Abstract
Abstract
The variety of products derived from milk, without or with lactose, encourages the development of more effective analytical techniques that can be applied to the quality control of both the production line and the final products. Thus, in this work an efficient and minimally invasive method for the detection of lactose was proposed, using a biosensor containing the enzyme lactase (LAC) immobilised on carbon nanotubes (CNTs) that, when reacting with lactose, emit an electrochemical signal. This biosensor was connected to a potentiostat, and its electrochemical cell was composed of the following three electrodes: reference electrode (Ag/AgCl), auxiliary electrode (platinum wire), and working electrode (biosensor) on which graphite (carbon) paste (CP), CNTs, and LAC were deposited. The transmission electron microscopy and scanning electron microscopy were used in the characterisation of the composite morphology, indicating excellent interactions between the CNTs and LAC. The sensitivity of the CP/LAC/CNT biosensor was determined as 5.67 μA cm−2.mmol−1 L and detection limits around 100 × 10−6 mol L−1 (electrode area = 0.12 cm2) and an increase in the stability of the system was observed with the introduction of CNTs because, with about 12 h of use, there was no variation in the signal (current). The results indicate that the association between the CNTs and LAC favoured the electrochemical system.
Graphic Abstract
Keywords: Electrochemistry, Nanomaterial, Lactosil®, Voltammetry, Image analysis, Biosensor
Introduction
Individuals that are lactose-intolerant avoid the intake of milk and its derivatives due to the negative side effects to their health (Troise et al. 2016), effects caused by the deficiency or absence of the intestinal lactase enzyme (β-galactosidase), which is responsible for the hydrolysis of disaccharide lactose, the major compound in dairy products, glucose, and galactose (Ruiz-Matute et al. 2012; Monti et al. 2017; Traffano-Schiffo et al. 2017).
However, due to the nutritional value of these products, there is significant demand for products with or without lactose, which has led the dairy industry to invest in the development and commercial production of products in this promising consumer market (Troise et al. 2016; Torres et al. 2017).
It is necessary to certify the efficiency of the hydrolysis process in these products. Currently, these analyses are performed by classical (titration) or instrumental techniques, such as high-performance liquid chromatography (HPLC) (Scheppingen et al. 2017), gas chromatography (GC) (Sharma and Leblanc 2017), infrared spectroscopy, and spectrophotometry (Junior et al. 2016; Monti et al. 2017; Trani et al. 2017). These methodologies present some laboratory limitations related to the high cost of instrumentation, long analysis time, high reagent consumption, and skilled labour, factors that make daily monitoring difficult (Erich et al. 2012).
To overcome these disadvantages, it is important to research and develop new lactose quantification methodologies, and electrochemical biosensors represent a promising and attractive tool compared to current techniques due to their simplicity, fast response, continuous monitoring, and ease of handling. Among the electrochemical biosensors, amperometric detection is a widely used method due to its sensitivity and selectivity (Thakur and Ragavan 2013; Mortari and Lorenzelli 2014; Khan et al. 2016).
In addition to traditional materials, such as conductive polymers (Malhotra et al. 2006) and modified silicas (Jesionowski et al. 2013), used in the development of amperometric biosensors, nanomaterials have the capacity to potentiate the surface of transduction of the sensors, which in turn increases the catalytic behaviour due to their excellent optical, electronic, and magnetic properties (Zeng et al. 2016; Zhang and Wei 2016; Lin et al. 2018).
Nanomaterials can be classified into groups, such as metals, metal oxides, and organic materials [especially those based on carbon, such as fullerenes, carbon nanotubes (CNTs), and graphene], according to their chemical composition in inorganic materials. (Barberis et al. 2015; Kurbanoglua et al. 2017).
The CNTs are potential material for the development of a new generation of bioelectronic devices with high sensitivity and stability, working in association with other materials. Yet, they are still able to improve electron transfer between the enzymes and electrode surfaces, acting as electron mediators, while supporting the immobilisation of the enzyme (Ansari et al. 2013; Santos et al. 2015; Garrido et al. 2016; Gupta et al. 2017). As examples of amperometric biosensors for food quality control, there is the CNT, tyrosinase, and LAC electrode developed by Vlamidis et al. (2017) used to determine catechol and other polyphenols in fruit juices, as well as the glucose oxidase, CNT, and graphene oxide electrode developed by Palanisamy et al. (2014) to detect glucose oxidation.
Thus, the objective of this work was to develop an amperometric biosensor based on carbon paste (CP) modified with CNTs for the detection of lactose.
