Abstract
Starch is the main sugar source present in staple foods. Understanding starch hydrolysis during digestion and the resulting glucose release can be important to strategically modulate starch digestion and glucose absorption. In vitro digestion methodologies are fundamental to evaluate starch hydrolysis length and rate, but the lack of uniformity between protocols prevent the comparison of results. In this context, three different Carolino rice varieties (i.e., Carolino white—Cw, Carolino brown—Cb and Carolino Ariete brown—CAb) were submitted to the INFOGEST harmonized in vitro digestion protocol for the evaluation of starch hydrolysis and subsequent glycemic index (GI) determination, and starch granules morphological study. Samples of Carolino rice presented total starch percentages between 64.52 (for Cb) to 71.52% (for Cw) with low amylose content (16.19–19.95%, varying in the following order Cb < Cab ≈ Cw). During digestion, between 39.43 (for CAb) to 44.48% (for Cb) of starch was hydrolyzed, classifying samples as medium GI foods (61.73–69.17). Starch hydrolysis was accompanied by a decrease of starch granules dimensions. For all samples, area decrease was higher than 59%, perimeter decrease was higher than 37%, feret diameter decrease was higher than 39% and minimum feret diameter decrease was higher than 32%. This work provides new insights to describe, both qualitatively and quantitatively, the fate of rice during digestion, and allowed establishing a comparative basis for the development of rice-based recipes with a lower GI.
Keywords: Rice starch hydrolysis, Rice starch morphology, Glycemic index, In vitro digestion, Digestion protocols
Introduction
Rice is among the most consumed staple foods, representing the main source of carbohydrates for an estimated 3.5 billion people, of which nearly 50% is represented by China and India (Muthayya et al. 2014). Rice grains have high starch content, representing up to 90% of the grain (dry weight basis) (Juliano 1993; Patindol et al. 2015). The hydrolysis of this polymeric carbohydrate is caused mainly by α-amylase and pancreatic enzymes during the digestion process, in which it is primarily released as maltose, that is further broken down into two glucose molecules thus becoming available for absorption (Tester et al. 2006).
Rice consumption can significantly raise blood glucose levels (Chang et al. 2014), being generally described as a high glycemic index (GI) food. The GI of a food is a kinetic parameter which is determined as the increment in the blood sugar concentration after the consumption of that food compared with a standard food (normally white bread) (Jenkins et al. 2002). GI is considered a more accurate measurement than the caloric content, because it contemplates the rate and extension of digestion and sugar absorption (Ludwig 2002).
One parameter of interest when studying GI of starchy foods is its amylose content (Srikaeo and Sangkhiaw 2014). Amylose and amylopectin are the components of starch, with linear and branched chain structures, respectively. Due to its more compact structure, amylose is more resistant to enzyme action. Therefore, the amylose content is also correlated with starch digestibility, which can be described as rapidly digestible starch (RDS), slowly digestible starch (SDS) and resistant starch (RS) (Deepa et al. 2010). In this sense, high amylose content is correlated with a higher percentage of RS (Srikaeo and Sangkhiaw 2014), that in consequence will result in a lower GI.
GI of a food product is also influenced by its form, post-harvesting processing, cooking procedure and even conservation process (Björck et al. 1994).
Rice with lower amylose content (i.e., waxy rice; Japonica varieties) will have higher retrogradation than rice with higher amylose content, thus presenting a pasty texture after cooking. The presence or absence of bran will affect the water absorption capacity of the grain and, therefore, brown rice requires longer cooking times and a higher water volume (Roy et al. 2008).
