening method (Raheb et al. 2009). The sophisticated method for the detection and quantification of 2-HBP such as reverse phase HPLC (Bahuguna et al. 2011) and GC–MS (Folsom et al. 1999) is quite laborious and time-consuming for large number of samples for screening purpose. Further, the present method facilitates the detection and quantification of DBT and 2-HBP simultaneously in a given sample. To the best of our knowledge, there is no other method for simultaneous detection and quantification of DBT and 2-HBP by UV–Vis spectrophotometer.
Screening of specific biodesulfurizing microbes
Two different pathways can be followed by microbes to remove sulfur from organosulfur compounds; Kodama pathway or 4S pathway. However, the specific biodesulfurizing microbes following 4S pathway are desirable for desulfurization study as these are efficient to remove sulfur from the organosulfur compounds without breaking the benzene ring leading to the retention of calorific value of the fuel (Verma et al. 2016). Dibenzothiophene (DBT) is considered a representative compound in fuel and 2-hydroxybiphenyl (2-HBP) is the most commonly produced end product of DBT desulfurization (Martínez et al. 2016). Therefore, the specific desulfurizing microbes can be screened by detecting 2-HBP in DBT desulfurized medium.
A total of 57 desulfurizing microbial cultures were isolated from 15 soil samples using DBT as a sole sulfur source in the minimal salt medium (MSM). On screening with developed spectrophotometric method, only two isolates (MS5 and BR31) were reported to produce 2-HBP on specific DBT desulfurization (Table 2). The isolates MS5 and BR31 presented 48.21 ± 0.319 and 45.91 ± 0.411% desulfurization of DBT (1 mM) with 0.230 ± 0.161 and 0.177 ± 0.005 mM 2-HBP production. The decrease in DBT was not found directly equivalent to 2-HBP production. It was attributed to either slow discharge of 2-HBP from the cells or conversion of 2-HBP into other metabolites like biphenyl or 2-methylbiphenyl (Li et al. 2005; Rath et al. 2012). The specific desulfurizing nature (4S pathway) of these isolates MS5 and BR31 was further confirmed by colorimetric Gibb’s assay as well as with HPLC analysis.
Table 2.
Screening of specific desulfurizing microbes with developed spectrophotometric method
| Microbial isolates | Conc. of residual DBT (mM) | DBT desulfurization (%) | Conc. of 2-HBP (mM) | 2-HBP production (%) |
|---|---|---|---|---|
| PAS-1 | 0.457 ± 0.015 | 54.26 ± 1.489 | – | – |
| MS-5 | 0.518 ± 0.003 | 48.21 ± 0.319 | 0.230 ± 0.161 | 23.033 ± 1.607 |
| MFS-3 | 0.658 ± 0.008 | 34.19 ± 0.840 | – | – |
| BR-31 | 0.541 ± 0.004 | 45.91 ± 0.411 | 0.177 ± 0.005 | 17.667 ± 0.472 |
| GR-5 | 0.522 ± 0.014 | 47.78 ± 1.410 | – | – |
Confirmation of 2-HBP production
Gibb’s assay
In Gibb’s assay, the production of 2-HBP on desulfurization with two isolates MS5 and BR31 was validated by the appearance of purple color on incubation with Gibb’s reagent as clearly shown in Fig. 4.
Fig. 4.

Screening results of desulfurizing microbes with Gibb’s assay. The purple color was developed on reaction of Gibb’s reagent with desulfurizing mixtures from Isolate BR-31 and MS-5 indicating 2-HBP production. No color change was observed with control and desulfurizing mixture from other isolates
High performance liquid chromatography
During HPLC analysis, the retention time of DBT and 2-HBP was recorded 5.7 (Fig. 5a) and 2.8 min (Fig. 5b), respectively, with their standard solutions. The 2-HBP production was confirmed from appearance of peaks at 2.8 min in desulfurizing reaction mixtures of isolate MS-5 (Fig. 5c) and isolate BR-31 (Fig. 5d). The spectra obtained from the analysis of desulfurizing mixtures of selected two isolates were quite similar to that obtained with procured specific desulfurizing microbe R. rhodochrous (Fig. 5e) which further proved the 4S pathway followed by isolates MS-5 and BR-31.
Fig. 5.
HPLC chromatograms of a standard solution of DBT (RT 5.7 min), b standard solution of 2-HBP (RT 2.8 min). c–e Desulfurization reaction mixtures of c Isolate MS5, d Isolate BR-31, and eRhodococcus rhodochrous MTCC 3552
The conversion of DBT to 2-HBP in 4S pathway occurs via three intermediates: DBT sulfoxide (DBTO), DBT sulfone (DBTO2), hydroxybiphenyl sulfinate (HBPS). These intermediates are usually consumed very fast (except HBPS) (Gray et al. 1996) and assumed to have no interference in the estimation of DBT and 2-HBP in this assay. Further, no major peak except those of DBT and 2-HBP was observed during HPLC analysis of samples indicating the absence of considerable concentration of other intermediates.
