Abstract
In this study, the efficacy of dragon fruit peel (DFP) powder as antioxidant dietary fibre (ADF), at two different concentrations (T1-1.5% and T2-3.0%), on quality improvement and susceptibility to lipid oxidation of chicken nuggets during 20 days of refrigerated storage was assessed. DFP, rich in dietary fibre (56.91%) with higher insoluble dietary fibre, phenolics (36–39 mgGAE/100 g) content and possessing good radical scavenging activity as well as reducing power, contained 10.36% protein, 4.48% fat and 2.34% ash. HPLC analysis revealed presence of high concentrations of gallic and ferulic acid, among the phenolics. Incorporation of DFP in nuggets although decreased the pH but improved emulsion stability as well as cooking yield and had higher protein, ash and lower fat content. Further, the treated nuggets had significantly (p < 0.05) higher dietary fibre and total phenolics content than control. Incorporation of DFP decreased the hardness, gumminess and chewiness and improved (p < 0.05) the products’ redness values. Sensory evaluation of the products revealed significant improvement in the appearance score and non-significant (p > 0.05) increase in the scores of other attributes compared to control samples. DFP significantly decreased lipid peroxidation, odour scores and microbial load in chicken nuggets during 20 days of storage period. From the study, it could be deduced that DFP rich in bioactive components had positive influence on the nutritional quality of chicken nuggets and could also be used as ADF in muscle food without affecting the quality and acceptability of products.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-04180-z) contains supplementary material, which is available to authorized users.
Keywords: Dragon fruit peel, Antioxidant dietry fibre, Lipid oxidation, Chicken nuggets
Introduction
Meat and meat products, with an excellent source of bioavailable proteins, essential minerals, trace elements, and vitamins, are considered as an important component in a healthy diet (Nohr and Biesalski 2007). In spite of being nutritious, meat especially chicken has some drawbacks as it is deficient in dietary fibre and more prone to oxidation of lipids due to relatively high content of polyunsaturated fatty acids. These fatty acids and their degree of unsaturation accelerate various oxidative processes resulting in deterioration of meat colour, flavour, texture and nutritive value (Sáyago-Ayerdi et al. 2009; Das et al. 2015). The use of antioxidants is an effective strategy to minimize or prevent lipid oxidation in food products. This in turn helps in retarding the formation of toxic oxidation products responsible for off-flavour development, thereby improving colour stability, nutritional quality and prolonging the shelf life of foods (Nam and Ahn 2003). Now a days, food processors prefer natural antioxidant additives over synthetic ones, which are viewed negatively due to toxicity, adverse effect on human health and related food safety issues (Das et al. 2016). On contrary, phenolic extract from plants as well as many phenolic compounds have been successfully demonstrated for their antioxidant and antimicrobial properties in retarding lipid oxidation and increasing the shelf life of different foods, including cooked pork nuggets (Chauhan et al. 2018), cooked beef (Rojas and Brewer 2007), chicken hamburgers (Sáyago-Ayerdi et al. 2009), goat meat (Das et al. 2011) etc.
Negative concerns about meat and associated additives and its influence on human health have accelerated research in the development of novel functional meat products using bioactive ingredients. Dietary fibre has been considered as a functional ingredient in meat products (Verma and Banerjee 2010). Dietary fibre, apart from easing the risk of important diseases promotes healthier life style (Verma and Banerjee 2010; Dhingra et al. 2012). The use of dietary fibre may improve texture (Sáyago-Ayerdi et al. 2009; Das et al. 2015), increase the cooking yield due to water-holding (Verma et al. 2013) and may also influence technological properties that improve physico-chemical and sensory attributes (Jimenez Colmenero et al. 2003). Because of these added advantages, researchers and meat processors are continuously searching for various natural functional ingredients having dual properties i.e., having a source of dietary fibre and antioxidant potential, commonly known as antioxidant dietary fibres (ADF). The incorporation of ADF in meat products not only increases the shelf life during storage by inhibiting lipid oxidation due to presence of phenolic antioxidants, but also improve the texture, physico-chemical and sensory properties of meat products (Jimenez Colmenero et al. 2003; Sáyago-Ayerdi et al. 2009; Verma et al. 2013; Das et al. 2015). In spite of these benefits, very few attempts have been made to enrich the ADF content in meat products using various natural ingredients like grape antioxidant dietary fibre (Sáyago-Ayerdi et al. 2009), guava powder (Verma et al. 2013), bael pulp residue (Das et al. 2015) and drumstick flower (Madane et al. 2019) extracted from plant materials including fruits, flowers and vegetables.
Dragon fruit (Hylocereus undatus), one of the tropical fruits, is very popular for its attractive bright red skin colour studded with green scales, juicy and sweet white flesh with tiny black seeds. Some studies have reported that dragon fruit contains considerable amount of total soluble solids, rich in organic acids, protein and other minerals and vitamins (Rebecca et al. 2010). Dragon fruit peel (DFP), which accounts for more than 20% by weight of the whole fresh fruit, is usually discarded as waste during processing. Recent studies have indicated dragon fruit peel as a potential natural colourant, having good antioxidant activities (Luo et al. 2014), rich in polyphenols and also an effective antibacterial agent (Wu et al. 2006). But as far as its application in muscle food products is concerned, reports available are scanty. The present study was, therefore, designed to assess the effectiveness of DFP on lipid stability, sensory qualities and acceptability of the chicken nuggets during refrigerated storage study.
Materials and methods
Preparation of dragon fruit peel powder and extract
Fully ripened dragon fruits (H. undatus) were taken from market and white pulp was carefully peeled and cleaned with tap water. After cleaning extraneous dirt, fruit peels were dried in a hot air oven at 45 ± 2 °C till complete drying. The dried peels were ground in a grinder (Kenstar, India) and sieved (#60 mesh sieves). The powder so obtained was stored in air-tight containers at room temperature until further use.
Dragon fruit peel (DFP) powder was extracted using either aqueous (AE) or aqueous ethanol (AEH) as solvent (60:40 v/v). To each 5 g powder accurately weighed into a flask, 100 mL solvent was added. The flasks were kept overnight at room temperature (27 °C ± 1 °C) with constant shaking (400 rpm) and vortexed at high speed for 10 min. After centrifugation at 5000 g for 10 min, the content was then passed through Whatman filter paper No. 1 (HiMedia® Mumbai, India). The resulting extract was kept in a container and stored at 2 °C for further studies such as total phenolics content (TPC), 1,1 diphenyl-2-picrylhydrazil (DPPH) radicals scavenging activity and ferric reducing antioxidant power (FRAP) assay. The efficacy of extracts was determined based on the weight of respective dry powders.
Analysis of total phenolics content
The total phenolics content in extracts were determined by the Folin–Ciocalteu (F–C) method. About 0.75 µL of F–C reagent was added to 100 µL of different dilutions of extract and the final volume was made ten times with distilled water (DW). After 5 min, 750 µL of sodium carbonate solution (7.5%) was added to each tube. The tubes were incubated for 90 min at room temperature in the dark, and absorbance (Eppendorf BioSpectrometer, USA) was measured against a blank at 725 nm. A standard curve was plotted using different concentrations of gallic acid and the amount of total phenolics was calculated as gallic acid equivalents (GAE) in mg/g of dried powder.