GI determination of starchy foods using an in vitro methodology was firstly described by Goñi et al. (1997) and since then, in vitro protocols have been applied in various products to study the effect of different characteristics and processing methods on the GI value. These protocols are often modified to fulfill the experiment requirements, thus lacking the necessary uniformity to allow comparison between different studies. More recently, a consensus protocol was proposed by the COST action INFOGEST (Minekus et al. 2014). This protocol was developed in order to mimic physiologic in vivo parameters of the human gastrointestinal tract, providing a rapid and easy standardized method for analysis of food or pharmaceuticals behavior during the digestion process. It has been applied to some food matrices (Egger et al. 2016), prebiotics (Nobre et al. 2018), probiotics (Ramos et al. 2016), compounds of interest (Rodrigues et al. 2017), different cereals (Bustos et al. 2017) and more recently, rice (Azizi et al. 2019).
In this context, this study aims at assessing of the GI of three different Carolino rice varieties, Carolino white (Cw), Carolino brown (Cb) and Carolino Ariete brown (Cab), by the application of the INFOGEST harmonized in vitro digestion protocol, for the first time in Carolino rice varieties, taking advantage of its robustness and the use of more realistic conditions. Three different Carolino rice varieties have been chosen for this study once it is the type of rice most consumed in Portugal, which is the Europe’s biggest rice consumer. Moreover, the morphology of the rice grains was also evaluated during the digestion process using fluorescence spectroscopy to assess starch digestion qualitatively. Finally, the results were compared with extensive literature data on starch digestion and conclusions were drawn about the compatibility of the existing in vitro digestion methodologies.
Materials and methods
Rice samples and their preparation
The samples used in this work were provided by Ernesto Morgado S.A. (Alqueidão, Portugal). The three rice varieties used were from Japonica species (Oryza sativa), specifically a commercially available cultivar Carolino white rice (Cw) branded “Pato Real Malandrinho”, cv. Carolino brown rice (Cb) and cv. Carolino Ariete brown rice (CAb) (newest Portuguese variety of cultivar Carolino rice).
Rice grains length and width were measured using a micrometer (No. 293-5, Mitutoyo, Japan) (n = 12), for size and shape characterization. Grain size was categorized by its length and its shape was categorized by its length–width ratio.
Prior to the application of the digestion protocol, rice samples were cooked and grinded. For the cooking process, an electric rice cooker was used (Ref. 968935000, Rice Chef Compact, Taurus, Spain) in order to maintain the same conditions in every cooking process. Preliminarily tests (data not shown) have been conducted to determine the adequate rice and water amounts for the complete cooking of each rice variety. According to these tests, 62.5 g of Cw rice plus 160 mL of water and 70 g of Cb or CAb rice plus 320 mL of water have been used. In order to mimic the mechanic degradation due to mastication while keeping the uniformity of this operation, rice was grinded in a food grinder (VM-4210, Tristar, Netherlands) mounted with the included fine cutting plate (XX-4210230, Tristar, Netherlands).
Total starch quantification
For the determination of the rice total starch, the Total Starch Assay Kit (AA/AMG), from Megazyme (K-TSTA, Ireland) was used. Briefly, samples were milled, weighed (100 mg), and incubated with 5 mL of ethanol 80% (v/v) at 80 °C for 5 min. Another 5 mL of ethanol 80% (v/v) were added before centrifugation at 1800 g for 10 min (EBA 20, Hettich, Germany). The supernatant was discarded and samples were washed and centrifuged again under the same conditions with 10 mL of ethanol. Then 2 mL of KOH 2 mol L−1 was added while stirring on cold water (at 4 °C), which helped to hydrolyze resistant starch. After 20 min of stirring, samples were neutralized with 8 mL of sodium acetate buffer 1.2 mol L−1 (i.e., pH 3.8) and 0.1 mL of thermostable α-amylase (3.000 U/mL on Ceralpha reagent at pH 6.5 and 40 °C or 1.600 U/mL on Ceralpha reagent at pH 5.0 and 40 °C, Megazyme, Ireland) and 0.1 mL of amyloglucosidase (3.300 U/mL on soluble starch or 200 U/mL on p-nitrophenyl β-maltoside at pH 4.5 and 40 °C, Megazyme, Ireland) were added. These enzymes hydrolyze starch into soluble maltodextrins (α-amylase enzymatic digestion) to be further hydrolyzed into d-glucose (amyloglucosidase enzymatic digestion). In the final step, d-glucose is oxidized to D-gluconate, by glucose oxidase–peroxidase (GOPOD), releasing hydrogen peroxide which is then consumed with p-hydroxybenzoic acid and 4-aminoantipyrine to produce quinoneimine dye, during incubation at 50 °C for 20 min. The absorbance of this colorimetric reaction was read at 510 nm (V560, Jasco, Italy) and percentage of d-glucose in samples (100 mg) was calculated. Total starch was obtained by multiplying the percentage of d-glucose by the conversion factor (162/180), that represents anhydrous d-glucose and free d-glucose molecular weight, respectively.
