Abstract
Powdered soft drinks (PSDs), fortified with antioxidants such as ascorbic acid (AA), are normally controlled by titration or chromatographic methods. This study evaluated the feasibility of using near-infrared spectroscopy (NIRS) and multivariate analysis to predict AA contents in PSDs as an alternative not-destructive method. The AA content of sixty-seven samples of commercial fortified grape and passion fruit PSDs was analyzed by the standard method (titration) and showed significant variance between flavors within the same brand. In addition, 75% of the samples required from 0.3 to 10.2 more cups of grape than passion fruit flavor to supply the AA Reference Nutrient Intake for children and adults. Spectral and reference data sets were split into calibration and validation sets. Partial least squares regression models were built and validated for the determination of AA in both PSDs. The model’s basic statistics for grape flavor PSDs (RMSEC = 0.49 mg g−1, R2cal = 0.84; RMSECV = 0.67 mg g−1, R2CV = 0.70; RMSEP = 0.50 mg g−1, R2pred = 0.84), and that for passion fruit flavor PSDs (RMSEC = 0.24 mg g−1, R2cal = 0.95; RMSECV = 0.56 mg g−1, R2CV = 0.76; RMSEP de 0.57 mg g−1, R2pred = 0.72) indicated that NIRS-PLS methodology produced reasonable results. The limits of detection and quantification obtained showed that the method is useful to detect and quantify AA in the studied samples. A new set of grape drinks was used for external prediction and the RMSEP was 0.62 mg g−1, R2pred was 0.72. Based on the results, NIRS–multivariate analysis proved to be useful for quality control of AA in commercialized grape and passion fruit in PSDs and a faster, objective and environmentally friendly method alternative to standard methods.
Keywords: PLS regression, Food quality control, Alternative green method
Introduction
Powdered soft drinks present a fine or granular appearance, specific smell and taste, and color dependable on its constituents, that can include sugar, maltodextrin, acidulants, flavoring, and starch, according to the National Surveillance Agency (ANVISA) (Brazil 1978, 2009). The Brazilian Decree n.6871, of June 4th, 2009, states that they can be prepared by dilution in water, and the Normative Instruction n.17, of June 19th, 2013, from the Ministry of Agriculture, Livestock and Supply requires that they should contain a minimum of 1% of fruit juice (Brazil 2009, 2013). Since this soft drink is easy to prepare, carry, store, and it has a low price and high yield, of about 1 L per 30 g, its consumption increased 3% from 2013 to 2014, reaching 24.6 L per capita in 2014, and consumption of approximately 120,339 tons in 2015 in Brazil (ABIR 2015).
According to Rosa et al. (2006), the most consumed flavors of juices in Brazil are: orange, mango, grape, passion fruit and peach, in decreasing preference order. There are some studies in the literature that have reported the ascorbic acid content in PSDs of orange, lemon, mango, pineapple and passion fruit flavors by standard methods (Silva et al. 2005; Souza 2007; Cruz et al. 2013).
The majority of commercial PSDs are fortified with AA, also called as vitamin C, which is water soluble, and an antioxidant that produces and keeps collagen, increases the body’s immune resistance against infections, and assists in the processes of healing and maintenance of cell integrity. Also, AA indicates the quality for consumption of its derived products, due to its sensitivity to degradation during the processing and storing stages, otherwise, it is stable when dry (Webb and Whitney 2003). The nutrition facts of these PSDs show, in average, 6.8 mg of AA added to each 6 g of product. The PSDs available in the markets of Brazil, United States, Hungary, Turkey, and United Kingdom are fortified with ascorbic acid.
Some parameters for the quality control of PSDs are regulated in countries such as Brazil and United States. Brazil regulates the titratable acidity, total sugars and soluble solids (Brazil 1998a), and classifies the addition of vitamins and minerals to food products by the minimum required to be a source of it or a product fortified with this nutrient, but there is no specific legislation for the AA added to PSDs (Brazil 1998b).
Ascorbic acid is commonly determined in foods by classic volumetric methods, such as volumetric titration, but it requires toxic reagents; thus, careful handling is necessary to avoid contamination. Besides, it produces chemical waste and it is time-consuming. One of the most used volumetric methods is the Tillmans method, described by the Association of Official Analytical Chemists (AOAC 1984), adapted by (Benassi and Antunes 1988), which is a titration for the reduction of the salt 2,6-dichlorophenolindophenol by AA, and applied by Silva et al. (2005) to determine AA in orange PSDs.
