Abstract
The effect of pectinolytic enzyme preparation (PEP) produced by the fungus Thermomucor indicae-seudaticae-N31 (PEP-N31) on total phenolic content, concentrations of methanol and color of grape juice was studied. Positive results were found when PEP-N31 was used to extract phenolic compounds after the grapes had been blanched for 3 min and macerated for 1 h. The resulting juice had better yield, color characteristics and higher phenolic content (1637.21 mg.L-1, as gallic acid equivalent, or GAE) than the conventionally prepared juice (1422.59 mg GAE.L-1), and it was very similar to the juice obtained through the treatment with a commercial enzyme (1682.10 mg GAE.L-1). The concentration of methanol in the juice produced with the PEP-N31 was less than 200 mg.L-1. These results encourage the use of PEP produced by Thermomucor indicae-seudaticae-N31 by the grape-processing industry.
Keywords: Pectinolytic enzyme, Juice, Phenolic content, Methanol, Grape
Introduction
Grape juices are important sources of bioactive compounds, including anthocyanins and other phenolic compounds that have been associated with improved human development and health, as well as with lower risk of degenerative diseases (Magro et al. 2016; Toaldo et al. 2015).
The different techniques employed in the production process (such as the extraction method or thermal and enzymatic treatments) can have a profound impact on the potential health benefits of this product. Some studies have reported that exposing whole or previously crushed fruits to steam blanching can increase the extraction of phenolic compounds and denature oxidase enzymes such as polyphenol oxidase (PPO) and peroxidase (POD). Temperature and the amount of time that the grape is exposed to heat are relevant parameters that must be controlled during the process (Brambilla et al. 2008; Vagiri and Jensen 2017; Xiao et al. 2017). Complex pectinolytic mixtures from microorganisms, some of which contain cellulases, hemicellulases and proteases, are being used to promote the hydrolysis of the polysaccharides that are present in the cell wall of grapes, to allow the releasing of the components present within the cell vacuoles into the grape must (Amin et al. 2019; Magro et al. 2016; Saharan and Sharma 2018).
Microbial enzymes which degrade pectin are classified according to their mode of attack on the pectin molecule. The pectin methylesterases (PME, EC 3.1.11.1) is classified as demethoxylating, which de-esterify pectins by removing methoxy groups (OCH3), and, consequently, release methanol and low methoxyl pectins (Pedrolli et al. 2009). The other group include the depolymerizing enzymes, which breakdown the α-1,4 glycosidic linkages between galacturonosyl (methyl ester) residues. Depolymerizing pectinases acting by trans-elimination results in galacturonide with an unsaturated bond between C4 and C5 at the non-reducing end of the galacturonic acid formed. The main representatives of this group are pectin lyase and pectate lyase. If the enzyme acts by hydrolysis, it can be polymethylgalacturonases (PMG), acting preferentially in highly esterified pectin or it can be polygalacturonases (PG), acting in pectic acid (polygalacturonic acid). In addition, depending upon the pattern of action on the polygalacturonan chain, these enzymes are termed as endo- or exo- enzymes. If the cleavage is random (endo-), the enzymes are called liquefying enzymes and, if the enzyme works by cleaving galacturonate units sequentially (exo-) of a polygalacturonan chain, the enzymes are called saccharifying enzymes (Silva et al. 2007). The use of enzyme preparations that contain pectin methylesterase (PME) is widely used in the fruit processing to increase juice yield (Magro et al. 2016; Siddiq et al. 2018), however, may result in an increased concentration of methanol in the products (Hou et al. 2008; Pedrolli et al. 2009; Saharan and Sharma 2018).
In the light of this information, efforts have been concentrated on developing pectinolytic enzyme preparations that safely and adequately perform their functions under specific processing conditions for juices (Amin et al. 2019; Barman et al. 2015; Magro et al. 2016; Siddiq et al. 2018) to developing functional foods (Khan et al. 2013; Saharan and Sharma 2018). Attention has been given to pectinases produced by thermophilic fungi which can exhibit important characteristics, such as higher thermostability, optimum activity at higher temperatures and high rates of hydrolysis (Bala and Singh 2018), which are all interesting in terms of industrial application (Amin et al. 2019).
The thermophilic fungus Thermomucor indicae-seudaticae N31 produced a mix of pectinases using solid state fermentation (Martin et al. 2010) which encouraged studies of its potential for industrial use. The goal of this study was to evaluate the effect of the pectinolytic enzyme preparation made with this fungus on the an improve in quality of produced juice, evaluating o total phenolic content (TPC), the concentration of methanol and the color of grape juice. In order to make comparisons, the commercial preparation Vinozym Vintage FCE was applied to the grapes under the same experimental conditions. This work presents, for the first time, studies on the application of pectinase produced by solid state fermentation on wheat bran by the thermophilic fungus Thermomucor indicae-seudaticae N31.