Materials and methods
Materials
The electrochemical measurements were obtained on a micro-autolab III model potentiostat (Metrohm Pensalab) with the use of an electrochemical cell composed of the following three electrodes: Ag/AgCl reference electrode, platinum wire auxiliary electrode, and working electrode containing the immobilised LAC. Cyclic voltammetry was performed with the General Purpose Electrochemical System (GPES), and the resulting data were treated with OriginPro v.8 software. A pH meter (3305, Jenway) and an analytical balance (AUW220D, Shimadzu) were also used.
Chemically modified electrodes based on carbon paste
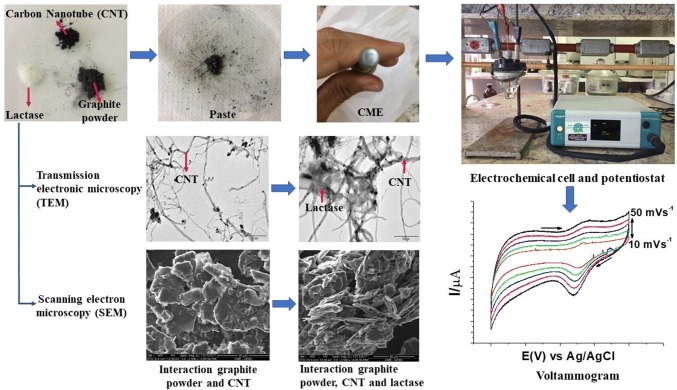
The working electrode was developed at the Electrochemical Laboratory Center (Santa Cruz State University, Ilhéus, Bahia, Brazil) and it was composed of a copper wire covered with a glass tube connected to the base of a gold disk, forming a lower cavity, 4.0 mm in inner diameter with 1.5 mm depth, for the insertion of the samples.
The commercial LAC-based preparation (Lactosil 4000 FCC ALU, from Aspergillus oryzae) was acquired from local pharmacies, and the Institute for Space Research (São José dos Campos, São Paulo, Brazil) kindly donated the multi-walled carbon nanotubes (MWCNTs). Mineral oil (Schering-Plough, 100% purity), graphite powder (Synth, 99% purity), lactose monohydrate P.A. (Synth, 99% purity), and other reagents, all analytical grade, were purchased from local suppliers.
Voltammetric experiments were performed in a range of − 0.2 to 0.6 V redox potential, varying the electrolyte, potential scanning speed, electrolyte concentration, and solution pH. Three different sensors were investigated:
(1) CP/LAC: 30.0 mg of graphite powder was mixed with 20 μL of mineral oil, forming a CP, and 1.5 mg of the LAC-based enzymatic preparation (LAC) was added and homogenised. (2) CP/CNT: the same CP described above was obtained and 2.7 mg of CNTs was added and homogenised. (3) CP/LAC/CNT: the same CP described above was obtained and 2.7 mg of CNTs and 1.5 mg of LAC was added and homogenised. The samples were placed in the lower cavity of the working electrode, being careful not to leave cause deformation on the surface of the electrode.
Electrochemical characterisation of the electrodes
Transmission electronic microscopy
Transmission electron microscopy (TEM) analysis was performed at the Electronic Microscopy Center (Santa Cruz State University, Ilhéus, Bahia, Brazil). The CNT/LAC samples were diluted in pure alcohol and then were dripped onto a 3-mm diameter copper grid, after evaporation of the alcohol, analysis was performed on a transmission electron microscope model Morgani 268D (FEI Company).
Scanning electron microscopy
Scanning electron microscopy (SEM) analysis was performed at the Electronic Microscopy Center (Santa Cruz State University, Ilheus, Bahia, Brazil) using a scanning electron microscope (SEM) (QUANTA 250). The CP/CNT and CP/LAC/CNT samples were fixed to a “stub” support, and the reading was performed in ambient mode, which is used to investigate samples that are incompatible with high vacuum and, therefore, difficult to analyse by other traditional SEM methods.
Supporting electrolyte and pH
The responses of the CP/LAC/CNT biosensor as a function of the supporting electrolyte were analysed using the following solutions (0.3 mol L−1, pH 6.5): potassium chloride, sodium phosphate, sodium acetate, Tris–HCl, and PBC (phosphate, bohrium, and citric acid). For each supporting electrolyte analysed, the anodic (IPa, µA) and cathodic (IPc, µA) peak currents, the IPa/IPc ratio, the peak separation (ΔE, V), and the formal potential (E°, V) were obtained. With the best supporting electrolyte chosen (PBC buffer, 0.3 mol L−1), the effect of pH on the sensor was evaluated in the pH range of 3.0 to 9.0.
