Abstract
The aim of this study was to develop the optimal conditions for supercritical fluid extraction (SFE) of bioactive trans-resveratrol from peanut kernels using an experimental design. The variables taken into account were extraction pressure, extraction temperature, extraction time and amount of modifier. The model was first set for significant factor screening by full factorial design, then, optimized by central composite designs. The optimal extraction parameters were a pressure of 7000 psi, temperature of 70 °C and time of 50 min while amount of modifier did not show significant effect. The quantity of trans-resveratrol was predictable by a full quadratic regression equation with R2(predict) = 95.56%. The predicted trans-resveratrol concentration in peanut samples was 0.7998 µg/g while the experimental concentration was 0.7884 ± 0.1553 µg/g. Conventional solvent extraction demonstrated less selectivity and needed more clean-up process prior to HPLC analysis. Our optimized SFE condition was effective to maximize trans-resveratrol extraction with less contaminants and gave the comparable amount of trans-resveratrol between actual and predicted values.
Keywords: Trans-resveratrol, Peanut, Supercritical fluid extraction, Optimization, Full factorial design, Central composite design
Introduction
Peanut (Arachis hypogaea) belongs to the botanical family Fabaceae. Peanut kernels are a common food in Thailand. They are consumed boiled, roasted or as a component in traditional Thai foods. They contain a variety of beneficial health constituents such as proteins, carbohydrates, fibers, vitamins and minerals (Limmongkon et al. 2017). Several papers have revealed that a large variety of phenolic compounds including arachidin-1, piceatannol and resveratrol are available in peanuts and peanut sprouts (Nepote et al. 2005; Wang et al. 2005; Lopes et al. 2011; Khang et al. 2016).
Resveratrol (3,5,4′-trihydroxystilbene), identified in grape skins, wine, Japanese knotweed, berries, Scots pines, peanuts, and processed peanuts, possesses many biological effects including cardio-protective, chemo-preventive, anti-inflammatory and anti-oxidative effects (Sanders et al. 2000; Lee et al. 2004; Rudolf et al. 2005; Sales and Resurreccion 2009; Chun-fu et al. 2013; Carrizzo et al. 2013).
Several extraction techniques have been reported to extract bioactive trans-resveratrol (t-resveratrol) from natural matrices including solvent extraction with solid phase extraction (Sanders et al. 2000; Lee et al. 2004; Rudolf et al. 2005; Sales and Resurreccion 2009; Chiva-Blanch et al. 2011), high-speed counter-current chromatography (Carrizzo et al. 2013), multi-stage counter current extraction (Zhang et al. 2015), microwave assisted extraction (Du et al. 2007) and supercritical fluid extraction (Berna et al. 2001). For conventional solvent extraction, a mixture of water and ethanol or acetonitrile have been used to extract t-resveratrol from peanuts which has been found in the range of 0.03–0.30 µg/g (Sanders et al. 2000; Lee et al. 2004) but the disadvantage of this method is that samples have to pass through a clean-up column or solid-phase extraction before analysis.
Supercritical fluid extraction (SFE), a green extraction technique, based on the specific properties of carbon dioxide gas that achieves in the supercritical state which can increase the mass transfer efficiency (Calero-Rubio et al. 2014) and has the advantages of high selectivity, expeditiousness, automation and environmental safety (Berna et al. 2001). Several papers have reported on the conditions of carbon dioxide supercritical fluid extraction. The ranges of pressure, temperature and time were 2100–6000 psi, 40–100 °C and 4–45 min respectively. Either ethanol or acetonitrile have been used as organic modifiers in the range of 5–7.5% (Berna et al. 2001; Pascual-Marti et al. 2001; Beňová et al. 2010; Casas et al. 2010). According to all of the studies, the extract samples were easy to quantify using HPLC without a sample purification step.
