Abstract
Potato snacks can be used as an ideal strategy for nutrient delivery, since they are one of the most widely consumed products in the world. Due to fried snacks are known to be a significant source of fat intake, consumption is changing towards healthier alternatives. The aim of this research is to evaluate the effect of vacuum impregnation and three dehydration techniques: heated air drying, freeze drying, and microwave vacuum drying of the potato snack that has been fortified with active components: calcium and vitamins C and E. Vacuum impregnation was evaluated using the response surface methodology that had a central composite experimental design with the following independent variables: vacuum pressure, vacuum stage time, and atmospheric stage time. The following were the dependent variables: fraction and volumetric deformation in the vacuum stage and at the end of the process and effective porosity. Finally, a sensorial analysis was carried out on the dehydrated potatoes. The results of the optimal vacuum impregnation process conditions were: a vacuum pressure of 77.3 kPa for 3.0 min followed by 4.0 min at atmospheric pressure. The content of calcium, vitamin C, and vitamin E in the impregnated potato were 956, 472, and 35 mg 100 g−1 dry solids, respectively. The highest retention of the active components in snacks was obtained by the combination of vacuum impregnation and the dehydration techniques in the following order: freeze drying, microwave vacuum drying, and then heated air drying. It can be concluded that the integration processes give an added value to potato snacks due to the increased content of the active components; additionally, the vacuum impregnation process together with microwave vacuum drying was the alternative that had the highest sensorial acceptability.
Keywords: Solanum tuberosum, Matrix engineering, Potato chips, Fortification, Functional foods
Introduction
The potato is one of the most important food crops in the world due to its high energy content; it plays a key role in the food security of many countries. Three-hundred million tons per year are produced globally, which is only exceeded by wheat, corn, and rice (Singh and Kaur 2016). Currently, the potato is processed in different ways such as being frozen, fried potato chips, dehydrated potatoes for mashed potato preparation, and potato flour as a food ingredient. Potato snacks in the form of crisps are one of the most popular consumer products around the world (Singh and Kaur 2016).
Traditionally, potato crisps are prepared by conventional frying using vegetable oils at a temperature between 170 and 190 °C. The obtained potato snack has good sensorial properties, however, it is a product with a fat content that, in many cases, is a third of the final snack (Belkova et al. 2018). This ensures a high satiety level for the consumer, but at the same time, involves a health risks as it contributes to the development of different diseases: obesity, hypertension, diabetes, and cardiovascular diseases (World Health Organization 2018). In this context, modern consumer trends gravitate towards healthier foods or foods that contribute to disease prevention (functional foods); therefore, it is necessary to explore new technologies to improve processes that promote a better quality of life.
The dehydration processes combines the heat and mass transfer to decrease the food’s water activity of, avoiding undesirable reactions by enzymatic or microbial action (Barbosa-Cánovas and Ibarz 2003). Hence, several authors have reported dehydrating potatoes through different drying methods including: freeze drying (Zvitov-Ya’ari and Nussinovitch 2014), convective drying (Aprajeeta et al. 2015), microwaving (Joshi et al. 2016a), and vacuum microwaving (Setiady et al. 2007). Additionally, combinations of these methods have also been reported (Setiady et al. 2009a, b).
Moreover, within the methodologies used to obtain functional foods, matrix engineering uses the vacuum impregnation technique to incorporate active components such as vitamins, minerals, and antioxidants into the structure through the hydrodynamic mechanism (Fito et al. 1996). This has been described as a fast mass transfer phenomenon which occurs when porous structures are immersed in a liquid phase. According to this process, when a product is submerged in an impregnation solution at a certain vacuum pressure, and then the atmospheric pressure is subsequently restored, pressure gradients are created in the system. This causes the external solution (that containing active components or other compounds including texture, colour, and flavour modifiers) to move towards inside the food structure. However, pressure changes can also promote product deformations because of the viscoelastic properties of the solid matrix. In some cases, deformation can lead to the size and shape of the pores being changed, resulting in different levels of impregnation. Therefore, the final response to the vacuum impregnation is defined by the coupling of the hydrodynamic mechanism and the deformation–relaxation phenomena that the matrix undergoes due to the pressure gradient (Fito et al. 1996). Thus, volume changes (deformation) and the effective porosity affects the volumetric fraction of the product that has been impregnated by the external liquid.
Several papers have reported the incorporation of active components via vacuum impregnation in potatoes, such as, ascorbic acid (Hironaka et al. 2011), calcium (Tiwari et al. 2018), calcium and zinc (Joshi et al. 2016b), iron (Erihemu et al. 2015), zinc (Hironaka and Koaze 2015), and phenolic compounds from beet (Moreira and Almohaimeed 2018). However, stabilization processes after impregnation and sensorial analysis are rarely included. Other products have also been studied including cape gooseberries (Cortés-Rodríguez et al. 2016) and pears (Chafer et al. 2011).
The objective of the present paper is to evaluate the effect that the integration of vacuum impregnation and the drying processes [heated air drying (HAD), freeze drying (FD), and microwave vacuum drying (MVD)] have on the sensorial and microstructural properties of potato snacks that have active components (calcium and vitamins C and E). It also analyses how these components are conserved.
Materials and methods
Materials
This research used the diacol capiro variety of potato (Solanum tuberosum) with no mechanical affectations and that were free of damage caused by pathogens and insects. The potatoes were medium calibre (45–64 mm diameter) based on Colombian Technical Standard NTC 341 (ICONTEC 1996) and were harvested in a small village called La Unión (5°58′22″N 75°21′40″O), located east of Medellín. The potatoes were washed and cut into slices 2.5 mm thick. Afterwards, they were blanched with a 1:10 potato:water ratio for 4 min at 80 ± 2 °C; then, they were cooled and dried with a paper towel to remove any excess moisture. Blanching could be conducted using less amount of water, in order to retain more water soluble nutrients. For the vacuum impregnation process, a NaCl (Bellchem®) isotonic solution was prepared. The precursors of active components were: calcium lactate (Bellchem®), Dl-α-tocopherol acetate (Bellchem®), and l-ascorbic acid (Bellchem®). Additionally, surface activity agents Tween 80 (Bellchem®) and Span 60 (Bellchem®) were used that had a hydrophilic–lipophilic balance of 14.9 and 4.7, respectively. Solvents acetonitrile and methanol were acquired from Merck®, and the Vitamin E standard was acquired from Sigma-Aldrich ®.