Radical scavenging activity using DPPH assay
Different concentrations (0.2, 0.4, 0.6, 0.8, 1.0 mL) of DFP extracts (1 mg/mL) were taken in test tube and the volume was made up to 4 mL with DW. 1 mL of 1 mM DPPH (prepared in methanolic solution) was added to each test tube, shaken well and allowed to stand for 30 min at room temperature. Control was prepared by adding 1 mL DPPH solution and 4 mL of DW and absorbance (Eppendorf BioSpectrometer, USA) was taken immediately at 517 nm (DW used as blank). The free radical scavenging activity (RSA) was calculated using the following formula—
Ferric reducing antioxidant power assay
The reducing power of the extracts was determined according to the method of Oyaizu (1986). Different concentrations of extracts were mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1% (w/v) potassium ferricyanide in 10 mL test tubes. The mixture was incubated for 20 min at 50 °C followed by addition of 2.5 mL of 10% trichloroacetic acid (TCA) and centrifuged at 700 g for 10 min. After that 2.5 mL supernatant was mixed with 2.5 mL of DW and 0.5 mL of ferric chloride (0.1% w/v), and the absorbance was measured at 700 nm (Eppendorf BioSpectrometer, USA). An increase in the absorbance of the reaction mixture indicated the reducing power of the sample.
Determination of dietary fibre content
Dietary fibre composition was analyzed following enzymatic–gravimetric method (Prosky et al. 1988). Briefly, samples were first dispersed in phosphate buffer before subjected to sequential enzymatic digestion by heat stable α-amylase, protease and amyloglucosidase. Subsequently, insoluble dietary fibre (IDF) was filtered and the residue was washed with warm DW. Combined solution of filtrate and washings were precipitated with four volumes of 95% ethanol for soluble dietary fibre (SDF) determination. Collected residues were then dried in the oven and weighed. Protein and ash contents of both IDF and SDF residues were determined for corresponding corrections. The total dietary fibre (TDF) was calculated as the sum of IDF and SDF.
Phenolic composition of DFP by HPLC
Phenolic composition of DFP was carried out by high-performance liquid chromatography with a diode-array detector (HPLC–DAD) as per the procedure outlined by Zeb (2015). The system used was Agilent 1260 Infinity HPLC consisting of quaternary pump, degasser, auto-sampler and DAD. The separation was achieved with the help of Agilent rapid resolution Zorbax Eclipse plus C18 (4.6 × 100 mm, 3.5 µm) column. The column was maintained at 25 °C. Phenolic compounds were analyzed in standard and sample solutions using gradient elution at 0.6 mL/min with gradient program (0–20 min 95–75% A, 20–40 min 75–50% A, 50–20% 40–50 min A, 50–60 min 20% A) and 1% formic acid in water as solvent A and methanol as solvent B. The chromatograms were obtained using 280 nm for analysis of phenolic compounds. The identification was carried out using available standards, retention times, and UV spectra. The quantification of identified compounds was based on the per cent peak area.
Preparation of chicken nuggets
Chicken nuggets were prepared following the procedure as described by Madane et al. (2019). Briefly, broiler meat was procured and minced twice (through a 6 mm grinding plate followed by 4 mm plate) in a meat mincer. After mincing, meat samples were divided into three (3) different batches. The composition of ingredients for each batch was based on the standard formulation (Supplementary data). The first batch was considered as control-C (meat without any ADF) whereas DFP at 1.5% and 3.0% level was used as ADF for chicken nuggets in formulations (T1 and T2) replacing equal per cent of breast trimmings. All the batches of minced meat samples were prepared separately in a bowl chopper. For meat emulsion preparation, salt, sugar, phosphate and nitrite were thoroughly mixed to the pre-weighed quantity of minced meat (breast trimmings and skin) in a bowl chopper and ice flakes were added during chopping to maintain lower temperature (8 ± 2 °C). Thereafter, condiments, dry spice mix, fine wheat flour was added and chopping was continued till uniform mixing of all the ingredients. The emulsion prepared was steam cooked for 40 min to get cooked chicken meat nugget. The blocks were sliced uniformly to obtain small cubes of chicken nuggets. The formulated nuggets (C, T1 and T2) were aerobically packed in LDPE pouches and kept at refrigerated temperature (4 ± 1 °C) to evaluate different physico-chemical parameters, including storage stability (0, 5, 10, 15 and 20 days) and sensory attributes. The whole experiment was conducted three times and samples were analysed in duplicate.
Cooking yield of nuggets
The cooking yield of nuggets was determined by recording the weight of each nugget block before and after cooking and expressed as percentage using the following calculation.
Determination of total phenolics content in cooked nuggets
The TPC in cooked nuggets was analyzed by using the method described by Escarpa and González (2001) with slight modification. Five gram (5 gm) of cooked product was homogenized with 25 mL of 70% acetone and kept overnight for extraction at refrigeration temperature (4 + 1 °C). Suitable aliquots of extracts were taken in a test tube and the volume was made to 0.5 mL with DW followed by addition of 0.25 mL F–C reagent (1 N) and 1.25 mL sodium carbonate solution (20%). The tubes were vortexed, mixed well and the absorbance was recorded after 40 min at 725 nm (Eppendorf BioSpectrometer, USA).
Proximate analysis
Moisture, protein, fat and ash content of the cooked meat nugget samples were determined by procedures prescribed by AOAC (1995) using hot air oven, Kjeldahl assembly, Soxhlet extraction apparatus and Muffle furnace, respectively.
pH
The pH of nugget samples was determined by combination electrode digital pH meter (Benchtop pH meter, BR Biochem, PHS-25CW, India). Briefly, 10 g of sample was homogenized with the help of tissue homogenizer (Omni, Germany) for about a minute in 50 mL of DW. Homogenized sample was kept for 5 min and then mixed again by shaking rod. pH was recorded by immersing the electrode directly into the suspension.
Expressible water
The expressible water percentage was estimated using the method of Das et al. (2008) with slight modification. About 5 g of cooked sample was weighed and placed on 2 layers of Whatman No. 1 filter paper. The sample was placed at the bottom of 50 mL centrifuge tube and centrifuged at 1500g (Remi, India) for 15 min. Immediately after centrifugation, the meat samples were re-weighed and the percentage of expressible water was calculated as initial weight − final weight/initial weight × 100.
Thiobarbituric acid reacting substances values
The evaluation of lipid stability was performed by measuring thiobarbituric acid reacting substances (TBARS) values following the method of Witte et al. (1970) with slight modification. Briefly, 10 g of sample was triturated with 25 mL of pre-cooled 20% TCA for 2 min. The content was then quantitatively transferred into a beaker by rinsing with 25 mL of chilled DW, mixed and filtered through Whatman filter paper No. 1. Three milliliters of TCA extract (filtrate) were mixed with 3 mL of thiobarbituric acid (TBA) reagent (5 mM) in test tubes and cooled in a running tap water after boiling in a water bath at 70 °C for 35 min. A blank sample was made by mixing 3 mL of 10% TCA and 3 mL of 5 mM TBA reagent. The absorbance was measured at a fixed wavelength of 532 nm (Eppendorf BioSpectrometer, USA). The TBA value was calculated as mg malonaldehyde per kg of the sample by multiplying the absorbance value with a factor of 5.2.
Odour score of chicken nuggets
Odour scores were evaluated by a panel of seven judges consisting of faculty and post-graduate students of the institute using a 5-point descriptive scale where 1- was denoted as very unpleasant, 2-moderately unpleasant, 3-moderately pleasant, 4-pleasant and 5-very pleasant (Das et al. 2011).
Instrumental colour of chicken nuggets
The colour profile of thick slice of chicken nugget or fresh chicken block was measured using Hunter Color Lab (Mini XE, Portable type) to record Hunter L*, a*, and b* values wherein L* denoted (brightness-100 or lightness-0), a* (+ redness/− greenness) and b* (+ yellowness/− blueness). The instrument was calibrated using light trap/black glass and white tile provided with the instrument. The instrument was directly put on the surface of meat block and reading was taken at three different points. The mean as well as standard error for each parameter was estimated.