Experimental design has been used to optimize the different operating conditions of various processes to achieve high effective extraction. It is composed of 2 steps: screening for significant factors and optimizing those selected variables. Full factorial design (FFD) and central composite design (CCD) are commonly known as a screening design and an optimization design, respectively (Sharif et al. 2014; Sahu et al. 2017). A full 23 design of experiment was used to find the significant factors including temperature, amount of ethanol and flow rate for SFE of phenolic compounds from Eucalyptus globulus (Santos et al. 2012). Additionally, CCD was used in the optimization study of polysaccharide separation from Adenophorae radix (Zhang et al. 2014). To the best of our knowledge, no study has conducted research on experimental design to optimize t-resveratrol extraction from peanut kernels using SFE.
The aim of this study was to develop SFE-CO2 (supercritical fluid extraction-carbon dioxide) conditions for extraction of t-resveratrol from peanut kernels. Experimental design, CCD, was used to determine the optimal conditions that should be accurate, reproducible and suitable for the extraction of t-resveratrol from peanut kernels. Finally, t-resveratrol extraction with SFE technique was compared to that with conventional solvent extraction technique.
Materials and methods
Materials and reagents
Fresh Thai peanuts (Arachis hypogaea L., var. Kalasin-2) for the experiment were purchased from a local market in Bangkok, Thailand. Pretreatment procedures included washing and removing the shell. The peanut kernels were then collected and blended in a high speed blender for 3 min. The coarse powder was dried in an oven at 60 °C for 24 h and ground into a fine powder with a mortar and pestle. The moisture content of dried peanut powder was found at 3.91% by weight and the particle size of peanut powder was 900.2523 µm determined by Horiba Partica LA-950, Japan. The fine peanut powder was kept in a refrigerator at 8 °C. Prior to extraction, the peanut powder was warmed up to room temperature and about 4 g was transferred into an SFE extraction thimble.
A t-resveratrol reference standard was obtained from Sigma Aldrich Co, USA. A 10 µg/mL standard stock solution was prepared by dissolving the t-resveratrol standard in ethanol and was kept in an amber vial at − 20 °C. Acetonitrile HPLC grade and ethanol AR grade were procured from JT Baker Co, USA. Formic acid (98%) AR grade was purchased from Merck Co, USA. Ultra-purified water was produced using a TKA-UPW40 (Thermo Scientific, UK). Commercial ultra-high purity grade carbon dioxide (Masser Specialty Gas Co., Ltd., Thailand) purity > 99.98% was used for SFE.
Extraction methods
Supercritical fluid extraction
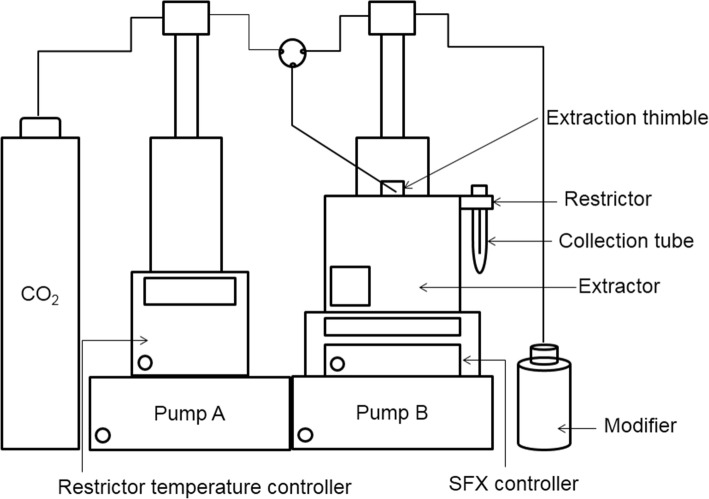
The procedure was carried out using an ISCO-SFX220 extractor (Lincoln, NE, USA). The schematic diagram of SFE equipment was shown in Fig. 1. The extractor consisted of an extraction chamber, a restrictor temperature controller, a central controller and two syringe pumps, with one syringe pump for the supercritical fluid and the other for the modifier. The restrictor flow rate was controlled at 0.8 mL/min.
Fig. 1.
SFX220 supercritical fluid extraction unit schematic diagram
During each extraction, the extraction thimble that contained about 4 g of dried peanut sample was loaded in the extraction chamber. The modifier was ethanol. The restrictor temperature was set to 40 °C and immersed in a tube with 5 mL of ethanol. The extraction parameters, including pressure, temperature, time and amount of modifier, were set according to the experimental design. The trapped solution was adjusted to 10 mL with ethanol and centrifuged at 9500 rpm for 5 min prior to injection into the HPLC.
