Abstract
The anti-amyloidogenic potential and cyclooxygenase anti-inflammatory activity of Lasianthus trichophlebus extracts were evaluated. The MeOH extract (LTM) and chloroform extract (LTC) exhibited significant cytotoxic inhibition against the neuroblastoma SH-SY5Y cell with an IC50 of 17.52 μg/mL and 12.28 μg/mL, respectively. Thioflavin T assay indicated the LTC extract inhibition (70.56% at 50 μg/mL) to be statistically comparable (p < 0.05) to the positive control. Cyclooxygenase inhibition against COX-1 and COX-2 enzymes gave IC50 values for the LTM extract to be 18.20 and 29.60 µg/mL, respectively; while, the LTC extract showed 4.11 and 2.78 µg/mL, respectively. LC–MS of the LTM extract identified 22 putative compounds, which may prove to be pharmacologically relevant. This study has provided potential insights into the utilization of L. trichophlebus to develop safer plant-based agents for anti-inflammatory or neurodegenerative diseases.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2145-2) contains supplementary material, which is available to authorized users.
Keywords: Lasianthus, Cytotoxicity, Neuroblastoma, Cyclooxygenase
Introduction
Genus Lasianthus Jack consists of 225 species, with the highest diversity in tropical and subtropical Asia (Arshed and Alejandro 2016). These plants are used in several types of traditional folk medicine, including treatments for fever and blood loss with Lasianthus lucidus (Choudhury et al. 2014) and as an unspecified antidote using the fruit of Lasianthus andamanicus (Sharief 2007). The root decoction of Lasianthus oblongus was used by post-partum mothers to accelerate the contraction of expanded organs (Ong et al. 2012). Recent pharmacological studies revealed the antioxidant potential of Lasianthus hartii (Yang et al. 2017), anti-inflammatory effect of L. oblongus (Saha et al. 2004), and cytotoxicity against human ovarian 2780 cells by Lasianthus acuminatissimus (Li et al. 2006). Secondary metabolites from Lasianthus species were isolated using several chromatographic methods and elucidated with spectroscopic techniques. These included the presence of anthraquinones and their glycosides and sesquiterpenes from L. acuminatissimus (Li et al. 2006), iridoid glucosides from Lasianthus wallichii (Takeda et al. 2002), Lasianthus hartii (Yang et al. 2017), and Lasianthus verticillatus (Al-Hamoud et al. 2019), megastigmane glucosides from Lasianthus fordii (Takeda et al. 2004), and triterpenoids and steroids from Lasianthus gardneri (Dallavalle et al. 2004).
As part of our research in studying the endemic and indigenous Rubiaceae plants of medicinal importance from the Philippines (Castro et al. 2016; Olivar et al. 2018; Tan et al. 2012, 2014), we investigated the Lasianthus trichophlebus Hemsl. ex. F.B. Forbes & Hemsl. This species is found in South China, Thailand, Vietnam, Malay Peninsula, Philippines, and Indonesia and grows from a short shrub to reach 1–2-m tall (Zhu 2002). No study on the phytochemicals or bioactivity of L. trichophlebus has been previously reported. Hence, the present study of L. trichophlebus describes its cytotoxicity against a neuroblastoma cell line (SH-SY5Y), amyloid-beta aggregation potential, inhibitory effects against COX-1 and COX-2, and the metabolite profile of its extracts.
Materials and methods
Plant materials
Fresh leaves of L. trichophlebus were collected from the province of Antique, Philippines (11° 24′ 33.59″ N, 122° 04′ 2.40″ E) in April 2016. The plant morphology was identified by Grecebio Jonathan Alejandro, a Philippine Rubiaceae specialist. After a year, this species was molecularly authenticated using five DNA barcoding loci (Arshed et al. 2017). A voucher specimen was kept at the UST-Herbarium (USTH 012462).
Extraction and fractionation of extracts
Air-dried, ground leaves of L. trichophlebus (2.7 kg) were subjected to MeOH extraction overnight. The filtrate was collected and concentrated under reduced pressure using a rotary evaporator set at 45 °C. The process was repeated thrice using a total of 22.0 L MeOH. The extraction yielded 247.34 g of the MeOH extract (LTM). A portion (190 g) of the marc was suspended in dist. H2O and partitioned successively with hexane and CHCl3. The hexane and CHCl3 layers were, respectively, dried with anhydrous Na2SO4 and concentrated in vacuo to obtain the hexane (LTH, 27.7 g) and CHCl3 extracts (LTC, 8.0 g). The remaining aqueous layer was freeze-dried using a lyophilizer to obtain the aqueous extract (LTA, 9.4 g).