Identification of selected cultures
The 16S rRNA gene sequences of isolates MS-5 and BR-31 were determined by Yaazh Xenomics, Mumbai, Maharashtra (India) and submitted in GenBank of NCBI with accession numbers MH557847 and MH557848, respectively. The 16S rRNA gene sequence of isolate MS5 was found closely related to B. flexus (MF800923) using NCBI BLAST and hence, it was identified as B. flexus MS-5. On the other hand, the isolate BR31 was identified as B. cereus BR-31 depending upon the highest sequence similarity with B. cereus (DQ518612).
Biodesulfurization study with selected microbes
DBT desulfurization profile in minimal salt media with 2% (4.0–4.5 × 107 cells/mL) fresh inoculum of B. flexus MS-5 and B. cereus BR-31 is depicted in Fig. 6. B. flexus MS-5 presented higher desulfurization efficiency as compared to B. cereus BR-31 throughout their study. The isolate MS-5 achieved its maximum desulfurization efficiency of 54.88 ± 1.12% (0.549 mM) with 1.0 mM DBT at 54 h, however, the desulfurization efficiency of isolate BR-31 remain increased till 72 h and became comparable to that of isolate MS-5, i.e., 55.72 ± 1.32% (0.557 mM). The desulfurization efficiency acquired with the present Bacillus strains was found higher than the existing one. For instance, B. subtilis WU-S2B presented 0.54 mM DBT desulfurization in 120 h (Kirimura et al. 2001). On the other hand, Bacillus sp. KS1 demonstrated 68.75% (0.206 mM) desulfurization of 0.3 mM DBT in 168 h (Raheb et al. 2009). Arabian et al. (2014) described the use of B. cereus HN for the desulfurization of 33% of kerosene with initial content of 2333 mg/L. However, the use of B. flexus for biodesulfurization has not been described in the literature so far, to the best of our knowledge.
Fig. 6.

Comparative DBT desulfurization (%) profile of B. flexus MS-5 and B. cereus BR-31 with time (h). Isolate MS-5 presented the higher desulfurization efficiency as compared to isolate BR-31 after 6 h, however achieved its maximum desulfurization efficiency at 54 h. Desulfurization efficiency of Isolate BR-31 continued to increase and became comparable after 70 h
Conclusion
A novel spectrophotometric method was developed facilitating the simultaneous analysis of DBT and 2-HBP in biodesulfurization study. The concentration of DBT and 2-HBP was found proportionate to the absorbance difference ΔA (λ320–λ247) and ΔA (λ286–λ324), respectively, in their mixed spectrum using dual wavelength method. The developed method was quick, simple and reliable to screen the specific desulfurizing microbes. Two microbes B. flexus MS-5 and B. cereus BR-31 were selected on the basis of 2-HBP production in the desulfurizing mixture which was confirmed by Gibb’s assay and HPLC. B. flexus MS-5 and B. cereus BR-31 presented 54.88 ± 1.12% and 55.72 ± 1.32% desulfurization of DBT (1.0 mM). The present strains have an excellent potential for biodesulfurization of fuels.
Acknowledgements
The author (Rajni Sharma) highly acknowledges University Grant Commission (UGC), New Delhi, India, for financial support in the form of Senior Research Fellowship (SRF).
Author contributions
RS: conceptualization, methodology, formal analysis and investigation, data curation, original draft preparation, review and editing, funding acquisition; JS: draft review and editing, resources; NV: conceptualization, methodology, formal analysis and investigation, draft review and editing, resources, supervision.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Rajni Sharma, Email: rajni.sharma18@gmail.com.
Jagdish Singh, Email: jagdish122@rediffmail.com.
Neelam Verma, Email: verma.neelam2@gmail.com.