Materials and methods
Materials
Grapes
During the 2010 harvest season, healthy Bordô grapes (Vitis labrusca) were collected at the optimum ripeness usually reached by this grape cultivar in the town of Jales in São Paulo state, Brazil. Once in the lab, the grapes were randomly separated into subsamples, and used for all the different types of analyses, which were each performed in triplicate. The results were given as the average of the data obtained for all batches of grapes (n = 3). The sampled grapes were found to have the following characteristics, measured following the official analysis methods proposed by the Association of Official Analytical Chemists (AOAC) (1990): soluble solids (approximately sugar content) of 12.92 ± 0.29° Brix (refractometer Abbe Model 2 Waj, Biobrix); a total acidity of 5.5 ± 0.1 g as tartaric acid.kg-1 of fresh grape weight; and, pH levels of 3.34 ± 0.05 (pHmeter, Marconi).
Enzymes preparations
The pectinase enzyme preparation (PEP-N31) was made using the thermophilic fungus Thermomucor indicae-seudaticae N31 through solid state fermentation according to Martin et al. (2010). Mycotoxin analyses were done in PEP-N31 and the results showed that PEP-N31 is free of Aflatoxin B1, B2, G1 and G2, ochratoxin A and zearalenone. The comercial pectinase preparation was Vinozym Vintage FCE (Novozyme, Germany) which is made with the fungi Aspergillus niger and Aspergillus aculeatu (according to manufacturer’s information).
Methods
Pectinases activities in the fungal enzyme preparations
Exopolygalacturonase (Exo-PG) activity was assayed with 0.9 mL of 1% solution of citric pectin 67% methoxylated (KELCO, SP, Brazil) in 0.2 M sodium acetate buffer (pH 4.5) and 0.1 mL of enzyme solution at 37 °C for 10 min. The number of reducing groups, expressed as galacturonic acid released by enzymatic action, was quantified by the DNS method (Miller 1959). One unit of enzyme activity (U) was defined as the amount of enzyme releasing one l μmol of galacturonic acid, per min. The results were expressed as the mean ± standard deviation (SD).
Endopolygalacturonase (Endo-PG) activity was determined by measuring the reduction in the pectin solution’s viscosity after an enzymatic reaction. The reaction mixture containing 6 mL of 3% solution of 67% methoxylated citrus pectin (KELCO, Sao Paulo, Brazil) in 0.2-M acetate buffer at pH 4.5 and 2 mL of pectinolytic enzyme preparation was incubated at 37 °C for 10 min. The viscosity of the reaction mixture was determined using a Fungilab basic viscometer at 25 °C. One unit of enzymatic activity (U) was defined as the amount of the enzyme necessary to reduce the viscosity of the solution by 50% under the testing conditions (Lopez et al. 1994). The results were expressed as the mean ± SD.
Pectin methylesterase (PME) was determined titrimetrically according to the method described by Deng, Wu and Li (2005): 5 mL of 1% solution of 93% methoxylated citrus pectin (Sigma) containing 0.6% NaCl was used as substrate and adjusted to pH 7.0 with 0.02 M NaOH at 35 °C before the addition of 1 mL of enzyme preparation. After the enzyme addition, the reaction mixture remained incubated at 35 °C and was stirred continuously. During the course of the reaction, the pH was maintained at 7.0 by adding 0.01 M NaOH solution using an automatic pH–stat titrator (Metrohm, Berchem, Belgium) for 10 min. The amount of NaOH consumed to keep the pH 7.0 was used to calculate the enzyme activity. One unit of PME was defined as μmol equivalent of acid per minute per mg or mL, under the test conditions. The results were expressed as the mean ± SD.
Endogenous enzymes activities in the grapes
In order to determine PPO and POD activity in Bordô grapes, an enzyme extract was first obtained using an extraction solution containing Triton-X-100, following the method described by Lago-Vanzela et al. (2011). PPO and POD activities were assessed in triplicate using a DU 640 spectrophotometer (BECKMAN COULTER) following the method of González et al. (2000). One unit of enzyme activity (U) was arbitrarily defined as the amount of enzyme that caused an increase in the absorbance of 0.001/min/100 g fresh fruit under the assay conditions. The results were expressed as the mean ± SD.
To determine pectinase activities in Bordô grapes, two samples of the fruit (100 g each) were homogenized with a 0.5 M Tris–HCl buffer solution (pH 8.0) that contained 1 mM of EDTA, 5% PVP and 2 M of NaCl at a 1:1 w/v ratio. The samples were submitted to an extraction procedure similar to that used for PPO and POD. The enzymatic activities of pectinases (Exo-PG, Endo-PG and PME) in grapes were determined as described before. All of the enzymatic activities values were converted to reflect enzymes in 100 g of fresh fruit. The results were expressed as the mean ± SD.
Effect of steam blanching on PPO, POD and PME endogenous enzymes, as well as on the extraction of phenolic compounds from grapes
Aiming to determine an efficient steam blanching method for inactivation of endogenous enzymes (PPO, POD and PME) in grapes, as well as to assist extraction of phenolic compounds from the grape juice, without excessive production of methanol due to a possible catalytic action of the endogenous enzyme PME, grape samples were washed with deionised water and exposed to the saturated steam at 94 ± 1 °C (the city of São Jose do Rio Preto, Brazil) for different amounts of time (0–10 min, with 1 min intervals) followed by cooling with the aid of an ice bath to terminate the heat treatment. They were then dried and set aside for later analysis.