ATP cell cytotoxicity assay
Fibroblast N9 and neuroblastoma SH-SY5Y cells were maintained in DMEM supplemented with 10% FBS, 1% kanamycin, and 1% penicillin. Cell cultures were maintained at 37 °C in 5% CO2 and passaged once per week. The cells were sub-cultured into a 96-well plate at 1 × 103 cells/well and incubated for 24 h. The cells were treated with plant extracts and incubated for 48 h for fibroblast N9 cells and 72 h for neuroblastoma SH-SY5Y cells. The media was removed, and the wells were washed with PBS. Fresh media (100 μL) were added and incubated for another 30 min. CellTiter-Glo® luminescent reagent (100 μL) was added and the luminescence was measured using a PerkinElmer Victor-3® multi-plate reader (PerkinElmer, Waltham, MA, USA).
Thioflavin T (ThT) fluorescence assay
The amyloid-β1–42 (10 μM) dissolved in PBS (pH 7.4) was incubated at 37 °C for 24 h in the presence or absence of the plant extracts. Then, ThT solution (20 μL, 50 μM) in glycine–NaOH buffer (pH 9) was added. The fluorescence signal was measured (excitation wavelength, 450 nm; emission wavelength, 510 nm) using a PerkinElmer Victor-3® multi-plate reader. The percentage of aggregation inhibition was calculated using the following equation: [(1 − IFi/IFc) × 100%], where IFi and IFc are the fluorescence absorbance with and without the inhibitors, respectively, after subtracting the background fluorescence of the ThT solution (Xia et al. 2019).
Cyclooxygenase assay
To a solution of 150 μL of 100-mM Tris, 10 µL of plant extracts dissolved in DMSO, 10 µL of 1000 µM Hemin, and 10 µL of 250 U/mL COX-2 or COX-1 enzyme were added. Indomethacin was used as the positive control, and DMSO served as the negative control. The mixture was incubated at 25 °C for 15 min. After incubation, 10 µL of 200-µM amplex red was added to the mixture. Then, 10 µL of 2000-µM arachidonic acid was added, and the reaction fluorescence absorbance was monitored for 2 min using Varioskan Flash at 535 nm (excitation) and 590 nm (emission).
The percent inhibition of the samples and the positive control was determined based on the averaged slope of each replicate using the following formula:
“Slope uninhibited” is the slope of the line from the fluorescence intensity vs. time plot of the negative control group, and “slope inhibited” is the slope of the line from the fluorescence intensity vs. time plot of the samples/positive control.
LC–MS metabolite profiling
LC–MS/MS analysis was performed using a Xevo G2-S Qtof (Waters Corp., Singapore). The separation was achieved using a BEH HSS T3 column (50 × 2.1 mm internal diameter). The system delivered a constant flow of 0.4 mL/min, and the mobile phase consisted of 5% CH3CN in MeOH and 0.1% HCOOH in H2O. The injection volume was 1 µL. For operation in MS/MS mode, a mass spectrometer with an electrospray interface (ESI) was used, and the parameters were set as follows: capillary voltage, 3.0 kV for negative mode; source temperature, 120 °C; desolvation temperature, 400 °C; cone gas flow, 100 L/h; and desolvation gas flow, 1000 L/h. Low collision energy at 6 V, high collision energy at 20–50 V, and lock mass solution at 1 ng/µL were used to calibrate mass accuracy. All LC–MS/MS data were processed by the MassLynx version 3.5 NT Quattro data acquisition software. For putative compound identification, accurate mass screening was carried out using the UNIFI data analysis software. The acquired MS spectra were subjected to library matching using the Traditional Chinese Medicine (TCM) library that is integrated within the UNIFI analysis software. Annotation of the candidate masses was based on the accurate mass match, isotopic ratio match, and precursor ion intensity counts. The criteria for a component ID to be considered a good match are as follows: a mass accuracy error ≤ 5 mDa or ≥ − 5 mDa, and a response precursor for precursor ion ≥ 2000.
Statistical analysis
All values were reported as means ± SD. Statistical significance was analyzed using one-way ANOVA and Levene’s test followed by Tukey’s HSD test. p < 0.05 was considered statistically significant.
Results and discussion
In vitro cell toxicity using fibroblast N9
Methanolic extraction and solvent partitioning of L. trichophlebus leaves resulted in obtaining the different extracts. All extracts were dried free of solvent prior to their use in the different assays. Initially, the toxicity of the L. trichophlebus crude methanolic extract (LTM) was determined in vitro using the fibloblast N9 cell line (Fig. 1). The LTM extract did not show any significant cytotoxicity after 48-h incubation even at the highest concentration of 50 μg/mL. Maximum cell growth inhibition of 15% was noted at 50 μg/mL. The percentage cell viability of the LTM extract was comparable to the negative control (0 μg/mL) at p < 0.05. The in vivo acute oral toxicity of the LTM extract is reported in the Online Resource material.
Fig. 1.