Characterisation of fresh potatoes and snacks
Moisture, protein, fat, ashes (AOAC 2000), and carbohydrates (Kim and Kim 2015) were evaluated in raw potatoes. The total starch was determined using the spectrophotometric method (AOAC 2000). The potato real porosity (εr) was determined according to Eq. 1 using the apparent density (ρa) (g cm3) and the real density (ρr) (g cm−3) (Barbosa-Cánovas and Ibarz 2003). For its part, the apparent density was determined by measuring the buoyant forces using n-heptane (Barreto et al. 2019) and the real density was calculated from the potato’s composition with the assumption that the sample is composed only by carbohydrates and water (Barbosa-Cánovas and Ibarz 2003). The water activity of the potato snacks (aw) was measured using a dewpoint hygrometer at 25 °C (AQUALAB-PRE) (AOAC 2000).
| 1 |
Vacuum impregnation (VI) procedure
Experiments were carried out using a vacuum impregnator (Centricol Ltda. Medellín, Colombia). This consists of a stainless-steel chamber, an electromechanical system that allows the movement of the sample vertically and vibrationally, and an analytical scale to determine the evolution of the mass of the sample and the impregnation liquid during the process (Fig. 1).
Fig. 1.
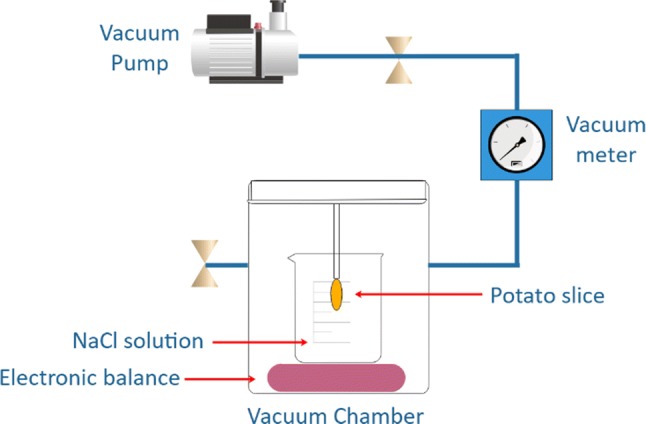
Schematic representation of the vacuum impregnation system, used to determine the characteristic parameters of the impregnation process
The response to potato vacuum impregnation was performed using NaCl (aw = 0.996) isotonic solutions and the parameters of impregnation (i.e. volumetric fraction and volumetric deformation) were determined in the vacuum stage (X1, ϒ1) and at the end of the process (X, ϒ), and the effective porosity (εe).
Equation 2 describes the relationship between Hydrodynamic Mechanism and Deformation–Relaxation Phenomena in Solid Porous Food–Liquid Systems that was previously modelled by Fito et al. (1996).
| 2 |
where X is the volumetric fraction at the end of the vacuum impregnation process, ϒ is the volumetric deformation at the end of the process, εe is the effective porosity (porosity available for the vacuum impregnation process), ϒ1 is the volumetric deformation at the end of the vacuum stage, and r is the compression ratio (ratio between vacuum pressure and atmospheric pressure).
Equation 3 is derived from Eq. 2 and is used to calculate the effective porosity (cm3 gas cm3 potato−1) from the impregnation parameters X, ϒ, ϒ1.
| 3 |
Finally, to calculate the parameters of impregnation: volumetric fractions (X, X1) (cm3 impregnation liquid cm3 potato −1) and volumetric deformations (ϒ, ϒ1) (cm3 potato cm3 potato−1) of potato for any time t, i.e. at the end of the vaccum stage (X1, ϒ1) or at the end of the vacuum impregnation process (X, ϒ), Eqs. 4 and 5 were used (Fito et al. 1996; Martínez-Navarrete et al. 1999):
| 4 |
| 5 |
where Xt is the volumetric fraction at time t, Mt is the mass of the sample at time t, Mo is the initial sample mass, is the impregnation solution density, Vo is the initial sample volume, Vt is the sample volume at time t, and is the volumetric deformation at time t. Volumetric fraction and Volumetric deformation are calculated according to the variations in weight experienced by the sample and the variations of upward thrust that it exerts at each moment in time (Fito et al. 1996; Martínez-Navarrete et al. 1999).
Formulation and characterisation of the fortification emulsion
The formulation of the impregnating emulsion was determined based on vacuum impregnation parameters. The components of impregnating emulsion (Dl-α-tocopherol acetate: 0.2%, l-ascorbic acid: 0.6%, and 9.27 mg Ca2+ g−1 emulsion) were emulsified on an aqueous and isotonic NaCl phase (0.8%) and surface activity agents Tween 80 (0.051%) and Span 60 (0.049%) were added (Cortés-Rodríguez 2004). The impregnating emulsion was prepared using a homogenizer (Ultraturrax Microdistech, MDT 1000, USA) at 20,000 rpm for four 3-min cycles that were each followed by a 3-min rest to control the system’s temperature.
The particle size of the impregnating emulsion was analysed using dynamic light scattering with a Zetasizer Nano-ZS90 equipped with zetasizer software (version 7.11, Malvern Instruments Ltd., UK). A solid-state laser with a wavelength of 677 nm and 15 mW was used. Scattered light was detected with a 90° scattered angle.
Moreover, the density of impregnation liquids was determined with the pycnometer method (AOAC 2000), and the apparent viscosity was ascertained by using a concentric cylinder rheometer (Bohlin Gemini–HR Nano) at 25 ± 2 °C. Quantifications were made in triplicate.
Dehydration methods
The potato slices impregnated with the active components were dehydrated by HAD, FD, and MVD, and the methodology in Setiady et al. (2009b) was slightly modified:
Heated air drying (HAD) Air drying was undertaken in a tray-type convection dryer, which consists of a fan connected to electrical resistance to produce air heating. The weight change over time was measured with a balance Vector® S-type scale, 5AX797, which had a ± 0.1 g error. The dryer was set to 60 °C and air velocity was 2.7 m s−1. The samples were placed on a 50 cm × 25 cm plate and dried to a constant weight, with a 6-h operation.
Freeze drying (FD) Labconco Freeze Dryer 3, Cat 75,200 equipment was used at − 90 kPa vacuum pressure and − 40 °C condenser temperature for 48 h. The samples were previously (and independently) frozen at − 80 °C for 20 h.
Microwave vacuum drying (MVD) Semi-industrial vacuum microwave equipment scale with magnetrons (CW 2450 MHz, Toshiba, China) and a vacuum pump (Lanphan 2XZ-4, Zhengzhou, China) was used. The system was equipped with 2 rotating trays at 6 rpm (50 cm diameter), input power: 1.6 kW, output power: 0.6 kW, and absolute operating pressure: 4.32 kPa. The microwaves were applied under a temperature controlled system that automatically regulates the power of the introduced microwaves (on–off cycles) depending on the temperature, which was set to 60 °C (Regier et al. 2017). Drying time was 20 min.