Textural properties of chicken nuggets
The textural properties of nuggets were evaluated using a texture analyzer (Stable Micro System, Model TA.HDi, UK). The texture profile analysis was performed using central cores of five pieces of each sample (2 cm × 2 cm × 2 cm), which were compressed twice to 80% of the original height by a compression probe (P 75). A crosshead speed of 2 mm/s was used. The following parameters were determined: hardness (N/cm2); springiness (cm); cohesiveness; gumminess (N/cm2) and chewiness (N/cm).
Total plate count
The total plate count was determined by using pour plate method. 10 g of meat sample was homogenized in 90 mL of sterile peptone water (0.1%). Appropriate serial dilutions were prepared in 0.1% sterile peptone water and plated in duplicate with plate count agar, incubated at 37 °C for 48 h. Microbial colonies from the plates were counted and expressed as log10 cfu/g.
Sensory evaluation of chicken nuggets
Sensory attributes such as appearance, flavour, texture, juiciness and overall acceptability of control and treated chicken nuggets were evaluated using 8-point descriptive scale (Das et al. 2016), where 8 = extremely desirable and 1 = extremely undesirable. The consent of trained and experienced panelists was taken prior to the sensory evaluation process. The nugget samples (C, T1 and T2) were coded and then warmed in a microwave oven for 20 s before serving the same to the panelists. Plain potable water was provided to rinse the mouth in between the samples.
Statistical analysis
The present study was repeated thrice and in each replication, measurements of all parameters were done in duplicate. The analysis was carried out using SPSS software (version 20.0) and for storage study, data was analyzed using two-way ANOVA with interaction using SPSS software (version 20.0) where treatment (control, T1 and T2) and storage time (0, 5, 10, 15 and 20 days) were considered as factors. The experiment was carried out in 3 × 5 factorial design. The obtained data were subjected to variance analysis, and Duncan’s multiple range tests was used for comparing the means to find out the effect of ADF on various parameters. The values were presented as mean along with standard error (Mean ± Standard Error).
Results and discussion
Chemical composition and dietary fibre of dragon fruit peel
Dragon fruit peel (H. undatus), which consists approximately 22% of the whole fruit weight, is often discarded during processing, especially by the beverage industries (Jamilah et al. 2011). The purpose of this study was to harness its nutritional values and evaluate the potential to recover value-added material (s), if any and incorporate the same as functional ingredient into muscle food products. Accordingly, the chemical composition and dietary fibre contents of the DFP were estimated and is presented in Table 1. The protein, ash and lipid content of DFP were 10.36%, 2.34% and 4.48%, respectively. In general, the chemical composition of a plant component varies and depends on the edible part that is analyzed. Our results on proximate composition of DFP powder could not be substantiated, as reports on the nutritional composition and fatty acid profile of dragon fruit and its parts are scanty.
Table 1.
Proximate composition, dietary fibre and total phenolics content, phenolic compounds and IC50 (µg/mL) of dragon fruit peel
| Proximate composition | TPC, IC50 (µg/mL) and phenolic compounds | ||
|---|---|---|---|
| Protein (%) | 10.36 ± 0.23 | TPC (mg GAE/g) | |
| AE | 0.36 ± 0.72 | ||
| Lipid (%) | 2.34 ± 0.18 | AEH | 0.39 ± 0.86 |
| Ash (%) | 4.48 ± 0.31b | IC50µg/mL | |
| AE | 652 ± 2.84 | ||
| TDF (%) | 56.91 ± 0.51 | AEH | 586 ± 3.76 |
| SDF (%) | 7.69 ± 0.56 | Phenolic compounds (mg/kg) | HPLC |
| IDF (%) | 49.22 ± 0.84 | Caffeic acid | 10.00 ± 0.24 |
| NSC (%) | 25.91 ± 0.74 | Ferulic acid | 40.20 ± 1.86 |
| Quercertin | 3.32 ± 0.34 | ||
| Gallic acid | ND | ||
TDF total dietary fibre, SDF soluble dietary fibre, IDF insoluble dietary fibre, NSC non-structural carbohydrates, AE aqueous extract, AEH aqueous ethanol extract, ND not detected, HPLC high performance liquid chromatography; n = 6
As presented in Table 1, TDF content (56.91%) of DFP powder in this study was lower than the results published by Jamilah et al. (2011) but higher than the by-product of pear (14.1% SDF, 22% IDF), orange (13.6% SDF, 24.2% IDF), peach (9.7% SDF, 26.1% IDF), artichoke (14.3% SDF, 44.5% IDF) and asparagus (10.4% SDF, 38.6% IDF) as reported by Grigelmo-Miguel and Martín-Belloso (1999). The presence of good amount of IDF and SDF in the DFP powder indicate that it is a promising source of dietary fibre with a very good physiological effect; better than some cereals (Grigelmo-Miguel and Martín-Belloso 1999), like wheat bran (2.9% SDF, 41.1% IDF), and oat bran (3.6% SDF, 20.2% IDF).
Phenolic compounds in dragon fruit peel
The amount of TPC extracted from DFP using different solvents is presented in Table 1. Results show that AEH extract of DFP contained higher TPC (39 mg GAE/100 g powder) than AE (36 mg GAE/100 g), though not statistically significant (p < 0.01). Nurliyana et al. (2010) reported similar TPC i.e. 36.12 mg GAE/100 g from H. undatus peel. Manihuruk et al. (2017) also recorded 31.12 mg GAE/100 g from red DFP extract. Different phenolics exhibit different antioxidant potential and may influence the antioxidant activities (Chen et al. 2008). In fact, flavonoids (Nurliyana et al. 2010) and phenols (Wu et al. 2006) found in DFP extract are responsible for antioxidant activity. In this study, the phenolic compounds in DFP were identified (Table 1) and quantified by HPLC as shown in supplementary data. Among the phenolics, ferulic acid was present in higher concentration followed by caffeic acid (10.0 mg/kg) but other compounds, including flavonoids, were present only in trace amounts. It could be due to the reason that fruits are the main source of hydroxycinnamic acid and its derivatives, including ferulic, sinapic, p-coumaric, chlorogenic and caffeic acid.
DPPH radical scavenging activity
DPPH radical scavenging activity and antioxidant capacity are proportional to the total phenol antioxidants. In this study, DFP powder extracted in AEH showed higher radical scavenging activity compared to AE extract (Fig. 1). However, both the extracts demonstrated purple bleaching reaction at increasing concentrations, showing the presence of compounds responsible as free radical scavengers which reduces the initial DPPH concentration. At a concentration of 1.0 mg/mL, DFP showed the highest percentage of scavenging activity (59.01–65.87%) but at the same concentration, radical scavenging activity of BHT was (74.44%). Manihuruk et al. (2017) reported 50.14–52.15% DPPH scavenging activity of DFP extract which is not much different from this present study. Nurliyana et al. (2010) stated that 1 mg/mL red dragon fruit peel can inhibit 83.48% free radical DPPH. As far as IC50 is concerned, the lowest value was shown by the positive control, BHT (0.15 mg/mL) followed by DFP powder (0.65 mg/mL). Nurliyana et al. (2010) reported a lower IC50 value of DFP extract than the present study. The variation might be due to the use of dragon fruits at different stages of maturity thereby influencing the phenolics and antioxidant capacity.
Fig. 1.