In these samples of grape, PPO and POD activities were determined as previously described in item 2.2.2 In addition, the total phenolic content (TPC) of the grapes in natura and blanched were determined using a modified version of the Folin–Ciocalteau calorimetric method (Singleton et al. 1999). The absorbance was measured at 765 nm (spectrophotometer, Beckman Coulter DU-640). The results were expressed as gallic acid equivalents (GAE) in mg.L-1. Calibration was performed by analyzing the standard gallic acid (Sigma-Aldrich CO, USA) at different concentrations (R2 = 0.99). The percentage of TPC in grapes exposed to steam blanching was calculated by comparing it to the grape in natura, which corresponds to 100%. The concentration of methanol in the grapes before and after steam blanching (for different times) was used as an indirect method to evaluate if the endogenous enzyme PME acted on the pectin present in the grape to produce methanol. Therefore, the concentration of methanol was determined according to Baffi et al. (2011), using an HP-5890 gas chromatograph equipped with a Flame Ionization Detector (FID) and a HP-FFAP fused silica capillary column (25 m × 0.2 mm × 0.3 μm). 3 mL aliquots of the grape must were kept at 40 °C for 10 min, and 20 μL of the volatile fraction was injected. In order to quantify the concentration of methanol (mg.L-1), standard methanol solutions were used at different concentrations.
In light of the results, the most adequate length of blanching time was standardized to be used in the pre-treatment of the grapes for juice production.
Juice preparation using pectinase enzyme preparation PEP-N31 and its effect in the juice quality
For the production of juices, 3 kg samples of grape berries were exposed to saturated vapor for 3 min and after cooled to 37 °C. At this temperature, the grapes were gently crushed and depectinization was performed for different amounts of time (0.5, 1, 2, 3, and 4 h) while being mixed at 37 °C using PEP-N31 (1 mL.kg-1 of grape) and Vinozym Vintage FCE (Novozyme, Germany) (1 mg.kg-1 of grape), at the fruit’s natural pH. It is important to note, though, that Vinozym is in a lyophilized form, unlike PEP-N31, which is an aqueous extract. The activities were normalized based on the activity of Exo-PG. In sequence, the grapes were pressed and the grape juices was filtered and centrifuged (10 min/6000 g/4 °C). The juices obtained were exposed to saturated vapor (94 °C) for 5 min, and then immediately bottled in 250 mL glass flasks and cooled to room temperature. The control juice was prepared in the same way, except for the use of the enzyme.
To evaluate the quality of the juice produced using the enzymatic preparations PEP-N31 and Vinozym (used as control for comparison) yield, concentration of methanol, TPC and color were determined. The determination of concentration of methanol and TPC were determined as previously described in the item 2.2.3 Juice yield was determined by weighing the pressed juice, which was compared to the initial sample weight. The color index of the juices were determined following Glories (1984) with the equation: CI = 0.1 * (abs|420 + abs|520 + abs|620), where 420 nm corresponded to yellow, 520 nm corresponded to red, and 620 nm corresponded to violet. Absorbance measurements were made in a Beckman DU-640 Spectrophotometer, USA with 1.0 cm path length glass cells. The results were expressed as the mean ± SD.
In order to characterize the juices produced under the best process conditions, with and without the ezimatic preparations, the following physico-chemical analyzes were performed: density at 20 °C (g.cm-3), non-reducing sugars as sucrose (g.100 g-1), reducing sugars as glucose (g.100 g-1), total sugars from the grape (g.100 g-1), total acidity as tartaric acid (g.100 g-1), volatile acid as acetic acid (g.100 g-1), soluble solids at 20 °C (°Brix), insoluble solids at 20 °C (% v/v), total dry extract (g.kg-1), ash content (g.100 g-1) and pH. All these analyses were made according to analytical methods of the AOAC (1990) and the results were expressed as the mean ± SD.
Statistical analysis
All of the results obtained through these analyses were applied to an analysis of variance (ANOVA), and the averages determined in each experiment were used in Tukey’s test with a 5% error rate in the ESTAT computer program, Version 2.0.
Results and discussion
Partial biochemical characterization of the fungal enzyme preparations and of the grape
The main pectinases in the fungal enzyme preparations are presented in Table 1. PEP-N31 had Endo-PG, Exo-PG and PME activities. For juice application the PME cannot be too high because it leads to an excess of methanol. The manufacturer, Vinozym, only declares the presence of PG and an activity of 7500 PGNU.g-1, which is an enzymatic Unit defined by the company. However, both PEP-N31 (4.5 U.mL-1) and Vinozym (6.5 U.mL-1) presented PME activity. Magro et al. (2016) characterized some enzymes, incluing the total pectinase, PG, pectinlyase, PME, and total cellulase, from two commercial pectinolytic enzyme preparations. They reported significant differences among both the compositions of the enzymes present and their concentrations. Thus, it would be reasonable to monitor concentrations of methanol in juices produced with grapes treated with enzymes in order to avoid the potential risk to consumer health, as has been advised by Hou et al. (2008).
Table 1.