Conventional solvent extraction
The method was adapted from Sales and Resurreccion (2009). Four grams of dried peanut powder was transferred to a 100-mL beaker. 15 mL of ethanol was added and sonicated on ice for 50 min. The solution was passed through a filter paper and dried under a rotary evaporator at 38 °C. The dry extract was then re-dissolved in 10 mL of ethanol. The solution was centrifuged at 9500 rpm for 5 min prior to injection into the HPLC.
Chromatographic system and determination of t-resveratrol
The t-resveratrol assay method was performed using an Agilent-1260 (Agilent Technologies, Waldbronn, Germany) equipped with a photo-diode-detector (G1315C). Chromatographic separation was achieved in a Mightysil RP18 GP column (4.6 × 250 mm; 5 µm; Kanto Corporation, Portland, OR, USA) with a 4 mm LC-18 guard column. The column oven was maintained at 30 °C. The mobile phase consisted of 0.1% formic acid in water (A) and acetonitrile (B) at a flow rate of 0.8 mL/min. A gradient elution program was used as follows: 22% B at 0–5 min, 22–100% B at 5–20 min, 100% B at 20–30 min, 100–22% B at 30–35 min, and 22% B at 35–45 min. The injection volume was 20 µL. The detection wavelength was set at 306 nm. The retention time of t-resveratrol was 13.9 min. The concentration of t-resveratrol in the extract was calculated from the calibration curve.
The analytical method for t-resveratrol was validated for specificity, linearity and range, and system repeatability, as well as limit of detection (LOD) and limit of quantification (LOQ).
Experimental design
The experimental design was used to evaluate the main and interaction effects of these SFE factors: pressure, temperature, extraction time and amount of modifier.
Screening design
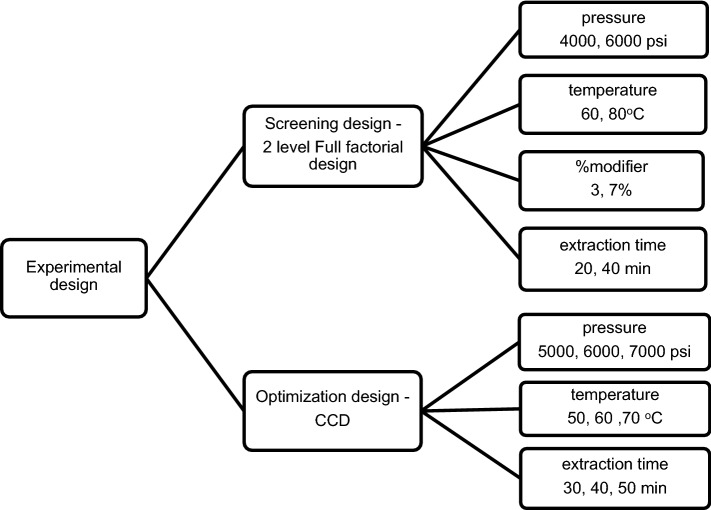
Four variable factors including pressure (A: 4000, 6000 psi), temperature (B: 60, 80 °C), amount of modifier (C: 3, 7%) and extraction time (D: 20, 40 min) were assigned by reviewing previous articles (Berna et al. 2001; Pascual-Marti et al. 2001; Beňová et al. 2010; Casas et al. 2010). Sixteen experiments (Table 1) were generated from the full factorial design and performed to locate a center point for the CCD study. The concentrations of t-resveratrol obtained from each extraction were entered into the MINITAB 17 software to determine the optimal point.
Table 1.
