Abstract
In this study, active poly lactic acid (PLA) films containing 0, 10, 20 and 40% w/w propolis extract (PE), as active agent, were developed. A high amount of phenolic content (PC) was measured in PE. The antioxidant effect of active PLA films was determined by measuring the PC of sausage slices after 0, 2 and 4 days storage at refrigerator. Results showed that phenolic compounds of PE were released from PLA films in quantities proportional to PE concentration. Disc diffusion test indicated that PE showed an inhibitory effect against Staphylococcus aureus and Pseudomonas aeruginosa bacterial species but was more effective against gram-positive species. PE containing PLA films had antimicrobial effect on S. aureus while in the case of P. aeruginosa, PLA/PE films needed polyethylene glycol (PEG)/CaCO3 content to show inhibitory effect. Addition of PE changed the tensile strength, elongation at break and elastic modulus of PLA films negatively. However, addition of PEG/CaCO3 improved the film mechanical properties and antimicrobial effect of films.
Keywords: Antimicrobial packaging, Natural antimicrobial, Antioxidant property, Biodegradable packaging, Propolis extract, Meat products packaging
Introduction
Packaging is believed to be an important means to extend food products shelf life, while maintaining food quality (Irkin and Esmer 2015). There are two methods of packaging namely passive and active packaging however, there may be seen a rapid rise in the use of active packaging over the last decades (Dini 2015). Active packaging is an emerging novel technology and increases the longevity of food products by protection against microbial spoilage and chemical deterioration by addition of proper components to the packaging material.
Biodegradable packaging materials has received much attention due to vast negative impact of conventional oil-based polymers on the environment (Choi et al. 2018). However, mechanical properties of biodegradable polymers are not comparable with traditional ones. Furthermore, certain features such as hydrophilicity of some kinds of bio-based polymers restrict their commercial usage (Choi et al. 2018). Several recent studies have focused on finding new bio-polymers which in addition to favorable properties, have other required features that make them suitable for active food packaging. One possible appropriate polymer could be Poly lactic acid (PLA) (Sanyang and Sapuan 2015). This is a promising thermoplastic biocompatible and biodegradable polymer which has proper mechanical, optical and barrier attributes in comparison to conventional petroleum based plastics (Lim et al. 2008). Monomers of PLA are produced by fermentation from non-toxic renewable resources such as starch derivatives (Lasprilla et al. 2012). Furthermore, alcoholysis or hydrolysis could recycle back PLA to lactic acid (Jamshidian et al. 2010). A striking feature of PLA polymer is its mechanical stability in a wide range of temperatures (Holm et al. 2006). It appears to be innocuous and has the potential to combine with a variety of components and materials in order to improve its chemical, physical and mechanical characteristics. Various approaches have been hypothesized by several studies to resolve some drawbacks of PLA like poor toughness and hydrophobicity (Farah et al. 2016), and even to develop antimicrobial/antioxidant packaging material without any adverse effect on food quality. All of these properties of PLA have provoked packaging researchers to find a beneficial active poly-lactic acid polymer by incorporation of diverse active agents like nisin (Jin and Zhang 2008), bacteriocins (Liu et al. 2007) and lysozyme (Nobile et al. 2009). According to many studies, utilization of natural antioxidant and antimicrobial agents, like plant extracts and herbal essential oils, is an alternative to synthetic active substances in active packaging (Siripatrawan and Vitchayakitti 2016). Among them, propolis extract has received much attention as it can be used as a safe strong antioxidant and antibacterial ingredient on broad range of bacteria (Mascheroni et al. 2010; Correa et al. 2019).
Depending on the regions from which propolis is developed, it may have different colors and complicated and even diverse chemical compounds containing flavonoids, terpenes, phenolic acids and derivatives, and fatty acids (de Oliveira Dembogurski et al. 2018; Rizzolo et al. 2016; Kalogeropoulos et al. 2009).
Various biological activities of propolis are primarily related to the flavonoids and the phenolic acid esters (de Oliveira Dembogurski et al. 2018) and have led to significant interest in the use of propolis or its extracts as a natural antioxidant/antibacterial agent in active packaging (Rizzolo et al. 2016; Siripatrawan and Vitchayakitti 2016). In the literature there are a growing number of examples of incorporation of various propolis extracts as a functional-active agent into bio-films like gelatin-based polymers and chitosan films (Siripatrawan and Vitchayakitti 2016; Bodini et al. 2013). But a little attention has been paid to PE derivatives application in PLA. As an example, the peroxide values and the shelf life of minced beef packed in PLA based films containing propolis ethanolic extract (PE) increased and microbial growth decreased during at least 11 days of storage without any adverse organoleptic effect (Shavisi et al. 2017). It was also demonstrated that PLA films containing PE and Zataria multiflora Essential oil (ZME) have appropriate mechanical properties as well as antimicrobial activities in vitro and even in vacuum-packed cooked sausages (Rezaeigolestani et al. 2017). However, there is still a need for determining antioxidant properties of PLA/PE films. Also, more research needs to be undertaken to improve the methods for enhancing mechanical and microbial attributes of PLA/PE polymers to be used in meat industry.
Recently a comprehensive review on the application of antioxidant films and a variety of active and intelligent packaging methods as an effective approach to eliminate lipid oxidation and microbial spoilage of meat products, has been published in the literature (Fang et al. 2017; Domnguez et al. 2018). However, no study appears to be on antioxidant and antimicrobial activities as well as mechanical properties of PLA film containing PE together.