Amylose and amylopectin quantification
Amylose content was determined by the standardized protocol ISO 6647-1, Rice—Determination of amylose content, for rice samples with amylose content ranging from 1 to 30%. Briefly, 100 mg of milled rice sample was weight and 1 mL of ethanol 95% (v/v), and 9.0 mL of sodium hydroxide solution 1 mol L−1 was added. Starch was dispersed by heating the samples on a boiling water bath for 10 min. The solution was made up to 100 mL. For color development, 5 mL of the previously treated sample was used and 1 mL of acetic acid 1 mol L−1 and 2 mL of iodine solution (iodine 8 mmol L−1 with potassium iodide 0.12 mol L−1) was added and made up to 100 mL. The absorbance of samples was read at 620 nm (V560, Jasco, Italy), and the corresponding concentration was calculated using a plotted calibration curve using pure amylose from potato (Sigma-Aldrich A0512; CAS 9005-82-7). The amylopectin content was indirectly determined, subtracting the amylose percentage to the total starch percentage of the sample.
Static in vitro digestion
The consensus INFOGEST protocol was followed for the static in vitro digestions (Minekus et al. 2014). The procedure is composed by an oral, gastric and small intestinal phase (with the possibility of continuation to a large intestinal phase). Simulated digestion fluids (i.e., Simulated Salivary Fluid (SSF: KCl 15.1 mmol L−1, KH2PO4 3.7 mmol L−1, NaHCO3 13.6 mmol L−1, MgCl2(H2O)6 0.15 mmol L−1, (NH4)2CO3 0.06 mmol L−1, HCl 1.1 mmol L−1); Simulated Gastric Fluid (SGF: KCl 6.9 mmol L−1, KH2PO4 0.9 mmol L−1, NaHCO3 25 mmol L−1, NaCl 47.2 mmol L−1, MgCl2(H2O)6 0.1 mmol L−1, (NH4)2CO3 0.5 mmol L−1, HCl 15.6 mmol L−1); Simulated Intestinal Fluid (SIF: KCl 6.8 mmol L−1, KH2PO4 0.8 mmol L−1, NaHCO3 85 mmol L−1, NaCl 38.4 mmol L−1, MgCl2(H2O)6 0.33 mmol L−1, HCl 8.4 mmol L−1)) were prepared 1.25 × concentrated, allowing the subsequent addition of enzyme solution and CaCl2(H2O)2 (i.e., 0.75 mmol L−1 in SSF, 0.075 mmol L−1 in SGF and 0.3 mmol L−1 in SIF). The experiments were performed in a water bath (B. BRAUN BIOTECH model CERTOMAT WR, Melsungen, Germany) with horizontal agitation (120 rpm) at 37 °C.