Fortified PSDs require control of AA on the final product, but the official analytical methods present undesirable features as previously mentioned. In this context, near-infrared spectroscopy (NIRS) can be an alternative technique applied to predict AA in foodstuff and presented advantage as it is a quick, non-destructive, environmentally friendly and reliable technique. NIRS can be applied in industrial quality control as a qualitative and quantitative analytical method. However, chemometric tools are required in the application of this technique. Chemometrics analyze complex chemical data through statistics and allows to develop multivariate calibration models to predict identity and quality parameters, among others, involving spectrum preprocessing (e.g. smoothing, first derivative and mean centering) and regression techniques (such as PLS, partial least squares; PCR, principal components regression; and LS-SVM, least square support vector machine) according to Ferreira (2015).
Ascorbic acid has been evaluated by NIRS in mango, oranges, frozen pulp of guava and passion fruit, and nectar of cashew fruit and guava (Betemps et al. 2011; Flores 2015; Alamar et al. 2016; Caramês et al. 2017). However, there is no study about the use of NIRS to control AA in PSDs.
Thus, this study evaluated the use of NIRS and chemometric tools for AA control in PDSs. The Tillmans method (AOAC 1984) with modifications (Benassi and Antunes 1988) was applied as a standard method for AA analysis in PDSs, which results were correlated with the same samples NIRS spectra to generate a validated prediction model that is an alternative technique for AA content control in this kind of sample. More than environmentally friendly, since no reagents are used, producing no analytical waste, NIR analysis requires minimum sample preparation, and offers instant results during industrial quality control, for example. The recommended intake of PSDs to meet the daily AA needs was also assessed in this study.
Materials and methods
Sampling
Sixty-seven samples of grape and passion fruit PSDs of twelve commercially available brands were collected. The samples were identified from A to L, in two to three different batches of these soft drinks in the local market of Campinas/SP, Brazil. Due to a visual granular heterogeneity among the sample granules, they were ground in an analytical mill (IKA® model A11 basic) to a granulometry of minimum 48 mesh and stored in polypropylene flasks inside a desiccator. Ascorbic acid was analyzed in all the samples.
According to the AA evaluation, a new set of samples was prepared for the grape flavor that contained the highest and lowest ascorbic acid contents to outcome in 5 concentration levels (20%, 30%, 50%, 60% and 70%). Such samples were analyzed in triplicate, totalizing 15 samples identified from M to Q and used as prediction set, to externally measure the predictability of the prediction model.
Ascorbic acid analysis by titration
The AA content was determined titrimetrically in triplicates, through the reduction of 2,6-dichlorophenolindophenol (0.01%) by the AA present in the samples, according to the Tillmans method, which is described in the reference method 967.21 of the Official Methods of Analysis (AOAC 1984) with modifications (Benassi and Antunes 1988).
Spectra acquisition
The NIR spectra were obtained by placing about 2 g of each sample into a vial, and quadruplicates were analyzed per batch, summing 112 samples in grape flavor and 108 samples in passion fruit PSDs.
Reflectance measurements were performed via the near-infrared reflectance accessory (Perkin Elmer-Waltham, USA, model NIRA) in the spectrometer FT-NIR (Perkin Elmer-Waltham, USA, model Spectrum 100 N) in the range of 10,000–4000 cm−1. Each spectrum was generated by averaging 32 scans with a 4 cm−1 increment.
Statistical data analysis
The AA determined by titration (mean ± standard deviation) was evaluated by analysis of variance (ANOVA) in the software Statistica version 10 (StatSoft, USA), which replicates were compared by the Tukey’s test.
The original spectra and AA content were organized in matrix format. The data set was split into two subsets: the calibration set consisting of approximately 70% of the samples and validation set with the remaining samples. The division was performed by analysis of the graph of leverage versus Studentized residuals and the graph of measured versus predicted AA content. For the ascorbic acid prediction models, there were 76 samples in the calibration set and 27 samples in the validation set for the grape flavor, besides 55 and 25 samples in the calibration and validation sets, respectively, for the passion fruit flavor. Partial least squares (PLS) method was chosen (Ferreira 2015) to build the regression models. Leave-one-out cross-validation was used to determine the number of latent variables (LV) in the final models. The optimal wavenumber range for correlations between the spectral data and the reference method to analyze AA content was selected by the regression vector, VIP scores and the selectivity of each model. Regression models were evaluated by calculating the Root-Mean-Square Error of Calibration (RMSEC), the Root-Mean-Square Error of Cross-Validation (RMSECV), the Root-Mean-Square Error of Prediction (RMSEP), the inverse of the analytical sensitivity, selectivity, limits of detection and quantification, and the coefficient of determination (R2). Finally, the external prediction set was used to confirm the predictability of the final model proposed for grape flavor.