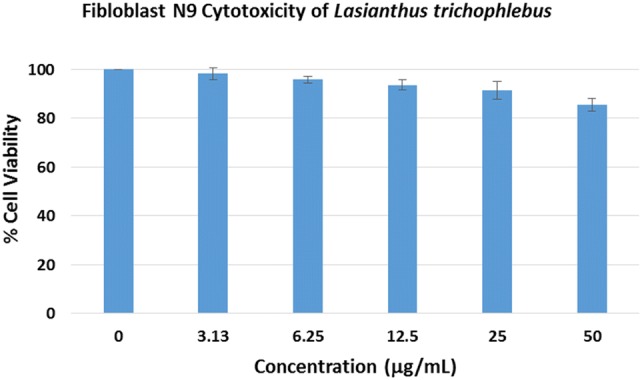
Cell viability of the Lasianthus trichophlebus crude methanolic extract (LTM) against fibroblast N9 cell line. The viability was determined by ATP assay and is expressed as % of the control (0 μg/mL). Results are expressed as means ± SD of three trial experiment
Cyclooxygenase-1 and -2 inhibition
The cyclooxygenase pathways have been widely accepted as major enzymatic routes dealing with inflammatory progression in mammalian cells. Hence, their clinical relevance has also been noted in several review articles (González-Périz and Clària 2007). Medicinal drugs exhibiting anti-inflammatory activity were also prioritized in pharmacological treatment dealing with degenerative inflammatory diseases (Malik et al. 2017). COX-1 inhibition in gastric mucosal cells led to decreased prostaglandin synthesis (Saha et al. 2004), which would be responsible for the significant gastric toxicities imposed by nonsteroidal anti-inflammatory drugs (NSAIDs) due to the loss of local protection. COX-2 inhibition was more desirable for delivering therapeutic outcomes for inflammation, pain, cancer, and neurological diseases (Blobaum and Marnett 2007).
The results of COX-1 and COX-2 screening assays of the L. trichophlebus extracts at 10 μg/mL are presented in Fig. 2. Among the four tested extracts, LTC showed the highest inhibition towards both COX-1 (61.63%) and COX-2 (57.75%). The LTM extract also showed enzyme inhibitions greater than 50%. The LTM extract gave 54.01% against COX-1 and 57.07% inhibition using COX-2. The other extracts, hexane (LTH) and aqueous (LTA), gave enzyme inhibitions of less than 50%. All extracts exhibited significantly lower inhibition of COX-1 and COX-2 compared to the standard drug, indomethacin (p < 0.05), which gave 88.61% inhibition for COX-1 and 92.28% inhibition for COX-2.
Fig. 2.
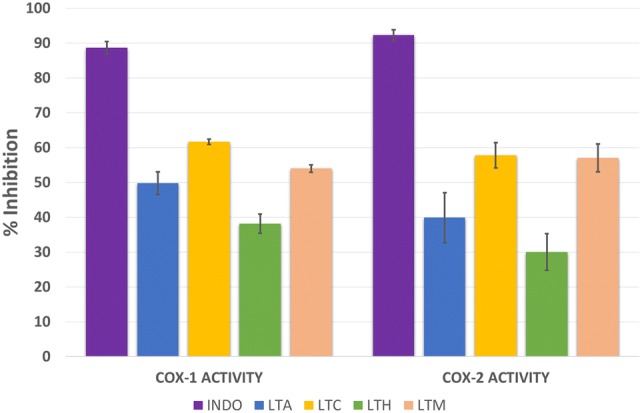
In vitro cyclooxygenase of Lasianthus trichophlebus extracts at 10 µg/mL. Indomethacin (INDO, 4.0 mM) was used as the positive control. The % inhibition was expressed as means ± SD of three trial experiment. Only LTM and LTC extracts exhibited > 50% inhibition to the COX-1 and COX-2 enzymes. At p < 0.05, there is a significant difference on the % inhibition of INDO to the plant extracts. LTA aqueous extract, LTC chloroform extract, LTH hexane extract, LTM crude methanol extract
As only LTM and LTC extracts gave greater than 50% inhibition to the cyclooxygenase enzymes, we determined their 50% maximal inhibitory concentration (IC50) using 0.5, 1, 5, 10, 40, 70, and 100 µg/mL concentrations. The LTM extract exhibited IC50 values of 18.20 and 29.60 µg/mL for COX-1 and COX-2, respectively. The IC50 values for LTC were 4.11 and 2.78 µg/mL for COX-1 and COX-2, respectively. Based on these values, the LTC revealed a more potent IC50 compared to LTM. Extensive literature search also indicated limited studies on the anti-inflammatory effect of Lasianthus extracts. The L. oblungus crude extract gave 85.97% inhibition (at 250 μg/mL) on nitric oxide production from macrophages (RAW 264.7 cells) (Saha et al. 2004).
Neuroblastoma SH-SY5Y cell viability
Different methods of in vitro cell viability and cytotoxicity assays were established (Ishiyama et al. 1996) and applied for drug screening and development as they are rapid, inexpensive, and allow low-cost animal study. Adenosine tri-phosphate (ATP) is the most important chemical energy source for all biological processes in cells. As such, measuring cellular ATP is the most sensitive method for probing cellular stages and determining their viability. Cells tended to limit their ability to produce ATP when they were stressed, mutilated, or subjected to limited nutrient availability. The ATP assay utilized the conversion of luciferin to oxyluciferin catalyzed by luciferase in the presence of Mg2+ ions and ATP. Hence, the luminescent signals correlated linearly with the ATP concentrations or cell numbers (Garcia and Massieu 2003; Mueller et al. 2004).