For the oral phase, a ratio of food to SSF of 50:50 (w/v) was considered and α-amylase was added (Sigma-Aldrich A1031, CAS 9000-90-2) to obtain 75 U/mL activity. The mixture was incubated for 2 min. The gastric phase consisted in the addition of SGF and pepsin (Sigma-Aldrich P7012; CAS 9001-75-6) to a final ratio of food to SGF of 50:50 (v/v) and enzyme activity of 2000 U/mL. At this point, pH was adjusted to 3.0 using HCl 1 mmol L−1 and the mixture was incubated for 120 min. The intestinal phase was simulated by adding SIF to the gastric chyme in a final ratio of 50:50 (v/v). Pancreatin (Sigma-Aldrich P7545; CAS 8049-47-6) was added to obtain 100 (TAME: one unit hydrolyses 1 µmol of p-toluene-sulfonyl-l-arginine methyl ester (TAME) per minute at 25 °C, pH 8.1) U/mL activity, as well as bile salts (Sigma-Aldrich B8631; CAS 8008-63-7) in a concentration of 80.0 mg/mL. pH was then adjusted to 7.0 using NaOH 1 mmol L−1 and the mixture was incubated for 120 min.
This protocol was applied to evaluate starch hydrolysis and also to evaluate morphological changes in the rice starch granules (RSG).
Starch hydrolysis evaluation
For the determination of the starch hydrolysis, 5 g of cooked and grinded rice were added into a 50 mL Falcon tube, and samples were collected from the liquid medium in the end of the oral phase (2 min) and subsequently each 30 min until the end of the digestion process (240 min). Collected samples were centrifuged at 1800 g for 10 min (EBA 20, Hettich, Germany) and supernatant was used for glucose quantification using GOPOD reagent as described in total starch determination. Samples were incubated with GOPOD reagent at 50 °C for 20 min and afterwards absorbance was read at 510 nm (V560, Jasco, Italy). The obtained percentages of d-glucose were converted to percentages of starch with a 0.9 factor (division of starch occurring anhydrous d-glucose by free d-glucose molecular weight). Obtained values of starch available were normalized to percentage of hydrolysed starch.
Calculation of glycemic index, rapidly digestible starch, slowly digestible starch and resistant starch
Glucose concentration quantified during the digestion process was normalized to percentage of total starch hydrolyzed, so that the GI index of the samples can be determined using the area under the curve (AUC). AUC of total starch hydrolyzed during the digestion was calculated manually as the sum (0–240 min) of areas between sample points, considering the area of the trapezium formed by the points in the graph (Eq. 1):
| 1 |
where AUC2–30 represents the area between 2 and 30 min, HS2 represents the percentage of total starch hydrolysed after 2 min, HS30 represents the percentage of total starch hydrolysed after 30 min and t represents the time between 2 and 30 min. The AUC of both sample and reference food (starch from potato; 121096.1211 Panreac, Spain) were used to calculate the hydrolysis index (HI) as described by Goñi et al. (1997), according to Eq. (2):
| 2 |
where AUCsample corresponds to the AUC of the rice sample and AUCref corresponds to the AUC of the reference food. GI was then determined according to Eq. (3):
| 3 |
Using the data obtained in the in vitro digestion, starch fractions corresponding to rapidly digestible starch (RDS), slowly digestible starch (SDS), and resistant starch (RS) were determined by Eqs. (4)–(6), based on those described by Englyst et al. (1999) with modifications, considering RDS as starch hydrolysed during the oral phase, SDS as the maximum amount of starch hydrolysed during gastric—intestinal phase and RS as starch that was not hydrolysed
| 4 |
| 5 |
| 6 |
where HS2 represents the percentage of total starch hydrolysed after 2 min, HS180 represents the percentage of total starch hydrolysed after 180 min, HS represents the percentage of total starch hydrolysed until the end of the digestion, and TS represents the total starch (i.e., 100%).