In this study, PLA-based films were developed containing different concentrations of PE. Active films were made by solvent casting method. Antimicrobial and antioxidant property of both propolis ethanolic extract and the PLA active films on two commonly found bacteria in meat products namely Gram-positive Staphylococcus aureus and Gram-negative Pseudomonas aeruginosa (Siripatrawan and Vitchayakitti 2016) were evaluated using dry meat sausage was chosen as a model. In addition, mechanical properties of PLA/PE polymers were studied. Moreover, this study considered effect of addition of additives such as calcium carbonate (CaCO3) and polyethylene glycol (PEG) on film mechanical characteristics as well as antimicrobial/antioxidant properties of its composite with propolis.
Materials and methods
Materials
All chemicals including calcium carbonate, sodium carbonate, ethanol, chloroform, polyethylene glycol 6000 (PEG), Folin–Ciocalteu reagent were obtained from international market (Merck, Germany). Gallic acid was from Riedel-De-Haen (Germany). MN 615 filter paper was provided from international market (Macherey–Nagel, Germany).
Raw dark brown propolis, samples of dry meat sausage and poly lactic acid NatureWork brand granules was provided by local market (Honey Mahvin Company, Karaj, Sigol-60% and Ista Polymer Sharif, Eshtehard industrial city, Iran respectively). Propolis was stored at − 20 °C until use.
Deionized water and bacterial species (S. aureus ATCC 33591 and P. aeruginosa ATCC 9027) were obtained locally (Sharif Central laboratory, university culture collection (BBRC, Tehran, Iran, respectively).
Methods
Ethanolic extraction of propolis
Propolis ethanolic extract (PE) was prepared following Kalogeropoulos et al. (2009) with some changes. A specified quantity of grounded crude propolis was extracted with tenfold volume of 70% ethanol solution into a closed Erlenmeyer flask. Then the solution was stirred on a stirrer for 3 days at ambient temperature and in the dark. The resulting ethanol-extract solution was frozen at − 20 °C for one day and then filtered with filter paper (MN 615). The freezing-filtration cycle was re-done three times in which the final filtrated solution was called PE. PE was stored at − 20 °C in dark place for further analyses.
Gravimetric analysis was used to evaluate the yield of PE. The PE stock solution was evaporated at 50 °C and then freeze-dried. Obtained yellowish powder referred to as PE powder which was used for producing active films.
Determination of total phenolic content (PC) of PE
The Folin–Ciocalteu method was adopted to determine the total phenolic content (PC) of PE (Rizzolo et al. 2016). In this procedure, the Folin–Ciocalteu reagent reacts with the hydroxyl group of phenols and results in a blue complex that has maximum absorbance at 770 nm. First, 100 µl of PE, 900 µl of deionized water and 75 µl of Folin–Ciocalteu reagent were mixed in an Eppendorf tube. After 2 min, 300 µl of 7.5% Na2CO3 was added and the mixture was thoroughly mixed and then the tube was kept in a dark place at ambient temperature for 2 h. Finally, the absorbance of the resulting mixture was measured at 770 nm with spectrophotometer (UNICO UV/Visible-2100 series). Blank solution contained all above components except the PE sample tested. Calibration curve of gallic acid was prepared using standard gallic acid solutions and Eq. (1) was obtained from this curve. Concentration of PC was calculated using this curve and result was expressed as mg equivalent weight of gallic acid per g dry mass of sample.1
| 1 |
Methanolic extracts of sausage (ME)
The methanolic extraction of sausage samples (at day 0 and after 2 and 4 days packed in films) was prepared as described by Rashidinejad et al. (2013). First, 0.5 g of sausage samples was homogenized and extracted with 3.5 ml of a methanolic solution (95:5 methanol: HCl 6 N) at 50 °C under stirring. After 30 min, solution was filtered with the filter paper (MN 615).
PC of ME was analyzed in accordance with the Folin–Ciocalteu method explained in literature (Rizzolo et al. 2016). PC of ME was expressed as mg Gallic acid/g sausage sample.
PLA-PE active film preparation
Active film making procedure used was taken from literature (Mascheroni et al. 2010). Several formulations of active PLA-based films were produced by solvent-casting method shown in Table 1. Three grams of PLA granules were ground and dissolved in 30 ml of chloroform under stirring at 40 °C. Various amounts of PE were dissolved in 10 ml of chloroform. Various amounts of CaCO3 as opening polymer structure agent and PEG as plasticizer were also added. Sample number 8 was considered as control sample.
Table 1.
Compositions and thicknesses of PLA-film samples
| Film sample | %PEWPE/WPLA | %CaCO3CaCO3/PLA | %PEGPEG/PLA | Film thickness (µm) |
|---|---|---|---|---|
| 1 | 10 | 10 | 15 | 100 ± 1 |
| 2 | 20 | 10 | 15 | 90 ± 1 |
| 3 | 40 | 10 | 15 | 92 ± 2 |
| 4 | 10 | 0 | 0 | 90 ± 2 |
| 5 | 20 | 0 | 0 | 98 ± 1 |
| 6 | 40 | 0 | 0 | 92 ± 1 |
| 7 | 0 | 10 | 15 | 100 ± 1 |
| 8 | 0 | 0 | 0 | 92 ± 2 |
Antimicrobial activity measurement
Disk diffusion method was used to analyze the antimicrobial activity of PE against both S. aureus and P. aeruginosa strains (Rezaeigolestani et al. 2017). Cultures of bacterial strains were cultivated in nutrient broth during 24 h at 37 °C up to 107CFU/ml.