References
- Ansari F, Prayuenyong P, Tothill I. Biodesulfurization of dibenzothiophene by Shewanella putrefaciens NCIMB 8768. J Biol Phys Chem. 2007;7:75–78. doi: 10.4024/20708.jbpc.07.02. [DOI] [Google Scholar]
- Arabian D, Najafi H, Farhadi F, Dehkordi AM. Biodesulfurization of simulated light fuel oil by a native isolated bacteria Bacillus cereus HN. J Pet Sci Technol. 2014;4:31–40. [Google Scholar]
- Bahuguna A, Lily MK, Munjal A, Singh RN, Dangwal K. Desulfurization of dibenzothiophene (DBT) by a novel strain Lysinibacillus sphaericus DMT-7 isolated from diesel contaminated soil. J Environ Sci. 2011;23:975–982. doi: 10.1016/S1001-0742(10)60504-9. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Sharma DK. Biodesulfurization of dibenzothiophene, its alkylated derivatives and crude oil by a newly isolated strain Pantoea agglomerans D23W3. Biochem Eng J. 2010;50:104–109. doi: 10.1016/j.bej.2010.04.001. [DOI] [Google Scholar]
- Bordoloi NK, Rai SK, Chaudhuri MK, Mukherjee AK. Proteomics and metabolomics analyses to elucidate the desulfurization pathway of Chelatococcus sp. PLoS ONE. 2016 doi: 10.1371/journal.pone.0153547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derikvand P, Etemadifar Z. Improvement of biodesulfurization rate of alginate immobilized Rhodococcus erythropolis R1 Jundishapur. J Microbiol. 2014;7:1–7. doi: 10.5812/jjm.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derikvand P, Etemadifari Z, Saber H. Sulfur removal from dibenzothiophene by newly isolated Paenibacillus validus strain PD2 and process optimization in aqueous and biphasic (model-oil) systems. Pol J Microbiol. 2015;64:47–54. doi: 10.33073/pjm-2015-006. [DOI] [PubMed] [Google Scholar]
- Folsom BR, Schieche DR, Digrazia PM, Werner J, Palmer S. Microbial desulfurization of alkylated dibenzothiophenes from a hydrodesulfurized middle distillate by Rhodococcus erythiopolis I-19. Appl Environ Microbiol. 1999;65:4967–4972. doi: 10.1128/AEM.65.11.4967-4972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcha S, Verma N, Brar SK. Isolation, characterization and identification of microorganisms from unorganized dairy sector wastewater and sludge samples and evaluation of their biodegradability. Water Resour Ind. 2016;16:19–28. doi: 10.1016/j.wri.2016.10.002. [DOI] [Google Scholar]
- Gray KA, Pogrebinsky OS, Mrachko GT, Xi L, Monticello DJ, Squires CH. Molecular mechanism of biocatalytic desulfurization of fossil fuels. Nat Biotechnol. 1996;14:1705–1709. doi: 10.1038/nbt1296-1705. [DOI] [PubMed] [Google Scholar]
- Jain JY, Patadia RI, Vanparia DI, Chauhan RE, Shah SH. Dual wavelength spectrophotometric method for simultaneous estimation of drotaverine hydrochloride and aceclofenac in their combined tablet dosage form. Int J Pharm Pharm Sci. 2010;2(4):76–79. [Google Scholar]
- Kirimura K, Furuya T, Nishii Y, Ishii Y, Kino K, Usami S. Biodesulfurization of dibenzothiophene and its derivatives through the selective cleavage of carbon–sulfur bond by moderately thermophilic bacterium Bacillus subtilis WU-S2B. J Biosci Bioeng. 2001;91:262–266. doi: 10.1016/S1389-1723(01)80131-6. [DOI] [PubMed] [Google Scholar]
- Labana S, Pandey G, Jain RK. Desulphurization of dibenzothiophene and diesel oils by bacteria. Lett Appl Microbiol. 2005;40:159–163. doi: 10.1111/j.1472-765X.2004.01648.x. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang Y, Wang MD, Shi Y. Biodesulfurization of dibenzothiophene and other organic sulfur compounds by a newly isolated Microbacterium strain ZD-M2. FEMS Microbiol Lett. 2005;247:45–50. doi: 10.1016/j.femsle.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Mabrouk MM, Hammad SF, El-Fatatry HM, El-Malla SF. Spectroscopic methods for determination of dexketoprofen trometamol and tramadol HCl. Inven Impact Pharm Anal Qual Assur. 2014;4:276–282. [Google Scholar]
- Martínez I, Mohamed ME, Rozas D, García JL, Díaz E. Engineering synthetic bacterial consortia for enhanced desulfurization and revalorization of oil sulfur compounds. Metab Eng. 2016;35:46–54. doi: 10.1016/j.ymben.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Nuhu AA. Bio-catalytic desulfurization of fossil fuels: a mini review. Rev Environ Sci Bio/Technol. 2013;12:9–23. doi: 10.1007/s11157-012-9267-x. [DOI] [Google Scholar]
- Raheb J, Hajipour MJ, Saadati M, Rasekh B, Memari B. The enhancement of biodesulfurization activity in a novel indigenous engineered Pseudomonas putida. Iran Biomed J. 2009;13:207–213. [PubMed] [Google Scholar]
- Rath K, Mishra B, Vuppu S. Biodegrading ability of organosulfur compound of a newly isolated microbe Bacillus sp. KS1 from the oil contaminated soil. Arch Appl Sci Res. 2012;4:465–471. [Google Scholar]
- Shibata S. Dual-wavelength spectrophotometry. Angew Chem Int Ed Engl. 1976;15(11):673–679. doi: 10.1002/anie.197606731. [DOI] [Google Scholar]
- Sousa M, Melo VMM, Rodrigues S, Santana HB, Gonçalves LRB. Screening of biosurfactant-producing Bacillus strains using glycerol from the biodiesel synthesis as main carbon source. Bioprocess Biosyst Eng. 2012;35:897–906. doi: 10.1007/s00449-011-0674-0. [DOI] [PubMed] [Google Scholar]
- Verma N, Sharma R, Kaur R. Microbial desulfurization study of dibenzothiophene and crude oil by a soil isolate. Int J Sci Res Methodol. 2016;4:133–145. [Google Scholar]