Experimental orders generated from the full factorial and center-faced central composite design
| Run order | Full factorial design | Center-faced central composite design | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pressure | Temp | % Modifier | Time (min) | t-resveratrol (µg/g) | Pressure | Temp | Time (min) | Block | t-resveratrol (µg/g) | |
| 1 | 4000 | 80 | 3 | 40 | 0.0820 | 5000 | 60 | 40 | 3 | 0.0269 |
| 2 | 4000 | 80 | 7 | 20 | 0.8014 | 6000 | 70 | 40 | 3 | 0.2503 |
| 3 | 6000 | 80 | 3 | 40 | 1.0419 | 6000 | 60 | 50 | 3 | 0.0256 |
| 4 | 6000 | 60 | 7 | 20 | 0.0071 | 6000 | 60 | 40 | 3 | 0.0285 |
| 5 | 4000 | 80 | 7 | 40 | 0.1723 | 6000 | 60 | 40 | 3 | 0.0218 |
| 6 | 6000 | 80 | 7 | 40 | 0.5636 | 6000 | 50 | 40 | 3 | 0.2019 |
| 7 | 4000 | 60 | 7 | 40 | 0.0696 | 6000 | 60 | 30 | 3 | 0.0081 |
| 8 | 4000 | 80 | 3 | 20 | 0.1401 | 7000 | 60 | 40 | 3 | 0.0178 |
| 9 | 6000 | 60 | 7 | 40 | 0.0315 | 5000 | 50 | 30 | 1 | 0.6457 |
| 10 | 6000 | 80 | 7 | 20 | 0.2116 | 7000 | 70 | 30 | 1 | 0.0398 |
| 11 | 4000 | 60 | 3 | 20 | 0.1177 | 6000 | 60 | 40 | 1 | 0.0338 |
| 12 | 4000 | 60 | 3 | 40 | 0.1048 | 5000 | 70 | 50 | 1 | 0.2554 |
| 13 | 4000 | 60 | 7 | 20 | 0.0274 | 6000 | 60 | 40 | 1 | 0.0376 |
| 14 | 6000 | 60 | 3 | 20 | 0.0590 | 7000 | 50 | 50 | 1 | 0.0914 |
| 15 | 6000 | 80 | 3 | 20 | 0.0988 | 6000 | 60 | 40 | 2 | 0.0248 |
| 16 | 6000 | 60 | 3 | 40 | 0.0885 | 7000 | 50 | 30 | 2 | 0.1743 |
| 17 | 5000 | 70 | 30 | 2 | 0.0176 | |||||
| 18 | 6000 | 60 | 40 | 2 | 0.0224 | |||||
| 19 | 5000 | 50 | 50 | 2 | 0.0171 | |||||
| 20 | 7000 | 70 | 50 | 2 | 0.8198 | |||||
Optimization design
Significant factors from the screening full factorial design were continuously examined in optimization step by Response Surface Methodology (RSM). The face-centered central composite design at 3-levels (low, middle and high levels) was utilized. Run orders were generated and divided into 3 blocks. Each block contained two replications at the center point and was performed on different days.
Statistical analysis
The full quadratic model was employed to study the main and interaction effects of the variables. The response surface equations, response surface and main effect plot were generated to determine the correlation of data and locate the optimal condition using MINITAB 17 software.
Results and discussion
Determination of t-resveratrol and method validation
Validation of t-resveratrol analysis method was performed to ensure that the method was suited to determine the amounts of t-resveratrol from the SFE extracts. Specificity was presented at the same retention time of the t-resveratrol from the standard and the extract. The chromatogram of the SFE extract showed no interference with the t-resveratrol peak. The peak purity factor of t-resveratrol peak in sample was more than 0.9 with resolution value of 1.3, and its UV spectrum corresponded to t-resveratrol in the standard chromatogram (Fig. 2a, b). Moreover, this HPLC method is capable to separate between trans- and cis-isomerism (Fig. 2c) which simply differentiated by specific UV absorption at 306 nm for trans-resveratrol and 288 nm for cis-resveratrol (Liu et al. 2013). The calibration curve presented a linear correlation equation between peak area and six concentrations of t-resveratrol in a range of 0.03–2.00 µg/mL, with the coefficient of determination (r2) of 0.9999. The % relative standard deviation (%RSD) of 5 replicated injections of the t-resveratrol standard was 0.93 and was reported for system repeatability. Regarding the signal-to-noise ratio, the LOD was calculated as 2 ng/mL (three times the signal to noise ratio) while the LOQ was 3 ng/mL (ten times the same ratio) which was lower than the reported LOD and LOQ of Rudolf et al. (2005), Grippi et al. (2008) and Beňová et al. 2010. To determine the extraction efficacy, the extraction recovery was quantified by adding standard solution of t-resveratrol at 2, 4 and 6 µg to cotton wool in the extraction thimble and drying it in a fume hood. It was then extracted using normal SFE procedures and the UV absorbance at 350 nm in 1st derivative mode was measured (Berna et al. 2001). The average recovery values of the spiked samples were 92.17–99.16% with less than 10% RSD from the overall process. This demonstrated that almost all of t-resveratrol was recovered at the optimized conditions. According to all of the validation results, this analysis approach is suitable for determination of t-resveratrol from the SFE extract.
