Abstract
Present study aimed to investigate the effect of preharvest bagging and bag colour on physico-chemical, nutraceutical quality and consumer acceptability of pomegranate arils. Fruits of 10-years-old Kandhari variety were covered with 15 × 20 cm three colored single layer cellulosic bags (35 µm thickness, 2700 cm3 24 h−1 GTR, WVTR 28.60 cm3 24 h−1) 60 days after flowering. Fruits were harvested 150 days after anthesis (average TSS 13° brix) and fruits were taken to various physical-biochemical parameters. Red colored bagging minimized fruit cracking (66%) and bacterial blight incidence (78%) over control. Ascorbic acid and total anthocyanin content was found maximum with red colour bagged (18.20 ± 0.87 mg 100 g−1 FW; 73.03 ± 3.97 mg 100 g−1 FW) fruits, while total soluble solids, total sugars and total antioxidant capacity were maximum in control fruits. Total phenols were found maximum in fruits which were bagged with blue colour bags (32.12 ± 1.53 mg GAE 100 g−1 FW). Respiration rate was recorded maximum in the red colored bags (17.93 ± 0.22 mL kg−1 h−1) followed by white, blue and control fruits. Overall acceptability was recorded maximum in the fruits which were covered with red colour bags (8.67) whereas sweetness was reported maximum in control fruits (8.30). For harnessing the benefit of bagging in pomegranate, fruit should be bagged 60 days after flowering with red color cellulosic bags. Red color cellulosic bags are much effective in producing quality fruits except compromising on few quality traits like aril Ca and total phenols content.
Keywords: Pomegranate, Fruit bagging, Aril firmness, Anthocyanin, Total antioxidant activity
Introduction
Pomegranate, a fruit of Lythraceae family has recently attracted huge interest of researchers worldwide due to its health promoting properties (Gumienna et al. 2016; Boussaa et al. 2019; Ozcan et al. 2019). Despite being a hardy crop, pomegranate suffers massive pre and post harvest losses due to sun burning, fruit cracking, internal breakdown, insect attack and mechanical damage. To address these problems growers use different chemicals through foliar application. Indiscriminate use of these chemicals poses a great risk to human health and ecosystem. Physical protection methods have been used for many years in different crops aiming to isolate partially or temporarily the fruits or even entire plants from a threatening environment. There is increasing consumers demand worldwide for pesticide free and intensely red colored fruits (Gadze et al. 2012). Among several alternatives, pre-harvest (on tree) fruit bagging has emerged one of the potential tools for several crops. This technique is getting commercial status in Japan, China, New Zealand and Australia in apple, Peach, pear and grapes (Kitagawa et al. 1992). Bagging affects pigment synthesis, days to maturity, cell histology, physiology, shelf-life, storage behavior, biochemical composition (Sharma et al. 2014; Jhang et al. 2015) pesticide residue content (Kitagawa et al. 1992; Amarante et al. 2002) and insect severity (Joyce et al. 1997; Hofman et al. 1997). Reported research on pre-harvest fruit bagging indicates that quality of bagged fruits influenced with types of bags, variety, time of bagging and de-bagging (Kim et al. 2010). Preharvest pomegranate fruit bagging has been reported to increase fruit yield and decrease pest incidence (Shalomo 2015), but with limited or unknown effects on other aspects of physico-chemical and nutraceutical qualities. Considering this gap on impact of colored bagging on fruit quality parameters with focus on nutraceutical qualities and aril softness, this study was undertaken.
Materials and methods
On tree fruit bagging
During two successive seasons of 2014–2015 healthy and uniform size fruits of 10 years old Kandhari variety were covered with 15 × 20 cm three colored single layer cellulosic bags (35 µm thickness, 2700 cm3 24 h−1 GTR, WVTR 28.60 cm3 24 h−1) 60 days after flowering at Horticulture research farm Bajoura (Himanchal Pradesh), India. As a prophylactic measure, trees were sprayed with fungicide (2 g L−1 copperoxichloride) 1 week prior bagging. Each treatment was replicated thrice and four bags of each individual color were tied per tree. Trees were randomly selected across all the directions and, also the bags were evenly distributed on whole tree canopy. Control fruits were left un-bagged (naked) and all the trees were subjected to uniform cultural practices during experiment. Fruits were harvested 150 days after anthesis (average TSS 13° brix). Total 360 fruits were selected for the experiment and divided into four lots of 90 fruits each for the treatments (3 replications of 30 fruits per treatment).
External quality attributes
Observation on fruit cracking, sun scalding, scratching, thrips incidence and bacterial spot were taken from the randomly selected five trees from each replication at the final harvesting stage. Affected fruits were with each individual melodies were sorted out and counted separately and presented in per cent.