Spread plate method were used to inoculate every diluted bacterial strain suspensions on nutrient agar. The paper disks (with 5 mm diameter) were sterilized by UV-light and then completely impregnated with PE. Four paper disks were placed on the surface of each plate. Inoculated plates were then incubated at 37 °C. After 24 h of incubation, the diameter of inhibition zones around disks was measured and the average of 4 diameters of them for each inoculation method and bacterial strain was reported.
In case of the films prepared, 2 cm diameter film sample discs were first sterilized by UV light and inhibition zones were measured.
Film mechanical properties
Tensile strength (TS), elongation percentage at break (%EB) and elastic modulus (EM) of films were measured in compliance with the ASTM-D882 method with some adjustments. 15 mm × 70 mm film specimens were mounted between grip head of the texture analyzer machine (HIWA 2126 Universal testing machine, HIWA ENGINEERING COMPANY, Tehran, Iran) with 23 mm initial grip separation length. The film sample was subjected to tension with 5 mm/min of cross-head speed until broken.
Replicability
All experiments were replicated three times and every measurement was taken in triplicate unless otherwise specified, and results were reported as the mean value ± standard deviation. Data management and analysis were performed using Minitab 18.0 software while significance levels were set at the 5% level (Tukey Method with 95% confidence interval was used).
Results and discussion
Early measurements showed that concentration of PE was 22.8 ± 0.8 mg/ml and its yield was 61.67%. Yield of PE relies on several factors including the solvent, method and in particular propolis characteristics. For example, yield of twelve types of propolis has been reported in literature between 23.9 and 61.2% (Kalogeropoulos et al. 2009).
PE antimicrobial activity
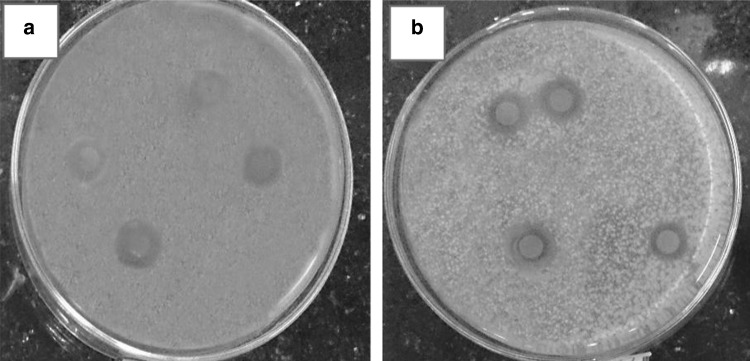
Figure 1 clearly shows the antimicrobial activity of PE against the two bacterial species. The average diameters of inhibition zones after 24 h incubation were 12.3 ± 1 mm for S. aureus and 6.9 ± 1 mm for P. aeruginosa. Obviously, PE was much more effective against gram-positive S. aureus than gram-negative P. aeruginosa. All plates were maintained at 35 °C for 30 days. No significant reduction in inhibition zone diameters was seen during this period. These findings verify stability of antimicrobial activity of PE in long-term storage. Also the initial strong inhibitory action of PE could be explained by antibacterial activity of volatile components in PE formulation.
Fig. 1.
Inhibition zones of PE-saturated discs against S. aureus (a) and P. aeruginosa (b)
Film antimicrobial activity
Table 2 demonstrates antimicrobial activity of the films for bacteria after 24 h at 35 °C. As expected, no antibacterial effect was detected for PLA-based films without PE. No significant inhibitory effect was also observed on P. aeruginosa. However, it may be seen that gram-positive strain was susceptible to active films similar to published data (Shavisi et al. 2017). Maximum inhibition effect was seen in sample film No. 3 in which the clear zone diameter was by 30 ± 2 mm against S. aureus. Available literature reveals that antimicrobial properties of propolis and PE against several bacteria is due to flavonoids and phenolic compounds and in particular polyphenol compounds were recognized as main active compounds of PE (Mascheroni et al. 2010). Furthermore, PE is characterized as a hydrophobic bioactive agent due to hydrophobic constituents of propolis (Torlak and Sert 2013). Considering all of this evidence, the PE antibacterial action against a broad spectrum of organisms, especially gram-positive ones, can be explained.
Table 2.
Average inhibition zone diameter at 35 °C
| Film sample | Inhibition zone diameter (mm) | |
|---|---|---|
| S. aureus | P. aeruginosa | |
| 1 | – | – |
| 2 | 26 ± 2b | – |
| 3 | 30 ± 2a,A | 22 ± 1a,B |
| 4 | – | – |
| 5 | 22 ± 2d | – |
| 6 | 25 ± 1c | – |
| 7 | – | – |
| 8 | – | – |
a–dDifferent lowercase letters within a column indicate significant differences (P value < 0.05)
A,BDifferent uppercase letters within a row indicate significant differences (P value < 0.05)
(–): means no inhibition zone around the disc detected
Inhibition against P. aeruginosa was only detected for film sample No.3. This finding that gram-positive bacterial strain was more susceptible to PE appears to be well substantiated by many previous research results (Correa et al. 2019). Chemical composition and in vitro antimicrobial activity of twelve different propolis extracts against 18 bacterial strains (including S. aureus) and two pathogenic fungi are investigated (Kalogeropoulos et al. 2009). They indicated that these extracts had stronger antimicrobial effects on gram-positive strains than gram-negative ones. Moreover, the direct correlation between concentration of terpenoids and antibacterial activity of propolis extract has been detected. In addition to identifying eighty-six different compounds from several Brazilian brown propolis extracts including flavonoids and phenyl propanoic acids, it has been found that propolis-fractions killed about 93% of S. aureus in biofilm but did not show any antibacterial activity against P. aeruginosa (de Oliveira Dembogurski et al. 2018).