Determination of calcium, vitamin C and vitamin E
The calcium analysis was performed by atomic absorption (AOAC 2000) and the Vitamin C analysis by titration using indicator 2.6 dichlorophenolindophenol (Contreras-Calderón et al. 2011). Vitamin E was quantified by high-performance liquid chromatography (HPLC, Agilent 1100) and the HPLC system was equipped with an autosampler, and the UV/Vis detector was set to 280 nm (Cortés-Rodríguez 2004). Separation was carried out using a reverse phase Zorbax C18 column (4.6 mm × 150 mm × 5 µm), a flow rate of 2 ml min−1, 45 °C and acetonitrile: methanol (70:30) as the mobile phase. The quantification of Vitamin E was carried out through calibration of Vitamin E standard (Dl-α-tocopherol acetate, Sigma-Aldrich®).
Microstructure
The microstructure analysis was performed using Scanning Electron Microscopy, the potato snack samples were fixed to metal cylinders through adhesion with double-sided graphite tape and then coated with gold. The cylinders containing the samples were placed in the JEOL JSM-64990LV microscope chamber (Duarte et al. 2017). Additionally, elemental analysis was carried out by means of an X-ray-EDX Microscope (INCA PentaFETx3 Oxford Instruments) to demonstrate the calcium present in the dry matrix.
Sensorial analysis
The sensorial analysis of potato snacks that had added active components was conducted in the Food Sensory Analysis Laboratory at the Universidad de Antioquia, Colombia using a multidimensional profile approach (NTC 3932,1996: ISO 11035:1994) (ICONTEC 1996) with an expert panel composed of five judges. The judges were able to identify the set of relevant descriptors: odour, taste, and texture to provide the maximum amount of information about the samples’ sensorial attributes to establish a sensory profile. The scale used to qualify intensities of the descriptors was from 0 to 5, where 0 is absence, 1 is very weak, 2 is weak, 3 is moderate, 4 is strong, and 5 is intense. The evaluation was carried out in individual cubicles with an ambient temperature of 24.0 ± 0.2 °C and relative humidity of 60 ± 1.0%. The overall quality was also evaluated on a scale from 1 to 3, where 1 is low, 2 is medium, and 3 is high. These analyses were carried out following international tenets and informed consents were obtained.
Statistical analysis
The evaluation of the vacuum impregnation process was carried out using a response surface methodology and a central composite experimental design with 4 central points (18 experiments) (Table 1). The independent variables are: vacuum pressure (50.8–77.9 kPa), vacuum stage time (3–5 min), and atmospheric stage time (3–5 min). The dependent variables are: X1, ϒ1 X, ϒ y εe, which are expressed as the average ± standard deviation of three replicates. The results were analysed using the Statgraphics Centurion XVI software (Statistical Graphics Corporation, Ver. 16.0.07, Rockville, USA). Also, a quadratic model was used to determine each dependent variable (Y) as a function of the independent variables (Xi y Xj) (Eq. 6), where b0 is a constant, bi are model coefficients linked to a linear effect, bii are the coefficients related to the quadratic effect, and bij are the constants for the interaction effect.
| 6 |
Table 1.
Results for the experimental design, conditions of impregnation
| Treatments | Factors | Impregnation parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| VP (kPa) | t1 (min) | t2 (min) | X1 (%) | ϒ1 (%) | X (%) | ϒ (%) | εe (%) | |
| 1 | 64.3 | 4 | 4 | − 9.7 ± 1.9 | 4.4 ± 3.1 | 10.0 ± 1.7 | 1.5 ± 0.6 | 12.8 ± 2.4 |
| 2 | 50.8 | 3 | 5 | − 5.5 ± 0.3 | 2.7 ± 0.3 | 5.7 ± 0.7 | 1.7 ± 0.5 | 8.7 ± 0.9 |
| 3 | 50.8 | 5 | 5 | − 4.5 ± 0.3 | 4.1 ± 0.4 | 5.4 ± 0.3 | 2.8 ± 0.6 | 7.2 ± 0.7 |
| 4 | 64.3 | 3 | 4 | − 8.0 ± 0.5 | 3.0 ± 2.0 | 8.7 ± 0.8 | − 0.9 ± 1.6 | 13.6 ± 1.3 |
| 5 | 50.8 | 3 | 3 | − 5.0 ± 0.9 | 3.1 ± 0.4 | 5.4 ± 0.4 | 1.4 ± 0.3 | 8.9 ± 0.8 |
| 6 | 64.3 | 4 | 4 | − 8.7 ± 2.4 | 1.9 ± 1.0 | 8.2 ± 0.9 | − 1.7 ± 0.5 | 13.8 ± 1.7 |
| 7 | 64.3 | 4 | 3 | − 7.1 ± 1.0 | 1.8 ± 1.6 | 6.8 ± 2.0 | − 0.1 ± 1.7 | 9.7 ± 1.0 |
| 8 | 77.9 | 3 | 3 | − 10.0 ± 0.5 | 1.9 ± 1.7 | 10.1 ± 0.7 | 0.6 ± 1.9 | 10.6 ± 1.5 |
| 9 | 77.9 | 3 | 5 | − 9.8 ± 0.9 | 1.2 ± 0.4 | 9.6 ± 0.9 | − 1.0 ± 0.5 | 11.8 ± 0.9 |
| 10 | 64.3 | 4 | 5 | − 5.8 ± 1.4 | 2.3 ± 1.2 | 6.8 ± 1.8 | 1.4 ± 1.3 | 7.9 ± 1.2 |
| 11 | 77.9 | 5 | 3 | − 8.0 ± 1.7 | 1.9 ± 0.4 | 9.0 ± 1.3 | 0.3 ± 0.6 | 9.7 ± 2.0 |
| 12 | 77.9 | 4 | 4 | − 8.1 ± 1.6 | 1.1 ± 0.7 | 9.2 ± 0.8 | − 0.2 ± 2.4 | 10.4 ± 2.3 |
| 13 | 64.3 | 5 | 4 | − 5.1 ± 1.0 | 1.9 ± 0.6 | 6.0 ± 1.0 | 0.7 ± 0.5 | 7.8 ± 1.3 |
| 14 | 64.3 | 4 | 4 | − 6.3 ± 1.4 | 1.4 ± 0.6 | 7.0 ± 0.9 | − 1.4 ± 0.9 | 11.6 ± 1.3 |
| 15 | 77.9 | 5 | 5 | − 7.1 ± 0.9 | 2.5 ± 1.1 | 8.5 ± 0.8 | 1.7 ± 2.0 | 7.7 ± 1.9 |
| 16 | 50.8 | 5 | 3 | − 5.0 ± 1.0 | 1.8 ± 0.4 | 5.1 ± 0.9 | − 0.4 ± 0.4 | 10.6 ± 1.1 |
| 17 | 64.3 | 4 | 4 | − 7.7 ± 1.5 | 0.8 ± 0.3 | 8.1 ± 0.9 | − 0.7 ± 0.4 | 12.0 ± 1.6 |
| 18 | 50.8 | 4 | 4 | − 5.2 ± 0.2 | 2.2 ± 0.3 | 5.8 ± 0.4 | 0.9 ± 0.5 | 9.7 ± 0.4 |
VP = Vacuum pressure; t1 = vacuum stage time; t2 = atmospheric stage time; X1 = volumetric fraction at the end of the vacuum stage; ϒ1 = volumetric deformation at the end of the vacuum stage; X = volumetric fraction at the end of the vacuum impregnation process; ϒ = volumetric deformation at the end of the vacuum impregnation process; εe = effective porosity
The analysis of variance (ANOVA) was developed with a 95% confidence level, which includes the statistical significance of each of the terms of the adjusted model (p value), the coefficients estimated in each term (αi), the model’s coefficient of determination (R2), and the lack of fit in order to establish the accuracy of the model. The models obtained in the experimental design were optimized to determine the levels of the factors that simultaneously optimize the response variables (maximum, minimum, or fixed depending on the case). For the optimization process, we used the desirability function. This transforms each response variable into an individual desirability function, which varies from 0 to 1. This value increases as the best value of the variable corresponding response is required (Gutiérrez Pulido 2012).