The cellular viability of neuroblastoma SH-SY5Y cells was explored in the presence of LTM and LTC extracts via the ATP assay. As depicted in Fig. 3, both extracts at various concentrations inhibited SH-SY5Y cell growth. There is no significant difference (p < 0.05) in the percentage cell viability of both extracts at the 0.39 μg/mL lowest concentration when compared to the negative control (0 μg/mL). However, significant difference (p < 0.05) was observed for the other percentage inhibitions for both extracts. The IC50 of the LTM extract was determined as 17.52 μg/mL, while the LTC extract gave an IC50 of 12.28 μg/mL. Since the neuroblastoma SH-SY5Y cells are used in neurological studies including Parkinson's disease (PD), Alzheimer's disease (AD), and traumatic brain injury (TBI) (Wu et al. 2011), the results of the cytotoxicity assay may offer promising leads to the discovery of pharmacologically active compounds in neurological diseases.
Fig. 3.
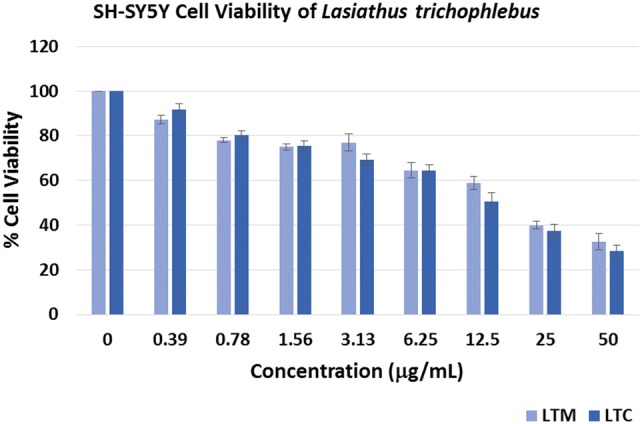
Cell viability of the Lasianthus trichophlebus crude methanolic extract (LTM) and chloroform extract (LTC) against the neuroblastoma SH-SY5Y. The viability was determined by ATP assay and is expressed as % of the control (0 μg/mL). Results are expressed as means ± SD of three trial experiment
Thioflavin T assay
Alzheimer’s disease (AD) is a fatal neurodegenerative disorder associated with dementia, deterioration of cognitive functions, and memory loss. Several factors have been identified to enhance AD progression, including abnormal β-amyloid (Aβ) deposition, tau protein aggregation, decreased acetylcholine, oxidative stress, and neuroinflammation of the nervous system (Xia et al. 2019). Currently, only five compounds (donezipil, tacrine, rivastigmine, galantamine, and memantine) are available and approved in the market to reduce the symptoms associated with AD (Alghazwi et al. 2019). The control of Aβ deposition is an important approach for AD treatment as it damages neuronal and mitochondrial function, leading to oxidative stress and neuroinflammation.
As part of our on-going program on research involving AD (Giau et al. 2019, Giau and An 2019), we have tested the potential of the LTM and LTC extracts (Table 1) to inhibit the aggregation of Aβ1–42 deposition using the thioflavin-T (ThT) fluorescence assay utilizing phenol red as the positive control. Moderate percentage inhibition was noted for the LTM extract at 50 μg/mL (44.02%) and 5 μg/mL (28.49%), and the LTC extract at 5 μg/mL (31.67%). A strong inhibition of 70.56% was observed for the LTC extract at 50 μg/mL. This value is also statistically comparable to the inhibition of the positive control at p < 0.05.
Table 1.