Peel weight, peel thickness, fruit—aril firmness, specific gravity and juice recovery
Every individual fruit was weighed and arils were separated by hands using knife and again weighed by using three digits standard weighing balance and denoted in gram per 100 g fruit weight (%).Thickness was taken from three parts (top, mid, bottom) of fruit peel by a digital caliper and average value were calculated accordingly. Peel thickness was measured and expressed in millimeter. Fruit firmness was measured by texture analyzer (Model: TA + Di, Stable micro systems, UK) trough penetrations and force measurement required for a probe of 2 mm diameter to penetrate 10 mm inside the fruit. The force during compression was measured using the load cell of 500 kg capacity. Puncture force measurement was done at three equatorial zones at interval of 90 0 angles and expressed in Newton (N). Each value of fruit firmness represents the mean of three individual measurement points (bottom, middle and top) taken on sample fruits (Jha et al. 2010). Aril firmness was determined through compression test by using P 75 probe and compression distance of 60%. Average of highest peak forces (out of multiple peaks) of the 3 arils was taken as firmness (N) of the arils. Fruits were weighed with the help of electronic balance followed by placing in water cane. Fruit volume was measured by displaced water and specific gravity was calculated as fruit weight divided by fruit volume. Juice was extracted through cold press method by using juicer and grinder and squeezed by using muslin-cloth, centrifuge it and measured the supernatant by using graduated measuring cylinder and expressed in % on fruit weight basis.
Total soluble solids (TSS) and total sugars
The TSS was determined with a handheld refractometer (PAL-3, Atago, Japan) calibrated using distilled water as suggested by Ranganna (1999). Juice was extracted by using pestle and mortar and straining with muslin cloth. Then two drops of juice was placed on prism and corresponding refraction index was read and value calculated according to the 21 °C and expressed as °Brix.
Total sugars were determined by taking 20 g aril extract, added 100 mL distilled water, 2 mL lead acetate (45%), kept the mixture for 1 h after added 2 mL potassium oxalate (22%), volume made to 250 mL with distilled water. Filtrate it and took 50 mL filtrate in a 100 mL volumetric flask and add 5 mL concentrated hydrochloric acid, kept for some minutes then neutralize it with sodium hydroxide (40%) till very light pink colour appear. After that make volume 100 mL with distilled water and used it for titrating the Fehling solution while boiling and methylene blue as indicator till brick red colour appear. Total sugars were expressed in (%).
Ascorbic acid and total phenols
Ascorbic acid was determined using 2,6-dichlorophenol-indophenol dye titrimetric method (Jhang et al. 2015). 10 g sample was grinded with 3% HPO3 to a final volume of 100 mL. Grind sample was centrifuged and an aliquot of 10 mL was taken for titration with the standard dye to a pink end point. Results were expressed as milligrams of ascorbic acid 100 g−1 FW arils.
Total phenols were estimated using Folin–Ciocalteau reagent (Singleton et al. 1999). None-refrigerated 20 g of arils were taken for juice extraction. In 200 μL of the aril aliquot extracted with 80% ethanol, added 2.8 mL of double distilled water, 0.5 mL of Folin–Ciocalteau reagent (1 N) and after three minutes waiting period 2.0 mL of 20% Na2CO3 solution was mixed. The mixture was allowed to stand for 60 min in dark conditions and absorption was measured at 760 nm against a reagent blank in UV–visible spectrophotometer (JASCO V-670, Ishikawa-machi, Hachioji-shi, Tokyo, Japan). Results were expressed in milligram gallic acid equivalent per 100 g of fresh aril weight (mg GAE 100 g−1 FW).
Total antioxidant activity and total anthocyanins
Ferric reducing antioxidant power (FRAP) of pomegranate arils was estimated by (Benzie and Strain 1996) method using 10 g arils. FRAP reagent of 3 mL was mixed with 100 μL of sample extract in a test tube, vortexes and incubated at 37 °C for 30 min in a water bath. Reduction in the ferric–tripyridyltriazine to the ferrous complex formed an intense blue color which was measured at a UV–visible spectrophotometer at 593 nm at the end of 4 min. Results were expressed in terms of μmol Trolox 100 g−1 FW.
Total anthocyanins content was determined by pH differential method using two buffer solutions; potassium chloride buffer (pH 1.0, 0.025 M) and sodium acetate buffer (pH 4.5, 0.4 M) (Wrolstad et al. 1982). Extract (1 mL) taken from 3 g arils was mixed with 9 mL of buffers and read against water as a blank at 520 and 700 nm. Results were expressed as milligrams of cyanidin-3-glucoside (molar absorptive coefficient = 26,900 and molecular weight = 449.2) equivalents per kilogram of fresh weight. Total anthocyanins content was calculated as follows; ([A × MW × DF × 1000]/MA × 1), where DF is the dilution factor, MW is molecular weight and MA is molar absorptive coefficient.
Ca content, polygalacturonase (EC 3.2.1.15) activity and respiration rate
Ash of the samples was dissolved in nitric acid till the final concentration reached up to 0.16 M. Peel and aril Ca content was measured by atomic absorption spectrophotometer and expressed in mg kg−1 FW (Sharma et al. 2014). Polygalacturonase (PG) activity was determined by following the method of (Lazan et al. 1995) and expressed in n ktal g−1 FW. Respiration rate was estimated by adopting the static head space technique using gas analyzer (Model: Checkmate 9900 O2/CO2, PBI Dansensor, Denmark) and results were expressed in mL CO2 kg−1 h−1. For this known quantity of pomegranate fruits were trapped in silicon septum fitted airtight containers. The containers were kept at 30 °C temperature for 3 h. After specified time, concentration of CO2 and O2 accumulated in container head space was recorded and respiration rate was measured by using following formula.