In some relevant studies, antibacterial activity of plant extracts and essential oils (EOs) have been studied and attributed to phytochemical substances like phenolic compounds (Negi 2012). Again, it has been stated that gram-positive organisms are generally more susceptible to ingredients of EOs and plant extracts than gram-negative ones (Turgis et al. 2008). It has been pointed out that relation between the concentration of polyphenols and antibacterial and antioxidant activities of plant extracts and phenolic compounds are the most important agents of bacterial membrane disruptor (Ennajar et al. 2009). The inhibitory mechanism of these bioactive ingredients may be explained by lipophilic properties of phenolic compounds which play a vital role in antibacterial effects by acting on the bacterial cell membrane which is causing an increase in permeability and cellular crucial components release (Azizkhani et al. 2013). It has been indicated that the Hydroxyl group (-OH) in phenolic compounds serves an inhibitory function (Gyawali and Ibrahim 2014). The multilayer membrane of gram-negative bacteria allows them to be more resistant to hydrophobic compounds. But the membrane of gram-positive bacteria consists of peptidoglycan and hydrophobic molecules can easily penetrate the cells (Turgis et al. 2008; Nazzaro et al. 2013).
As shown in Table 2, there was a significant positive correlation (P value < 0.05) between PE content in active films and the antimicrobial activity of the films against S. aureus. Films containing 40% PE (with or without PEG/CaCO3) caused larger inhibition zone while films with 10% PE did not show inhibition at all. Lack of inhibition zone around films containing 10% of PE can be attributed to low migration rate of active agents from molecular network of PLA-film into the culture medium that has been reported similarly before (Mascheroni et al. 2010). Migration of propolis into food has been modeled and found that the diffusivity values of propolis polyphenols in the PLA matrix were very low. Low concentration of PE in PLA based film as well as low internal mass transfer rate could lead to poor antimicrobial activity of 10%PLA/PE samples.
On the other hand, films containing PEG and CaCO3, increase antimicrobial effect of active polymers (Table 2). It has been reported that incorporation of plasticizers and modifiers into PLA polymers can open the molecular network and eventually results in increased migration rate of active substances (Mascheroni et al. 2010).
Few studies have examined the antimicrobial activity of a PLA active packaging containing PE. Antimicrobial activity of a gelatin-based film containing different concentrations of PE (0, 5, 40, 200 gPE/100 ggelatin) against S. aureus by 20 mm disc diffusion method during 180 days has been studied (Bodini et al. 2013). Besides promotion of water vapor permeability and some improvement in mechanical properties by adding PE, gelatin films with 40 and 200 gPE/100 ggelatin showed inhibitory action against S. aureus. The diameters of inhibition zones, during 180 days, for 40 gPE/100 ggelatin and 200 g of PE/100 g of gelatin film were 21.2–25.4 mm and 25.4–29.3 mm, respectively. It has been demonstrated that there was a minimum concentration of PE incorporated into active films to exhibit the inhibitory action. Moreover, the results confirmed that films with 40% and 200% kept polyphenol content and antimicrobial effects for 180 days.
A number of similarities with the above findings may be seen in this research. In our work, discs showed a stability of antimicrobial activity during up to 30 days. Furthermore, it was shown that the minimum inhibitory concentration of PE in PLA-based films was 20% wPE/wPLA against S. aureus, with the diameter of inhibition zone ranging from 22 ± 2 to 30 ± 2 mm, and 40% wPE/wPLA against P. aeruginosa, with the diameter of inhibition zone of 22 ± 1 mm.
PC and antioxidant effect
Total phenolic content (PC) of PE
A direct correlation between PC of PE and their free radical scavenging ability2 as well as their antioxidant power3 has been reported in literature. Both DPPH and FRAP assay measure the antioxidant capacity of bioactive samples. It has also been verified that pinocembrin, pinobanksin, pinobanksin-3-O-acetate, chrysin and galangin, are the major flavonoids components among the majority of the propolis samples studied. However, in most of cases, terpenes were the substantial substances (Kalogeropoulos et al. 2009). Based on this type of reports, it is generally believed that terpenes and phenolic compounds account for biological activities of PE and flavonoids exert considerable influence on its antioxidant and antimicrobial properties (Ahn et al. 2004). The antioxidant action and radical scavenging role of flavonoids rely on their formulations and the structure of hydroxyl groups (Wojdyo et al. 2007). Thus, in broad terms, there seems to be a positive correlation between the amount of PC and antioxidant activity of PE.