Stability and calibration curve of the electrodes
The stabilities of the CP/LAC and CP/LAC/CNT working electrodes were analysed with the PBC buffer supporting electrolyte (0.3 mol L−1, pH 6.5), applying a scanning speed of 20 mV s−1 in a total of 360 cycles, equivalent to 12 working hours.
For construction of the calibration curve, the chemically modified electrode (CME) CP/LAC/CNT and a solution of lactose (0.3 mg mL−1) were used. Measurements were performed nine times, from 0 to 2000 μL, by adding 250 μL of the lactose solution eight times. Buffer solutions, instead of the lactose solution, were used as the blank solutions.
Results and discussion
Electrode development
Preliminary voltammetric experiments, which were performed with a redox potential of − 0.2 to 0.6 V in a solution of KCl (0.3 mol L−1, pH 6.5), were performed in order to investigate the electrochemical behaviour of the developed electrodes. According to the data obtained (Fig. 1), all sensors (CP/LAC, CP/CNT, and CP/LAC/CNT) presented electrochemical activity on the surface of the CP (blank condition). For CP/CNT and CP/LAC/CNT, a clear current gain and a well-defined redox pair were observed. CP/LAC presented a relatively low current gain, without a redox pair, which proves that LAC immobilisation on the surface of the CNTs (the CP/LAC/CNT sensor) improved the system. This system operates at low energy (E0 = 0.33 V) and with low peak separation (ΔE = 0.1 V), which are important characteristics for the good performance of an electrochemical biosensor. Thus, this behaviour can be related to the use of CNTs associated with the enzyme, acting as an electron mediator, increasing the electron transfer rate on the surface of the electrode and decreasing the energy for the operation of the electrochemical cell (Lawal 2016).
Fig. 1.

Cyclic voltammogram of the sensors. a Carbon paste (CP); b carbon paste and lactase (CP/LAC); c carbon paste and carbon nanotubes (CP/CNTs); and d carbon paste, lactase, and carbon nanotubes (CP/LAC/CNTs). The experiments were conducted in a KCl solution (0.3 mol L−1) with a scanning velocity of 30 mV s−1
The pair of redox peaks found in voltammogram “Fig. 1c” for CP/CNT are due to the oxidation/reduction of trace ferrocene incorporated into the MWCNTs, which was used as a catalyst for the preparation of the MWCNTs. It is noteworthy that no other post-treatment process was used to remove the metal catalyst in the CNTs because did not introduce oxidatives functional groups. In addition, the aim of this study was to investigate the interaction of the enzyme with carbon (Almeida et al. 2008; Edwards et al. 2011).
Similar behaviour was observed by Ansari et al. (2013) when immobilising β-galactosidase in CNTs. Due to the displacement of oxy-reduction potentials and the increase in the currents, improvements in the electrocatalytic and conductive characteristics of the system, as well as the reversibility of the system, were obtained. In another example, Romero-Arcosa et al. (2017) immobilised LAC in CNTs and observed an increase in electron transfer and a reduction in the resistance to transfer charges between the of the enzyme and the electrode.
The CP/LAC/CNT biosensor was also subjected to different potential scanning velocities, and it was observed (Fig. 2) that with an increase in the velocities there was an increase in the cathodic and anodic currents, with clear peak separation, operating at low formal potentials (demonstrating that a redox reaction occurred and that the system had good reversibility). The anodic and cathodic peak currents were linearly proportional to the scanning velocities (from 10 to 50 mV s−1), as presented in Fig. 2. Therefore, the behaviour of the biosensor was governed by the adsorption of the enzyme on the surface of the electrode since no other component was added to the system.
Fig. 2.

Cyclic voltammograms of the CP/LAC/CNT sensor at different scanning velocities in a KCl electrolyte (0.3 mol L−1). The graph for the anodic and cathodic peak currents as a function of the scan velocity (mV s−1) is presented
Electrochemical characterisation of the electrodes
Image analysis
Through the transmission electronic microscopy (TEM), it was possible to observe (Fig. 3a) the elongated structure of the CNTs, with some darker points corresponding to the iron contained in interiors. When adding LAC to the system (Fig. 3b), it was possible to visualise the change in the CNT morphology, with darker areas corresponding to the interaction between the two components. Similar results were observed for Khan et al. (2017) in CNT images with cobalt atoms and with the interaction of CNTs with β-galactosidase (LAC), immobilised by adsorption.