Results and discussion
Physicochemical characterization of potato and impregnation liquids
Table 2 shows the physicochemical parameters obtained for the diacol capiro potato.
Table 2.
Physicochemical characterization of potato
| Parameter (wet basis) | Potato var. diacol capiro |
|---|---|
| Moisture | 77.07 ± 0.57 |
| Protein (N × 6.25) | 4.36 ± 0.23 |
| Fat | 0.01 ± 0.00 |
| Ash | 1.31 ± 0.04 |
| Carbohydrates | 17.25 ± 1.18 |
| Starch | 16.73 ± 0.14 |
| Real density (g cm−3) | 1.08 ± 0.57 |
| Apparent density (g cm−3) | 0.92 ± 0.06 |
| Real porosity (%) | 14.5 |
The value obtained for moisture content agrees with that reported by Gobernación de Antioquia (2012). This variety stands out from all the others in the country because of its high percentage of dry matter and greater use in agroindustrial processes. The values obtained for protein, ash, and starch contents are within previously reported ranges (Singh and Kaur 2016).
The real porosity of the potato was greater than that found by Madiouli et al. (2012) and Singh et al. (2015), who reported values between 1 and 9% for unspecified varieties. This is due to the fact that the quality of potatoes used for processing is affected by cultural and environmental conditions including: soil, humidity, temperature, morphology, composition, internal distribution, and other related factors (Singh and Kaur 2016).
The value of real porosity obtained showed an availability of intercellular spaces for the incorporation of the impregnation solution; thus, it allows the diacol capiro potato to be identified as a suitable matrix for the Vacuum Impregnation process to be used. Real porosity represents the maximum volume that can be occupied by a liquid medium in a vacuum impregnation process.
The values obtained for the densities of the impregnation liquids, 0.998 ± 0.003 g cm−3 for isotonic solution and 1.019 ± 0.000 g cm−3 for the impregnation emulsion, facilitate the entry of liquids into the structure due to a lower drop in pressure (Cortés-Rodríguez 2004). These values are similar to those reported by Betalleluz (2015) during the vacuum impregnation of apples.
Vacuum impregnation
Table 1 shows the results of the vacuum impregnation parameters for diacol capiro potatoes at the different conditions described by the experimental design. Table 3 shows the ANOVA results.
Table 3.
ANOVA of experimental design
| p value | |||||
|---|---|---|---|---|---|
| X1 | ϒ1 | X | ϒ | εe | |
| A: vacuum pressure, VP (kPa) | 0.029 | 0.368 | 0.017 | 0.352 | 0.19 |
| B: vacuum stage time, t1 (min) | 0.154 | 0.949 | 0.256 | 0.534 | 0.04 |
| C: atmospheric stage time, t2 (min) | 0.64 | 0.674 | 0.918 | 0.371 | 0.131 |
| AA | 0.852 | 0.806 | 0.93 | 0.607 | 0.366 |
| AB | 0.434 | 0.798 | 0.668 | 0.507 | 0.157 |
| AC | 0.789 | 0.677 | 0.672 | 0.417 | 0.391 |
| BB | 0.799 | 0.641 | 0.921 | 0.998 | 0.956 |
| BC | 0.718 | 0.426 | 0.981 | 0.238 | 0.101 |
| CC | 0.713 | 0.918 | 0.465 | 0.435 | 0.05 |
| R2 | 78.27 | 45.5 | 83.15 | 71.80 | 70.38 |
| Lack of fit | 0.716 | 0.99 | 0.781 | 0.977 | 0.146 |
X1 = volumetric fraction at the end of the vacuum stage; ϒ1 = volumetric deformation at the end of the vacuum stage; X = volumetric fraction at the end of the vacuum impregnation process; ϒ = volumetric deformation at the end of the vacuum impregnation process; εe = effective porosity
At the beginning of the impregnation process, the vacuum promotes the expansion of the gas with the subsequent output. The native liquid contained inside the potato structure and the water absorbed during the blanching process can also come out due to the vacuum applied. This liquid outlet is reflected in the negative values of volumetric fraction at the end of the vacuum stage (X1), which were significantly affected (p < 0.05) by the vacuum pressure applied during the process. The largest liquid outlet was X1 = − 10.0 ± 0.5% at 77.9 kPa. This produced an increase in the sample volume and generated positive deformation values at the end of the vacuum stage (ϒ1), which oscillated between 0.8 and 4.4%. This range covers the deformation values reported when apples, strawberries, and pears were impregnated with isotonic solutions (Salvatori et al. 1998). The parameter ϒ1, was not significantly affected by any of the factors studied (p > 0.05).
In general, the effective porosity for the vacuum impregnation process (εe), defined as the fraction of the sample volume available for the hydrodynamic mechanism, oscillated between 7.2 and 13.8%. These values are lower than the calculated real porosity (14.5%) and they indicate that after the impregnation process, not all the available volume of the sample is occupied by the isotonic solution (Salvatori et al. 1998). The tissue structure plays an important role not only for its total porosity but also for the size, shape, and distribution of the pores, as well as for the method of communicating with the outer liquid (Betalleluz 2015). Similar results have been presented when working on the impregnation of other food matrices such as mangos, kiwis, pears, and strawberries (Radziejewska-kubzdela and Kido 2014).