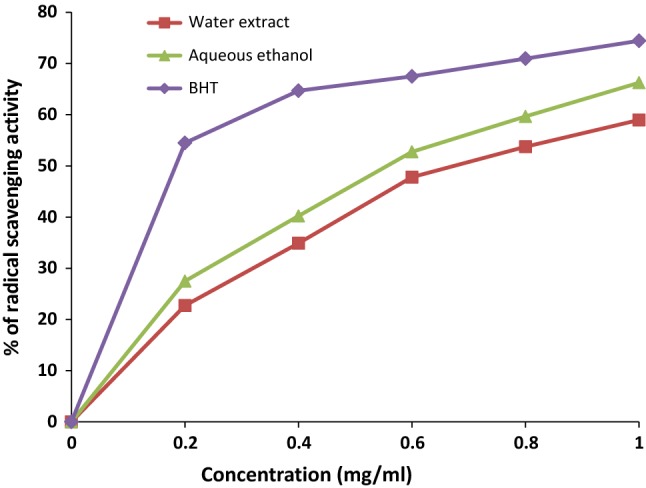
DPPH radical scavenging activity of different solvent extracts of dragon fruit peel
Ferric reducing antioxidant power assay
Ferric reducing antioxidant power (FRAP), a relatively simple and quick method, is used to determine antioxidant activity. As FRAP value is based on reducing the ferric ion, where antioxidants are the reducing agent, higher FRAP values give higher antioxidant capacity. In this study, extracts (AE and AEH) of DFP showed good reducing activity, but on a dose-dependent manner. However, AE extracted DFP showed lower ferric reducing properties than AEH. Maximum activity of AEH and AE extracted DFP was noticed at the concentration of 20 mg/mL and 25 mg/mL, respectively (supplementary data). Further, FRAP offers a positive correlation with the TPC, which is a good measure of the antioxidant activity of plant extracts. In this study, AEH extraction was more efficient in extracting antioxidants of DFP compared to AE.
Effect of DFP powder on physico-chemical properties of chicken nuggets
Effect on pH of chicken meat emulsion
The pH value of control (C) and DFP powder incorporated chicken meat emulsion (T1 and T2) is presented in Table 2. A significant (p < 0.05) decrease in pH of chicken emulsion was observed on incorporation of DFP powder. The highest pH value was observed in control (6.37) followed by T1 (6.31) and T2 (6.18), however a non-significant (p > 0.05) difference was recorded in control and T1. The decrease in pH may be attributed to the acidic pH (5.54) of DFP powder as also observed by Jamilah et al. (2011). Similarly, Manihuruk et al. (2017) reported that higher level of red DFP extract incorporation was responsible for lowering the pH of beef sausage. Madane et al. (2019) have also reported a decrease in pH in chicken meat nuggets incorporated with drum stick (Moringa oleifera) flower powder.
Table 2.
Effect of DFP powder on pH, emulsion stability, cooking yield and proximate composition of chicken nuggets
| Measurements | Control | T1 (DFP 1.5%) | T2 (DFP 3.0%) |
|---|---|---|---|
| pH | 6.37 ± 0.04a | 6.31 ± 0.01a | 6.18 ± 0.01b |
| Emulsion stability (%)** | 94.64 ± 0.17c | 95.42 ± 0.01b | 96.41 ± 0.03a |
| Cooking yield (%) | 96.63 ± 0.03c | 97.15 ± 0.02b | 98.34 ± 0.07a |
| Moisture (%) | 61.67 ± 2.38 | 62.07 ± 2.89 | 63.48 ± 2.58 |
| Protein (%)** | 14.95 ± 0.47 | 15.16 ± 0.04 | 15.46 ± 0.39 |
| Fat (%) | 17.20 ± 2.49 | 15.40 ± 2.22 | 14.58 ± 1.80 |
| Ash (%) | 2.58 ± 0.01 | 2.83 ± 0.11 | 2.86 ± 0.19 |
| TDF (%)** | 0.79 ± 0.13c | 1.94 ± 1.81b | 2.37 ± 0.19a |
| TPC (mg GAE/g)** | 0.053 ± 0.02b | 0.134 ± 0.04a | 0.163 ± 0.05a |
| Expressible water (%) | 28.16 ± 1.29 | 26.42 ± 3.74 | 25.61 ± 0.49 |
DFP dragon fruit peel, TDF total dietary fibre, TPC total phenolics content; n = 6
Means bearing different superscript row wise differ significantly (p < 0.05), **p < 0.01
Effect on emulsion stability
Incorporation of DFP powder significantly (p < 0.05) improved the emulsion stability of chicken meat compared to control sample (Table 2). The emulsion stability of control group was 94.64% where as it was 95.42% and 96.41% in T1 and T2 groups, respectively. Das et al. (2015) reported significantly improved (p < 0.05) stability of goat meat nuggets with addition of bael pulp residue at 0.5% level. The probable reason for the increased emulsion stability could be the presence of a higher amount of dietary fibre in DFP powder. Reddy and Rao (2000) reported higher emulsion stability as well as lower cooking losses of chicken loaves prepared with 20% Bengal gram and black gram flour. Likewise, an increase in the emulsion stability and cooking yield in meat batters was reported by incorporating dietary fibre extracted from rice bran (Choi et al. 2007) and drumstick flower (Madane et al. 2019) at different levels.
Effect on cooking yield
Cooking yield is one of the most practical tests to predict the influence of non-meat ingredients. A significant (p < 0.05) improvement in cooking yield was observed on the incorporation of DFP powder at different levels (Table 2). The cooking yield was higher in DFP incorporated nuggets (97.15% and 98.34%) compared to control (96.63%). The increased cooking yield due to DFP powder inclusion could mainly be attributed to the presence of high amount of dietary fibre and its ability to bind more water (Das et al. 2015). There are many reports stating that non-meat ingredients having high dietary fibre content improve the cooking yield when used in emulsion type of meat products. Non-meat ingredients, such as macromolecular hydrocolloids, starches and fibres, are known to have water binding properties (Pietrasik and Janz 2010). In fact, presence of insoluble fibre favours water binding properties and fat absorption capacity, thus helping to reduce cooking loss (Verma and Banerjee 2010).
Proximate composition of nuggets
The proximate analysis of chicken nuggets revealed that moisture, protein, fat and ash contents did not differ significantly between control and treated groups (Table 2). However, treated chicken nuggets had slightly higher moisture content and this could be due to absorption of added water by DFP during emulsion preparation. Das et al. (2015) reported that use of bael pulp residue in goat meat nuggets formulation non-significantly improved (p > 0.05) the moisture content. Incorporation of DFP powder non-significantly increased the protein content of cooked chicken nuggets. Control nugget had 14.95% protein content whereas it was 15.16% and 15.46% in treated nuggets (T1 and T2), respectively. Yılmaz (2004) reported higher protein and ash content in low fat meatballs with an increasing content of rye bran. The mean crude fat content of chicken nuggets prepared with DFP powder (1.5 and 3.0%) was not significantly (p > 0.05) different from control nuggets which had higher total fat content (17.20). Incorporation of DFP powder replacing breast trimmings probably decreased fat contents of chicken nuggets. Yasarlar et al. (2007) reported that fat content in meatballs gradually decreased with an increase in level of wheat and oat bran. There was no significant (p > 0.05) difference in ash content between control and treated nuggets. The ash content was higher in the treated nuggets with increased levels of dietary fibres which could be due to higher ash content of DFP powder. Similarly, Verma et al. (2013) reported increased ash content in goat meat patties prepared with guava powder as ADF.