Fig. 3.
Transmission electron microscopy (TEM) images for a CNTs and b the interaction between carbon nanotubes and lactase (CNTs/LAC). Scanning electron microscopy (SEM) images for c carbon paste and carbon nanotubes (CP/CNTs) and D) the interaction between carbon paste, carbon nanotubes, and lactase (CP/LAC/CNTs)
When analysing the proposed system by scanning electron microscopy (SEM) it was possible to observe (Fig. 3c) that the mixture of the carbon paste (CP) with the CNTs results in a presence at laminar structure, with larger, irregularly shaped particles. With the addition of LAC, it was noted (Fig. 3d) that the laminar structure is more defragmented with smaller aggregates, confirming the successful process of immobilisation of lactase by adsorption (which is a simple method, without the use of chemicals). Similar images were obtained in work by Khan et al. (2017).
Selection of the supporting electrolyte and the pH
The supporting electrolyte must be able to provide electroneutrality to the system, minimising the generation of capacitive current and providing the high conductivity of the solution. Thus, KCl, sodium phosphate, sodium acetate, Tris–HCl, and PBC electrolytes were investigated, and the voltammetric behaviour of the biosensor in each situation can be observed in the voltammogram presented in Fig. 4. The results indicated that, for this system, any of the supporting electrolytes (at 0.3 mol L−1 and with a scanning velocity of 30 mV s−1) could be used for the electrochemical measurements. Data extracted from this voltammogram (Fig. 4) are presented in Table 1, showing that the system was more favourable for the KCl solution (E0 = 0.31 V, ΔE = 0.11 V) and PBC buffer (E0 = 0.22 V, ΔE = 0.34 V). In these two cases, there was significant current gain (ΔE > 0), indicating better transfer of electrons. In addition, both presented the best responses, with the evident presence of anodic and cathodic peaks (Fig. 4). In general, the system responded well for all supporting electrolytes obeerved for the formal potential (Eo) and for the separation of peaks (∆E).
Fig. 4.
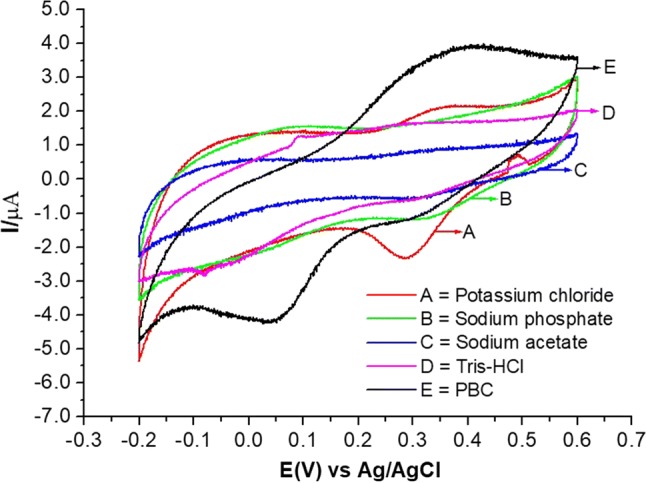
Voltammogram of the modified carbon paste biosensor with lactase and carbon nanotubes (CP/LAC/CNTs) in different electrolytes with a scanning speed of 30 mV s−1
Table 1.