Colour
Colour characteristics were measured using a colour meter (Colour Tec PCM/PSM, USA), the instrument generates a set of Cartesian coordinate the measured colour in a three dimensional colour space. In the CIE (L*, a*, b*) colour space abbreviated CIELAB, the lightness co-efficient, L*, ranges from black (0) to white (100).
Sensory evaluation
Sensory evaluation of arils was performed with a slight modification in the method as described by Gadže et al. (2011). Coded samples were given to 10 judges (5 women and 5 men, aged 30–60 years) familiar with the quality of pomegranate arils. Minimum basic information on sensory quality of arils was provided to the judges in order to get unbiased results. They were asked to rinse their mouth with room temperature water before or in between testing the given sample. Each sample was sensory scored for parameter like overall acceptability, color texture, juiciness and sweetness using 9-point hedonic scale with 1, dislike extremely; 2, dislike very much; 3, dislike moderately; 4, dislike slightly; 5, neither like nor dislike; 6, like slightly; 7, like moderately; 8, like very much and 9, like extremely. Scores of above 6 out of 9 is considered as acceptable for commercial purposes. To draw the spider web diagram, statistically computed sensory score of different traits were used.
Statistical analysis
The experiment was conducted in a Completely Randomized Design (CRD) with four treatments and three replications. Data recorded from each treatment under different parameters were subjected to analysis of variance (ANOVA). Duncan’s multiple range test was used to compare means among different treatments at a significance level of P ≤ 0.05. All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC, USA).
Results and discussion
External quality attributes
Fruit bagging with different color (white, blue and red) significantly influence the external quality traits over un-bagged fruits (Table 1). Among bags, red color bags were found most efficient in reducing fruit cracking incidence to 1/3rd (66%) over control, while blue color bagged proved much better in reducing sun scald incidence. Similarly, 78% reduction in bacterial blight was also achieved with red color bagging, compared to non-bagged fruits. Irrespective to color, bagging totally checked thrips incidence and provided 12% reduction in fruit scratches (skin friction). Following bagging, earlier workers also reported reduction in fruit cracking in pomegranate (Yuan et al. 2012), mould and insects in apple (Sharma et al. 2014).
Table 1.
Effect of bagging and bag color on external quality attributes of pomegranate
| Treatment (bag color) | Fruit cracking (%) | Sun scalding (%) | Scratching (%) | Thrips incidence (%) | Bacterial spot (%) |
|---|---|---|---|---|---|
| White | 5.21 ± 0.20b | 4.00 ± 0.10b | 3.50 ± 0.26b | 00 | 5.18 ± 0.07b |
| Blue | 2.52 ± 0.22c | 0.00 | 3.50 ± 0.26b | 00 | 4.09 ± 0.07c |
| Red | 3.25 ± 0.26c | 3.54 ± 0.10b | 3.50 ± 0.26b | 00 | 3.50 ± 0.05d |
| Control | 9.52 ± 0.24a | 6.82 ± 0.12a | 25.44 ± 0.66a | 5.36 ± 0.03 | 16.20 ± 0.81a |
Data are mean ± S.E. of three replicate determination (n = 3)
Treatment values with similar letters not significant different (p < 0.05)
Peel weight and peel thickness
All three coloured bags significantly influenced peel weight of pomegranate fruit. The red colour bagged fruit yielded low peel weight (33.00 ± 0.32%) among other bags while control fruits showed maximum peel weight (47.15 ± 0.43%). Similarly red colour bags fruit gave minimum peel thickness (0.35 ± 0.01 cm) while other bagged and non-bagged fruits produced thicker peel (0.50 ± 0.01 cm, Table 2). Previously, it has been demonstrated that the plant cuticle thickness reduced with low light intensity and high moisture prevalence in growing environment (Oren-Shamir 2009). So, this decrease in peel thickness and peel weight in bagged fruit may be due to more humidity inside bag, which could have affected cell structure, configuration and cuticle thickness (Amarante et al. 2002).
Table 2.