ThT assay results of Lasianthus trichophlebus extracts
| Sample | Aβ1–42 aggregation inhibition (%)a |
|---|---|
| Phenol red (18 μg/mL)b | 68.03 ± 2.33 |
| LTM extract (50 μg/mL) | 44.02 ± 1.26 |
| LTM extract (5 μg/mL) | 28.49 ± 1.68 |
| LTC extract (50 μg/mL) | 70.56 ± 2.46* |
| LTC extract (5 μg/mL) | 31.67 ± 3.98 |
*Statistically comparable to the positve control at p < 0.05
aThe values are expressed as mean ± SD of three trial experiments
bThe positive control
Metabolite profiling
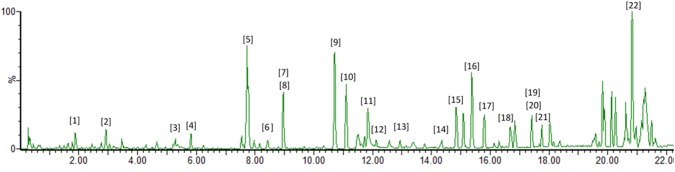
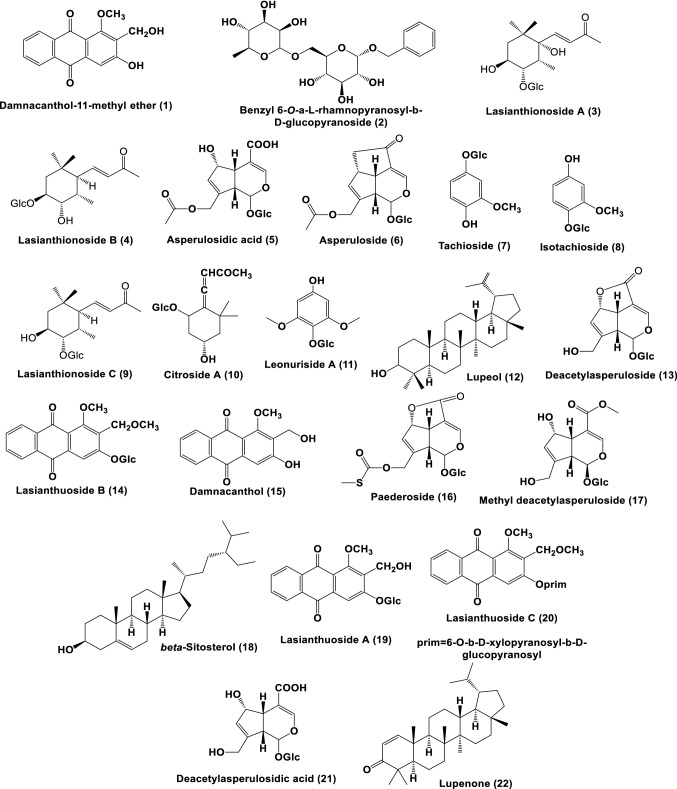
LC–MS metabolite profiling of the LTM extract has putatively identified 22 secondary metabolites (Figs. 4, 5, Table 2). Based on the literature, these compounds could be categorized as “good match” standards in the TCM library (Chen 2011; He et al. 2019), as listed in Table 2. The following compounds were previously isolated and identified from different Lasianthus species: benzyl 6-O-α-l-rhamnopyranosyl-β-d-glucopyranoside (2), asperuloside (6), citroside A (10), and paedroside (16) from L. wallichii (Takeda et al. 2002); lasianthionoside A (4), B (3), and C (9), asperuloside (6), citroside A (10), deacetylasperuloside (13), and methyl deacetylasperuloside (17) from L. fordii (Takeda et al. 2004); damnacanthol 11-methyl ether (1), asperulosidic acid (5), asperuloside (6), tachioside (7), isotachioside (8), lasianthuoside A (19), B (14), and C (20), damnacanthol (15), and deacetyl asperulosidic acid (21) from L. acuminatissimus (Li et al. 2006); lupeol (12), β-sitosterol (18), and lupenone (22) from L. gardneri (Dallavalle et al. 2004); and leonuriside A (11), asperuloside (6), and asperulosidic acid (5) from L. hartii (Yang et al. 2017). Interestingly, several compounds were found to be bioactive (Table 2). These bioactive compounds might be responsible for the observed biological activity of the L. trichophlebus extracts. Although these compounds were putatively identified using untargeted LC–MS, all compounds were present in other Lasianthus species, thus supporting their common biogenetic pathway in the genus Lasianthus. The antioxidant activity of several compounds and the phenolic functionality have been reported to have an effect on the inhibition of amyloid-beta aggregation. In particular, the presence of the aromatic moiety forms a non-covalent interaction with the amino acid residues, while the hydroxyl groups are capable of hydrogen bonding in amyloid-beta (Dhouafli et al. 2019).
Fig. 4.
Chromatogram of Lasianthus trichophlebus MeOH extract (LTM)
Fig. 5.
Structures of putative compounds of Lasianthus trichophlebus MeOH extract (LTM)
Table 2.