Regarding the ANOVA (Table 3), the effective porosity was significantly affected by the vacuum stage time (p < 0.05), which resulted in greater effective porosity with the shortest vacuum time. This is due to the fact that the time at the required vacuum pressure must be long enough to reach the mechanical equilibrium within the product (equal internal pressure and applied external pressure); and, according to the thickness used, this can be quickly reached (Betalleluz 2015). Therefore, a higher vacuum time could deform the food matrix by consequently clogging the pores due to the deformation–relaxation phenomena caused by changes in system pressure (Derossi et al. 2012).
In terms of the volumetric fraction at the end of the process (X), the positive values obtained were the result of restoring the atmospheric pressure in the system, which generated the impregnation liquid inflow. The pressure difference acts as a driving force for the vacuum impregnation, and the higher the vacuum pressure, the greater the liquid income (Cortés-Rodríguez 2004). According to the values shown in Table 1, the highest income was attained at a vacuum pressure of 77.9 kPa, with an X of 10.1 ± 0.7%. This X value is higher than what was found for peaches (5%), beetroots (7%), and zucchini slices (8%) but less than what was found for apples (15%), carrots (16%), aubergines (24%), and mushrooms (34%) (Salvatori et al. 1998).
Finally, Table 1 shows the deformation values at the end of the process (ϒ), which presented positive and negative values, associated with the expansion and contraction of the matrix, respectively,. This value was between − 1.7 and 2.8%. The negative values represent a contraction associated with the depression of the gases present in the matrix while the positive results indicate that the matrix expanded as a consequence of the initial release of the gas and internal liquids (Cortés-Rodríguez 2004). Additionally, a multivariate analysis (data not shown) established that there was a significant correlation between the effective porosity (εe) and the volumetric fractions (X, X1) and the deformation at the end of the process (ϒ). This demonstrated that the greater the amount of liquid that flows to and from the matrix, as well as a lower amount of deformation, leads to higher effective porosity values.
In general, it can be concluded that all the response variables could be adjusted to the general model (see Eq. 6) since, according to Table 3, the lack of fit had p values > 0.05. As such, Eqs. 7–9, show the models that describe the behaviour of the effective porosity (εe) (R2 = 70.38), volumetric fraction on the vacuum stage (X1) (R2 = 78.26), and volumetric fraction at the end of the process (X) (R2 = 83.15), respectively:
| 7 |
| 8 |
| 9 |
where A is the vacuum pressure, B is vacuum stage time, and C is atmospheric stage time.
Equations 7–9 can be used for quantification in the case that similar set of experiments are conducted by other researcher using the same range of impregnation conditions i.e. Vacuum pressure: (50.8–77.9 kPa), vacuum stage time: (3–5 min) and atmospheric stage time: (3–5 min), for the same sample material with identical pretreatments.
Lastly, for the optimization process with multi-response optimization methodology, the impregnation parameters with R2 > 70 were used, which means that optimal combination was obtained: Vacuum pressure = 77.3 kPa; vacuum stage time = 3.00 min; and atmospheric stage time = 4.05 min, with a 0.89 desirability. These conditions were used in the subsequent impregnations, prior to dehydration. The parameters estimated by the model were X1 = − 10.04%, X = 10.42%, ϒ = − 1.09%, and εe = 13.26%, and they were in agreement with the experimentally obtained parameters: X1 = − 11.14%, X = 11.57%, ϒ = 1.06%, and εe = 11.89%.
Fortification emulsion
The particle size of the emulsion had an average diameter of 234.9 nm with particle sizes ranging from 137.9 to 331.9 nm. These nanoscale values represented an advantage in the impregnation process since they contributed to greater incorporation due to the fact that the blockages that may be generated by larger particles could be avoided (Derossi et al. 2012). Regarding the apparent viscosity, the value obtained was 1.887 mPa s for the fortification emulsion. According to Betalleluz (2015), viscosity values lower than 10 mPa s do not affect the vacuum impregnation process, therefore, the value obtained contributed to a rapid impregnation kinetic.
Dehydration methods
Table 4 shows the moisture content and water activity for the fresh and impregnated potato as well as for the potato after different dehydration methods. According to the results, the impregnation process caused a 1% decrease in the moisture content of the potato, which was probably due to the gain in solutes; meanwhile, the water activity parameter did not change. As expected, for all the dehydration processes, the moisture content of the samples decreased, and the lowest value was obtained with a 3.09% FD process. Additionally, the lowest aw was achieved with FD, which ensured greater stability. On the other hand, the values reported in Table 4 for dehydrated potatoes obtained by HAD and MVD were located in an area of enzymatic activity where hydrolytic and oxidation reactions could occur (Barbosa-Cánovas and Ibarz 2003). However, due to the fact the enzymes were inactivated during blanching process as well as the low-fat content of the potato (Table 1), it is unlikely that these phenomena will occur. Therefore, the resulting products also had good stability.
Table 4.
Moisture and water activity of potatoes subjected to different treatments
| Water activity (Aw) | Moisture (%) | |
|---|---|---|
| Fresh potato (FP) | 0.996 ± 0.000 | 77.07 ± 0.81 |
| Impregnated Potato (IP) | 0.996 ± 0.002 | 76.02 ± 0.27 |
| IP + hot air drying (HAD) | 0.561 ± 0.016 | 5.43 ± 0.31 |
| IP + freeze drying (FD) | 0.176 ± 0.004 | 3.09 ± 0.53 |
| IP + microwave vacuum drying (MVD) | 0.444 ± 0.006 | 6.49 ± 0.67 |
Determination of calcium, vitamin C, and vitamin E
Figure 2 shows the calcium, vitamin E, and vitamin C content for fresh potatoes, impregnated potatoes, and dehydrated potatoes that are expressed on a dry basis. After the impregnation process, the following values were achieved: 35.92 mg Vitamin E, 472.79 mg Vitamin C, and 956.87 mg Calcium in 100 g dry solids. After the drying processes, the vitamin E content was reduced by 8%, 63%, and 67% for potatoes undergoing the FD, MVD, and HAD processes, respectively. The vitamin C reduction after FD, MVD, and HAD were 9%, 33%, and 38%, respectively. Similar results for the retention of vitamin C with FD, MVD, and HAD has been reported for strawberries (Kowalska et al. 2018). These reductions of Vitamin E and Vitamin C content were due to the thermolabile nature of these vitamins. As the main degradation pathway, ascorbic acid is oxidized to dehydroascorbic acid in a reversible reaction. The resulting dehydroascorbic acid has full vitamin C activity. However, dehydroascorbic acid is more labile than the reduced form and is readily, and irreversibly, oxidized to 2,3-diketogulonic acid consequently loosing vitamin C activity (Woolife 1987). On the other hand, even though the esterification process improves a-tocopherol stability, the molecule is still liable degradation due to environmental sensitivity to oxygen and temperature (Pereira et al. 2015). Degradation products include tocopherylquinone, tocopherylhydroquinone and dimers, trimers and other polar metabolites (Eitenmiller and Lee 2004). Regarding the calcium content, all drying methods had significant differences (p < 0.05) with the impregnated potatoes before the drying processes. This was reduced by 9%, 9%, and 29% when the potatoes were dried using FD, MVD, and HAD, respectively. These results can be explained in terms of the losses of a part of the internal liquid phase due to the sample collapse, which promotes the expulsion of non-compartmented liquid in the tissue that contains calcium ion as well as other soluble solids (Torres et al. 2008).