Fig. 2.
Chromatograms and UV spectra of t-resveratrol in standard (a), SFE sample (b), identification of trans- and cis-isomerism (c) and solvent extraction sample (d)
Supercritical fluid extraction of t-resveratrol from peanut kernels
Screening design study
Sixteen experiments covering four factors (pressure, temperature, amount of modifier and extraction time) were conducted by means of a 2 level full factorial design and t-resveratrol contents of each experimental order were shown in Table 1. The optimal condition was selected according to our previous study (Jitrangsri et al. 2015). The appropriate condition for further optimization by central composite design (CCD) was pressure of 6000 psi, temperature of 60 °C, modifier at 3% and time of 40 min. All factors were found statistically significant (p < 0.050) except for the amount of modifier, therefore the amount of modifier was excluded from the variables of interest in the CCD study.
Central composite design study
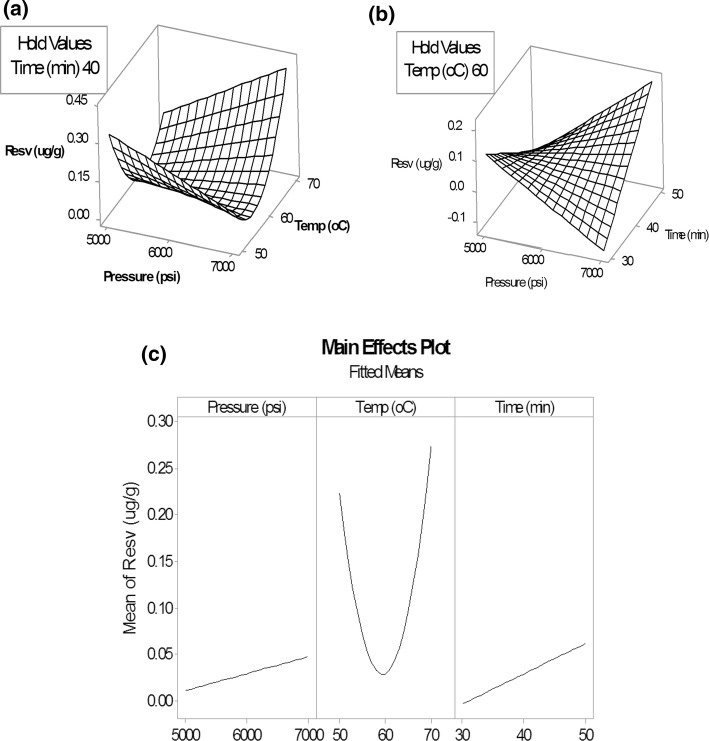
A center-face central composite design (CCF) of 3 significant factors; pressure (X1), temperature (X2) and extraction time (X3) at 3 different levels was utilized with the ranges of X1: 5000, 6000 and 7000 psi, X2: 50, 60 and 70 °C, X3: 30, 40 and 50 min, respectively. The center points of each variable were derived from the screening study results. A total of twenty experiments were separated into 3 blocks and carried out on different days (Table 1). The quadratic regression was acquired from the statistical significant model (p < 0.001). The optimized response surface equation in un-coded units obtained from the analysis was Y = 20.423 − 0.001264 X1 − 0.4212 X2 − 0.20805 X3 + 0.002196 X22 + 0.000012 X1X2 + 0.000014 X1X3 + 0.002162 X2X3 where Y was the t-resveratrol yields, X1, X2, and X3 were the extraction pressure, temperature, and time respectively. The Analysis of Variance (ANOVA) for the investigated parameters was shown in Table 2. It showed a good relationship between response and variables with R2(adjusted) = 99.30%. The model showed the ability to forecast extraction yields with R2(predicted) = 95.56%. The results obtained from the three different analysis days were not different (p > 0.050). The optimized extraction conditions were a pressure of 7000 psi, temperature of 70 °C and extraction time of 50 min.