In this work, PC of PE was measured as 27.08 ± 2.4 mggallic acid/gdry PE sample. This is while this value has been reported within the range of 80.2–338.5 mgcaffeic acid/gpropolis extract for twelve samples of PE collected from Greece and Cyprus (Kalogeropoulos et al. 2009). Composition and biological activities of propolis and PE depends on their geographic region and the source of plants collected (Ahn et al. 2004). Generally there are two major groups of propolis based on their composition i.e. Brazilian-type (Baccharis-type), which main constituents are terpenoids and p-coumaric acid derivatives, and European-type (poplar-type) mainly containing flavonoids. Propolis samples originated from populous species (found in temperate climate), are rich in flavonoids, phenolic acids and their esters while tropical propolis species produced from other kinds of plants primarily contain prenylated benzophenons, diterpenes and flavonoids (Kalogeropoulos et al. 2009; Ahn et al. 2004).
PC of ethanolic, glyceric and dry extracts of raw propolis in addition to commercial fluid extract, has been examined and found that commercial fluid extract had the highest total polyphenol content (1.24 ggalangin/mlextract). PC of PE and glyceric extract were 0.54 ggalangin/mlextract and 0.14 ggalangin/mlextract, respectively, while it was shown that the dry extraction resulted in minimum yield and the PC of dry extract was found to be 74.07 mggalangin/gextract, the lowest one. Composition of polyphenols in extracts was approximately similar with the higher concentration of galangin, pinocembrin, apigenin and pinobanksin (Juliano et al. 2007).
PC of sausage samples and antioxidant effect of active films
Meat products contain a high amount of fat and unsaturated fatty acids (Domnguez et al. 2018). Accordingly, one of the most important types of meat spoilage is lipid oxidation. This phenomenon produces unpleasant odors, flavors. Sometimes toxic products also change product color, texture and quality. Therefore, it would be useful if one could control lipid oxidation via active packaging containing antioxidant agents.
Table 3 presents PC of dry meat sausage samples packed by PLA based films at different storage times. Dry meat sausage inherently has some phenolic components (0.904 ± 0.002 mg gallic acid/g sausage sample) that could be attributed to its raw materials including such as onion and garlic. Total phenolic content in treated sausage samples considerably increased (P value < 0.05) throughout the storage time when they were packed by films containing PE, except for samples packed by PLA/PE 10% at day 2. These data implied that phenolic constituents were released from polymer network into the surface of sausage slices. Our results share a number of similarities with Mascheroni et al.’s findings which reported that polyphenolic acids, especially those having more hydrophilic property such as p-coumaric acid released properly from PLA structure into the food (Mascheroni et al. 2010).
Table 3.
PC of dry meat sausage samples packed by PLA based films
| Film sample | PC (mggallic acid/gsausage sample) | ||
|---|---|---|---|
| Day 0 | Day 2 | Day 4 | |
| 1 | 0.904 ± 0.002H,I | 1.195 ± 0.005D,E,F,G* | 1.356 ± 0.001C,D |
| 2 | 1.240 ± 0.04D,E | 1.555 ± 0.002B | |
| 3 | 1.251 ± 0.25D,E | 1.726 ± 0.001A | |
| 4 | 1.055 ± 0.015F,G,H | 1.280 ± 0.02D,E | |
| 5 | 1.149 ± 0.001E,F,G | 1.518 ± 0.002B,C | |
| 6 | 1.204 ± 0.001D,E,F | 1.660 ± 0.003A,B | |
| 7 | 1.044 ± 0.001F,G,H | 1.039 ± 0.001G,H | |
| 8 | 0.820 ± 0.01I | 0.640 ± 0.02J | |
*Means within a column that do not share a letter are significantly different
Antioxidant content and rate of release from film network are principal factors that lead to a film performance. They in turn are influenced by antioxidant agent and polymer attributes as well as method of incorporation of antioxidant agent into the structure of polymer. It has been demonstrated that active paper sheets produced by propolis surface spreading raised the amount of phenolic content and DPPH-assay of packed cooked ham slices, and also changed the sensory characteristics of samples after 4 day storage. Propolis incorporated in films did not affect the PC, DPPH-assay and organoleptic properties of cooked ham (Rizzolo et al. 2016).
According to action, Antioxidants are classified as primary, secondary and multifunctional based on their mechanism of function. Primary antioxidants are chain-breaker or free-radical scavengers. They react with radicals (e.g. lipid radicals) and convert them to stable non-radical molecules hindering their ability to initiate and propagate reactions. Metal chelates, oxygen scavengers, UV absorbers and single oxygen quenchers are secondary or preventive antioxidants. They can reduce rate of oxidation or inhibit their occurrences (Vilela et al. 2018). Phenolic compounds—particularly those which have more than one hydroxyl group—are multifunctional and act as free radical scavengers by transferring hydrogen atoms to free radicals and prevent lipid oxidation. For example, it has been shown that PC and antioxidant activity of chitosan films correlated fairly well with its content of PE (Siripatrawan and Vitchayakitti 2016). It was suggested that polyphenols in chitosan/PE film possessed scavenging function and could prevent lipid oxidation in many food products.
Concentration of PE and addition of PEG/CaCo3 presented no meaningful difference (P value > 0.05) in the amount of PC, at day 2 (Table 3). But at day 4, the PC of sausage samples further increased while more PE was incorporated into PLA films. However, modified active PLA films (containing PEG/CaCO3) could not statistically improve the antioxidant effect of active polymers (P value > 0.05). PC of sausage samples was essentially constant in inactive PLA film modified by PEG/CaCO3. It means that molecular network of PLA/PEG/CaCO3 film did not allow oxidizing agents to diffuse into the packaging atmosphere and react with phenolic and other reducing and antioxidant compounds during storage time. Moreover, PLA/PEG/CaCO3 polymer was a barrier against volatile phenolic constituents, hence making PC of samples remain constant. Comparing PC at day 0 with that of day 2 and 4 for PLA/control film showed that PC decreased steadily in this period. This may be noticed as a proper feature of modified PLA to be used in food packaging.