Fig. 2.
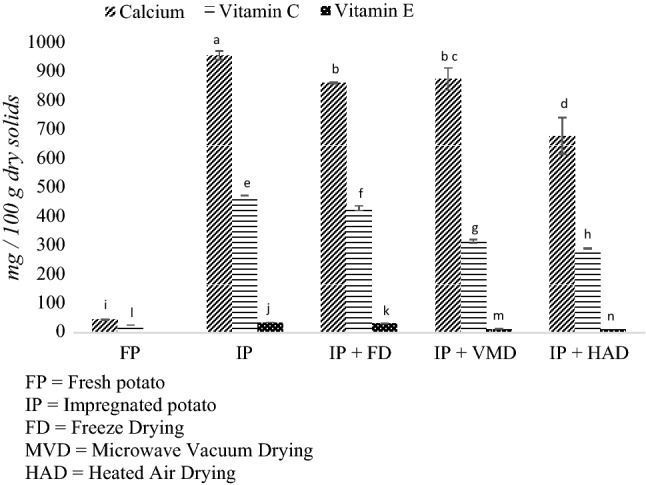
Content of calcium, vitamin C and vitamin E in fresh (FP), impregnated (IP) and dehydrated potatoes
Despite the fact that with the three dehydration processes, some amount of the added compounds was maintained, the greatest conservation was achieved with the FD process and then the MVD process; the lowest conservation of compounds was when the HAD process was used. This may be attributed to the long duration of the HAD process (Barbosa-Cánovas and Ibarz 2003). The advantages presented by the FD process resulted in a low temperature during sublimation and the maintenance of product structure. For this reason, FD has been widely used to obtain high quality and high-value dehydrated fruit and vegetables (Barbosa-Cánovas and Ibarz 2003). Finally, although there were more added compounds with FD than with MVD, the latter has the advantage of providing more rapid heat transfer (Setiady et al. 2007), so it can be executed in shorter processing times (Huang et al. 2011).
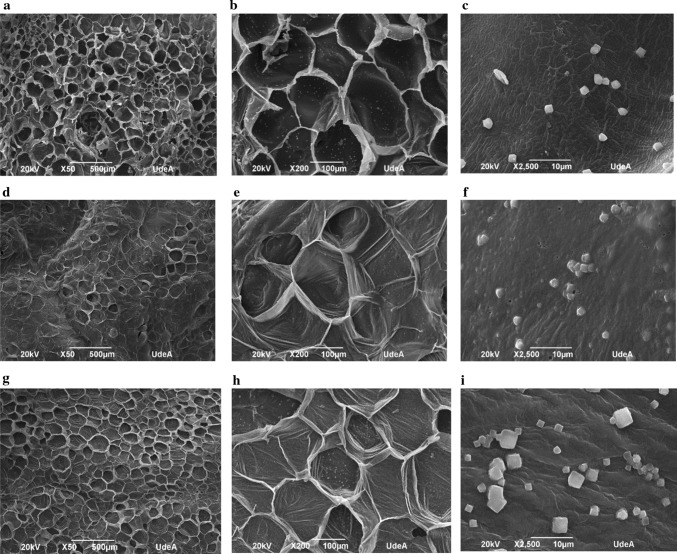
Microstructure
Figure 3 shows the micrographs obtained by Scanning Electron Microscopy of fortified potato that is dehydrated by the three techniques: FD (Fig. 3a–c), MVD (Fig. 3d–f), and HAD (Fig. 3g–i). In all cases, the heating causes stress in the cellular material; this, along with the loss of water, leads to the formation of pores, so porosity increases with the elimination of water (Fig. 3b, e, h) (Singh et al. 2015). Moreover, the micrographs also show the different degrees of porosity that were reached according to the drying process used. The highest porosity was for the potatoes processed by FD (Fig. 3a, b), followed by MVD (Fig. 3d, e). The potatoes dried by HAD (Fig. 3g, h) were the least porous. This behaviour was also reported by (Setiady et al. 2009b) for Russet potatoes when the microstructure of dehydrated potatoes was analysed by FD, MVD, and HAD. This is because, for the FD process, the outer ice crystals in the materials sublimated, and the space originally containing the ice was retained, which formed a highly porous structure in the final product (Barbosa-Cánovas and Ibarz 2003).
Fig. 3.
SEM Micrographs for impregnated and dehydrated potatoes obtained by FD (a–c), MVD, (d–f) and HAD (g–i)
Figure 3b shows the higher rupture in the cell walls for the FD process, which could be caused by the freezing process as this also produces greater damage to the tissues previously subjected to blanching: which is what happened in this case (Duarte et al. 2017). On the other hand, MVD micrographs exhibit signs of cell expansion (Fig. 3e), which show the effect of a vacuum on creating a puffed characteristic in the MVD process. This effect that is known as puffing, has already been shown for strawberries, carrots, and apples and occurs when the evaporation rate inside the product is higher than the transportation rate of steam to the outside the product (Scaman et al. 2014). The resulting overpressure leads to product expansion, which results in higher volume retention of the final product (Setiady et al. 2009b). By contrast, HAD samples are dense and collapsed (Fig. 3g, h). However, for all dehydration processes, the tuber parenchyma potato retained cell wall outlines after blanching as has been previously reported (Bordoloi et al. 2012). Finally, micrographs (Fig. 3c, f, i) for elemental analysis allowed the presence of incorporated calcium to be demonstrated, which can be observed as crystals on the surface of the tissue (Mimouni et al. 2007).