Table 2.
Analysis of variance (ANOVA) table in un-coded units for response surface quadratic model
| Source | Adjusted sum of squares | Adjusted mean square | F value | p value |
|---|---|---|---|---|
| Model | 0.920855 | 0.102317 | 302.25 | 0.000 |
| Blocks | 0.001294 | 0.000647 | 1.91 | 0.198 |
| Pressure (psi) | 0.003254 | 0.003254 | 9.61 | 0.011 |
| Temp (°C) | 0.006376 | 0.006376 | 18.83 | 0.001 |
| Time (min) | 0.010485 | 0.010485 | 30.97 | 0.000 |
| Temp (°C) * temp (°C) | 0.200908 | 0.200908 | 593.49 | 0.000 |
| Pressure (psi) * temp (°C) | 0.120958 | 0.120958 | 357.32 | 0.000 |
| Pressure (psi) * time (min) | 0.147941 | 0.147941 | 437.02 | 0.000 |
| Temp (°C) * time (min) | 0.37381 | 0.37381 | 1104.25 | 0.000 |
The estimated surface plots of t-resveratrol extraction and the main effect plots that show factors influencing responses are presented in Fig. 3. The relationship between the response and variables was the same as the screening design. The higher the extraction pressure as well as the longer the time, the higher the amount of t-resveratrol obtained. These results were comparable to the study of Berna et al. (2001) and Yothipitak et al. (2008). They are explained by the basic theory of SFE. The elution strength of the supercritical fluid depends on the density. At higher pressures, the supercritical solvent density is increased and this results in higher solubility of the substance (Yothipitak et al. 2008). However, the extraction pressures are restricted by operational cautions which leaded the unaffordable experiment beyond 7000 psi. Moreover, increasing extraction time results in more t-resveratrol dissolved in the supercritical fluid because of the greater contact time between the supercritical fluid and the solute. With regard to temperature, either increasing or lowering the temperature might result in higher yields of extraction. Additionally, considering pressure, the amount of extracted t-resveratrol decreased slightly when the pressure was increased in a low temperature and time, but it significantly increased in higher regions. This might be because solubility of the substance in the supercritical fluid depends on the complex system of supercritical fluid density and solute vapor pressure (Yothipitak et al. 2008). Too high or low a temperature can affect the supercritical fluid density so the appropriate adjustment of pressure and temperature variables can maximize the extraction yields. Verification of the predictive model was tested under optimized conditions. The average t-resveratrol amount of 0.7998 µg/g and 0.7844 ± 0.1553 µg/g were obtained from prediction and experiments respectively. This indicated that the model was capable of predicting the extraction yield. Moreover, the amount of t-resveratrol found in raw peanuts in this study was higher than those in conventional solvent extraction studies of Sanders et al. (2000) and Lee et al. (2004); 0.03–0.30 µg/g. The model of experimental design of this research was summarized in Fig. 4.
Fig. 3.
3D-surface plot of t-resveratrol extraction yield (µg/g) versus pressure (psi) and temp (°C) (a), and versus pressure (psi) and time (min) (b) including main effect plots of variables (c)
Fig. 4.
Summary of experimental design
Conventional solvent extraction
Six peanut samples were extracted using ethanol, and quantified. The chromatogram of the extracts (Fig. 2d) demonstrates incomplete separation of t-resveratrol from other contaminants. This was confirmed by the UV spectra at the retention time of t-resveratrol. The results showed that the ethanol extract of conventional solvent extraction contained more contaminations than SFE technique. This indicated the advantage of SFE over a conventional extraction method in the selectivity of the expected compound. To improve selectivity of t-resveratrol, the solvent extract samples needed an additional purification step to separate the t-resveratrol from the co-elution compounds. This was found in many studies of t-resveratrol extraction from peanuts using a solvent extraction method (Sanders et al. 2000; Lee et al. 2004; Sales and Resurreccion 2009).