The statistical and multivariate analyses were performed using PLS Toolbox v. 5.8 (Eigenvector Research, Wenatchee, WA, USA) for Matlab version R2013a (MathWorks, South Natick, MA, USA). Some samples were considered outliers and removed after analyzing the graph of leverage and Studentized residuals (Ferreira 2015).
Results and discussion
Determination of ascorbic acid by standard method
All the AA analyses were performed in triplicate for each of the two or three batches of the commercial brands (A to L) of grape and passion fruit PSDs, which are listed as 6 or 9 samples per brand, respectively, in Table 1. The mean ascorbic acid values of each batch per brand and for both flavors were evaluated by analysis of variance (ANOVA). Table 1 presents this data, including the range among the batches which are expressed below their mean values.
Table 1.
ANOVA for ascorbic acid in grape and passion fruit powdered soft drinks per brand
| Samples | Ascorbic acid (mg g−1) | Labeled concentration (mg g−1) | Moisture (%) | pH |
|---|---|---|---|---|
| A | ||||
| Passion fruit** | 3.13 ± 0.71a | 3.50 | 1.38 ± 1.66a | 2.88 ± 0.06a |
| 2.44–3.85 | 0.49–2.73 | 2.83–2.97 | ||
| Grape** | 5.23 ± 0.14b | 3.50 | 0.68 ± 0.20a | 2.70 ± 0.03b |
| 5.13–5.39 | 0.59 = 0.82 | 2.68–2.74 | ||
| B | ||||
| Passion fruit** | 5.31 ± 0.37a | 4.53 | 1.22 ± 0.23a | 2.61 ± 0.14a |
| 4.88–5.53 | 1.02–1.19 | 2.43–2.74 | ||
| Grape** | 4.86 ± 0.07a | 3.78 | 0.78 ± 0.27a | 3.02 ± 0.02b |
| 4.78–4.92 | 0.60–1.09 | 3.00–3.04 | ||
| C | ||||
| Passion fruit** | 3.41 ± 0.23a | 1.13 | 0.38 ± 0.15a | 2.80 ± 0.04a |
| 3.25–3.58 | 0.31–0.46 | 2.76–2.83 | ||
| Grape** | 0.60 ± 0.02b | 1.13 | 0.36 ± 0.14a | 2.93 ± 0.03b |
| 0.58–0.63 | 0.20–0.50 | 2.91–2.97 | ||
| D | ||||
| Passion fruit* | 5.85 ± 0.01a | 2.8 | 0.63 ± 0.06a | 3.00 ± 0.04a |
| 5.85–5.85 | 0.61–0.66 | 2.94–3.03 | ||
| Grape** | 3.42 ± 0.09b | 2.8 | 0.55 ± 0.25a | 2.89 ± 0.03b |
| 3.37–3.52 | 0.49–0.62 | 2.86–2.91 | ||
| E | ||||
| Passion fruit** | 4.55 ± 0.01a | 2.8 | 0.31 ± 0.22a | 2.95 ± 0.01a |
| 4.55–4.55 | 0.12–0.47 | 2.94–2.96 | ||
| Grape** | 2.96 ± 0.18b | 2.8 | 1.05 ± 0.52a | 3.04 ± 0.03b |
| 2.94–3.16 | 0.58–1.54 | 3.01–3.06 | ||
| F | ||||
| Passion fruit** | 2.87 ± 0.52a | 1.67 | 0.43 ± 0.07a | 3.02 ± 0.02a |
| 2.28–3.25 | 0.38–0.48 | 3.00–3.03 | ||
| Grape** | 1.71 ± 0.07b | 1.67 | 0.49 ± 0.22a | 2.85 ± 0.02b |
| 1.64–1.77 | 0.42–0.59 | 2.82–2.87 | ||
| G | ||||
| Passion fruit** | 3.90 ± 1.97a | 2.50 | 0.86 ± 1.13a | 2.76 ± 0.06a |
| 2.11–6.02 | 0.34–1.89 | 2.68–2.81 | ||
| Grape** | 2.34 ± 0.20b | 2.50 | 1.69 ± 0.31a | 2.65 ± 0.01b |
| 2.22–2.57 | 1.39–1.86 | 2.65–2.66 | ||
| H | ||||
| Passion fruit** | 2.44 ± 0.01a | 1.36 | 0.29 ± 0.25a | 3.07 ± 0.06a |
| 2.44–2.44 | 0.04–0.59 | 3.00–3.13 | ||
| Grape** | 2.45 ± 0.22a | 1.36 | 0.67 ± 0.18a | 2.78 ± 0.02b |
| 2.36–2.69 | 0.54–0.82 | 2.76–2.79 | ||
| I | ||||
| Passion fruit* | 5.04 ± 0.92a | 3.50 | 0.45 ± 0.20a | 2.68 ± 0.10a |
| 4.39–5.69 | 0.42–0.49 | 2.59–2.77 | ||
| Grape* | 1.89 ± 0.03b | 3.50 | 0.85 ± 0.33a | 2.52 ± 0.02b |
| 1.87–1.91 | 0.77–0.93 | 2.51–2.54 | ||
| J | ||||
| Passion fruit* | 2.44 ± 0.23a | 2.50 | 0.36 ± 0.08a | 2.98 ± 0.01a |
| 2.28–2.60 | 0.34–0.37 | 2.97–2.99 | ||
| Grape* | 3.92 ± 0.40b | 2.50 | 0.45 ± 0.09a | 3.02 ± 0.04b |
| 3.64–4.21 | 0.41–0.49 | 2.99–3.05 | ||
| K | ||||
| Passion fruit** | 5.93 ± 1.47a | 1.00 | 0.64 ± 0.22a | 2.96 ± 0.02a |