Total dietary fibre and total phenolics content in nuggets
The TDF and TPC of control and treated chicken nuggets are presented in Table 2. Addition of DFP significantly (p < 0.05) increased the dietary fibre and phenolics content in chicken nuggets. The TDF content (2.37%) was highest in nuggets (T2 group) with 3.0% DFP, followed by in T1 group (1.94%) with 1.5% DFP while it was lowest in control (0.79%). Likewise, the TPC content was significantly higher in treated nuggets (0.134 and 0.163 mg GAE/g) than control (0.57 mg GAE/g). The results indicate that chicken nuggets became nutritionally enriched due to the inclusion of DFP. In a study, Yasarlar et al. (2007) reported increased dietary fibre content in meat balls prepared with cereals. In a similar line of work, Verma et al. (2013) found significantly increased (p < 0.05) TDF and TPC in meat products incorporated with guava powder in the formulation. Such higher dietary fibre and TPC level in treated chicken nuggets could be due to high dietary fibre (56.91%) and phenolics content (36–48 mg/100 g dry powder) of DFP. So, in brief it can be deduced that ADF of DFP not only reduced the fat, increased the protein, ash, TDF and TPC but also had positive influence in improving the functional quality of chicken nuggets.
Expressible water content
The water holding capacity (WHC) and cooking yield are considered as important physico-chemical properties of meat products. Good WHC is essential, as it influences the desirable sensory characteristics to meat products. Results of the present study revealed that the expressible water of chicken nugget samples added with DFP had better performance on holding water compared to control (Table 2), but not significantly (p > 0.05). This could be due to incorporation of DFP as ADF in meat products and its ability to modify texture, retain more water, reduce cooking loss as also reported by (Verma et al. 2013; Das et al. 2016), besides having antioxidant properties.
Textural properties of nuggets
DFP powder had no significant effect (p > 0.05) on various textural parameters such as hardness, cohesiveness, gumminess and chewiness values of the control and treated groups, though all the values decreased with increasing level of DFP (supplementary data). The present findings are in accordance with results published by different researchers. Verma et al. (2009) reported decrease in the textural properties of low salt, low fat chicken nuggets incorporated with high fibre ingredients. Ham et al. (2017) reported that textural properties like hardness, cohesiveness, and gumminess of cooked sausages were unaffected (p > 0.05) when formulated with different levels of lotus rhizome powder. Likewise, the hardness, adhesiveness, cohesiveness, gumminess and chewiness values of sheep meat nuggets were not significantly affected (p > 0.05) by addition of guava antioxidant fibre (Verma et al. 2013). In a study, Choi et al. (2011) found significantly lower textural properties such as hardness, springiness, cohesiveness, gumminess and chewiness in gels with added rice bran fibre than the control samples.
Effect of DFP on quality changes of nuggets during storage
pH
Initially, the mean pH value of treatment groups was significantly lower than the control (Table 3). As storage period progressed, a significant (p < 0.05) increase in pH was observed in both control and treated nuggets. The increase in pH during the storage period of meat product might be due to the accumulation of metabolites due to the growth of Gram-negative bacteria like Pseudomonas, Moraxella, Acinetobacter etc. (Mcdowell et al. 1986). However, the increase in pH during storage was significantly (p < 0.05) less in treated samples (1.5% DFP and 3.0% DFP) as compared to control, which might be due to inhibition of oxidative changes of protein and lipid and its antimicrobial properites.
Table 3.
Effect of DFP powder on pH, odour score and microbial load of chicken nuggets during refrigerated storage
| Treatment | Storage days | ||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |
| pH | |||||
| Control | 6.28 ± 0.01dx | 6.38 ± 0.01cdx | 6.43 ± 0.06ex | 6.51 ± 0.03bx | 6.63 ± 0.04ax |
| T1 (DFP 1.5%) | 6.24 ± 0.02dy | 6.31 ± 0.02cdy | 6.34 ± 0.01bcxy | 6.36 ± 0.01aby | 6.39 ± 0.01ay |
| T2 (DFP 3.0%) | 6.21 ± 0.01cy | 6.26 ± 0.02bey | 6.30 ± 0.02aby | 6.32 ± 0.03aby | 6.37 ± 0.02ay |
| Odour | |||||
| Control | 4.44 ± 0.17a | 4.08 ± 0.17a | 3.59 ± 0.1by | 2.44 ± 0.09cy | 1.67 ± 0.11dy |
| T1 (DFP 1.5%) | 4.31 ± 0.21a | 4.19 ± 0.12ab | 4.11 ± 0.18abx | 3.81 ± 0.15bcx | 3.38 ± 0.11cx |
| T2 (DFP 3.0%) | 4.28 ± 0.24a | 4.06 ± 0.19ab | 4.11 ± 0.15abx | 3.72 ± 0.14bcx | 3.47 ± 0.10cx |
| Total plate count (log10 CFU/g) | |||||
| Control | 2.68 ± 0.02e | 4.13 ± 0.07dx | 4.62 ± 0.10ex | 5.82 ± 0.08bx | 6.20 ± 0.09ax |
| T1 (DFP 1.5%) | 2.70 ± 0.09e | 3.62 ± 0.08dy | 3.99 ± 0.08cy | 4.38 ± 0.07by | 4.82 ± 0.09ay |
| T2 (DFP 3.0%) | 2.71 ± 0.05e | 3.53 ± 0.12dy | 3.84 ± 0.11ey | 4.22 ± 0.06by | 4.77 ± 0.13ay |
DFP dragon fruit peel; n = 6
Means bearing different superscripts (a–e) row wise differ significantly (p < 0.05)
Means bearing different superscripts (x–y) column wise differ significantly (p < 0.05)
Thiobarbituric acid reactive substances of chicken nuggets
The TBARS values of chicken nuggets, irrespective of treatments, increased significantly (p < 0.05) from day 0 to day 20 in both control and treated groups (Fig. 2).The increase in TBA value might be due to the lipid oxidation and production of volatile metabolites in presence of oxygen during aerobic storage (Sáyago-Ayerdi et al. 2009; Verma et al. 2013; Das et al. 2015). Although TBARS value of all the groups increased, but the rate of increase was comparatively slower in case of treated nuggets. The reason could be due to the presence of phenolic compounds in the DFP having strong antioxidant ability as reported by (Manihuruk et al. 2017). Out of the treated groups, chicken nuggets in T2 (3.0% DFP) retarded the oxidation process more efficiently by maintaining TBARS values during 20 days storage. Sáyago-Ayerdi et al. (2009) reported similar findings using 1 and 2% grape antioxidant dietary fibre in chicken hamburgers. Likewise, guava powder, besides supplementing the dietary fibre to sheep meat nuggets, also retarded lipid peroxidation in the product as measured by TBARS number during refrigerated storage (Verma et al. 2013).
Fig. 2.
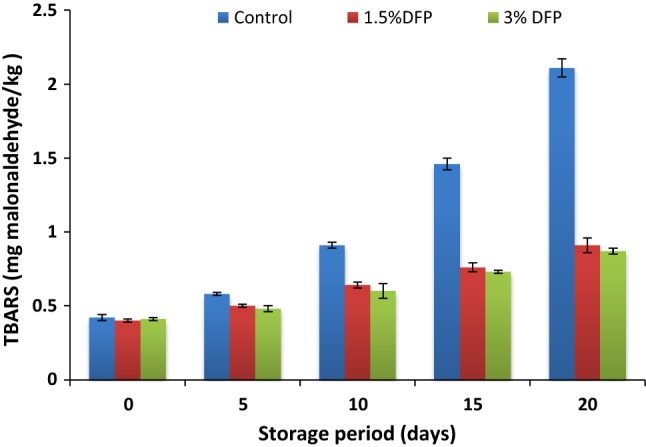
Effect of dragon fruit peel powder on thiobarbituric acid values of chicken nuggets during refrigerated storage
Sensory odour score of chicken nuggets
The analysis of variance revealed highly significant difference (p < 0.01) between storage days and in between treatments. At the beginning of storage, no difference in odour scores was observed between control and DFP treated nuggets. However, odour scores decreased for all treatments as storage time increased. On 15th day of storage, control nuggets received lower scores than the treated samples, although not statistically significant. On the other hand, the DFP treated nuggets were acceptable even on 20th day of storage period with odour score greater than 3.0 on contrary to below an acceptable score (1.67) of control sample indicating development of rancid odour (Table 3). This could possibly be due to the incorporation of DFP in treated nuggets facilitating inhibition of lipid oxidation. Das et al. (2011) reported a similar trend of odour score in ground goat meat during refrigerated storage. Rojas and Brewer (2007) observed reduced off-odour intensities in beef and pork treated with natural antioxidants like grape seed extract.