Enzyme activities of the main pectinolytic enzymes
| PEP | Endo-PG | Exo-PG | PME |
|---|---|---|---|
| PEP-N31 (U.mL-1) | 0.65 ± 0.01 | 6.97 ± 0.24 | 4.50 ± 0.16 |
| Vinozym (U.mg-1) | 3.65 ± 0.12 | 7.25 ± 0.12 | 6.25 ± 0.10 |
Analyses were performed in triplicate
When analyzing the presence of endogenous enzymes in Bordô grapes, was found to have PPO and POD activities, as shown in Table 2, this grape. In knowledge of the presence of these endogenous enzymes is of paramount importance, since theses enzymes which may oxidize the phenolic compounds during grape maceration (Cheynier et al. 2013).
Table 2.
Partial biochemical characteristics of Bordô grapes
| Enzymes | Average ± SD |
|---|---|
| PPO | 21.42 ± 1.15 |
| POD | 44.19 ± 0.12 |
| Endo-PG | 64.54 ± 0.34 |
| Exo-PG | 547.09 ± 2.87 |
| PME | 15.90 ± 0.98 |
Analyses were performed in triplicate. Activities are expressed as U.100 g-1 of fresh weight
During the processing of the Bordô grape to obtain the juice, the enzymatic reactions usually occur during the initial technological operations. The endogenous enzymes polyphenol oxidase and peroxidase of Bordô grape can catalyze reactions in the crushing stage, were the phenolic compounds come into contact with oxygen, potentiating also chemical reactions, that can lead to the formation of new compounds with different color and biological activity from those originally present in the grapes (de Freitas et al. 2008; Xiao et al. 2017).
Regarding the prospect of the pectins present in the Bordô grape, it was possible to detect the activity of the three pectinases (Exo-PG, Endo-PG and PME), as reported by Deytieux-Belleau et al. (2008) and de Freitas et al. (2008). Due to these results, a steam bleaching method was needed to minimize the possible deleterious effects caused by these enzymes, especially PPO, POD and PME. Therefore, a study about the effect of steam blanching as the pretreatment to obtain grape juice on these endogenous enzymes as well as on the extraction of phenolic compounds present in the grape was carried out.
Effect of steam blanching as pretreatment to obtain grape juice
Among the endogenous enzymes with activity in the Bordô grape, POD is among the most heat stable, along with PME, being used in the juice processing industry as a reference to indicate the efficiency of the blanching process (de Freitas et al. 2008). Due to the fact that this enzyme presents high thermal stability, its ease of assay and for providing a good margin of safety in that, if peroxidase is inactivated, other quality-related enzymes have been inactivated. On the other hand, thermal treatment even under rigorous temperature–time regimes may cause undesirable changes, on physical and chemical properties, such as colour, texture and nutrient content. Therefore, particular attention has to be paid to minimize the heat treatment impact while, however, keeping the peroxidase activity to a suitable residual level (Etzbach et al. 2019). Thus, to determine the most efficient blanching process to be employed, the residual activity of the PPO enzymes and, mainly, of the POD were monitored at each minute of blanching for 6 min, as well as the effect of this pre-treatment in the phenolic compounds at the same intervals.
Figure 1 shows that residual PPO and POD activities decreased with an increase in the length of time the grape berries were exposed to steam blanching and that the respective enzymes were inactivated in about 2 and 5 min. It is important to note that, within 3 min of blanching, the grape residual PPO activity was completely inactivated while the grape residual POD activity decreased slightly (14%). These results are consistent with others papers reporting oxidoreductase inactivation in other fruits, in which the time required varied between 2 and 8 min (Llano et al. 2003; Ndiaye et al. 2009). The use of steam blanching as a pre-treatment for inactivating these enzymes in grapes proved to be an almost linear process for PPO and a non-linear one for POD. This is a little different from the results of de Freitas et al. (2008), who reported a non-linear process for both enzymes from each of the Benitaka and Rubi varieties of grapes.
Fig. 1.

PPO, POD and phenolic content of grapes exposed to steam blanching. Total phenolic content (bars), residual activity PPO (squares) and residual activity POD (circles) in Bordô grapes using different steam blanching times
Grape juice obtained from fresh grapes showed a methanol concentration of 73.5 mg.L-1, a value that was lower than the value reported by Rizzon and Link (2006), in another study on Bordô grape juice (135.3 mg.L-1). After incubating the macerated Bordô grape at 37 °C for 1 h, the concentration of methanol increased significantly, reaching 673.5 mg.L-1. This result suggests that, under these processing conditions, endogenous PME may act upon the pectin in grape by releasing methanol into the juices. Similar results were reported by Anthon and Barrett (2010). They have shown that PME can be induced to attack cell wall pectins by heating the tissue, mainly in a low-temperature blanch, as shown by a rapid increase in methanol. However, after thermal treatment (steam blanching) for 3–6 min, only traces of methanol were found in the grape juices (data not shown). It suggests that a 3-min thermal treatment is enough to minimize PME action. In the production of tomato paste, for example, the tomato is rapidly heated to 95 °C immediately after homogenization (the “hot-break” process), to inactivate enzymes rapidly, particularly those involved in pectin degradation (Luh and Daouf 1971). Probably the same occurred in the present study. Better understanding of the relationship between these endogenous enzymes in the grape and the control of residual levels of the methanol during processing will improve the quality of the processed products.