| 4.23–6.83 | 0.46–090 | 2.95–2.97 | ||
| Grape** | 1.90 ± 0.33b | 1.00 | 0.99 ± 1.73a | 2.92 ± 0.03b |
| 1.59–2.24 | 0.33–2.16 | 2.89–2.93 | ||
| L | ||||
| Passion fruit** | 1.68 ± 1.50a | 1.46 | 1.04 ± 0.32a | 2.98 ± 0.02a |
| 0.81–3.41 | 0.96–1.09 | 2.96–3.01 | ||
| Grape** | 0.61 ± 0.08b | 1.46 | 1.83 ± 3.55a | 3.13 ± 0.01b |
| 0.55–0.70 | 0.58–4.33 | 3.12–3.14 | ||
Values expressed as mean ± standard deviation (*n = 6; **n = 9). CV = coefficient of variation. Equal letters for the flavors of each brand correspond to no significant variance between them
The mean concentration of AA in the samples was higher than the concentration labeled by their producers in 75% of them. Their labels have reported the same AA content for both flavors, except to brand B. According to the results obtained, AA concentrations were higher in passion fruit flavor than grape. The batches analyzed presented low coefficient of variation (CV), which were 0–11.7% for grape and 0–2.8% for passion fruit. Besides, when the CV was analyzed among the batches, it was observed high variation such as CV of 89.4% when just one batch contained AA, which strengths the importance of controlling the AA content in these products. Also, the Tukey’s test showed significant variance between most of the flavors in each brand. In this way, there was a variation between the batches and the flavors in the same brand, which requires better control of the AA content in these products.
The AA content in the passion fruit flavor was higher than the reported in the same kind of sample by Souza (2007), which ranged 152.2–219.86 mg 100 g−1, while our range was wider compared to the one for PSDs after dilution in water, for which we obtained 3.88–14.63 mg 100 mL−1 for the passion fruit flavor and 1.41–11.77 mg 100 mL−1 for the grape flavor by volumetric transformation. Souza (2007) also obtained lower AA content in orange PSDs (from 5.66 to 10.31 mg 100 mL−1) and in the lemon flavor (from 5.66 to 10.19 mg 100 mL−1), which differed mostly between brands rather than flavors according to Cruz et al. (2013). In orange PSDs, Caleguer et al. (2006) obtained from 4.3 to 20.9 mg 100 mL−1, which is closer to the results in this study mainly for the passion fruit flavor. Compared to fresh orange juices, Silva et al. (2005) reported higher AA content than our study, which was of 33.4 mg 100 mL−1, while it was lower than the AA in fresh purple passion fruit reported by Maniwara et al. (2014), which averaged 20.01 mg 100 g−1.
A significant difference (p < 0.05) in the AA content was verified among the batches of most of the samples, except to B, C, H and L in grape flavor and D in passion flavor, and a significant difference among approximately 25 to 75% of the brands. Regarding the labeled values, 75% of samples presented higher AA content than the labeled value, from 2.4 to 493% higher AA content. This failure in controlling AA was also reported by Granato et al. (2012), who have compared laboratorial and labeled values for the AA in PSDs and industrialized juices. They reported that 76.7% of the samples did not resemble the labeled values, but they were from 12 to 90% lower, while we observed mostly higher AA content. In this way, batches and brands also did not resemble one another for the values of AA, which require the control of this parameter.