Data extracted from the voltammetric behaviour (Fig. 4) of the modified carbon paste sensor with lactase and carbon nanotube (CP/LAC/CNT) as a function of the variation of the supporting electrolyte solution
| Eletrólito suporte | IPa (µA) | IPc (µA) | ΔE (v) | E° (V) | IPa/IPc |
|---|---|---|---|---|---|
| KCl | 2.20 | − 2.32 | 0.11 | 0.31 | 0.92 |
| Sodium phosphate | 1.68 | − 1.14 | 0.08 | 0.32 | 1.48 |
| Sodium acetate | 0.84 | − 0.50 | 0.05 | 0.32 | 1.67 |
| Tris–HCl | 1.62 | − 0.61 | 0.01 | 0.28 | 2.64 |
| PBC | 3.87 | − 4.17 | 0.34 | 0.22 | 0.93 |
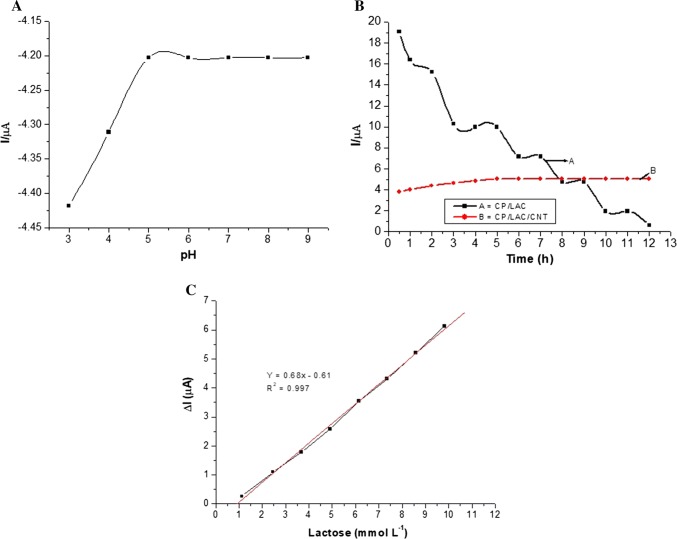
Another important factor is the pH of the selected supporting electrolyte (PBC buffer) since the pH can strongly affect the ionisable groups in an enzyme. Hence, the electrocatalytic response of the biosensor is influenced by the variation in pH. Figure 5a presents the results obtained for different pH values, and it can be observed that the response of the sensor (current) and its potential remained almost constant between pH 5 and 9. In this pH range, the formal potential remained small (E0 = 0.2–0.3 V). This behaviour suggests good stability for the CP/LAC/CNT sensor in a wide pH range. According to Kim et al. (2006), the CP has exerting protection to the variation of the pH, and increases system stability regardless of the eletrochemical environment.
Fig. 5.
a Response of the modified carbon paste sensor with lactase and carbon nanotubes (CP/LAC/CNTs) as a function of the pH of the supporting electrolyte (PBC buffer, 0.3 mol L−1 mol L−1), with a scanning velocity of 30 mV s−1. b The experiments were conducted with a KCl solution (0.3 mol L−1) and a scanning velocity of 20 mV s−1. c Calibration curve for lactose using the carbon paste biosensor modified with the lactose and carbon nanotube (CP/LAC/CNT) matrix
Stability and sensitivity studies
This was achieved by applying several voltammetric cycles with and without the CNTs. From the data in Fig. 5b, stability of the CP/LAC/CNT sensor was observed after 12 h of work (more than 360 cycles), indicating chemical and electrochemical stability as a result of the addition of CNTs. For the CP/LAC system, more pronounced instability (Fig. 5b) was observed, with an accentuated variation in the current.
It is worth mentioning that the adsorption of LAC on the CP/CNT matrix is a simple technique, capable of providing excellent improvements to the enzymatic stability, without the necessity of promoting covalent links (commonly cited in literature) which requires more chemical components and longer immobilisation times. The 12-h stability presented in this work is greater than that reported by Ansari and co-workers (2013), for example, who modified the CNT matrix with glutaraldehyde for β-galactosidase immobilisation, obtaining a stability of 6 h. Varmira et al. (2018) obtained a stability of 3 h, which is also smaller than the time obtained in our work, for a bimetallic alloy of Pd/Pt, chitosan, graphene, and CNTs for the immobilisation of tyrosine hydroxylase (another food-related enzyme). Jedrzak et al. (2018) which was considered good performance for a CP biosensor modified with silica/lignin used for glucose determination, even though it was much lower than that presented in this work (360 cycles).
In relation to the sensitivity, which is an important factor for any analytical measurement since it concerns the variation of the response signal in relation to the variation of the analyte concentration, Fig. 5c shows the calibration curve obtained for the detection of lactose. Based on this data, the sensitivity of the developed biosensor was determined to be 5.67 μA cm−2 mmol−1 L (considering an electrode area of 0.12 cm2), with a correlation coefficient (R2) of 0.997, and detection limits around 100 × 10−6 mol L−1 lactose could be estimated considering 3σB. Thus, the electrode presented a quick as the lactose was added to the supporting electrolyte.