The steam blanching treatment of grapes in about 1–5 min increased the TPC of the grape musts (Fig. 1). These results suggest that the steam blanching process is likely to result in softer tissues, leading to a loss in cell cohesion and mechanical strength of the cell wall, and therefore to a increasing extraction efficiency these bioactive compounds of the grape skin for grape juice. Similar results were reported in study on black chokeberry, where heating of the mash prior to enzyme addition led to the production of a juice with higher yield and content of anthocyanins, regardless of enzyme addition and pre-processing treatments (Vagiri and Jensen 2017). Rossi et al. (2003) also observed that the inactivation of PPO before milling of blueberry fruits by steam blanching resulted in a significant increase in the recovery of the most soluble anthocyanin pigments in blueberry juice. Brambilla et al. (2008) reported a positive effect on the use of steam bleaching on the quality attributes of blueberry purées frozen in terms of color, anthocyanin monomeric pigments and total phenolic compounds. According to these authors, the steam blanching could be considered a valid step along the chain of processing of the juice to improve the extraction rate of the phenolic compounds and, therefore, contributing to obtain a product similar to the original fruit. The results in this study indicate that a standard steam blanching time of 3 min for Bordô grapes as a pretreatment for juice extraction is appropriate. This makes it possible to more consistently evaluate and without interference if any PME present both in the PEP-N31 and the commercial enzyme (control) could produce methanol during the extraction of the grape juice.
Effect of pectinase enzymes preparations PEP-N31 on grape juice quality
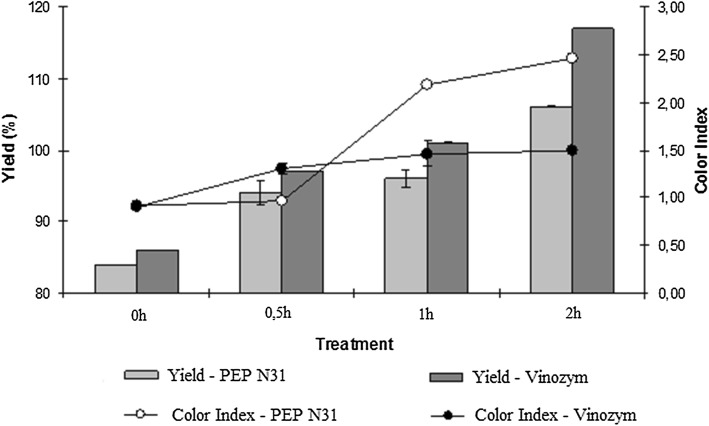
All the juices were produced from blanched grapes. The grape juice yields increased significantly in enzyme-treated grapes in comparison to control treatment (no enzyme added). This increase is more pronounced in the samples that had been treated with Vinozym. The results show that, after 2 h of treatment, the juices that had been prepared with either PEP-N31 and Vinozym presented maximum values of the yields (106% and 117%, respectively) (Fig. 3). These yields are next those reported by Magro et al. (2016) and Siddiq et al. (2018).
Fig. 3.
Yield and color index of juices. Juices were prepared with blanched grapes without enzymes (0 h) and blanched grapes treated with N31 or Vinozym Vintage FCE preparations for different times
Figure 2 shows that the juice obtained at time zero did not contain methanol. Over time both enzymes, PEP-N31 and Vinozym, produce higher concentrations of methanol in the juices. Maximum values were reached after 3 h of treatment for both pectinolytic enzyme preparations and posterior stabilization was reached after 4 h of treatment. Maximum limits for daily methanol consumption that are specific to juices have yet to be determined. While the maximum limits for wines established by the International Organization of Wine—OIV (1990) are 150 mg.L-1 in white wine and rosé and 300 mg.L-1 in red wine, this study found that the introduction of the enzymatic treatment into the production process resulted in products with concentrations of methanol that were less than 200 mg.L-1 (Fig. 2 and Table 3). These results are important because they suggest that products prepared with PEP-N31 under these conditions do not present any potential health risks from excessive levels of methanol. Figure 2 also shows that the use of PEP-N31 caused an increase in TPC in the juice compared with the juice which was bleached only (0 h). The results show that, after 1 h of treatment, the juices that had been prepared with either PEP-N31 (1677.25 mg.L-1) or Vinozym (1682.10 mg.L-1) already possessed significant TPC and the results from both enzyme preparations were very similar. However, higher phenolic levels were obtained after 2, 3 and 4 h of contact between Vinozym and the grape than with similar between PEP-N31 and the grape.
Fig. 2.
Phenolic content and methanol concentration in juices. The juices were prepared with blanched grapes without (0 h) or with the PEP-N31 or Vinozym Vintage FCE preparations for different times
Table 3.