Effect of bagging and bag color on physical attributes of pomegranate
| Treatments (bag color) | Peel weight (%) | Peel thickness (mm) | Fruit firmness (N) | Juice recovery (%) | Specific gravity | Aril Firmness (N) |
|---|---|---|---|---|---|---|
| White | 38.12 ± 0.62c | 0.40 ± 0.01c | 18.92 ± 0.20a | 48.20 ± 0.50b | 1.02 ± 0.01a | 206.15 ± 1.44b |
| Blue | 40.70 ± 0.30b | 0.45 ± 0.01b | 17.25 ± 0.20b | 47.12 ± 0.70b | 0.93 ± 0.01c | 191.45 ± 1. 15c |
| Red | 33.00 ± 0.32d | 0.35 ± 0.01d | 17.53 ± 0.26b | 55.09 ± 0.45a | 1.05 ± 0.01a | 206.15 ± 1.25b |
| Control | 47.15 ± 0.43a | 0.50 ± 0.01a | 19.21 ± 0.22a | 42.17 ± 0.61c | 0.98 ± 0.01b | 208.63 ± 1.34a |
Data are mean ± S.E. of three replicate determination (n = 3)
Treatment values with similar letters not significant different (p < 0.05)
Fruit firmness, aril firmness, juice recovery and specific gravity
Pre-harvest bagging with three types of coloured bags significantly influenced the fruit and arils firmness. Fruit covered with blue bags produced softer peel and arils (191.45 ± 0.15 N) than fruit bagged with red and white colour bags, while non-bagged fruit had the highest mean peel and arils firmness value (19.21 ± 0.22 N; Table 2). Calcium accumulation in fruit affects seed and peel firmness and water exchange from fruit surface due transpiration, exudation and vapour pressure (Simmons et al. 1998). Earlier, Hofman et al. (1997) also reported less calcium accumulation in bagged Keitt mango fruits. Non-bagged fruits yielded significantly lesser juice (42.17 ± 0.61%) than bagged fruits, among bagged fruits red bags fruits recorded maximum juice recovery (55.09 ± 0.45%, Table 2.). The apparent reasons for higher juice recovery in red colored bag fruits may be attributed to low peel weight and thickness. In our findings, there was less peel weight and thickness in red bagged fruits over control. Highest specific gravity (1.05 ± 0.01) was recorded with red and white (at par) color bagged fruits followed by blue and control. Comparative early maturation in red bag fruits might have induced rapid disintegration of aril tissues, which has possibly facilitated escaping of cell air and resulted in higher specific gravity. Specific gravity has a strong correlation with physical state of fruit like juice percentage and aril firmness (Supe and Saitwal 2016).
Total soluble solids and total sugars
Total soluble solids (TSS) contribute significantly to the sweetness, flavor and consumer preference. Irrespective to bag color, bagged fruit had significantly lower value of TSS (≈ 13.10 °Brix; Table 3) than non-bagged fruit (14.20 ± 0.12 °Brix). Non-bagged fruits recorded significantly higher (10.40% ± 0.21) sugar over bagged one being lower in blue colour bags fruits (9.45 ± 0.54%). Lower respiration rate in non-bagged fruits could have helped in maintaining higher sugar compare to bagged fruits. In earlier finding too bagging had shown the lower accumulation of sugars and organic acids in ‘Red Fuji’ Apples (Chen et al. 2017). With bagging, fruits are partially protected from sun light, heat and moisture deficit, which intern favored excess water accumulation and lowering down fruit juice TSS. Reduction in TSS was reported in bagged blood orange by Sun et al. (2015). Contrary to these findings, improvement in TSS and total sugars was reported in bagged apple (Sharma et al. 2014), pear (Lin et al. 2012) and mango (Watanawan et al. 2008).
Table 3.
Effect of bagging and bag color on biochemical attributes of pomegranate aril
| Treatments (bag color) | Total soluble solids (°B) | Total sugars (%) | Ascorbic acid (mg 100 g−1 FW) | Total phenols (mg GAE 100 g−1 FW) | Total antioxidant capacity (µmole Trolox 100 g−1 FW) | Total anthocyanin (mg 100 g−1 FW) |
|---|---|---|---|---|---|---|
| White | 13.13 ± 0.09b | 10.22 ± 0.74b | 14.56 ± 0.30b | 9.06 ± 0.48c | 17.80 ± 0.63a | 70.00 ± 3.52a |
| Blue | 13.10 ± 0.21b | 9.45 ± 0.54b | 15.74 ± 0.62b | 32.12 ± 1.53a | 12.91 ± 0.60c | 44.04 ± 2.18b |
| Red | 13.30 ± 0.12b | 10.20 ± 0.22a | 18.20 ± 0.87a | 15.20 ± 1.05b | 15.34 ± 0.12b | 73.03 ± 3.97a |
| Control | 14.20 ± 0.12a | 10.40 ± 0.21b | 15.62 ± 0.67b | 17.18 ± 0.91b | 19.72 ± 0.95a | 71.01 ± 4.48a |
Data are mean ± S.E. of three replicate determination (n = 3)
Treatment values with similar letters not significant different (p < 0.05)
Ascorbic acid and total phenols
Fruit covered with red colour bags had higher ascorbic acid content (18.20 ± 0.87 mg/100 g FW), while control, blue and white bags exhibited lower ascorbic acid (14.56–15.74 mg 100 g−1 FW). Blue colored bag fruits had higher TPC values (32.12 ± 1.53 mg GAE100 g−1 FW) over control, red and white-coloured bags respectively. Both the ascorbic acid and phenolics are temperature labile (Borochov-Neori et al. 2011) phyto-nutrients. Selective permeability of colored bags to sun light may be the probable reason for higher ascorbic acid in red bagged fruit and total phenolics in blue colour bagged fruits (Chonhenchob et al. 2011).