Mechanical properties of PLA-based films
Some important mechanical properties of polymers, are TS, %EB and EM. Table 4 presents measurements of properties of PLA based films.
Table 4.
TS, EB and EM of PLA based films
| Film sample | TS (MPa) | EB (%) | EM (GPa) |
|---|---|---|---|
| 1 | 18.74 ± 0.82d* | 30.30 ± 0.52c | 1.91 ± 0.04e |
| 2 | 17.86 ± 0.11d | 19.21 ± 1.23d,e | 2.31 ± 0.02c |
| 3 | 17.62 ± 0.44d | 20.49 ± 0.64d | 2.75 ± 0.03a |
| 4 | 20.81 ± 0.28c | 15.82 ± 1.22f | 2.10 ± 0.04d |
| 5 | 21.45 ± 0.5b,c | 12.90 ± 0.8g | 1.65 ± 0.03f |
| 6 | 22.58 ± 0.34b | 18.13 ± 0.29e | 1.66 ± 0.03f |
| 7 | 18.11 ± 0.17d | 42.22 ± 1.11a | 1.64 ± 0.02f |
| 8 | 27.28 ± 0.79a | 34.98 ± 0.97b | 2.37 ± 0.02b |
*a–dDifferent lowercase letters within a column indicate significant differences (P value < 0.05)
Tensile strength of control PLA film was measured to be 27.28 ± 0.79 MPa. That was the highest amount (Table 4). Incorporation of PEG and CaCO3 caused a serious fall (P value < 0.05) in TS value (18.11 ± 0.1 MPa). A similar trend was observed by adding PE to the film. Moreover, EM dropped sharply from 2.37 ± 0.02 GPa to 1.64 ± 0.02 GPa (P value < 0.05) when PEG/CaCO3 was added.
Properties of a polymer are under influence of many parameters such as degree of crystallinity, production procedure, chemical composition, steric features including L/D ratio (enantiomers of lactic acid), additives and in particular plasticizers, blending other materials, copolymerization, etc. (Farah et al. 2016).
These findings suggest that addition of PEG, CaCO3 or PE destroyed the crystalline structure of PLA film and weakened the intermolecular interactions within its matrix. Thus, their resistance to fracture diminished. High degree of crystallinity makes a more compact polymer and restricts the chains mobility, therefore leading to higher tensile strength and elastic modulus (Middleton and Tipton 2000).
There was no significant difference (P value > 0.05) between TS values of PLA/PEG/CaCO3 film without PE and PLA/PEG/CaCO3 films with various concentration of PE. But the TS value almost increased 10% when concentration of PE changed from 10 to 40% in PLA/PE films. This suggested that if more PE is added, the interaction between PLA and PE molecules boosts. Also, EM values for PLA polymers containing PEG/CaCO3 were enhanced by adding PE and a positive correlation (P value < 0.05) was revealed between amount of PE and EM. Possibly, there were effective crosslinks between PEG, PLA and PE which reduced the discontinuity of free volume of polymer matrix and subsequently enhanced stiffness. PLA/PEG/CaCO3/PE 40% was described as the stiffest film with EM of 2.75 ± 0.03 GPa.
Films possessing PEG, had higher EB. Besides improving biodegradability of PLA by blending with PEG, this high molecular weight plasticizer is miscible and compatible with PLA, making it more flexible and increasing its EB (Farah et al. 2016). It is worth noting that there is an optimum concentration (10–20% w/w) of plasticizer to improve EB of polymers and not to have negative effects on other mechanical attributes. Previously, it has been shown that elastic modulus and stress at break of PLA film decreased when PEG was used as plasticizer, but EB increased. Similar observations also happened when other kinds of plasticizers were blended (Farah et al. 2016).
Addition of PE considerably decreased (P value < 0.05) the EB of films. Moreover, no meaningful trends were observed between the amount of PE in films and the values of EB. These data are in contradiction with some reported data that PE acted as plasticizer and improved the flexibility of polymers and subsequently increased EB, while decreasing TS (Rezaeigolestani et al. 2017). Initial TS, EM and EB of PLA in this work have been reported as of 16.1 ± 0.41 MPa, 1.00 ± 0.09 GPa and 49.3 ± 1.2%, respectively. Upon addition of PE, TS, EM and EB values have been reported to change as 14.3 ± 0.27 MPa, 0.82 ± 0.08 GPa and 54.3 ± 1.2%, respectively.
In broad terms, PLA is characterized as a brittle polymer with poor toughness. Its low elongation at break limits its application in some fields. However, tensile strength and elastic modulus of PLA are favorable and comparable to poly ethylene terephthalate (PET) (Farah et al. 2016). Many studies have been conducted to modify properties of PLA to suit it for packaging. For example, using some modifiers such as citrate esters and polyglycerol esters or blending it with polyvinyl acetate can improve its EB while copolymerization of PLA with polyvinyl chloride improves strength and toughness (Farah et al. 2016).