A variety of sensitivities are reported in the literature. Antiochia and Gorton (2007), for example, developed a biosensor for the determination of glucose in sweet wines, with glucose dehydrogenase immobilised on CNTs, mediated by the osmium polymer, obtaining a sensitivity of 13.4 μA cm−2 μmol−1 L and a detection limit of 10 × 10−6 mol L−1. In another example, Santos et al. (2015) immobilised horseradish peroxidase (HRP) in CNTs, fullerene, and hydroxylated fullerene and obtained sensitivities for phenol of 33, 21, and 16.3 μA cm−2 μmol−1 L, respectively, and a detection limit of 5–200 × 10−6 mol L−1. In addition, the immobilisation of β-galactosidase on films with poly(ethylene imine) (PEI) polyelectrolyte and poly(vinyl solvent) (PVS) on a modified tin-oxide electrode with a layer of Prussian Blue (PB), developed by Campos et al. (2014), resulted in a sensitivity for lactose of 0.31 µA cm−2 mmol−1 L and a detection limit of 113 × 10−6 mol L−1. Therefore, as can be observed, the use of CNTs provides the ability to accommodate different enzymes, particularly the LAC enzyme evaluated in this work, resulting in good performance as a signal transduction material.
It is important to emphasise that in this work a non-pure enzyme preparation (pharmaceutical commercial product) was used which has ingredients that could interfere in some analyses, hindering the potential of the electrode. However, the enzymatic activity in this preparation presented excellent results for the development of the biosensor for lactose determination, with excellent stability and sensitivity.
Conclusion
The biosensor proposed in this work, containing the enzyme lactase adsorbed to carbon paste with carbons nanotubes has presented a detection limit around 100 × 10−6 mol L−1 lactose. The obtained results indicate some advantages in relation to traditional instrumental methods, such as the use of a simpler and cheaper procedure to prepare the biosensor (adsorption of a non-purified enzyme) and shorter analysis times with low detection limits and high operational stability. The results presented in this work may encourage the development of more affordable biosensors applicable to food industries.
Acknowledgements
The authors would like to thank the important financial support of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), National Council for Scientific and Technological Development (Bolsas de Produtividade em Pesquisa 302259/2018-0, CNPq, Brazil), Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB, Brazil), the State University of Southwest Bahia (UESB, Itapetinga, Bahia Brazil) and the State University of Santa Cruz (UESC, Ilhéus, Bahia, Brazil) for the equipment and facilities.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Almeida EC, Baldan MR, Rosolen JM, Ferreira NG. Impedance characteristics of the diamond/carbon fiber electrodes for electrical double-layer capacitor. Diam Relat Mater. 2008;17:1529–1533. doi: 10.1016/j.diamond.2008.03.006. [DOI] [Google Scholar]
- Ansari SA, Satar R, Chibber S, Khan MJ. Enhanced stability of Kluyveromyces lactis β-galactosidase immobilized on glutaraldehyde modified multiwalled carbon nanotubes. J Mol Catal B Enzym. 2013;97:258–263. doi: 10.1016/j.molcatb.2013.09.008. [DOI] [Google Scholar]
- Antiochia R, Gorton L. Development of a carbon nanotube paste electrode osmium polymer-mediated biosensor for determination of glucose in alcoholic beverages. Biosens Bioelectron. 2007;22:2611–2617. doi: 10.1016/j.bios.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Barberis A, Spissu Y, Fadda A, Azara E, Bazzu G, Marceddu S, Angioni A, Sanna D, Schirra M, Serra PA. Simultaneous amperometric detection of ascorbic acid and antioxidant capacity in orange, blueberry and kiwi juice, by a telemetric system coupled with a fullerene- or nanotubes-modified ascorbate subtractive. Biosens Bioelectron. 2015;67:214–223. doi: 10.1016/j.bios.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Campos PP, Moraes ML, Volpati D, Miranda PB, Oliveira ON, Jr, Ferreira M. Amperometric detection of lactose using β-galactosidase immobilized in layer-by-layer films. ACS Appl Mater Interfaces. 2014;6:11657–11664. doi: 10.1021/am5024463. [DOI] [PubMed] [Google Scholar]