Total antioxidant capacity and total anthocyanins
Non- bagged fruits exhibited higher total antioxidant capacity (19.72 ± 0.95 μmol Trolox g−1 FW); than bagged fruits (12.91 ± 0.60–17.80 ± 0.63 μmol Trolox 100 g−1 FW). Anthocyanin, ascorbic acid and phenolic acids, either alone or in combination, are responsible for the antioxidant activity in pomegranate arils (Tehranifar et al. 2010). Additionally, there are numerous unknown components which contribute for total antioxidants in fruits. Therefore, despite having higher total phenols and total anthocyanin, colored bag fruits proved laggard in total antioxidant count over non-bagged fruits. Total anthocyanins values were highest in fruits bagged with red colour bags (73.03 ± 3.97 mg cyanidin-3-glucoside 100 g−1 FW, Table 3) compared to other bagged or non-bagged fruits. Biosynthesis and accumulation of anthocyanin in fruits is sensitive to crop growing environment (Seeram et al. 2005). Earlier, detrimental effect sunshine period and radiation level on anthocyanin accumulation has been reported by other researchers (Oren-Shamir 2009; Schwartz et al. 2009). The differential effects of bag colour on fruit total anthocyanins may be due to differences in the light reflectance, absorbance, or transmission patterns of each bag in the visible, far-red, and/or infra-red regions of the spectrum (Chonhenchob et al. 2011).
Ca content, polygalacturonase activity and respiration rate
Elemental calcium (Ca) is essential for cell wall formation and its integrity retention which helps in better fruit firmness and shelf-life during storage. Preharvest fruit bagging significantly reduced the aril and peel Ca content over non-bagged fruits (Table 4). The difference was much prominent in arils. Earlier researchers have also reported decrease in Ca content of bagged fruits in Red Fuji apple (Chen et al. 2017) and pear (Han et al. 2012). Contrary to these findings, Sharma et al. (2013) observed increase in Ca content in bagged Royal Delicious apple. In some other experiments, bagging yielded no effect on fruit Ca content in pear (Amarante et al. 2002) and mango (Joyce et al. 1997). It is presumed that bags are likely to subject a resistance to free movement of water vapour from fruit peel surface (Witney et al. 1991), thus reduce rate of transpiration and ultimately less Ca accumulation particularly in blue bagged fruits. Comparing to non-bagged fruits, bagging has significantly suppressed (> 50% in peel) PG activity being lowest with blue color bagged fruits. The activity of indigenous polygalacturonase (PG) is mainly responsible for the breakdown of carbohydrate and tissue softening during fruit ripening. As we have recorded lower TSS (indicator of onset of maturity) in bagged fruits over control fruits, it could have helped in suppression of PG activity (Asrey et al. 2013). The rate of starch modification (expected to be slow in bagged fruits) during fruit ripening may be another reason for lower PG activity in bagged fruits due to shading effect of bags. Sharma et al. (2013) also reported lower enzyme activities in bagged apple fruits. Marked difference was recorded in fruit respiration rate among all the treatments (Fig. 1a). Highest respiration rate (17.93 ± 0.22 mL CO2 kg−1 h−1) was recorded under red bagged fruit and lowest (14.44 ± 0.31 mL CO2 kg−1 h−1) in non bagged fruits. Comparatively higher respiration rate in red bagged fruit may be attributed to early onset of fruit maturity and enhanced (less cuticle formation) gas interchange and consequently high oxygen availability to the fruit tissues for respiration (Debnath and Mitra 2008).
Table 4.
Effect of bagging and bag colour on Ca content, PG activity and colour value of peel and aril
| Treatment (bag color) | Ca (mg kg−1) FW | PG (n ktal g−1) FW | L value | ‘a’ value | ‘b’ value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peel | Aril | Peel | Aril | Peel colour | Aril colour | Peel colour | Aril colour | Peel colour | Aril colour | |
| White | 118.19 ± 1.10b | 141.00 ± 1.21b | 1.00 ± 0.03b | 1.85 ± 1.13c | 42.7 ± 0.20a | 16.41 ± 0.04b | 35.06 ± 1.10b | 40.0 ± 2.10b | 35.0 ± 1.10a | 30.15 ± 1.13a |
| Blue | 110.56 ± 1.10c | 120.14 ± 1.01c | 0.81 ± 0.01c | 1.60 ± 0.67c | 35.5 ± 0.22b | 16.02 ± 0.02a | 32.12 ± 1.76a | 36.8 ± 2.22c | 37.16 ± 1.10b | 26.18 ± 0.67b |
| Red | 115.00 ± 1.00b | 138.25 ± 1.20b | 1.23 ± 0.03a | 2.01 ± 1.53b | 40.1 ± 0.26a | 18.25 ± 0.01b | 38.00 ± 1.10c | 45.11 ± 2.10a | 33.14 ± 1.20a | 33.00 ± 1.72a |
| Control | 135.12 ± 1.21a | 156.24 ± 1.33a | 2.03 ± 0.05b | 3.67 ± 0.62a | 35.8 ± 0.24b | 15.13 ± 0.02c | 34.18 ± 0.73b | 38.16 ± 2.80b | 34.00 ± 0.78a | 32.15 ± 1.73a |
Treatment values with similar letters not significant different (p < 0.05)
Fig. 1.