Secondary metabolites from Lasianthus trichophlebus MeOH extract (LTM)
| RT | Exact mass | Elemental composition | Error (ppm) | Putative identity | Associated biological activity | |
|---|---|---|---|---|---|---|
| Calculated | Observed | |||||
| 1.88 | 298.08412 | 298.08540 | C17H14O5 | 4.35 | Damnacanthol-11-methyl ether (1) | |
| 2.92 | 326.12131 | 326.12120 | C12H22O10 | − 0.31 | Benzyl 6-O-α-l-rhamnopyranosyl-β-d-glucopyranoside (2) | |
| 5.29 | 388.20310 | 388.20340 | C19H31O8 | − 1.03 | Lasianthionoside B (3) | |
| 5.81 | 404.19560 | 404.19680 | C19H31O9 | − 2.96 | Lasianthionoside A (4) | |
| 7.73 | 432.12677 | 432.12820 | C18H24O12 | 3.29 | Asperulosidic acid (5) | |
| 8.43 | 414.11621 | 414.11570 | C18H22O11 | − 1.2 | Asperuloside (6) |
Treatment of rheumatoid arthritis (Li et al. 2006) Antiangiogenic (Camero et al. 2018) |
| 8.97 | 302.10016 | 302.10050 | C13H18O8 | 1.13 | Tachioside (7) | Antioxidant (DPPH) (Simlai and Roy 2013) |
| 8.97 | 302.10016 | 302.10050 | C13H18O8 | 1.13 | Isotachioside (8) | Antioxidant (DPPH) (Simlai and Roy 2013) |
| 10.71 | 388.19980 | 388.19940 | C19H31O8 | 0.77 | Lasianthionoside C (9) | |
| 11.09 | 386.19406 | 386.19370 | C19H30O8 | − 1.03 | Citroside A (10) | |
| 11.84 | 332.11073 | 332.11180 | C14H20O9 | 3.2 | Leonuriside-A (11) | Anti-oxidant (FTC) (Sugaya et al. 1998) |
| 12.11 | 390.18311 | 390.18190 | C25H26O4 | − 3.07 | Lupeol (12) |
Antiinflammatory (Geetha and Varalakshimi. 2001) Antiangiogenic (You et al. 2003) |
| 12.93 | 372.10565 | 372.10540 | C16H20O10 | − 0.8 | Deacetylasperuloside (13) | |
| 14.35 | 460.13700 | 460.13840 | C23H24O10 | 3.04 | Lasianthuoside B (14) | Antitumor (Li et al. 2006) |
| 14.84 | 284.06848 | 284.06700 | C16H12O5 | − 5.26 | Damnacanthol (15) | Antibacterial (Comini et al. 2011) |
| 15.37 | 446.08829 | 446.08620 | C18H22O11S | − 4.7 | Paedroside (16) | |
| 15.80 | 404.13187 | 404.13100 | C17H24O11 | − 2.22 | Methyl deacetylasperuloside (17) | |
| 16.84 | 414.38617 | 414.38650 | C29H50O | 0.72 | β-Sitosterol (18) | Antiinflammatory, Antipyretic (Gupta et al. 1980) Anthelminthic, antimutagenic, analgesic (Villasenor et al. 2002) |
| 17.41 | 446.12100 | 446.12080 | C22H22O10 | − 0.45 | Lasianthuoside A (19) | Antitumor (Li et al. 2006) |
| 17.41 | 592.17900 | 592.17760 | C28H32O14 | − 2.36 | Lasianthuoside C (20) | Antitumor (Li et al. 2006) |
| 17.67 | 390.11621 | 390.11720 | C16H22O11 | 2.56 | Deacetylasperulosidic acid (21) | In vivo antioxidant (Ma et al. 2013) |
| 20.83 | 424.37051 | 424.36950 | C30H48O | − 2.35 | Lupenone (22) | Antidiabetic (Xu et al. 2014) |
RT retention time in minutes
Conclusions
This is the first report on the pharmacological activities associated with cyclooxygenase inhibition and amyloid-beta aggregation potential of Lasianthus trichophlebus. The crude methanolic and semi-polar chloroform extracts exhibited significant inhibition against neuroblastoma SH-SY5Y cells and cyclooxygenase-1 and -2 enzymes; while, the chloroform extract exhibited strong inhibition using the ThT assay. The present results demonstrated that L. trichophlebus could be a source of pharmacologically relevant compounds with potentially new biologically active materials obtained from its extracts. Studies of L. trichophlebus also provided potential insights into the utilization of plant materials to develop safer plant-based agents for anti-inflammatory or neurodegenerative diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The Pascual Pharma Corporation is gratefully acknowledged for the LC–MS analysis and the Philippine DOST-NSC-SEI for the scholarship to M.W.D.L. This research was partly funded by the National Research Foundation of Korea (NRF) Grants awarded by the Korean government (MEST, No. 2017R1A2B4012636).
Author contribution
MAT and SSAN conceptualized the study; MWDL performed the experiments; GJDA collected and identified the plant materials; MAT, SSAN, GJDA wrote and revised the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The in vivo acute oral toxicity in the Online Resource material was approved by the Institutional Animal Care and Use Committee (IACUC) at the Research Center for Natural and Applied Sciences, University of Santo Tomas, and the Philippine Bureau of Animal Industry and Animal Research Permit.