Total plate count of chicken nuggets
The total plate count of treated chicken nuggets was significantly lower as compared to the control during refrigeration storage study (Table 3). Chicken nuggets in T2 group (3.0% DFP powder) had lower total plate count compared to T1 group (1.5% DFP powder), which might be due to its richness in polyphenolic compounds having an antimicrobial effect along with the antioxidant property. Das et al. (2008) also observed similar trend in results while studying the quality attributes and shelf life of goat meat extended with soy granules. Likewise, incorporation of bael pulp residue as ADF in goat meat nuggets was very much effective (p < 0.05) in controlling the microbial counts throughout the 21 days of storage period (Das et al. 2015). It has been well documented by some researchers that polyphenols from DFP have microbiocidal activities against a number of pathogenic bacteria (Nurmahani et al. 2012; Manihuruk et al. 2017).
Instrumental colour stability
The instrumental colour [lightness (L*), yellowness (b*) and redness (a*)] values of treated chicken nuggets were recorded and compared to control (Table 4). On 0 day, a significant increase (8.91–11.55%) in the lightness (L*) values was observed in treated chicken nuggets (T1 and T2) in comparison to control. The reduction in lightness value of treated chicken nuggets was even more (up to 27%) after 10th day onwards of refrigerated storage study, relative to control. This effect could be attributed to the dilution of meat pigment in other meat products due to the presence of non-meat ingredients. Similar trend of lightness values has been reported in chicken hamburgers using grape antioxidant dietary fibre during storage (Sáyago-Ayerdi et al. 2009). The redness (a*) values of treated chicken nuggets were not significantly different in comparison to control on 0 day. These a* values were stabilized in nuggets with DFP resulting in a significant increase in redness values relative to controls, after 10 days of storage. This could be due to the presence of phenolics in DFP powder stabilizing the redness of treated nuggets during storage. A similar effect has been observed by (Sáyago-Ayerdi et al. 2009) using grape as ADF in cooked chicken hamburgers.
Table 4.
Effect of DFP powder on instrumental colour analysis of chicken nuggets during refrigerated storage
| Treatment | Storage period | ||||
|---|---|---|---|---|---|
| 0 day | 5 day | 10 day | 15 day | 20 day | |
| L (lightness) | |||||
| Control | 29.95 ± 1.21ay | 26.21 ± 0.28by | 25.49 ± 0.24bcx | 23.89 ± 0.37cdx | 22.52 ± 0.43dx |
| T1 (DFP 1.5%) | 32.62 ± 1.25ayx | 26.33 ± 0.22by | 23.49 ± 0.29cy | 18.08 ± 0.26dy | 17.00 ± 0.21dy |
| T2 (DFP 3.0%) | 33.41 ± 0.20ax | 28.29 ± 0.23bx | 18.57 ± 0.18cz | 18.51 ± 0.26cy | 17.11 ± 0.43dy |
| a (redness) | |||||
| Control | 13.77 ± 0.26a | 11.59 ± 0.20by | 5.17 ± 0.06cz | 5.10 ± 0.06cz | 5.02 ± 0.12cy |
| T1 (DFP 1.5%) | 13.34 ± 0.72a | 12.25 ± 0.20bx | 7.94 ± 0.12cx | 5.89 ± 0.18dy | 5.33 ± 0.13dy |
| T2 (DFP 3.0%) | 13.19 ± 0.12a | 12.20 ± 0.06bx | 8.39 ± 0.07cx | 7.85 ± 0.09dx | 7.26 ± 0.16ex |
| b (yellowness) | |||||
| Control | 13.11 ± 0.26a | 11.81 ± 0.25bx | 7.98 ± 0.06cx | 7.33 ± 0.13dx | 6.92 ± 0.17dx |
| T1 (DFP 1.5%) | 13.01 ± 0.72a | 10.65 ± 0.18by | 7.38 ± 0.10cy | 5.28 ± 0.16dy | 5.15 ± 0.09dz |
| T2 (DFP 3.0%) | 13.13 ± 0.19a | 11.43 ± 0.12bx | 6.45 ± 0.10cz | 6.18 ± 0.09cz | 6.12 ± 0.11cy |
DFP dragon fruit peel; n = 6
Means bearing different superscripts (a–e) row wise differ significantly (p < 0.05)
Means bearing different superscripts (x–z) column wise differ significantly (p < 0.05)
There was no variation in yellowness values between control and treated samples up to 10th day of storage, but values significantly decreased thereafter in all. The findings in this study are similar to that reported by Mitsumoto et al. (2005) for chicken patties added with tea catechins. A decrease in (b*) and (a*) values of beef patties containing natural antioxidants has been reported by Ashour et al. (2014) who reported that over storage time, the a* values of all patties samples decreased and became less red or brown due to metmyoglobin formation.
Sensory attributes of chicken nuggets
Sensory evaluation is an important tool to know the acceptability of meat products. Various sensory parameters viz. appearance, flavour, juiciness, texture etc. of control and treated chicken nuggets were recorded during refrigerated storage study and is presented in Table 5. Although sensory score of all the treatments decreased with time, control group received much lower score and was acceptable only up to 10th day of storage. The later develop rancid odour on 15th day and was, therefore, rejected by the panellists. On the other hand, treated nuggets were acceptable even on 20th day of storage. This could be due to the reason that DFP as ADF might have acted as stabilizing agent for retaining the flavour by inhibiting the lipid oxidation. These findings are in agreement with Sáyago-Ayerdi et al. (2009) who reported that chicken hamburgers added with grape ADF improved stability in flavour and tenderness.
Table 5.