Conclusion
The developed analytical method was suitable for quantifying t-resveratrol without any purification step. After considering the chromatograms, SFE showed greater selectivity of t-resveratrol over conventional solvent extraction methods. Only the extraction pressure, temperature and time were found to be significant factors for the extraction yields. The optimized SFE conditions at a pressure of 7000 psi, temperature of 70 °C and time of 50 min provided higher extraction amounts of t-resveratrol which was comparable to previous studies. Moreover, under the optimal conditions, the experimental yield was consistent with the predicted value. Therefore, the optimization of SFE through the experimental design could maximize both the selectivity and t-resveratrol yield which could be useful as an effective alternative method to extract t-resveratrol from peanut kernels.
Acknowledgements
The authors acknowledge the financial support from Faculty of Pharmacy, Silpakorn University (via Research and Creative Fund) and Silpakorn University Research and Development Institute through Research Grant No. SURDI 610109 as well as the instrumental provision from the Faculty of Pharmacy, Silpakorn University throughout the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Beňová B, Adam M, Pavlíková P, Fischer J. Supercritical fluid extraction of piceid, resveratrol and emodin from Japanese knotweed. J Supercrit Fluids. 2010;51:325–330. doi: 10.1016/j.supflu.2009.10.009. [DOI] [Google Scholar]
- Berna A, Chafer A, Monton JB. High-pressure solubility data of the system resveratrol (3) + ethanol (2) + CO2 (1) J Supercrit Fluids. 2001;19:133–139. doi: 10.1016/S0896-8446(00)00088-7. [DOI] [Google Scholar]
- Calero-Rubio C, Stashenko E, Martínez JR, López-Giraldo LJ. Formulation of a new generic density-based model for modeling solubility of polyphenols in supercritical carbon dioxide and ethanol. J Supercrit Fluids. 2014;85:116–122. doi: 10.1016/j.supflu.2013.11.004. [DOI] [Google Scholar]
- Carrizzo A, Forte M, Damato A, et al. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem Toxicol. 2013;61:215–226. doi: 10.1016/j.fct.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Casas L, Mantell C, Rodríguez M, et al. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J Food Eng. 2010;96:304–308. doi: 10.1016/j.jfoodeng.2009.08.002. [DOI] [Google Scholar]
- Chiva-Blanch G, Urpi-Sarda M, Rotchés-Ribalta M, et al. Determination of resveratrol and piceid in beer matrices by solid-phase extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2011;1218:698–705. doi: 10.1016/j.chroma.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Chun-fu WU, Jing-yu Y, Fang W, Xiao-xiao W. Resveratrol: botanical origin, pharmacological activity and applications. Chin J Nat Med. 2013;11:1–15. doi: 10.1016/S1875-5364(13)60001-1. [DOI] [Google Scholar]
- Du FY, Xiao XH, Li GK. Application of ionic liquids in the microwave-assisted extraction of trans-resveratrol from Rhizma Polygoni Cuspidati. J Chromatogr A. 2007;1140:56–62. doi: 10.1016/j.chroma.2006.11.049. [DOI] [PubMed] [Google Scholar]
- Grippi F, Crosta L, Aiello G, et al. Determination of stilbenes in Sicilian pistachio by high-performance liquid chromatographic diode array (HPLC-DAD/FLD) and evaluation of eventually mycotoxin contamination. Food Chem. 2008;107:483–488. doi: 10.1016/j.foodchem.2007.07.079. [DOI] [Google Scholar]