In addition, according to the AA contents obtained in passion fruit and grape PSDs, it was possible to estimate the Reference Nutrient Intake of AA for children and adults, which is 30 mg day−1 and 45 mg day−1, respectively (FAO/WHO 2004), in cups of 200 mL. The AA RNI for children ranged from 1 to 10.6 cups, while it was from 1.5 to 16 for adults, among which 9 brands required higher consumption of the grape PSDs than of the passion fruit flavor to supply the daily needs of AA for children (from 0.3 to 6.7 more cups) and adults (from 0.6 to 10.2 more cups). It can be explained by a difference in the AA concentration added into the samples.
Ascorbic acid RNI in these PSDs, mainly in the passion fruit flavor, resembled the study with the lemon and orange flavors by Cruz et al. (2013), who did not observe significant difference between flavors to fulfill the RNI for adults (45 mg day−1), which were from 2.1 to 4 cups of 200 mL. However, according to Silva et al. (2005), male adults required ingestion of 4 to 67 cups of 200 mL of orange PSDs to fulfill their RNI (90 mg day−1), which was greater than our RNI for the passion fruit flavor, but part of the RNI for the grape flavor fits in their range. It was also due to the RNI they used, different to the value considered in this study.
Based on the characterization of a food as an AA source (Brazil 1998c), it should provide a minimum of 7.5% of the mineral RNI per 100 mL of the product ready for consumption. Thus, most of the reported samples were a source of AA, due to a range of 4.7 to 48.8% for children and of 3.1 to 32.9% for adults, except to two brands in the grape flavor.
NIRS and PLS for ascorbic acid control
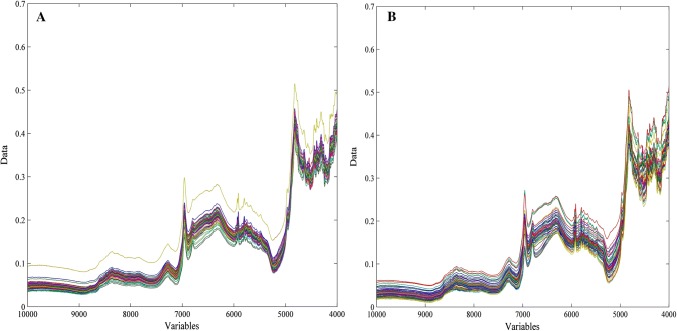
A total of 108 samples in the passion fruit flavor and 112 in the grape flavor were considered for ascorbic acid control via NIRS and PLS. The original NIR absorbance spectra (10,000–4000 cm−1) are in Fig. 1 for both fruit flavors.
Fig. 1.
Raw near-infrared spectra of grape (a) and passion fruit (b) powdered soft drinks
The spectral region ranging from 10,000 to 8704 cm−1 was removed because it did not present significant information for the analysis of ascorbic acid.
The selected spectral range were pretreated by applying smoothing (11 points, 2nd order) to remove random spectral noise, standard normal variate (SNV) for scatter correction, the second derivative using Savitzky-Golay algorithm (15 points) to remove spectral baselines, then, they were mean centered (Nicolaï et al. 2007). Cross-validation by leave-one-out was applied and outliers were removed by analyzing the graph of leverage and Studentized residuals, which were 28 for the passion fruit flavor model and 9 for the grape model, reducing the number of samples to 103 and 80 for the grape and passion fruit flavors, respectively.
For the ascorbic acid prediction models, PLS regression models were built and evaluated through their coefficient of determination (R2), number of latent variables (LV), RMSEC, RMSEP, RMSECV, analytical sensitivity, selectivity, and limits of detection and quantification, which are shown in Table 2.
Table 2.
PLS results for ascorbic acid of grape and passion fruit powdered soft drinks
| Sample flavor | LV | RMSEC (R2cal) | RMSEP (R2pred) | RMSECV (R2CV) | SEN | SEN−1a | SEL | LD | LQ |
|---|---|---|---|---|---|---|---|---|---|
| Grape (mg g−1) | 3 | 0.49 (0.84) | 0.50 (0.84) | 0.67 (0.70) | 0.001 | 0.06 | 0.16 | 0.19 | 0.59 |
| Passion fruit (mg g−1) | 5 | 0.24 (0.95) | 0.57 (0.72) | 0.56 (0.76) | 0.001 | 0.09 | 0.08 | 0.31 | 0.95 |
LV, latent variables; RMSEC, root-mean-square error of calibration, RMSECV, root-mean-square error of cross-validation; RMSEP, root-mean-square error of prediction; R2cal, coefficient of determination of calibration; R2pred, coefficient of determination of prediction; R2CV, coefficient of determination of cross validation; SEN, sensitivity; SEN−1a, inverse of the analytical sensitivity; SEL, selectivity; LD, limit of detection; LQ, limit of quantification
According to a correlation among the regression vector, VIP scores and selectivity of the model, the optimal wavenumbers range for correlations between the spectral data and the reference method to analyze AA content were 6821–6742 cm−1 (first overtone of O–H stretching), 6002–5901 cm−1 and 5860–5759 cm−1 (first overtone of C–H stretching), 5024–4283 cm−1 (combination of O–H stretching and second harmonics of C=O stretching) and 4215–4029 cm−1 (combination of C–H stretching) for the grape flavor, and 7505–7093 cm−1 (C–H stretching), 6034–5857 cm−1 (first overtone of C–H stretching), 5460–5368 cm−1 (combination of N–H and O–H stretching and second harmonics of C=O stretching), 4428–4269 cm−1, 4179–4074 cm−1 and 4068–4007 cm−1 (combination of C–H stretching) for the passion fruit flavor (Workman and Weyer 2012; Stuart 2004).