Conclusion
Regarding the result of this study, it is suggested that an effective PLA-based active film could be developed using PE as a bioactive agent. PE showed a strong antimicrobial activity against both gram-positive (S. aureus) and gram-negative (P. aeruginosa) bacteria, while its inhibitory effect against S. aureus was more effective. At least 20% PLA/PE has to be added to PLA/PE film to limit the growth of S. aureus. However, almost all active PLA/PE films presented antioxidant effect on dry meat sausage. Experiments showed that incorporating PEG/CaCO3 into PLA/PE films, remarkably improved the antimicrobial activity of films, enhanced flexibility and stiffness of polymers, and reduced their tensile strength. Though low, TS was still acceptable and comparable to conventional polymers in food packaging industry. Therefore, it seems that PLA/PE film provides a possible active packaging that could prolong meat products shelf life. This study may work as a basis for further studies to optimize the amount of PE and PEG/CaCO3 in PLA and develop an active PLA-based film which could be applied to meat products packaging.
Footnotes
Abs770: Absorbance at 770 nm.
Assayed via 2,2-diphenyl-1-picrylhydrazyl, DPPH.
Assayed via ferric reducing antioxidant power, FRAP.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahn MR, Kumazawa S, Hamasaka T, Bang KS, Nakayama T. Antioxidant activity and constituents of propolis collected in various areas of Korea. J Agric Food Chem. 2004;52(24):7286–7292. doi: 10.1021/jf048726s. [DOI] [PubMed] [Google Scholar]
- Azizkhani M, Misaghi A, Basti AA, Gandomi H, Hosseini H. Effects of Zataria multiflora Boiss. essential oil on growth and gene expression of enterotoxins A, C and E in Staphylococcus aureus ATCC 29213. Int J Food Microbiol. 2013;163(2–3):159–165. doi: 10.1016/j.ijfoodmicro.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Bodini RB, Sobral PJA, Favaro-Trindade CS, Carvalho RA, et al. Properties of gelatin-based films with added ethanol-propolis extract. LWT Food Sci Technol. 2013;51(1):104–110. doi: 10.1016/j.lwt.2012.10.013. [DOI] [Google Scholar]
- Choi I, Lee SE, Chang Y, Lacroix M, Han J. Effect of oxidized phenolic compounds on cross-linking and properties of biodegradable active packaging film composed of turmeric and gelatin. Lwt Food Sci Technol. 2018;93:427–433. doi: 10.1016/j.lwt.2018.03.065. [DOI] [Google Scholar]
- Correa FT, de Souza AC, de Souza Júnior EA, Isidoro SR, Piccoli RH, Dias DR, de Abreu LR. Effect of Brazilian green propolis on microorganism contaminants of surface of gorgonzola-type cheese. J Food Sci Technol. 2019;56(4):1978–1987. doi: 10.1007/s13197-019-03664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Dembogurski DS, Trentin DS, Boaretto AG, Rigo GV, da Silva RC, Tasca T, Macedo AJ, Carollo CA, Silva DB. Brown propolis-metabolomic innovative approach to determine compounds capable of killing Staphylococcus aureus biofilm and Trichomonas vaginalis. Food Res Int. 2018;111:661–673. doi: 10.1016/j.foodres.2018.05.033. [DOI] [PubMed] [Google Scholar]
- Dini I. Essential oils in food preservation, flavor and safety. Use of essential oils in food packaging. Amsterdam: Elsevier Inc.; 2015. [Google Scholar]
- Domnguez R, Barba FJ, Gómez B, Putnik P, Kovačević DB, Pateiro M, Santos EM, Lorenzo JM. Active packaging films with natural antioxidants to be used in meat industry: a review. Food Res Int. 2018;113:93–101. doi: 10.1016/j.foodres.2018.06.073. [DOI] [PubMed] [Google Scholar]
- Ennajar M, Bouajila J, Lebrihi A, Mathieu F, Abderraba M, Raies A, Romdhane M. Chemical composition and antimicrobial and antioxidant activities of essential oils and various extracts of Juniperus phoenicea l. (Cupressacees) J Food Sci. 2009;74(7):364–371. doi: 10.1111/j.1750-3841.2009.01277.x. [DOI] [PubMed] [Google Scholar]
- Fang Z, Zhao Y, Warner RD, Johnson SK. Active and intelligent packaging in meat industry. Trends Food Sci Technol. 2017;61(2):60–71. doi: 10.1016/j.tifs.2017.01.002. [DOI] [Google Scholar]
- Farah S, Anderson DG, Langer R. Physical and mechanical properties of PLA, and their functions in widespread applications: a comprehensive review. Adv Drug Deliv Rev. 2016;107:367–392. doi: 10.1016/j.addr.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Gyawali R, Ibrahim SA. Natural products as antimicrobial agents. Food Control. 2014;46:412–429. doi: 10.1016/j.foodcont.2014.05.047. [DOI] [Google Scholar]
- Holm VK, Ndoni S, Risbo J. The stability of poly(lactic acid) packaging films as influenced by humidity and temperature. J Food Sci. 2006;71(2):40–44. doi: 10.1111/j.1365-2621.2006.tb08895.x. [DOI] [Google Scholar]
- Irkin R, Esmer OK. Novel food packaging systems with natural antimicrobial agents. J Food Sci Technol. 2015;52(10):6095–6111. doi: 10.1007/s13197-015-1780-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidian M, Desobry S, Imran M, Jacquot M, Tehrany E. Poly-lactic acid: production, applications, nanocomposites, and release studies. Compr Rev Food Sci Food Saf. 2010;9(5):552–571. doi: 10.1111/j.1541-4337.2010.00126.x. [DOI] [PubMed] [Google Scholar]