- Edwards R, Antunes EF, Botelho EC, Baldan MR, Corat EJ. Evaluation of residual iron in carbon nanotubes purified by acid treatments. Appl Surf Sci. 2011;258:641–648. doi: 10.1016/j.apsusc.2011.07.032. [DOI] [Google Scholar]
- Erich S, Anzmann T, Fischer L. Quantification of lactose using ion-pair RP-HPLC during enzymatic lactose hydrolysis of skim milk. Food Chem. 2012;135:2393–2396. doi: 10.1016/j.foodchem.2012.07.059. [DOI] [PubMed] [Google Scholar]
- Garrido JMPJ, Rahemi V, Borges F, Brett CMA, Garrido EMPJ. Carbon nanotube b-cyclodextrin modified electrode as enhanced sensing platform for the determination of fungicide pyrimethanil. Food Control. 2016;60:7–11. doi: 10.1016/j.foodcont.2015.07.001. [DOI] [Google Scholar]
- Gupta S, Murthy CN, Prabha CR. Recent advances in carbon nanotube based electrochemical biosensors. Int J Biol Macromol. 2017;108:687–703. doi: 10.1016/j.ijbiomac.2017.12.038. [DOI] [PubMed] [Google Scholar]
- Jedrzak A, Rebis T, Klapiszewski L, Zdarta J, Milczarek G, Jesionowski T. Carbon paste electrode based on functional GOx/silica-lignin system to prepare an amperometric glucose biosensor. Sens Actuators B-Chem. 2018;256:176–185. doi: 10.1016/j.snb.2017.10.079. [DOI] [Google Scholar]
- Jesionowski T, Klapiszewski L, Milczarek G. Kraft lignin and silica as precursors of advanced composite materials and electroactive blends. J Mater Sci. 2013;49:1376–1385. doi: 10.1007/s10853-013-7822-7. [DOI] [Google Scholar]
- Junior PHR, Oliveira KS, Almeida CER, Oliveira LFC, Stephani R, Pinto MS, Carvalho AF, Perrone IT. FT-Raman and chemometric tools for rapid determination of quality parameters in milk powder: classification of samples for the presence of lactose and fraud detection by addition of maltodextrin. Food Chem. 2016;196:584–588. doi: 10.1016/j.foodchem.2015.09.055. [DOI] [PubMed] [Google Scholar]
- Khan AY, Noronha SB, Bandyopadhyaya B. Superior performance of a carbon-paste electrode based glucose biosensor containing glucose oxidase enzyme in mesoporous sílica powder. Adv Powder Technol. 2016;27:85–92. doi: 10.1016/j.apt.2015.11.003. [DOI] [Google Scholar]
- Khan M, Husaim Q, Bushra R. Immobilization of β-galactosidase on surface modifiedcobalt/multiwalled carbon nanotube nanocomposite improves enzyme stability and resistance to inhibitor. Int J Biol Macromol. 2017;105:693–701. doi: 10.1016/j.ijbiomac.2017.07.088. [DOI] [PubMed] [Google Scholar]
- Kim J, Grate JW, Wang P. Nanostructures for enzyme stabilization. Chem Eng Sci. 2006;61:1017–1026. doi: 10.1016/j.ces.2005.05.067. [DOI] [Google Scholar]
- Kurbanoglua S, Ozkan SA, Merkoçia M. Nanomaterials-based enzyme electrochemical biosensors operating through inhibition for biosensing applications. Biosens Bioelectron. 2017;89:886–898. doi: 10.1016/j.bios.2016.09.102. [DOI] [PubMed] [Google Scholar]
- Lawal AT. Synthesis and utilization of carbon nanotubes for fabrication of electrochemical biosensors. Mater Res Bull. 2016;73:308–350. doi: 10.1016/j.materresbull.2015.08.037. [DOI] [Google Scholar]
- Lin H, Li S, Xu C, Pang M, Wang S. Simultaneous determination of galactose, glucose, lactose and galactooligosaccharides in galactooligosaccharides raw materials by highperformance anion-exchange chromatography with pulsed amperometric detection. Food Chem. 2018;263:29–36. doi: 10.1016/j.foodchem.2018.04.092. [DOI] [PubMed] [Google Scholar]
- Malhotra BD, Chaubey A, Singh SP. Prospects of conducting polymers in biosensors. Anal Chim Acta. 2006;578:59–74. doi: 10.1016/j.aca.2006.04.055. [DOI] [PubMed] [Google Scholar]
- Monti L, Negri S, Meucci A, Stroppa A, Galli A, Contarin G. Lactose, galactose and glucose determination in naturally ‘‘lactose free”. Food Chem. 2017;220:18–24. doi: 10.1016/j.foodchem.2016.09.185. [DOI] [PubMed] [Google Scholar]