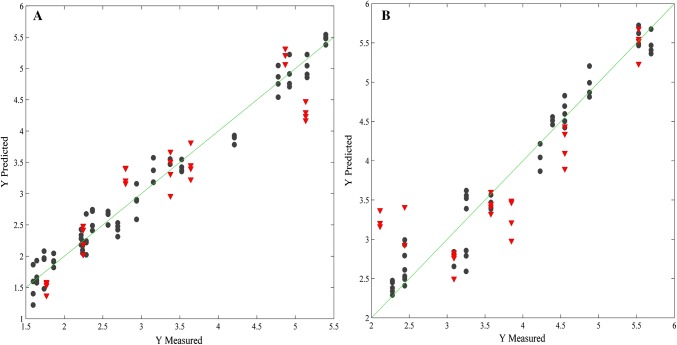
The PLS models for AA in grape and passion fruit PSDs (Fig. 2) predicted well according to similar magnitude in the errors for cross-validation and prediction. It ensures that the models present a good fitting and predictability. In addition to it, they also presented high coefficient of determination in calibration, cross-validation and prediction, satisfactory inverse of the analytical sensitivity, which expressed the minimum AA content that can be distinguished by the analytical method applied on these samples, adequate selectivity for AA prediction, and satisfactory limits of detection and quantification, since all the samples presented AA content higher than these limits. Besides, the passion fruit flavor presented more anomalous samples than the grape flavor.
Fig. 2.
Plot of the reference versus predicted values for ascorbic acid. a PLS model with 3 LV for Grape powdered soft drink; b PLS model with 5 LV for Passion fruit powdered soft drink. Calibration set (black circle) and validation set (inverted red triangle)
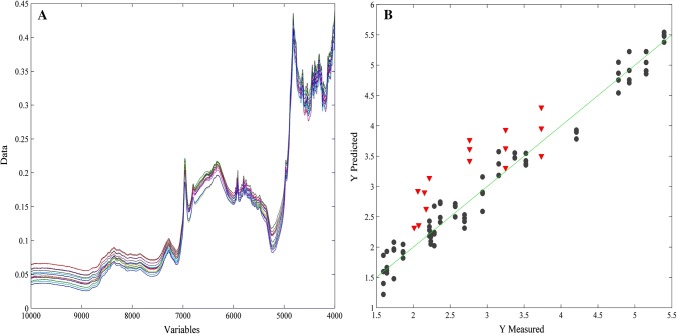
Also, a group of 5 samples, named M, N, O, P and Q, of the grape flavor was prepared in laboratory, as explained in the sampling section, and later tested in triplicates for external prediction to test one of the prediction models, which was the grape representative. Their AA content were determined also in triplicates and analyzed by the Tukey’s test (2.05 ± 0.03, 2.18 ± 0.03, 2.76 ± 0.01, 3.25 ± 0.01 and 3.73 ± 0.01 mg g−1 for M, N, O, P and Q, respectively), presenting no significant variance among the replicates according to their standard deviation, although there was significant difference between samples O, P and Q by the Tukey’s test, which corresponds to different composition between them.
According to this test (Fig. 3), the prediction model for the grape flavor was accurate and reliable for testing unknown samples because it resulted in RMSEC of 0.20 mg g−1 and R2cal of 0.97, RMSECV of 0.52 mg g−1 and R2CV of 0.81, RMSEP of 0.62 mg g−1 and R2pred of 0.72, which presented high R2 and errors in approximate magnitude order.
Fig. 3.