Effect of DFP powder on sensory attributes of chicken nuggets during refrigerated storage
| Treatment | Storage period | ||||
|---|---|---|---|---|---|
| 0 day | 5 day | 10 day | 15 day | 20 day | |
| Appearance | |||||
| Control | 7.11 ± 0.10a | 6.86 ± 0.14ab | 6.56 ± 0.18b | 6.14 ± 0.11c | 5.58 ± 0.11d |
| T1 (1.5% DFP) | 7.11 ± 0.13a | 7.02 ± 0.20ab | 6.89 ± 0.13ab | 6.83 ± 0.14ab | 6.64 ± 0.13b |
| T2 (3.0% DFP) | 6.94 ± 0.17a | 6.88 ± 0.20ab | 6.81 ± 0.12ab | 6.76 ± 0.19ab | 6.42 ± 0.10b |
| Flavour | |||||
| Control | 6.86 ± 0.18a | 6.83 ± 0.21a | 6.05 ± 0.19by | ND | ND |
| T1 (1.5% DFP) | 6.94 ± 0.16a | 6.83 ± 0.12a | 6.77 ± 0.16ax | 6.31 ± 0.09b | 6.17 ± 0.08b |
| T2 (3.0% DFP) | 6.99 ± 0.17a | 6.92 ± 0.16a | 6.75 ± 0.12abx | 6.53 ± 0.12ab | 6.36 ± 0.11b |
| Juiciness | |||||
| Control | 6.91 ± 0.20 | 6.70 ± 0.14 | 6.44 ± 0.16 | ND | ND |
| T1 (1.5% DFP) | 7.13 ± 0.16a | 6.81 ± 0.12ab | 6.77 ± 0.15ab | 6.68 ± 0.14ab | 6.39 ± 0.10b |
| T2 (3.0% DFP) | 7.05 ± 0.17a | 6.75 ± 0.10ab | 6.72 ± 0.19ab | 6.61 ± 0.12ab | 6.47 ± 0.12b |
| Texture | |||||
| Control | 6.98 ± 0.16 | 6.78 ± 0.20 | 6.72 ± 0.14 | ND | ND |
| T1 (1.5%DFP) | 6.82 ± 0.17a | 6.68 ± 0.20ab | 6.59 ± 0.14ab | 6.53 ± 0.10ab | 6.38 ± 0.10b |
| T2 (3.0% DFP) | 6.80 ± 0.06a | 6.70 ± 0.18ab | 6.63 ± 0.13ab | 6.62 ± 0.10ab | 6.51 ± 0.10b |
| Overall acceptability | |||||
| Control | 6.92 ± 0.19a | 6.39 ± 0.12ab | 6.32 ± 0.35b | ND | ND |
| T1 (1.5% DFP) | 6.87 ± 0.13a | 6.69 ± 0.10a | 6.53 ± 0.12ab | 6.53 ± 0.11ab | 6.38 ± 0.11b |
| T2 (3.0% DFP) | 6.92 ± 0.15a | 6.92 ± 0.15a | 6.81 ± 0.09ab | 6.64 ± 0.11ab | 6.39 ± 0.11b |
DFP dragon fruit peel; n = 18, ND not done
Means bearing different superscripts (a–d) row wise differ significantly (p < 0.05)
Means bearing different superscripts (x–y) column wise differ significantly (p < 0.05)
During storage study, a significant decrease (p < 0.05) in juiciness score was observed in both control and treated (T1 and T2) products from 10th day onwards. However, chicken nuggets containing DFP were found to be more juicer than the control, which could be attributed to the increased moisture retention of the product during cooking. Our findings are in line with those of Desmond et al. (1998) and Piñero et al. (2008) who found that use of oat fibre aided in water retention and produced juicier low-fat beef patties. The texture scores of both the control and treated nuggets, although decreased with storage time, were comparable up to 10th day. As far as overall acceptability of chicken nugget is concerned, the products also followed the same pattern that was observed for other sensory attributes. Control sample received lower overall acceptability score, although non-significantly (p < 0.05), on day 5 than treated and was found to have rancid odour on 15th day of storage. On the other hand, overall acceptability scores in products of both the treatment (T1 and T2) groups remained stable and were acceptable even on 20th day of storage. This could be due to the incorporation of DFP powder which might have prolonged the storage life. It is well documented by many researchers that meat products incorporated with natural antioxidant (s) have higher flavour and overall acceptability scores during storage owing to the colour and flavour stabilizing effect of them by inhibiting lipid and protein oxidation (Nam and Ahn 2003; Rojas and Brewer 2007; Chauhan et al. 2018) or ADF (Sáyago-Ayerdi et al. 2009; Verma et al. 2013; Das et al. 2015). Our findings are in agreement to the reports on role of ADF published earlier.
Conclusion
The results of the present study indicated that DFP is a rich source of dietary fibre and also posseses good antioxidant potential such as radical scavenging activity and ferric reducing antioxidant power. The addition of extracted DFP in chicken meat nuggets improved the cooking yield and dietary fibre content without affecting acceptability of the product. Further, DFP increased lipid and colour stability, improved odour score and shelf-life of chicken nuggets during 20 days of refrigeration storage by inhibiting lipid oxidation and microbial growth compared to control. Therefore, DFP, apart from offering its functional health promoting benefits, could be used as a safe, natural and valuable antioxidant in the meat food industry.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are thankful to the Director, ICAR-Indian Veterinary Research Institute (IVRI), Izatnagar, Bareilly and the Station In-charge, ERS, ICAR-IVRI, Kolkata for providing necessary facilities for conducting this research work.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pratap Madane, Email: pratapmadane8@gmail.com.
Arun K. Das, Email: arun.das@icar.gov.in
P. K. Nanda, Email: pramod.nanda@icar.gov.in
Samiran Bandyopadhyay, Email: samiranvet@gmail.com.
Prasant Jagtap, Email: dr.prashant@shalimarcorp.in.
Akshay Shewalkar, Email: dr.shewalkar.akshay@gmail.com.
B. Maity, Email: bmaity@shalimarcorp.in
References
- AOAC . Official methods of analysis. 16. Washington, DC: Association of Official Analytical Chemists; 1995. [Google Scholar]
- Ashour MMS, Moawad RK, Bareh GF. Quality enhancement and shelf-life extension of raw beef patties formulated with lactate/thyme essential oil during refrigerated storage. J Appl Sci Res. 2014;9(13):6699–6709. [Google Scholar]
- Chauhan P, Pradhan S, Nanda P, Bandyopadhyay S, Das A. Inhibition of lipid and protein oxidation in raw ground pork by Terminalia arjuna fruit extract during refrigerated storage. Asian Aust J Animal Sci. 2018;32(2):265–273. doi: 10.5713/ajas.17.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Fan J, Yue X, Wu X, Li L. Radical scavenging activity and phenolic compounds in persimmon (Diospyros kaki L. cv. Mopan) J Food Sci. 2008;73(1):24–28. doi: 10.1111/j.1750-3841.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- Choi Y-S, Jeong J-Y, Choi J-H, Han D-J, Kim H-Y, Lee M-A, Shim S-Y, Paik H-D, Kim C-J. Quality characteristics of meat batters containing dietary fiber extracted from rice bran. Korean J Food Sci Animal Resour. 2007;27(2):228–234. doi: 10.5851/kosfa.2007.27.2.228. [DOI] [Google Scholar]
- Choi Y-S, Choi J-H, Han D-J, Kim H-Y, Lee M-A, Kim H-W, Jeong J-Y, Kim C-J. Effects of rice bran fiber on heat-induced gel prepared with pork salt-soluble meat proteins in model system. Meat Sci. 2011;88(1):59–66. doi: 10.1016/j.meatsci.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Das AK, Anjaneyulu ASR, Verma AK, Kondaiah N. Physicochemical, textural, sensory characteristics and storage stability of goat meat patties extended with full-fat soy paste and soy granules. Int J Food Sci Technol. 2008;43(3):383–392. doi: 10.1111/j.1365-2621.2006.01449.x. [DOI] [Google Scholar]
- Das AK, Rajkumar V, Dwivedi DK. Antioxidant effect of curry leaf (Murraya koenigii) powder on quality of ground and cooked goat meat. Int Food Res J. 2011;18(2):563–569. [Google Scholar]
- Das AK, Rajkumar V, Verma AK. Bael pulp residue as a new source of antioxidant dietary fiber in goat meat nuggets. J Food Process Preserv. 2015;39:1626–1635. doi: 10.1111/jfpp.12392/. [DOI] [Google Scholar]