- Mortari A, Lorenzelli L. Recent sensing technologies for pathogen detection in milk: a review. Biosens Bioelectron. 2014;60:8–21. doi: 10.1016/j.bios.2014.03.063. [DOI] [PubMed] [Google Scholar]
- Palanisamy S, Cheemalapati S, Chen S-M. Amperometric glucose biosensor based on glucose oxidase dispersed in multiwalled carbon nanotubes/graphene oxide hybrid biocomposite. Mater Sci Eng C. 2014;34:207–213. doi: 10.1016/j.msec.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Romero-Arcosa M, Garnica-Romob MG, Martínez-Floresc HE. Characterization of Amperometric laccase biosensor based on carbon nanotube. Process Technol. 2017;27:279–281. [Google Scholar]
- Ruiz-Matute AI, Corzo-Martinez AM, Olano A, Copovi P, Corzo N. Presence of mono-, di- and galactooligosaccharides in commercial lactose-free UHT dairy products. J Food Compos Anal. 2012;28:164–169. doi: 10.1016/j.jfca.2012.06.003. [DOI] [Google Scholar]
- Santos AS, Costa VC, Felício RC. Comparative study of nanostructured matrices employed in the development of biosensors based on HRP enzyme for determination of phenolic compounds. Electroanalysis. 2015;27:1572–1578. doi: 10.1002/elan.201400730. [DOI] [Google Scholar]
- Scheppingen WB, Hilten PH, Vijverberg MP, Duchateau ALL. Selective and sensitive determination of lactose in low-lactose dairy products with HPAEC-PAD. J Chromatogr B. 2017;1060:395–399. doi: 10.1016/j.jchromb.2017.06.024. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Leblanc RM. Biosensors based on β-galactosidase enzyme: recent advances and perspectives. Anal Biochem. 2017;535:1–11. doi: 10.1016/j.ab.2017.07.019. [DOI] [PubMed] [Google Scholar]
- Thakur MS, Ragavan KV. Biosensors in food processing. J Food Sci Technol. 2013;50:625–641. doi: 10.1007/s13197-012-0783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JKF, Stephani R, Tavares GM, Carvalho AF, Costa RGB, Almeida CER, Almeida MR, Oliveira LFC, Schuck P, Perrone IT. Technological aspects of lactose-hydrolyzed milk powder. Food Res Int. 2017;101:45–53. doi: 10.1016/j.foodres.2017.08.043. [DOI] [PubMed] [Google Scholar]
- Traffano-Schiffo MV, Castro-Giraldez M, Fito PJ, Santagapita PR. Encapsulation of lactase in Ca(II)-alginate beads: effect of stabilizers and drying methods. Food Res Int. 2017;100:296–303. doi: 10.1016/j.foodres.2017.07.020. [DOI] [PubMed] [Google Scholar]
- Trani A, Gambacorta G, Loizzo P, Cassone A, Fasciano C, Zambrini AC, Faccia M. Comparison of HPLC-RI, LC/MS-MS and enzymatic assays for the analysis of residual lactose in lactose-free milk. Food Chem. 2017;233:385–390. doi: 10.1016/j.foodchem.2017.04.134. [DOI] [PubMed] [Google Scholar]
- Troise AD, Bandini E, De Donno R, Meijer G, Trezzi M, Fogliano V. The quality of low lactose milk is affected by the side proteolytic activity of the lactase used in the production process. Food Res Int. 2016;89:514–525. doi: 10.1016/j.foodres.2016.08.021. [DOI] [PubMed] [Google Scholar]
- Varmira K, Mohammadi G, Mahmoudi M, Khodarahmi R, Rashidi K, Hedayatid M, Goicoechea HC, Jalalvand AR. Fabrication of a novel enzymatic electrochemical biosensor for determination of tyrosine in some food samples. Talanta. 2018;181:165–181. doi: 10.1016/j.talanta.2017.12.087. [DOI] [PubMed] [Google Scholar]
- Vlamidis Y, Gualandi I, Tonelli D. Amperometric biosensors based on reduced GO and MWCNTs composite for polyphenols detection in fruit juices. J Electroanal Chem. 2017;799:285–292. doi: 10.1016/j.jelechem.2017.06.012. [DOI] [Google Scholar]
- Zeng Y, Zhu Z, Dan D, Lin Y. Nanomaterial-based electrochemical biosensors for food safety. J Electroanal Chem. 2016;781:147–154. doi: 10.1016/j.jelechem.2016.10.030. [DOI] [Google Scholar]
- Zhang Y, Wei K. The role of nanomaterials in electroanalytical biosensors: a mini review. J Electroanal Chem. 2016;781:401–409. doi: 10.1016/j.jelechem.2016.09.011. [DOI] [Google Scholar]