Physical-chemical characteristics of grape juices
| Analysis | Control | Vinozym | PEP-N31 |
|---|---|---|---|
| Density at 20 °C (g.cm-3) | 1.07 | 1.07 | 1.07 |
| The relationship between °Brix and total acidity (g.kg-1) | 29.92 | 29.91 | 31.70 |
| Non-reducing sugars in sacarose (g.100 g-1) | 3.77 | 3.29 | 3.64 |
| Reducing sugars in glucose (g.100 g-1) | 10.38 | 11.40 | 10.63 |
| Total natural sugars from the grape (g.100 g-1) | 14.30 | 14.69 | 14.26 |
| Total acidity in tartaric acid (g.100 g-1) | 0.56 | 0.56 | 0.51 |
| Volatile acid in acetic acid (g.100 g-1) | 0.01 | 0.01 | 0.01 |
| Alcohol concentration (% vol) | ND | ND | ND |
| Methanol concentration (mg.L-1) | ND | 104 | 128 |
| Soluble solids at 20 °C (°Brix) | 14.20 | 14.12 | 13.98 |
| Insoluble solids at 20 °C (% v/v) | 2.60 | 2.30 | 3.14 |
| Total dry extract (g.Kg-1) | 18.90 | 18.98 | 18.40 |
| Ash content (g.100 g-1) | 0.66 | 0.55 | 0.64 |
| pH | 3.17 | 3.18 | 3.18 |
Juices were analyzed in triplicate and averages of the results were presented
ND not detected
Magro et al. (2016) also found positive results regarding the use of pectinolytic enzymes and their role in the extractability of phenolic compounds from fruits during the process of obtaining juice. The TPC found in juices produced with Bordô grapes is in agreement to reported data by Margraf et al. (2016) in a study on commercial juices obtened of diferents purple Brazilian grapes from diferentes producing regions, between them the Bordô grapes (2311 mg GAE.L-1), and also by those reported by Toaldo et al. (2015) who studied juices made from the V. labrusca L., being found close values to the convencional juice prepared with Isabel and Bordô grapes at a juice proportion of 1:1 (v/v) (2015 mg GAE.L-1) and lower values to the organic juice prepared with Bordô organic grape (3378 mg GAE.L-1). This increase in TPC of the samples that had been treated with enzymes is associated with a positive effect on the color (Fig. 3).
Figure 3 shows that, for the juice prepared with PEP-N31, the color index increased throughout the treatment reaching the maximum after 2 h although the result after 1 h was not significantly different from the 2-h result. The color index for juice prepared with Vinozym increased reaching the maximum after 1 h of treatment and it stabilized after this time. The value of color index reached by Vinozym was lower than that reached by PEP-N31. The results of TPC (Fig. 2) and color (Fig. 3) of the juices suggest that 1 h of enzymatic treatment with PEP-N31 is sufficient to obtain products rich in phenolic compounds and color index.
Finally, the physical–chemical characteristics of the conventional grape juices (i.e. blanched but not treated with PEP) and those prepared after the use of pectinolytic enzyme preparations PEP-N31 and Vinozym (commercial enzyme preparation, used for comparison), for 1 h are presented in Table 3 and present results close to those found for other grape (Rizzon and Link 2006; Magro et al. 2016). Among the physicochemical parameters analyzed, close values were found for all the samples, except for the reducing and non-reducing sugar contents. Magro et al. (2016) also observed changes in physicochemical characteristics, such as pH, soluble solids total acidity and reducing sugars, among the Concord grape juice samples extracted with two commercial enzymatic extracts and the control. In view of the results it can be concluded that the use of blanching as a pretreatment to aid in the inactivation of undesirable endogenous enzymes for the processing of the grape in the juice form, together with the use of this new pectinolytic enzyme preparation (PEP-N31) on grapes during maceration to assist in the extraction of phenolic compounds from the grape for the juice has been shown to be promising alternative to improve the juice quality.
Conclusion
Bordô grape juice obtained after pre-treatment by steam blanching (3 min) followed by 1 h of treatment with the pectinolytic enzyme preparation produced by the fungus Thermomucor indicae-seudaticae-N31 (PEP-N31) was found to have concentrations of methanol and TPC, as well index color close to the values obtained from the product prepared with the commercial preparation (Vinozym FCE). In addition, this juice produced with this new preparation had physicochemical characteristics close to the juice of grapes commonly consumed and a better quality with respect to the content of phenolic compounds and methanol. These results support the proposal that pectinolytic enzyme preparation produced by the fungus Thermomucor indicae-seudaticae-N31 (PEP-N31) is appropriated for application to the grape juice processing industry.
N31 (PEP-N31) was found to have concentrations of methanol and TPC as well as index color close to the values obtained from the product prepared with the commercial preparation (Vinozym FCE).
Acknowledgements
The authors are grateful to CAPES (Finance Code 001 and grant number PDSE 99999.012757/2013-06), CNPq (grant number 486967/2012-3) and FAPESP (grant numbers and 2017/16482-5, 2013/19057-2 and 2010/09998-6) (Brazil) for financial support and for scholarships.
Abbreviations
- CI
Color index
- Endo-PG
Endopolygalacturonase
- Exo-PG
Exopolygalacturonase
- FID
Flame ionization detector
- GAE
Gallic acid equivalent
- OCH3
Methoxy groups
- PME
Pectin methylesterases
- PG
Polygalacturonases
- PMG
Polymethylgalacturonases
- POD
Peroxidase
- PPO
Polyphenol
- TPC
Total phenolic content
Authors’ contributions
All authors approved the final article.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Iasnaia Maria de Carvalho Tavares, Email: iasnaiamct@gmail.com.
Marcelo Andrés Umsza-Guez, Email: marcelo.umsza@ufba.br.
Natália Martin, Email: nataliamartin09@hotmail.com.
Thaise Mariá Tobal, Email: thaisetobal@ufgd.edu.br.