Sensorial analysis
Figure 4, shows descriptors found for the texture (Fig. 4a), flavour (Fig. 4b), and odour (Fig. 4c) of fortified and dehydrated potato chips. Additionally, they were evaluated in terms of their general quality as follows, HAD: 1-low, FD: 2-medium, and MVD: 3-high. The lower score for the HAD potatoes was mainly due to parameters related to its texture, which was evaluated as hard, dry, and fibrous. On the other hand, despite the fact the FD potatoes presented better textural evaluation, their medium-quality score was due to the fact that they possessed a marked flavour and odour of starch. This is because it is the main component of the dry matter of the potato (Singh and Kaur 2016). The saline odour and flavour is attributable to the addition of NaCl to the isotonic solution, and the acid descriptor to the addition of ascorbic acid in the fortification emulsion. The attribute that stands out in terms of the flavour and odour of potato chips is fresh, which is an important sensorial characteristic for the product. Finally, the MVD potatoes were at an equilibrium in terms of their descriptors, so they were categorized as high-quality, Likewise, Setiady et al. (2009a) reported that there was a tendency for panellists to prefer MVD, while HAD was awarded a the low-quality score. Other studies (Joshi et al. 2016a) found that microwave processing was suitable to process the potato chips with acceptable sensorial and textural scores (7.6 and 8.0, respectively on a 9.0 point hedonic scale). Huang et al. (2011), using re-structured mixed potato with apple chips, reported that, despite the fact that chips made by microwave freeze drying had the highest sensory rating, those produced by MVD also had a high score that demonstrated the consumer’s acceptability.
Fig. 4.
Results sensory analysis by multidimensional approach for a texture, b flavor and c odor
Conclusion
It was possible to obtain potato chips dehydrated and fortified with vitamins C and E and calcium, which are low-fat snacks. In addition, it was determined that the optimal vacuum impregnation conditions for the potato were: Vacuum pressure = 77.3 kPa; Vacuum stage time = 3.00 min; Atmospheric stage time = 4.05 min, with a 0.89 desirability. The independent variable that had the greatest influence on the impregnation parameters was vacuum pressure; greater impregnation was achieved when a higher vacuum pressure was used. It was established that the three drying methods evaluated retained the added compounds; however, the MVD stands out because it requires less processing time compared to FD and HAD. Using MVD, levels of vitamin E, vitamin C, and calcium of 13 mg, 314 mg, and 875 mg in 100 g dry solids, were reached, respectively. Therefore, MVD is a promising alternative for the production of fortified potato chips that are capable of retaining the added compounds. It has the advantage of a shorter processing time and high sensory acceptance compared to the other drying methods evaluated; hence, it deserves further study. It is also important to evaluate the MVD processing conditions that favour the quality of the chips.
Acknowledgements
The authors would like to acknowledge the LABCCA Laboratory (Universidad Nacional de Colombia), the BIOALI Research group (Universidad de Antioquia), and COLCIENCIAS, National Doctorates 727 of 2015. We would also like to thank the sustainability programs 2016–2018 Research Groups at the Universidad de Antioquia and Jaimie Brzezinski (London Metropolitan University) for the critical review of the manuscript in English.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AOAC . AOAC oficial methods of analysis. Washington: Association of Offical Analytical Chemists; 2000. [Google Scholar]
- Aprajeeta J, Gopirajah R, Anandharamakrishnan C. Shrinkage and porosity effects on heat and mass transfer during potato drying. J Food Eng. 2015;144:119–128. doi: 10.1016/j.jfoodeng.2014.08.004. [DOI] [Google Scholar]
- Barbosa-Cánovas G, Ibarz A. Unit operations in food engineering. Washington: CRC Press; 2003. [Google Scholar]
- Barreto IMA, Tribuzi G, Marsaioli Junior A, et al. Oil-free potato chips produced by microwave multiflash drying. J Food Eng. 2019;261:133–139. doi: 10.1016/j.jfoodeng.2019.05.033. [DOI] [Google Scholar]
- Belkova B, Hradecky J, Hurkova K, et al. Impact of vacuum frying on quality of potato crisps and frying oil. Food Chem. 2018;241:51–59. doi: 10.1016/j.foodchem.2017.08.062. [DOI] [PubMed] [Google Scholar]
- Betalleluz I (2015) Estudio del enriquecimiento de manzana con prebióticos, probióticos y componentes antioxidantes provenientes de zumo de mandarina por impregnación a vacío para el desarrollo de aperitivos altamente funcionales y con bajo contenido calórico. Universidad Politécnica de Valencia
- Bordoloi A, Kaur L, Singh J. Parenchyma cell microstructure and textural characteristics of raw and cooked potatoes. Food Chem. 2012;133:1092–1100. doi: 10.1016/j.foodchem.2011.11.044. [DOI] [Google Scholar]
- Chafer M, Chiralt A, Perez-Cabrera L, et al. Effectiveness of antibrowning agents applied by vacuum impregnation on minimally processed pear. LWT Food Sci Technol. 2011;44:2273–2280. doi: 10.1016/j.lwt.2011.04.007. [DOI] [Google Scholar]
- Contreras-Calderón J, Calderón-Jaimes L, Guerra-Hernández E, García-Villanova B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res Int. 2011;44:2047–2053. doi: 10.1016/j.foodres.2010.11.003. [DOI] [Google Scholar]
- Cortés-Rodríguez M (2004) Desarrollo de productos de manzana deshidratados enriquecidos con vitamina E. Universidad Politécnica de Valencia
- Cortés-Rodríguez M, Herrera-Herrera EA, Gil-Gonzáles JH. Semi-spherical shape of cape gooseberry (Physalis peruviana L.) impregnated with a fortificated emulsion. Biotecnol en el Sect Agropecu y Agroind. 2016;14:27–36. doi: 10.18684/BSAA(14)27-36. [DOI] [Google Scholar]
- Derossi A, De Pilli T, Severini C. The application of vacuum impregnation techniques in food industry. In: Valdez B, editor. Scientific, health and social aspects of the food industry. London: IntechOpen; 2012. pp. 26–56. [Google Scholar]
- Duarte Y, Chaux A, Lopez N, Largo E, Ramírez C, Nuñez H, Simpson R, Vega O. Effects of blanching and hot air drying conditions on the physicochemical and technological properties of yellow passion fruit (Passiflora edulis Var. Flavicarpa) by-products. J Food Process Eng. 2017;40:e12425. doi: 10.1111/jfpe.12425. [DOI] [Google Scholar]