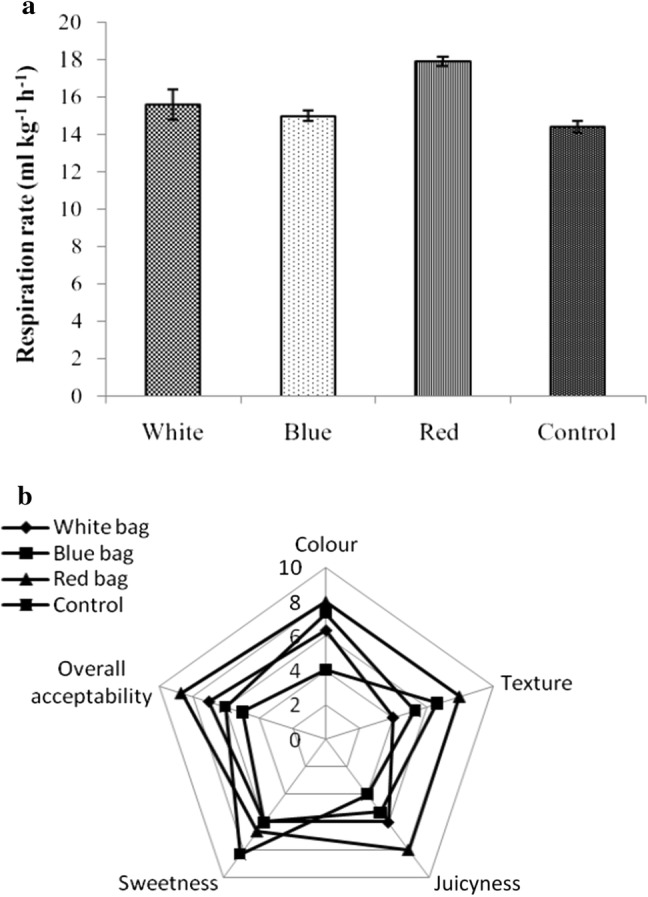
Effect of bag color on—a respiration rate, b sensory score of freshly harvested bagged pomegranate arils. Data are mean ± S.E. of three replicate determination (n = 3)
Peel and aril color (L, a, b value)
A significant variation in L* a and b values was recorded both in peel and aril color (Table 4). It is important to note that color variation (L*, a, b) was much contrasting in case of peel than aril. The L* value is a useful indicator of darkening either from oxidative browning reactions or from increasing pigment concentrations (Rocha and Morais 2003). Aril and peel of red bagged fruits exhibited higher L* values (18.25 and 40.1). This indicates positive impact of red bags on pigment synthesis in particular and protective role in general to minimize anthocyanins breakdown from sunlight (Kim et al. 2010).
Sensory evaluation
To ascertain the quality of any produce—colour, sweetness, texture, juiciness and aroma are major criteria used by the consumers (Nunes et al. 2007). The sensory score for quality parameters and overall acceptance for blue bagged arils was 5 which are lower than the commercial acceptance level of 6 (Fig. 1b). Red color bagged fruit arils showed highest overall acceptance score (8.7) over non-bagged, blue and white bagged fruits. As shown earlier, red color bagged fruit retained higher anthocyanins (color), juiciness, higher seed softness and these attributes might have contributed towards better overall aril appearance and thereby higher sensory score.
Conclusion
Fruit bagging in pomegranate improves most of the desired characters such as thin and attractive red peel, soft red juicy arils and rich anthocyanin. Red bag favored most of the quality attributes of pomegranate fruits except Ca content and total antioxidant activity. However, response of bagging varies from crop to crop depending on stage of fruit growth and color of the fruit bag. For harnessing the benefit of bagging in pomegranate, fruit should be bagged 60 days after flowering with red color cellulosic bags. More work should be carried out to explore its application in producing functionally rich fruits, vegetables and other food products.