Contributor Information
Mario A. Tan, Email: matan@ust.edu.ph
Seong Soo A. An, Email: seong.an@gmail.com
References
- Alghazwi M, Smid S, Musgrave I, et al. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (Aβ1–42) toxicity and aggregation. Neurochem Int. 2019;124:215–224. doi: 10.1016/j.neuint.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Al-Hamoud GA, Orfali RS, Perveen S, et al. Lasianosides A–E: new iridoid glucosides from the leaves of Lasianthus verticillatus (Lour.) Merr. and their antioxidant activity. Molecules. 2019;24:3995. doi: 10.3390/molecules24213995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshed MJC, Alejandro GJD. A new Philippine endemic species and new records of Lasianthus (Lasiantheae, Rubiaceae) Phytotaxa. 2016;288:296–300. doi: 10.11646/phytotaxa.288.3.12. [DOI] [Google Scholar]
- Arshed MJC, Valdez MB, Alejandro GJD. Evaluating the feasibility of five candidate DNA Barcoding Loci for Philippine Lasianthus Jack (Lasiantheae: Rubiaceae) Phcog Mag. 2017;13:553–558. doi: 10.4103/pm.pm_1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobaum AL, Marnett LJ. Structural and functional basis of cyclooxygenase inhibition. J Med Chem. 2007;50:1425–1441. doi: 10.1021/jm0613166. [DOI] [PubMed] [Google Scholar]
- Camero CM, Germano MP, Rapisarda A, et al. Anti-angiogenic activity of iridoids from Galium tunetanum. Rev Bras Farmacogn. 2018;28:374–377. doi: 10.1016/j.bjp.2018.03.010. [DOI] [Google Scholar]
- Castro SG, Cid JE, Ibañez WA, et al. GC–MS metabolite profiling of the hexane extract and antimicrobial characterization of the Philippine endemic Rubiaceae species Uncaria cordata var. circa, Psychotria luzoniensis, and Psydrax puberula. Acta Manilana. 2016;64:9–16. [Google Scholar]
- Chen C-Y. TCM database in Taiwan: the world’s largest traditional Chinese medicine database for drug screening in silico. PLoS ONE. 2011;6:e15939. doi: 10.1371/journal.pone.0015939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury KD, Choudhury MD, Baruah MK. Hepatoprotective potential of Lasianthus lucidus leaf extracts against carbon tetrachloride induced liver damage in Swiss albino mice. World J Pharma Pharm Sci. 2014;3:1536–1547. [Google Scholar]
- Comini LR, Nuńez Montoya SC, Paez PL, et al. Antibacterial activity of anthraquinone derivatives from Heterophyllaea pustulata (Rubiaceae) J Photoch Photobio B. 2011;102:108–114. doi: 10.1016/j.jphotobiol.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Dallavalle S, Jayasinghe L, Kumarihamy B, et al. A new 3,4-seco-lupane derivative from Lasianthus gardneri. J Nat Prod. 2004;67:911–913. doi: 10.1021/np030374g. [DOI] [PubMed] [Google Scholar]
- Dhouafli Z, Jannet HB, Mahjoub B, et al. 1,2,4-trihydroxynaphthalene-2-O-β-d-glucopyranoside: a new powerful antioxidant and inhibitor of Aβ42 aggregation isolated from the leaves of Lawsonia inermis. Nat Prod Res. 2019;33:1406–1414. doi: 10.1080/14786419.2017.1419229. [DOI] [PubMed] [Google Scholar]
- Garcia O, Massieu L. Glutamate uptake inhibitor l-transpyrrolidine 2,4-dicarboxylate becomes neurotoxic in the presence of subtreshold concentrations of mitochondrial toxin 3-nitropropionate: involvement of mitochondrial reducing activity and ATP production. J Neurosci Res. 2003;74:956–966. doi: 10.1002/jnr.10825. [DOI] [PubMed] [Google Scholar]
- Geetha T, Varalakshmi P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. J Ethnopharm. 2001;76:77–80. doi: 10.1016/S0378-8741(01)00175-1. [DOI] [PubMed] [Google Scholar]
- Giau VV, Senanarong V, Bagyinszky E, et al. Analysis of 50 neurodegenerative genes in clinically diagnosed early-onset Alzheimer’s Disease. Int J Mol Sci. 2019;20:1514. doi: 10.3390/ijms20061514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giau VV, An SSA. Epitope mapping immunoassay analysis of the interaction between β-amyloid and fibrinogen. Int J Mol Sci. 2019;20:496. doi: 10.3390/ijms20030496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Périz A, Clària J. New approaches to themodulation of cyclooxygenase-2 and 5-lipoxyenase pathways. Curr Top Med Chem. 2007;7:297–309. doi: 10.2174/156802607779941378. [DOI] [PubMed] [Google Scholar]
- Gupta MB, Nath R, Srivastava N, et al. Anti-inflammatory and antipyretic activities of β-sitosterol. Planta Med. 1980;39:157–163. doi: 10.1055/s-2008-1074919. [DOI] [PubMed] [Google Scholar]
- He M, Grkovic T, Evans JR, et al. The NCI library of traditional Chinese medicinal plant extracts—preliminary assessment of the NCI-60 activity and chemical profiling of selected species. Fitoterapia. 2019;137:104285. doi: 10.1016/j.fitote.2019.104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama M, Tominaga H, Shiga M, et al. Combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull. 1996;19:1518–1520. doi: 10.1248/bpb.19.1518. [DOI] [PubMed] [Google Scholar]