Maurício Boscolo, Email: boscolo@ibilce.unesp.br.
Eleni Gomes, Email: eleni.gomes@unesp.br.
Roberto Da-Silva, Email: roberto.silva@unesp.br.
Ellen Silva Lago-Vanzela, Email: ellen.sl.vanzela@unesp.br.
References
- Amin F, Bhatti HN, Bilial M. Recent advances in the production strategies of microbial pectinases—a review. Int J Biol Macromol. 2019;122:1017–1026. doi: 10.1016/j.ijbiomac.2018.09.0480141-8130. [DOI] [PubMed] [Google Scholar]
- Anthon GE, Barrett DM. Changes in pectin methylesterification and accumulation of methanol during production of diced tomatoes. J Food Eng. 2010;97:367–372. doi: 10.1016/j.jfoodeng.2009.10.031. [DOI] [Google Scholar]
- AOAC . Association of official analytical chemists. Official methods of analysis. Arlington: AOAC; 1990. [Google Scholar]
- Baffi MA, Tobal T, Henrique J, Lago G, Leite RS, Boscolo M, Gomes E, Da-Silva R. A novel β-glucosidase from Sporidiobolus pararoseus: characterization and application in winemaking. J Food Sci. 2011;76:C997–C1002. doi: 10.1111/j.1750-3841.2011.02293.x. [DOI] [PubMed] [Google Scholar]
- Bala A, Singh B. Cellulolytic and xylanolytic enzymes of thermophiles for the production of renewable biofuels. Renew Energy. 2018 doi: 10.1016/j.renene.2018.09.100. [DOI] [Google Scholar]
- Barman S, Sit N, Badwaik LS, Deka SC. Pectinase production by Aspergillus niger using banana (Musa balbisiana) peel as substrate and its effect on clarification of banana juice. J Food Sci Technol. 2015;52:3579–3589. doi: 10.1007/s13197-014-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla A, Lo Scalzo R, Bertolo GB, Torreggiani D. Steam-blanched highbush blueberry (Vaccinium corymbosum L.) juice: phenolic profile and antioxidant capacity in relation to cultivar selection. J Agric Food Chem. 2008;56:2643–2648. doi: 10.1021/jf0731191. [DOI] [PubMed] [Google Scholar]
- Cheynier V, Comte G, Davies KM, Lattanzio V, Martens S. Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol Biochem. 2013;72:1–20. doi: 10.1016/j.plaphy.2013.05.009. [DOI] [PubMed] [Google Scholar]
- de Freitas AA, Francelin MF, Hirata GF, Clemente E, Schmidt FL. Atividades das enzimas peroxidase (POD) e polifenoloxidase (PPO) nas uvas das cultivares benitaka e rubi e em seus sucos e geléias. Ciênc Tecnol Alim. 2008;28:172–177. doi: 10.1590/S0101-20612008000100025. [DOI] [Google Scholar]
- Deng Y, Wu Y, Li Y. Changes in firmness, cell wall composition and cell wall hydrolases of grapes stored in high oxygen atmospheres. Food Res Int. 2005;38:769–776. doi: 10.1016/j.foodres.2005.03.003. [DOI] [Google Scholar]
- Deytieux-Belleau C, Vallet A, Donèche B, Geny L. Pectin methylesterase and polygalacturonase in the developing grape skin. Plant Physiol Biochem. 2008;46:638–646. doi: 10.1016/j.plaphy.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Etzbach L, Pfeiffer A, Schieber A, Weber F. Effects of thermal pasteurization and ultrasound treatment on the peroxidase activity, carotenoid composition, and physicochemical properties of goldenberry (Physalis peruviana L.) puree. LWT Food Sci Technol. 2019;100:69–74. doi: 10.1016/j.lwt.2018.10.032. [DOI] [Google Scholar]
- Glories Y (1984) 1a partie: Les equilibres des anthocyanes et des tannins. In: La couleur des vins rouges, 18th edn. Connaiss, Vigne Vin, pp 95–217
- González EM, deAncos B, Cano MP. Partial characterization of peroxidase and polyphenol oxidase activities in blackberry fruits. J Agric Food Chem. 2000;48:5459–5464. doi: 10.1021/jf000169w. [DOI] [PubMed] [Google Scholar]
- Hou C-Y, Lin Y-S, Wang YT, Jiang C-M, Wu M-C. Effect of storage conditions on methanol content of fruit and vegetable juices. J Food Compos Anal. 2008;21:410–415. doi: 10.1016/j.jfca.2008.04.004. [DOI] [Google Scholar]
- Khan M, Nakkeeran E, Umesh-Kumar S. Potential application of pectinase in developing functional foods. Annu Rev Food Sci Technol. 2013;4:21–34. doi: 10.1146/annurev-food-030212-182525. [DOI] [PubMed] [Google Scholar]
- Lago-Vanzela ES, Pavezzi FC, Martin N, Gomes E, Silva R. Isolation and characterization of latent and active polyphenoloxidase in BRS Clara (CNPUV 154-147 × Centennial seedless) and BRS Morena (Marroo seedless × Centennial seedless) seedless table grapes. Plant Physiol Biochem. 2011;49:1251–1258. doi: 10.1016/j.plaphy.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Llano KM, Haedo AS, Gerschenson LN, Rojas AM. Mechanical and biochemical response of kiwifruit tissue to steam blanching. Food Res Int. 2003;36:767–775. doi: 10.1016/S0963-9969(03)00071-1. [DOI] [Google Scholar]