Acknowledgements
We are highly thankful to Dr. J. Kumar and Kumar Nand Lal, division of Food Science & Postharvest Technology, ICAR-Indian Agricultural Research Institute New Delhi (India).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amarante C, Banks NH, Max S. Effect of preharvest bagging on fruit quality and postharvest physiology of pears (Pyrus communis) New Zealand J Crop Hort Sci. 2002;30(2):99–107. doi: 10.1080/01140671.2002.9514204. [DOI] [Google Scholar]
- Asrey R, Patel VB, Barman K, Pal RK. Pruning affects fruit yield and postharvest quality in mango (Mangifera indica L.) cv. Amrapali. Fruits. 2013;68:367–381. doi: 10.1051/fruits/2013082. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Borochov-Neori H, Judeinstein S, Harari M, Bar-Ya’Akov I, Patil BS, Lurie S, Holland D. Climate effects on anthocyanin accumulation and composition in the pomegranate (Punica granatum L.) fruit arils. J Agric Food Chem. 2011;59:5325–5334. doi: 10.1021/jf2003688. [DOI] [PubMed] [Google Scholar]
- Boussaa F, Zaouay F, Burlo-Carbonell F, Nuncio-Jáuregui N, Gamati M, El Arbi B, Melgarejo P, Hernandez F, Mars M. Combined effects of cropping system and harvest date determine quality and nutritional value of pomegranate fruits (Punica granatum L. cv. Gabsi) Sci Hortic. 2019;249:419–431. doi: 10.1016/j.scienta.2019.02.007. [DOI] [Google Scholar]
- Chen B, Mao J, Huang B, Baoqin YM, Liu Zijing H, Zonghuan M. Effect of bagging and time of harvest on fruit quality of ‘Red Fuji’ apple in high altitude area in China. Fruits. 2017;72(1):36–46. doi: 10.17660/th2017/72.1.4. [DOI] [Google Scholar]
- Chonhenchob V, Khamhangwang D, Kurunate J, Singh SP. Preharvest bagging with wavelength-selective materials enhances development and quality of mango (Mangifera indica L.) cv. Nam Dok Mai. J Sci Food Agril. 2011;91(4):664–671. doi: 10.1002/jsfa.4231. [DOI] [PubMed] [Google Scholar]
- Debnath S, Mitra SK. Panicle bagging for maturity regulation, quality improvement and fruit borer management in litchi (Litchi chinensis) Acta Hort. 2008;773:201–208. doi: 10.17660/ActaHortic.2008.773.29. [DOI] [Google Scholar]
- Gadze J, Voća S, Čmelik Z, Mustać I, Ercisli S, Radunić M. Physico-chemical characteristics of main pomegranate (Punica granatum L.) cultivars grown in Dalmatia region of Croatia. J Applied Bot Food Quality. 2012;85:202–206. [Google Scholar]
- Gadže J, Prlić M, Bulić M, Leko M, Barbarić M, Vego D, Raguž M. Physical and chemical characteristics and sensory evaluation of pomegranate fruit of (Punica granatum L.) cv. “Glavaš”. Pomologia Croatica. 2011;17(3–4):87–97. [Google Scholar]
- Gumienna M, Szwengiel A, Gorna B. Bioactive components of pomegranate fruit and their transformation by fermentation processes. European Food Res Tech. 2016;242:631–640. doi: 10.1007/s00217-015-2582-z. [DOI] [Google Scholar]
- Han Y, Wang Y, Liu S. Effect of bagging on contents of mineral elements in Huangguan pear fruit. J Acta Agri Boreali-sinica. 2012;27(S1):144–148. [Google Scholar]
- Hofman PJ, Smith LG, Joyce DC, Johnson GL, Meiburg GF. Bagging of mango (Mangifera indica cv. “Keitt”) fruit influences fruit quality and mineral composition. Postarvest Biol Technol. 1997;12:83–91. doi: 10.1016/S0925-5214(97)00039-2. [DOI] [Google Scholar]
- Jha SK, Sethi S, Srivastav M, Dubey AK, Sharma RR, Samuel DVK, Singh AK. Firmness characteristics of mango hybrids under ambient storage. J. Food Engineering. 2010;97:208–212. doi: 10.1016/j.jfoodeng.2009.10.011. [DOI] [Google Scholar]
- Jhang BB, Guo JY, Ma RJ, Cai JX, Yan J, Zhang CH. Relationship between the bagging microenvironment and fruit quality in ‘Guibao’ peach [Prunus persica (L.) Batsch] J Hort Sci Biotech. 2015;90(3):303–310. doi: 10.1080/14620316.2015.11513187. [DOI] [Google Scholar]
- Joyce DC, Beasley DR, Shorter AJ. Effect of preharvest bagging on fruit calcium levels, and storage and ripening characteristics of ‘Sensation’ mangoes. Australian J Exp Agric. 1997;37:383–389. doi: 10.1071/EA96074. [DOI] [Google Scholar]
- Kim YK, Kang SS, Cho KS, Jeong SB. Effects of bagging with different pear paper bags on the color of fruit skin and qualities in “Manpungbae”. Kor J Hortic Sci Technol. 2010;28:36–40. [Google Scholar]
- Kitagawa H, Manabe K, Esguerra EB. Bagging of fruit on the tree to control disease. Acta Hort. 1992;321:870–875. [Google Scholar]
- Lazan H, Selamat M, Ali ZM. Β-Galactosidase, polygalacturonase and pectinesterase in differential softening and cell wall modification during papaya fruit ripening. Physiol Plant. 1995;95:106–112. doi: 10.1111/j.1399-3054.1995.tb00815.x. [DOI] [Google Scholar]