- Jitrangsri K, Chaidedgumjorn A, Satiraphan M. Optimization of trans-resveratrol extraction from peanut kernels using supercritical fluid extraction. Asian J Pharm Sci. 2015 doi: 10.1016/j.ajps.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khang D, Dung T, Elzaawely A, Xuan T. Phenolic profiles and antioxidant activity of germinated legumes. Foods. 2016;5:27. doi: 10.3390/foods5020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Lee SM, Kim M, et al. Analysis of trans-resveratrol in peanuts and peanut butters consumed in Korea. Food Res Int. 2004;37:247–251. doi: 10.1016/j.foodres.2003.11.007. [DOI] [Google Scholar]
- Limmongkon A, Janhom P, Amthong A, et al. Antioxidant activity, total phenolic, and resveratrol content in five cultivars of peanut sprouts. Asian Pac J Trop Biomed. 2017;7:332–338. doi: 10.1016/j.apjtb.2017.01.002. [DOI] [Google Scholar]
- Liu C, Wang L, Wang J, et al. Resveratrols in Vitis berry skins and leaves: their extraction and analysis by HPLC. Food Chem. 2013;136:643–649. doi: 10.1016/j.foodchem.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Lopes RM, Da Silveira A-CT, Gimenes MA, Silveira D. Chemical composition and biological activities of Arachis species. J Agric Food Chem. 2011;59:4321–4330. doi: 10.1021/jf104663z. [DOI] [PubMed] [Google Scholar]
- Nepote V, Grosso NR, Guzmán CA. Optimization of extraction of phenolic antioxidants from peanut skins. J Sci Food Agric. 2005;85:33–38. doi: 10.1002/jsfa.1933. [DOI] [Google Scholar]
- Pascual-Marti MC, Salvador A, Chafer A, Berna A. Supercritical fluid extraction of resveratrol from grape skin of Vitis vinifera and determination by HPLC. Talanta. 2001;54:735–740. doi: 10.1016/S0039-9140(01)00319-8. [DOI] [PubMed] [Google Scholar]
- Rudolf JL, Resurreccion AVA, Saalia FK, Phillips RD. Development of a reverse-phase high-performance liquid chromatography method for analyzing trans-resveratrol in peanut kernels. Food Chem. 2005;89:623–638. doi: 10.1016/j.foodchem.2004.05.033. [DOI] [Google Scholar]
- Sahu PK, Ramisetti NR, Cecchi T, et al. An overview of experimental designs in HPLC method development and validation. J Pharm Biomed Anal. 2017 doi: 10.1016/j.jpba.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Sales JM, Resurreccion AVA. Maximising resveratrol and piceid contents in UV and ultrasound treated peanuts. Food Chem. 2009;117:674–680. doi: 10.1016/j.foodchem.2009.04.075. [DOI] [Google Scholar]
- Sanders TH, McMichael RW, Hendrix KW. Occurrence of resveratrol in edible peanuts. J Agric Food Chem. 2000;48:1243–1246. doi: 10.1021/jf990737b. [DOI] [PubMed] [Google Scholar]
- Santos SAO, Villaverde JJ, Silva CM, et al. Supercritical fluid extraction of phenolic compounds from Eucalyptus globulus Labill bark. J Supercrit Fluids. 2012;71:71–79. doi: 10.1016/j.supflu.2012.07.004. [DOI] [Google Scholar]
- Sharif KM, Rahman MM, Azmir J, et al. Experimental design of supercritical fluid extraction: a review. J Food Eng. 2014;124:105–116. doi: 10.1016/j.jfoodeng.2013.10.003. [DOI] [Google Scholar]
- Wang KH, Lai YH, Chang JC, et al. Germination of peanut kernels to enhance resveratrol biosynthesis and prepare sprouts as a functional vegetable. J Agric Food Chem. 2005;53:242–246. doi: 10.1021/jf048804b. [DOI] [PubMed] [Google Scholar]
- Yothipitak W, Thana P, Goto M, Shotipruk A. Experiments and statistical analysis of supercritical carbon dioxide extraction. Chiang Mai J Sci. 2008;35:109–115. [Google Scholar]
- Zhang X, Chen J, Mao M, et al. Extraction optimization of the polysaccharide from Adenophorae Radix by central composite design. Int J Biol Macromol. 2014;67:318–322. doi: 10.1016/j.ijbiomac.2014.03.039. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Bian Y, Shi Y, et al. An economical and efficient technology for the extraction of resveratrol from peanut (Arachis hypogaea) sprouts by multi-stage countercurrent extraction. Food Chem. 2015;179:15–25. doi: 10.1016/j.foodchem.2015.01.113. [DOI] [PubMed] [Google Scholar]