- Eitenmiller R, Lee J. Vitamin E, food chemistry, composition and analysis. New York: Marcel Dekker Inc.; 2004. [Google Scholar]
- Fito P, Andrb A, Chiralt A, Pardo P. Coupling of hydrodynamic mechanism and phenomena during vacuum treatments in solid porous food-liquid systems. J Food Eng. 1996;21:229–240. doi: 10.1016/0260-8774(95)00005-4. [DOI] [Google Scholar]
- Gobernación de Antioquia (2012) Manual técnico del cultivo de papa bajo buenas prácticas agírcolas
- Gutiérrez Pulido H. Análisis y diseño de experimentos. 3. Ciudad de México: McGraw-Hill; 2012. [Google Scholar]
- Hironaka K, Koaze H. Zinc enrichment of whole potato tuber by vacuum impregnation. J Food Sci Technol. 2015;52:2352–2358. doi: 10.1007/s13197-013-1194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hironaka K, Kikuchi M, Koaze H, et al. Ascorbic acid enrichment of whole potato tuber by vacuum-impregnation. Food Chem. 2011;127:1114–1118. doi: 10.1016/j.foodchem.2011.01.111. [DOI] [PubMed] [Google Scholar]
- Hironaka K, Koaze H, et al. Iron enrichment of whole potato tuber by vacuum impregnation. J Food Sci Technol. 2015;52:2352–2358. doi: 10.1007/s13197-013-1194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Zhang M, Mujumdar A, Lim RX. Comparison of four drying methods for re-structured mixed potato with apple chips. J Food Eng. 2011;103:279–284. doi: 10.1016/j.jfoodeng.2010.10.025. [DOI] [Google Scholar]
- ICONTEC . Norma Técnica Colombiana. Colombia: Instituto Colombiano de Normas Técnicas y Certificación; 1996. [Google Scholar]
- Joshi A, Kar A, Rudra SG, et al. Vacuum impregnation: a promising way for mineral fortification in potato porous matrix (potato chips) J Food Sci Technol. 2016;53:4348–4353. doi: 10.1007/s13197-016-2424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Rudra SG, Sagar VR, et al. Development of low fat potato chips through microwave processing. J Food Sci Technol. 2016;53:3296–3303. doi: 10.1007/s13197-016-2304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kim HS. Physicochemical properties of dehydrated potato parenchyma cells with ungelatinized and gelatinized starches. Carbohydr Polym. 2015;117:845–852. doi: 10.1016/j.carbpol.2014.10.038. [DOI] [PubMed] [Google Scholar]
- Kowalska J, Kowalska H, Marzec A, et al. Dried strawberries as a high nutritional value fruit snack. Food Sci Biotechnol. 2018;27:799–807. doi: 10.1007/s10068-018-0304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madiouli J, Sghaier J, Lecomte D, Sammouda H. Determination of porosity change from shrinkage curves during drying of food material. Food Bioprod Process. 2012;90:43–51. doi: 10.1016/j.fbp.2010.12.002. [DOI] [Google Scholar]
- Martínez-Navarrete N, Andrés Grau AM, Chiralt Boix A, Fito P (1999) Termodinámica y cinética de sistemas alimento-entorno. Servicio de Publicaciones. Universidad Politécnica de Valencia, Valencia, España
- Mimouni A, Bouhallab S, Famelart MH, et al. The formation of calcium lactate crystals is responsible for concentrated acid whey thickening. J Dairy Sci. 2007;90:57–65. doi: 10.3168/jds.S0022-0302(07)72608-5. [DOI] [PubMed] [Google Scholar]
- Moreira RG, Almohaimeed S. Technology for processing of potato chips impregnated with red rootbeet phenolic compounds. J Food Eng. 2018;228:57–68. doi: 10.1016/j.jfoodeng.2018.02.010. [DOI] [Google Scholar]
- Pereira GG, Detoni CB, Da Silva TL, et al. α-Tocopherol acetate-loaded chitosan microparticles: stability during spray drying process, photostability and swelling evaluation. J Drug Deliv Sci Technol. 2015;30:220–224. doi: 10.1016/j.jddst.2015.10.018. [DOI] [Google Scholar]
- Radziejewska-kubzdela E, Kido M. Applicability of vacuum impregnation to modify physico-chemical, sensory and nutritive characteristics of plant origin products — a review. Int. J. Mol. Sci. 2014;15:16577–16610. doi: 10.3390/ijms150916577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier M, Knoerzer K, Schubert H. The microwave processing of foods. 2. Amsterdam: Elsevier Ltd; 2017. [Google Scholar]
- Salvatori D, Andrés A, Chiralt A, Fito P. The response of some properties of fruits to vacuum impregnation. J Food Process Eng. 1998;21:59–73. doi: 10.1111/j.1745-4530.1998.tb00439.x. [DOI] [Google Scholar]
- Scaman CH, Durance TD, Drummond L, Sun D. Combined microwave vacuum drying. In: Sun Da-Wen., editor. Emerging technologies for food processing. Dublin, Ireland: Academic Press; 2014. pp. 427–445. [Google Scholar]
- Setiady D, Clary C, Younce F, Rasco BA. Optimizing drying conditions for microwave-vacuum (MIVAC®) drying of russet potatoes (Solanum tuberosum) Dry Technol. 2007;25:1483–1489. doi: 10.1080/07373930701537187. [DOI] [Google Scholar]
- Setiady D, Rasco B, Younce F, Clary C. Rehydration and sensory properties of dehydrated russet potatoes (Solanum tuberosum) using microwave vacuum, heated air, or freeze dehydration. Dry Technol. 2009;27:1116–1122. doi: 10.1080/07373930903221382. [DOI] [Google Scholar]
- Setiady D, Tang J, Younce F, et al. Porosity, color, texture and microscopic structure of russet potatoes dried using microwave vacuum, heated air, and freeze drying. Appl Eng Agric. 2009;25:719–724. doi: 10.13031/2013.28844. [DOI] [Google Scholar]
- Singh J, Kaur L. Advances in potato chemistry and technology. 2. Palmerston North: Elsevier; 2016. [Google Scholar]
- Singh F, Katiyar VK, Singh BP. Mathematical modeling to study influence of porosity on apple and potato during dehydration. J Food Sci Technol. 2015;52:5442–5455. doi: 10.1007/s13197-014-1647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari P, Joshi A, Varghese E, Thakur M. Process standardization and storability of calcium fortified potato chips through vacuum impregnation. J Food Sci Technol. 2018;55:3221–3231. doi: 10.1007/s13197-018-3254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JD, Castello ML, Escriche I, Chiralt A. Quality characteristics, respiration rates, and microbial stability of osmotically treated mango tissue (Mangifera indica L.) with or without calcium lactate. Food Sci Technol Int. 2008;14:355–365. doi: 10.1177/1082013208097276. [DOI] [Google Scholar]
- Woolife JA. The potato in the human diet. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- World Health Organization (2018) Healthy diet. https://www.who.int/news-room/fact-sheets/detail/healthy-diet
- Zvitov-Ya’ari R, Nussinovitch A. Browning prevention in rehydrated freeze-dried non-blanched potato slices by electrical treatment. LWT - Food Sci Technol. 2014;56:194–199. doi: 10.1016/j.lwt.2013.10.017. [DOI] [Google Scholar]