- Lopez P, Delafuente JL, Burgos J. Continuous determination of endopolygalacturonase activity by means of rotational viscosimeters. Anal Biochem. 1994;220:346–350. doi: 10.1006/abio.1994.1348. [DOI] [PubMed] [Google Scholar]
- Luh BS, Daouf HN. Effect of break temperature and holding time on pectin and pectic enzymes in tomato pulp. J Food Sci. 1971;36:1030–1043. doi: 10.1111/j.1365-2621.1971.tb03341.x. [DOI] [Google Scholar]
- Magro LD, Dalagnol LMG, Manfoi V, Hertz PF, Klein MP, Rodrigues RC. Synergistic effects of Pectinex Ultra Clear and Lallzyme Beta on yield and bioactive compounds extraction of Concord grape juice. LWT Food Sci Technol. 2016;72:157–165. doi: 10.1016/j.lwt.2016.04.0460023-6438. [DOI] [Google Scholar]
- Margraf T, Santos ENT, Andrade EF, Ruth SMV, Granato D. Effects of geographical origin, variety and farming system on the chemical markers and in vitro antioxidant capacity of Brazilian purple grape juices. Food Res Int. 2016;82:145–155. doi: 10.1016/j.foodres.2016.02.003. [DOI] [Google Scholar]
- Martin N, Guez MAU, Sette LD, et al. Pectinase production by a Brazilian thermophilic fungus Thermomucor indicae_seudaticae N31 in solid-state and submerged fermentation. Microbiology. 2010;79:306–313. doi: 10.1134/S0026261710030057. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Ndiaye C, Xu S-Y, Wang Z. Steam blanching effect on polyphenoloxidase, peroxidase and colour of mango (Mangifera indica L.) slices. Food Chem. 2009;113:92–95. doi: 10.1016/j.foodchem.2008.07.027. [DOI] [Google Scholar]
- OIV . Recueil des methods internationals d’analyse des vins et desmouts. Paris: Office International de La Vigne et du Vin; 1990. [Google Scholar]
- Pedrolli DB, Monteiro AC, Gomes E, Carmona EC. Pectin and pectinases: production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol J. 2009;3:9–18. doi: 10.2174/1874070700903010009. [DOI] [Google Scholar]
- Rizzon LA, Link M. Composição do suco de uva caseiro de diferentes cultivares. Ciênc Rural. 2006;36:689–692. doi: 10.1590/S0103-84782006000200055. [DOI] [Google Scholar]
- Rossi M, Giussani E, Morelli R, Scalzo RL, Nani RC, Torreggiani D. Effect of fruit blanching on phenolics and radical scavenging activity of highbush blueberry juice. Food Res Int. 2003;36:999–1005. doi: 10.1016/j.foodres.2003.07.002. [DOI] [Google Scholar]
- Saharan R, Sharma KP. Industrial applications of thermophilic pectinase: a review. Int J Curr Res. 2018;10:70762–70770. doi: 10.24941/ijcr.31096.06.2018. [DOI] [Google Scholar]
- Siddiq M, Dolan KD, Perkins-Veazie P, Collins JK. Effect of pectinolytic and cellulytic enzymes on the physical, chemical, and antioxidant properties of blueberry (Vaccinium corymbosum L.) juice. LWT Food Sci Technol. 2018;92:127–132. doi: 10.1016/j.lwt.2018.02.008. [DOI] [Google Scholar]
- Silva D, Martins ES, Leite RSR, et al. Purification and characterization of an exo-polygalacturonase produced by Penicillium viridicatum RFC3 in solid-state fermentation. Process Biochem. 2007;42:1237–1243. doi: 10.1016/j.procbio.2007.05.025. [DOI] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM, et al. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. In: Sies H, et al., editors. Methods in enzymology: polyphenols and flavonoids. San Diego: Elsevier; 1999. pp. 152–178. [Google Scholar]
- Toaldo IM, Cruz FA, Alves TL, Gois JS, Borges DLG, Cunha HP, Silva ELS, Bordignon-Luiz MT. Bioactive potential of Vitis labrusca L. grape juices from the Southern Region of Brazil: phenolic and elemental composition and effect on lipidperoxidation in healthy subjects. Food Chem. 2015;173:527–535. doi: 10.1016/j.foodchem.2014.09.171. [DOI] [PubMed] [Google Scholar]
- Vagiri M, Jensen M. Influence of juice processing factors on quality of black chokeberry pomace as a future resource for colour extraction. Food Chem. 2017;217:409–417. doi: 10.1016/j.foodchem.2016.08.121. [DOI] [PubMed] [Google Scholar]
- Xiao H-W, Pan Z, Deng L-Z, El-Mashad HM, Yang X-H, Mujumdar AS, Gao Z-J, Zhang Q. Recent developments and trends in thermal blanching—a comprehensive review. Inf Process Agric. 2017;4:101–127. doi: 10.1016/j.inpa.2017.02.001. [DOI] [Google Scholar]