- Das AK, Rajkumar V, Nanda PK, Chauhan P, Pradhan SR, Biswas S. Antioxidant efficacy of litchi (Litchi chinensis sonn.) pericarp extract in sheep meat nuggets. Antioxidants. 2016;5(2):16. doi: 10.3390/antiox5020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond EM, Troy DJ, Buckley DJ (1998) The effects of tapioca starch, oat fibre and whey protein on the physical and sensory properties of low-fat beef burgers. LWT - Food Sci Technol 31:653–657. 10.1006/fstl.1998.0415
- Dhingra D, Michael M, Rajput H, Patil R. Dietary fibre in foods: a review. J Food Sci Technol. 2012;49(3):255–266. doi: 10.1007/s13197-011-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigelmo-Miguel N, Martín-Belloso O. Comparison of dietary fibre from by-products of processing fruits and greens and from cereals. LWT-Food Sci Technol. 1999;32(8):503–508. doi: 10.1006/fstl.1999.0587. [DOI] [Google Scholar]
- Ham Y-K, Hwang K-E, Song D-H, Kim Y-J, Shin D-J, Kim K-I, Lee H-J, Kim N-R, Kim C-J. Lotus (Nelumbo nucifera) rhizome as an antioxidant dietary fiber in cooked sausage: effects on physicochemical and sensory characteristics. Korean J Food Sci Animal Resour. 2017;37(2):219. doi: 10.5851/kosfa.2017.37.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamilah B, Shu C, Kharidah M, Dzulkily M, Noranizan A. Physico-chemical characteristics of red pitaya (Hylocereus polyrhizus) peel. Int Food Res J. 2011;18(1):279–286. [Google Scholar]
- Jimenez Colmenero F, Serrano A, Ayo J, Solas MT, Cofrades S, Carballo J. Physicochemical and sensory characteristics of restructured beef steak with added walnuts. Meat Sci. 2003;65(4):1391–1397. doi: 10.1016/s0309-1740(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Luo H, Cai Y, Peng Z, et al (2014) Chemical composition and in vitro evaluation of the cytotoxic and antioxidant activities of supercritical carbon dioxide extracts of pitaya (dragon fruit) peel. Chem Cent J. 10.1186/1752-153X-8-1 [DOI] [PMC free article] [PubMed]
- Madane P, Das AK, Pateiro M, Nanda PK, Bandyopadhyay S, Jagtap P, Barba FJ, Shewalkar A, Maity B, Lorenzo JM. Drumstick (Moringa oleifera) flower as an antioxidant dietary fibre in chicken meat nuggets. Foods. 2019;8(8):307. doi: 10.3390/foods8080307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manihuruk FM, Suryati T, Arief II. Effectiveness of the red dragon fruit (Hylocereus polyrhizus) peel extract as the colorant, antioxidant, and antimicrobial on beef sausage. Media Peternakan. 2017;40(1):47–54. doi: 10.5398/medpet.2017.40.1.47. [DOI] [Google Scholar]
- Mcdowell DA, Hobson I, Strain JJ, Owens JJ. Bacterial microflora of chill stored beef carcasses. Environ Health. 1986;94:65–68. [Google Scholar]
- Mitsumoto M, O’Grady MN, Kerry JP, Joe Buckley D (2005) Addition of tea catechins and vitamin C on sensory evaluation, colour and lipid stability during chilled storage in cooked or raw beef and chicken patties. Meat Sci 69:773–779. 10.1016/j.meatsci.2004.11.010 [DOI] [PubMed]
- Nam K, Ahn D. Use of antioxidants to reduce lipid oxidation and off-odor volatiles of irradiated pork homogenates and patties. Meat Sci. 2003;63(1):1–8. doi: 10.1016/S0309-1740(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Nohr D, Biesalski HK. ‘Mealthy’ food: meat as a healthy and valuable source of micronutrients. Animal. 2007;1(2):309–316. doi: 10.1017/s1751731107657796. [DOI] [PubMed] [Google Scholar]
- Nurliyana R, Syed Zahir I, Mustapha Suleiman K, Aisyah M, Kamarul Rahim K. Antioxidant study of pulps and peels of dragon fruits: a comparative study. Int Food Res J. 2010;17(2):367–375. [Google Scholar]
- Nurmahani M, Osman A, Hamid AA, Ghazali FM, Dek MP. Antibacterial property of Hylocereus polyrhizus and Hylocereus undatus peel extracts. Int Food Res J. 2012;19(1):77. [Google Scholar]
- Oyaizu M. Studies on products of the browning reaction. Antioxidative activities of browning reaction products prepared from glucosamine. Jpn J Nutr. 1986;44(6):307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Pietrasik Z, Janz J. Utilization of pea flour, starch-rich and fiber-rich fractions in low fat bologna. Food Res Int. 2010;43(2):602–608. doi: 10.1016/j.foodres.2009.07.017. [DOI] [Google Scholar]
- Piñero MP, Parra K, Huerta-Leidenz N, et al (2008) Effect of oat’s soluble fibre (β-glucan) as a fat replacer on physical, chemical, microbiological and sensory properties of low-fat beef patties. Meat Sci 80:675–680. 10.1016/j.meatsci.2008.03.006 [DOI] [PubMed]
- Prosky L, Asp N-G, Schweizer T, DeVries J, Furda I. Determination of insoluble, soluble, and total dietary fiber in foods and food products: interlaboratory study. J Assoc Off Anal Chem. 1988;71(5):1017–1023. [PubMed] [Google Scholar]
- Rebecca O, Boyce A, Chandran S. Pigment identification and antioxidant properties of red dragon fruit (Hylocereus polyrhizus) Afr J Biotechnol. 2010;9(10):1450–1454. doi: 10.5897/AJB09.1603. [DOI] [Google Scholar]
- Reddy PK, Rao JB. Effect of binders and pre-cooking meat on quality of chicken loaves. J Food Sci Technol. 2000;37(5):551–553. [Google Scholar]
- Rojas MC, Brewer MS. Effect of natural antioxidants on oxidative stability of cooked, refrigerated beef and pork. J Food Sci. 2007;72(4):S282–S288. doi: 10.1111/j.1750-3841.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Sáyago-Ayerdi S, Brenes A, Goñi I. Effect of grape antioxidant dietary fiber on the lipid oxidation of raw and cooked chicken hamburgers. LWT Food Sci Technol. 2009;42(5):971–976. doi: 10.1016/j.lwt.2008.12.006. [DOI] [Google Scholar]
- Verma AK, Banerjee R. Dietary fibre as functional ingredient in meat products: a novel approach for healthy living—a review. J Food Sci Technol. 2010;47(3):247–257. doi: 10.1007/s13197-010-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma AK, Sharma BD, Banerjee R (2009) Quality characteristics and storage stability of low fat functional chicken nuggets. Fleischwirtsch Int 24:52–57
- Verma AK, Rajkumar V, Banerjee R, Biswas S, Das AK. Guava (Psidium guajava L.) powder as an antioxidant dietary fibre in sheep meat nuggets. Asian Aust J Animal Sci. 2013;26(6):886–895. doi: 10.5713/ajas.2012.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte VC, Krause GF, Bailey MF. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. J Food Sci. 1970;35:582–585. doi: 10.1111/j.1365-2621.1970.tb04815.x. [DOI] [Google Scholar]
- Wu L-c, Hsu H-W, Chen Y-C, Chiu C-C, Lin Y-I, J-aA Ho. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006;95(2):319–327. doi: 10.1016/j.foodchem.2005.01.002. [DOI] [Google Scholar]
- Yasarlar EE, Daglioglu O, Yilmaz I. Effects of cereal bran addition on chemical composition, cooking characteristics and sensory properties of Turkish meatballs. Asian J Chem. 2007;19(3):2353. [Google Scholar]
- Yılmaz I. Effects of rye bran addition on fatty acid composition and quality characteristics of low-fat meatballs. Meat Sci. 2004;67(2):245–249. doi: 10.1016/j.meatsci.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Zeb A. A reversed phase HPLC-DAD method for the determination of phenolic compounds in plant leaves. Anal Methods. 2015;7(18):7753–7757. doi: 10.1039/C5AY01402F. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