- Jin T, Zhang H. Biodegradable polylactic acid polymer with nisin for use in antimicrobial food packaging. J Food Sci. 2008;73(3):127–134. doi: 10.1111/j.1750-3841.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- Juliano C, Pala CL, Cossu M. Preparation and characterisation of polymeric films containing propolis. J Drug Deliv Sci Technol. 2007;17(3):177–181. doi: 10.1016/S1773-2247(07)50033-X. [DOI] [Google Scholar]
- Kalogeropoulos N, Konteles SJ, Troullidou E, Mourtzinos I, Karathanos VT. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem. 2009;116(2):452–461. doi: 10.1016/j.foodchem.2009.02.060. [DOI] [Google Scholar]
- Lasprilla AJR, Martinez GA, Lunelli BH, Jardini AL. Poly-lactic acid synthesis for application in biomedical devices: a review. Biotechnol Adv. 2012;30(1):321–328. doi: 10.1016/j.biotechadv.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Lim LT, Auras R, Rubino M. Processing technologies for poly(lactic acid) Progr Polym Sci (Oxford) 2008;33(8):820–852. doi: 10.1016/j.progpolymsci.2008.05.004. [DOI] [Google Scholar]
- Liu LS, Finkenstadt VL, Liu CK, Jin T, Fishman ML, Hicks KB. Preparation of poly(lactic acid) and pectin composite films intended for applications in antimicrobial packaging. J Appl Polym Sci. 2007;106(2):801–810. doi: 10.1002/app.26590. [DOI] [Google Scholar]
- Mascheroni E, Guillard V, Nalin F, Mora L, Piergiovanni L. Diffusivity of propolis compounds in polylactic acid polymer for the development of anti-microbial packaging films. J Food Eng. 2010;98(3):294–301. doi: 10.1016/j.jfoodeng.2009.12.028. [DOI] [Google Scholar]
- Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21(23):2335–2346. doi: 10.1016/S0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- Nazzaro F, Fratianni F, Martino LD, Coppola R, Feo VD. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6(12):1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi PS. Plant extracts for the control of bacterial growth: efficacy, stability and safety issues for food application. Int J Food Microbiol. 2012;156(1):7–17. doi: 10.1016/j.ijfoodmicro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Nobile MAD, Conteab A, Buonocorec GG, Incoronatob AL, Massaroc A, Panza O. Active packaging by extrusion processing of recyclable and biodegradable polymers. J Food Eng. 2009;93(1):1–6. doi: 10.1016/j.jfoodeng.2008.12.022. [DOI] [Google Scholar]
- Rashidinejad A, Birch EJ, Sun-Waterhouse D, Everett DW. Effects of catechin on the phenolic content and antioxidant properties of low-fat cheese. Int J Food Sci Technol. 2013;48(12):2448–2455. doi: 10.1111/ijfs.12234. [DOI] [Google Scholar]
- Rezaeigolestani M, Misaghi A, Khanjari A, Basti AA, Abdulkhani A, Fayazfar S. Antimicrobial evaluation of novel poly-lactic acid based nanocomposites incorporated with bioactive compounds in-vitro and in refrigerated vacuum-packed cooked sausages. Int J Food Microbiol. 2017;260:1–10. doi: 10.1016/j.ijfoodmicro.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Rizzolo A, Bianchi G, Povolo M, Migliori CA, Contarini G, Pelizzola V, Cattaneo TM. Volatile compound composition and antioxidant activity of cooked ham slices packed in propolis-based active packaging. Food Packag Shelf Life. 2016;8:41–49. doi: 10.1016/j.fpsl.2016.03.002. [DOI] [Google Scholar]
- Sanyang ML, Sapuan SM. Development of expert system for biobased polymer material selection: food packaging application. J Food Sci Technol. 2015;52(10):6445–6454. doi: 10.1007/s13197-015-1759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavisi N, Khanjari A, Basti AA, Misaghi A, Shahbazi Y. Effect of PLA films containing propolis ethanolic extract, cellulose nanoparticle and Ziziphora clinopodioides essential oil on chemical, microbial and sensory properties of minced beef. Meat Sci. 2017;124:95–104. doi: 10.1016/j.meatsci.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Siripatrawan U, Vitchayakitti W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016;61:695–702. doi: 10.1016/j.foodhyd.2016.06.001. [DOI] [Google Scholar]
- Torlak E, Sert D. Antibacterial effectiveness of chitosan–propolis coated polypropylene films against foodborne pathogens. Int J Biol Macromol. 2013;60:52–55. doi: 10.1016/j.ijbiomac.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Turgis M, Han J, Borsa J, Lacroix M. Combined effect of natural essential oils, modified atmosphere packaging, and gamma radiation on the microbial growth on ground beef. J Food Prot. 2008;71(6):1237–1243. doi: 10.4315/0362-028X-71.6.1237. [DOI] [PubMed] [Google Scholar]
- Vilela C, Kurek M, Hayouka Z, Röcker B, Yildirim S, Antunes MDC, Nilsen- Nygaard J, Pettersen MK, Freire CS. A concise guide to active agents for active food packaging. Trends Food Sci Technol. 2018;80:212–222. doi: 10.1016/j.tifs.2018.08.006. [DOI] [Google Scholar]
- Wojdyo A, Oszmiaski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105(3):940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]