Raw spectra (a) and prediction model of ascorbic acid (b) for mixtures in the grape flavor. Calibration set (black circle) and validation set (inverted red triangle)
No other studies have evaluated AA by NIRS in PSDs as an alternative quality control, but there are some studies that did it in fruits or pharmaceutical tablets. Since some authors differed in the concentration units used, a comparison between studies was possible by calculating a percentage of error of prediction as a ratio between RMSEP and total AA. In the present work, a minimum of 9.6% error of prediction was obtained, what it was lower than that from Malegori et al. (2016), who obtained 12.0% for acerola. Sinelli et al. (2009), reported 31.3% for blueberries, and Maniwara et al. (2014), obtained 12.3% for passion fruit. However, our results were worse than that from Amodio et al. (2017), who achieved 2.4% of RMSEP on the prediction of AA content in strawberries, and Santos (2015), who achieved a minimum of 7.9% of error of prediction of AA with fruit nectars.
Thus, both models were accurate for predicting AA in passion fruit PSDs (RMSEP = 0.57 mg g−1) from a content of 2.11 to 5.53 mg g−1, and grape PSDs (RMSEP = 0.50 mg g−1) from 1.77 to 5.13 mg g−1, which high RMSEP could be explained by the low concentration of AA in the samples. Also, the samples presented AA content higher than the limits of detection and quantification. In this way, it is recommendable that further studies apply these models in and out the line of powdered soft drinks production.
Conclusion
The PSDs evaluated in this study presented variation in ascorbic acid contents across different batches of grape and passion fruit powdered soft drinks in a single brand, mostly higher than the labeled AA content of the samples, which requires the control of this nutrient in the final product. The use of a rapid, green and objective method together with multivariate calibration models predicted well the ascorbic acid content in both grape and passion fruit samples by showing satisfactory values of R2 and errors of calibration, cross validation and prediction, inverse of the analytical sensitivity and limits of detection and quantification. Thus, near-infrared spectroscopy is an alternative to standard methods of controlling ascorbic acid in powdered soft drinks, and it is also feasible for applications on the production line of PSDs by suppliers, producers and food quality control laboratories.
Funding
CAPES (Grant Number 001) and CNPq (Grant Number 132497/2017-4).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- ABIR (2015) Powdered soft drinks. Brazilian Association of soft drink and non-alcoholic beverages Industry. http://abir.org.br/o-setor/dados/refrescos-em-po/. Accessed 29 May 2016
- Alamar PD, Caramês ETS, Poppi RJ, Pallone JAL. Quality evaluation of frozen guava and yellow passion fruit pulps by NIR spectroscopy and chemometrics. Food Res Int. 2016;85:209–214. doi: 10.1016/j.foodres.2016.04.027. [DOI] [PubMed] [Google Scholar]
- Amodio ML, Ceglie F, Chaudhry MMA, Piazzolla F, Colelli G. Potential of NIR spectroscopy for predicting internal quality and discriminating among strawberry fruits from different production systems. Postharvest Biol Technol. 2017;125:112–121. doi: 10.1016/j.postharvbio.2016.11.013. [DOI] [Google Scholar]
- AOAC . Official Methods of Analysis. 14. Washington: Association of Official Analytical Chemistry; 1984. [Google Scholar]
- Benassi MT, Antunes AJA. Comparison of metaphosphoric and oxalic acids as extractants solutions for the determination of vitamin C in selected vegetables. Arquivos de Biologia e Tecnologia. 1988;31(4):507–513. [Google Scholar]
- Betemps DL, Fachinello JC, Galarça SP. Visible spectroscopy and near infrared (VIS/NIR), in assessing the quality of mangoes Tommy Atkins. Revista Brasileira de Fruticultura. 2011;33:306–313. doi: 10.1590/S0100-29452011000500038. [DOI] [Google Scholar]
- Brazil (1978) Regulation n. 12 from July 24, 1978 (Regulação n.12, de 24 de julho de 1978). Diário Oficial da União, Brasília (DF): July 24, 1978. Section 1
- Brazil (1998a) Ordinance n.544 from November 16, 1998 (Portaria n.544, de 16 de novembro de 1998). Ministry of Agriculture, Livestock, and Food SupplyDiário Oficial da União, Brasília (DF): November 17, 1998
- Brazil (1998b) Ordinance n.31 from January 13, 1998 (Portaria n.31, de 13 de janeiro de 1998). Ministry of Health, National Health Surveillance Agency. Diário Oficial da União, Brasília (DF): March 30, 1998. Section I-E