- Lin J, Wang JH, Li XJ, Chang YH. Effects of bagging twice and room temperature storage on quality of “Cuiguan” pear fruit. Acta Hort. 2012;934:837–840. doi: 10.17660/ActaHortic.2012.934.110. [DOI] [Google Scholar]
- Nunes MCN, Emond JP, Brecht JK, Dea S, Proulx E. Quality curves for mango fruit (cv. Tommy Atkins and Palmer) stored at chilling and non-chilling temperatures. J Food Qual. 2007;30:104–120. doi: 10.1111/j.1745-4557.2007.00109.x. [DOI] [Google Scholar]
- Oren-Shamir M. Does anthocyanin degradation play a significant role in determining pigment concentration in plants. Plant Sci. 2009;177:310–316. doi: 10.1016/j.plantsci.2009.06.015. [DOI] [Google Scholar]
- Ozcan MM, Aljuhaimi F, Uslu N, Ahmed IM, Osman MA, Gassem MA, Salih HA. Effect of oven drying on antioxidant activity, phenolic compounds, fatty acid composition and to copherol contents. J. Food Processing and Preservation. 2019 doi: 10.1111/jfpp.13885. [DOI] [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruit and vegetable products. New Delhi: Tata McGraw-Hill Education Publication; 1999. [Google Scholar]
- Rocha AMCN, Morais AMMB. Shelf life of minimally processed apple (cv. Jonagored) determined by color changes. Food Cont. 2003;14:13–20. doi: 10.1016/S0956-7135(02)00046-4. [DOI] [Google Scholar]
- Schwartz E, Zulker R, Glazer I, Bar-Ya’Akov I, Wiesman Z, Tripler E, Bar-Ilan I, Fromm H, Borochov-Neori H, Holland D, Amir R. Environmental conditions affect the color, taste and antioxidant capacity of 11 pomegranate accessions fruits. J Agric Food Chem. 2009;57:9197–9209. doi: 10.1021/jf901466c. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D. In vitro anti-proliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Shalomo M. Efficiency of bagging pomegranate fruits. Acta Hort. 2015;1089:485–488. doi: 10.17660/ActaHortic.2015.1089.66. [DOI] [Google Scholar]
- Sharma RR, Pal RK, Ram Asrey, Sagar VR, Dhiman MR, Rana MR. Pre-harvest fruit bagging influences fruit color and quality of apple cv. Delicious. Agri Sci. 2013;4(9):443–448. [Google Scholar]
- Sharma RR, Pal RK, Sagar VR, Pramanick KK, Paul V, Gupta BK. Impact of pre-harvest fruit-bagging with different coloured bags on peel colour and the incidence of insect pests, disease and storage disorders in ‘Royal Delicious’ apple. J Hort Sci Biotech. 2014;89(6):613–618. doi: 10.1080/14620316.2014.11513128. [DOI] [Google Scholar]
- Simmons SL, Hofman PJ, Whiley AW, Hetherington SE. Effects of leaf:fruit ratios on fruit growth, mineral concentration and quality of mango (Mangifera indica L. cv. Kensington Pride) J Hort Sci Biotechnol. 1998;73:367–374. doi: 10.1080/14620316.1998.11510987. [DOI] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Ranventos RM. Analysis of total phenols other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Meth Enzymol. 1999;99:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Sun Q, Zheng L, Wan-Peng X, Shao-Lan H. Transcriptome analysis of blood orange (Citrus sinensis) following fruit bagging treatment by digital gene expression profiling. J Horti Sci Biotechnol. 2015;89(4):397–407. doi: 10.1080/14620316.2014.11513098. [DOI] [Google Scholar]
- Supe VS, Saitwal YS. Morphological, biochemical and qualitative changes associated with growth and development of pomegranate fruit (Punica granatum L.) Indian J Agric Res. 2016;50(1):80–83. [Google Scholar]
- Tehranifar EA, Zarei M, Nemati Z, Esfandiyari BVB, Vazifeshenas AMR. Investigation of physico-chemical properties and antioxidant activity of twenty Iranian pomegranate (Punica granatum L.) cultivars. Sci Horti. 2010;126:180–185. doi: 10.1016/j.scienta.2010.07.001. [DOI] [Google Scholar]
- Watanawan A, Watanawan C, Jarunate J. Bagging “Nam Dok Mai #4” mango during development affects color and fruit quality. Acta Hort. 2008;787:325–328. doi: 10.17660/ActaHortic.2008.787.40. [DOI] [Google Scholar]
- Witney GW, Kushad MM, Barden JA. Induction of bitter pit in apple. Scientia Hort. 1991;47:173–176. doi: 10.1016/0304-4238(91)90039-2. [DOI] [Google Scholar]
- Wrolstad RE, Culbertson JD, Cornwell CJ, Mattick LR. Detection of adulteration in blackberry juice concentrates and wines. Journal-Association of Official Analytical Chemists. 1982;65:1417–1423. [PubMed] [Google Scholar]
- Yuan ZH, Yin YL, Feng LJ, Zhao XQ, Hou LF, Zhang YX. Evaluation of Pomegranate bagging and fruit cracking in Shandong, China. Acta Hort. 2012;940:125–130. doi: 10.17660/ActaHortic.2012.940.15. [DOI] [Google Scholar]