- Li B, Zhang D-M, Luo Y-M, et al. Three new and antitumor anthraquinone glycosides from Lasianthus acuminatissimus Merr. Chem Pharm Bull. 2006;54:297–300. doi: 10.1248/cpb.54.297. [DOI] [PubMed] [Google Scholar]
- Ma D-L, Chen M, Su C-X, et al. In vivo antioxidant activity of deacetylasperulosidic acid in Noni. J Anal Methods Chem. 2013 doi: 10.1155/2013/804504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik J, Tauchen J, Landa P, et al. In vitro antiinflammatory and antioxidant potential of root extracts from Ranunculaceae species. S Afr J Bot. 2017;109:128–137. doi: 10.1016/j.sajb.2016.12.008. [DOI] [Google Scholar]
- Mueller H, Kassack MU, Wiese M. Comparison of the usefulness of the MTT, ATP and calcein assays to predict the potency of cytotoxic agents in various human cancer cell lines. J Biomol Screen. 2004;9:506–515. doi: 10.1177/1087057104265386. [DOI] [PubMed] [Google Scholar]
- Olivar JE, Sy K, Villanueva C, et al. Alkaloids as chemotaxonomic markers from the Philippine endemic Uncaria perrottetii and Uncaria lanosa f. philippinensis. J King Saud Univ Sci. 2018;30:283–285. doi: 10.1016/j.jksus.2017.12.008. [DOI] [Google Scholar]
- Ong HC, Faezah AW, Milow P. Medicinal plants used by the Jah Hut Orang Asli at Kampung Pos Penderas, Pahang, Malaysia. Ethno Med. 2012;6:11–15. doi: 10.1080/09735070.2012.11886414. [DOI] [Google Scholar]
- Saha K, Lajis NH, Israf DA, et al. Evaluation of antioxidant and nitric oxide inhibitory activities of selected Malaysian medicinal plants. J Ethnopharm. 2004;92:263–267. doi: 10.1016/j.jep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Sharief MU. Plants folk medicine of Negrito tribes of Bay Islands. Indian J Trad Know. 2007;6:468–476. [Google Scholar]
- Simlai A, Roy A. Biological activities and chemical constituents of some mangrove species from Sundarban estuary: an overview. Pharmacogn Rev. 2013;7:170–178. doi: 10.4103/0973-7847.120518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya K, Hashimoto F, Ono M, et al. Anti-oxidative constituents from Leonurii Herba (Leonurus japonicus) Food Sci Technol Int Tokyo. 1998;4:278–281. doi: 10.3136/fsti9596t9798.4.278. [DOI] [Google Scholar]
- Takeda Y, Shimizu H, Masuda T, et al. An iridoid glucoside dimer and a non-glycosidic iridoid from the leaves of Lasianthus wallichii. Chem Pharm Bul. 2002;50:1395–1397. doi: 10.1248/cpb.50.1395. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Shimizu H, Masuda T, et al. Lasianthionosides A–C, megastigmane glucosides from leaves of Lasianthus fordii. Phytochem. 2004;65:485–489. doi: 10.1016/j.phytochem.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Tan MA, Eusebio JA, Alejandro GJ. Chemotaxonomic implications of the absence of alkaloids in Psychotria gitingensis. Biochem Syst Ecol. 2012;45:20–22. doi: 10.1016/j.bse.2012.07.016. [DOI] [Google Scholar]
- Tan MA, Villacorta RA, Alejandro GJ, et al. Iridoids and a norsesquiterpenoid from the leaves of Villaria odorata. Nat Prod Commun. 2014;9:1229–1230. [PubMed] [Google Scholar]
- Villasenor IM, Angelada J, Canlas AP, et al. Bioactivity studies on β-sitosterol and its glucoside. Phytother Res. 2002;16:417–421. doi: 10.1002/ptr.910. [DOI] [PubMed] [Google Scholar]
- Wu G-J, Chen W-F, Hung H-C. Effects of propofol on proliferation and anti-apoptosis of neuroblastoma SH-SY5Y cell line: new insights into neuroprotection. Brain Res. 2011;1384:42–50. doi: 10.1016/j.brainres.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Xia C-L, Tang G-H, Guo Y-Q, et al. Mulberry Diels-Ader-type adducts from Morus alba as multi-targeted agents for Alzheimer’s disease. Phytochem. 2019;157:82–91. doi: 10.1016/j.phytochem.2018.10.028. [DOI] [PubMed] [Google Scholar]
- Xu F, Wu H, Wang X, et al. RP-HPLC characterization of lupenone and β-sitosterol in Rhizoma musae and evaluation of the anti-diabetic activity of lupenone in diabetic Sprague-Dawley rats. Molecules. 2014;19:14114–14127. doi: 10.3390/molecules190914114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhang C, Liu X, et al. Chemical constituents and antioxidant activity of Lasianthus hartii. Chem Nat Compd. 2017;53:390–393. doi: 10.1007/s10600-017-2002-7. [DOI] [Google Scholar]
- You YJ, Nam NH, Kim Y, et al. Antiangiogenic activity of lupeol from Bombax ceiba. Phytother Res. 2003;17:341–344. doi: 10.1002/ptr.1140. [DOI] [PubMed] [Google Scholar]
- Zhu H. A revision of the Genus Lasianthus (Rubiaceae) from China. Syst Geogr Pl. 2002;72:63–110. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.