- Brazil (1998c) Ordinance n.27 from January 13, 1998 (Portaria n.27, de 13 de janeiro de 1998). Ministry of Health, National Health Surveillance Agency. Diário Oficial da União, Brasília (DF): July 17, 1998
- Brazil (2009) Decree n. 6871, from July 4, 2009 (Decreto n. 6871, de 4 de Junho de 2009). Ministry of Agriculture, Livestock, and Food Supply. Diário Oficial da União, Brasília (DF): June 5, 2009. Section 1
- Brazil (2013) Normative Instruction n.17 from June 19, 2013 (Instrução Normativa n.17, de 19 de Junho de 2013). Ministry of Agriculture, Livestock, and Food Supply. Diário Oficial da União, Brasília (DF): June 20, 2013 - Seção 1
- Caleguer VF, Toffoli EC, Benassi MT. Acceptance of commercial powdered orange flavored refreshment and their correlation to physicochemical parameters. Semina: Ciências Agrárias. 2006;27(4):587–598. [Google Scholar]
- Caramês ETS, Alamar PD, Poppi RJ, Pallone JAL. Quality control of cashew apple and guava nectar by near infrared spectroscopy. J Food Compos Anal. 2017;56(Supplement C):41–46. doi: 10.1016/j.jfca.2016.12.002. [DOI] [Google Scholar]
- Cruz RAN, Lobato LP, Santos JS. Ascorbic acid in powdered soft drinks in lemon and orange flavors (Ácido ascórbico em preparados sólidos para refresco sabores limão e laranja) Scientia Plena. 2013;9(11):5p. [Google Scholar]
- Ferreira MMC. Chemometrics—concepts, methods and applications (Quimiometria—Conceitos, Métodos e Aplicações) Campinas: Editora da Unicamp; 2015. [Google Scholar]
- Flores DWM (2015) Non-invasive systems for oranges classification by means of physical and chemical parameters. Dissertation, Luiz de Queiroz College of Agriculture (ESALQ)
- Granato D, Piekarski FVBW, Masson ML. Assessing the ascorbic acid contents in beverages and powdered juices: comparison between the experimental data and the values displayed on the product label. Revista do Instituto Adolfo Lutz (Impresso) 2012;71:331–336. [Google Scholar]
- Malegori C, Grassi S, Marques EJN, De Freitas ST, Casiraghi E. Vitamin C distribution in acerola fruit by near infrared hyperspectral imaging. J Spectr Imaging. 2016;5(a6):1–4. doi: 10.1255/jsi.2016.a6. [DOI] [Google Scholar]
- Maniwara P, Nakano K, Boonyakiat D, Ohashi S, Hiroi M, Tohyama T. The use of visible and near infrared spectroscopy for evaluating passion fruit postharvest quality. J Food Eng. 2014;143:33–43. doi: 10.1016/j.jfoodeng.2014.06.028. [DOI] [Google Scholar]
- Nicolaï BM, Beullens K, Bobelyn E, Peirs A, Saeys W, Theron KI, Lammertyn J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: a review. Postharvest Biol Technol. 2007;46:99–118. doi: 10.1016/j.postharvbio.2007.06.024. [DOI] [Google Scholar]
- Rosa SES, Cosenza JP, Leão LTS. Overview of the Brazilian beverage sector. Rio de Janeiro: BNDES Setorial; 2006. pp. 101–150. [Google Scholar]
- Santos DA (2015) Multiproduct multivariate calibration model development to quantify the acidity and vitamin C in industrialized fruit nectar and soy juices. Dissertation, Federal University of Technology - Paraná
- Silva PT, Fialho E, Lopes MLM, Valente-Mesquita VL. Industrialized orange juices and powdered soft drinks: chemical and physical-chemical stabilities (Sucos de laranja industrializados e preparados sólidos para refrescos: estabilidade química e físico-química) Ciênc Tecnol Aliment. 2005;25(3):597–602. doi: 10.1590/S0101-20612005000300033. [DOI] [Google Scholar]
- Sinelli N, Spinardi A, Egidio V, mIGnani I, Casiraghi E. Evaluation of quality and nutraceutical content of blueberries (Vaccinium corymbosum L.) by near and mid-infrared spectroscopy. Postharvest Biol Technol. 2009;50(1):31–36. doi: 10.1016/j.postharvbio.2008.03.013. [DOI] [Google Scholar]
- Webb FS, Whitney EN (2003) Nutrition: concept and controversies, 9th edn. Wadsworth Thomson Learning, Australia, pp 211–237, 277–290
- Souza AP (2007) Hygroscopic behavior and physical evaluation, physical-chemical and mineral of solid preparation to drink in flavors mango, orange, passion fruit and pineapple. Dissertation, Federal University of Ceará
- Stuart BH. Infrared spectroscopy: fundamentals and applications. Chichester: Wiley; 2004. [Google Scholar]
- Workman J, Weyer L. Practical guide and spectral atlas for interpretive near-infrared spectroscopy. 2. Florida: Taylor & Francis Group, LLC; 2012. [Google Scholar]