Starch hydrolysis rate (HR) throughout the digestion procedure was determined by Eq. (7).
| 7 |
Fluorescence microscopy
For the observation of RSG with fluorescence microscopy, staining agents’ safranin O (Acros Organics AC146640250; CAS 477-73-6), rhodamine B (Alfa Aesar A13572-18; CAS 81-88-9) and fluorescein-5,6-isothiocyanate (FITC) (Fluka 46950; CAS 27072-45-4) were tested since they were previously used by other authors to stain starch (van de Velde et al. 2002). Briefly, and according to these previous works, rice samples were stained (i.e., 20 µL of FITC/rhodamine in a concentration of 2.0 mg/mL and 30 µL of safranin in a concentration of 5.0 mg/mL), and washed 3 times with distilled water (i.e., centrifugation at 1800 g for 10 min (EBA 20, Hettich, Germany), discarding supernatant between washes). To assess the morphological changes in RSG, 500 mg of cooked and grind rice were added into ten individual 15 mL Falcon tubes and submitted to static in vitro digestion. Samples correspondent to the content of one Falcon tube were collected in the same time periods as for the starch hydrolysis experiment for posterior microscopic observation and correlation. RSG were visualized in an Olympus BX51 (Olympus Europa, Hamburg, Germany) fluorescence microscopy with a wavelength filter (λex = 488 nm; λem = 500 to 525 nm) and magnification of 10 × and 40 ×. For RSG dimensions analysis, 10 pictures with 40 × magnification were taken for each point of the digestion process (i.e., 2 min and each 30 min afterwards). RSG pictures were analyzed and measured (area, perimeter, Feret diameter and minimum Feret diameter) using ImageJ software.
Statistical analysis
The statistical analyses related to the current experimental data were performed using the statistical software GraphPad Prism software version 7.04 (GraphPad, USA). The statistical significance of different samples (at p ≤ 0.05) was determined using one-way ANOVA followed by post hoc. Tukey’s honestly significant difference (HSD) test. Unless otherwise stated, all the following experiments were run at least in triplicate and all measured parameters are means of experimental points.
Results and discussion
Rice properties
Kernels measurements (data not shown) demonstrates that all rice samples can be classified as long grain rice (length > 6.0 mm and length/width ratio ≥ 3) taking into consideration the reported standard for rice (Codex Alimentarius 1995). Dimensions observed are in accordance with the values found in literature for Japonica rice species (Carolino) (Santos et al. 2015). Percentages of total starch, amylose and amylopectin are depicted in Table 1.
Table 1.
Percentages of total starch and amylose content of Cw, Cb and CAb rice samples
| Total starch (%) | Amylose (%) | Amylopectin (%) | % RDS | % SDS | % RS | GI | |
|---|---|---|---|---|---|---|---|
| Cw | 71.52 ± 0.74a | 19.95 ± 1.03a | 51.57 ± 1.27a | 3.82 ± 0.32a | 37.00 ± 1.56a | 59.72 ± 1.53a | 67.84 ± 3.59a |
| Cb | 64.52 ± 3.55b | 16.20 ± 0.67b | 48.32 ± 3.61a | 5.19 ± 1.73b | 39.29 ± 2.77a | 55.53 ± 2.17b | 69.60 ± 5.94a |
| CAb | 65.47 ± 3.15b | 19.45 ± 0.57a | 46.03 ± 3.20a | 1.50 ± 1.49c | 37.93 ± 3.89a | 60.57 ± 3.59a | 62.28 ± 5.51a |
RDS, SDS, RS and GI values determined for Cw, Cb and CAb rice samples. Values presented correspond to mean and standard deviations deviation on a dry basis
Mean values within the same column, labelled with the same superscript letter (a, b or c) do not statistically differ from each other (p > 0.05)
It is possible to observe that, as expected, Cw sample exhibits the higher percentage of total starch, once their kernels are mainly composed by the endosperm of the seed as a result of the milling process, and it is in the endosperm that RSG are stored. Amylose content values vary between 16 and 20%, characterizing all samples as low-amylose cultivars (Juliano 1993). Due to the higher percentage of total starch, Cw also presented the highest amylopectin content. These values are expected for Carolino rice varieties, since this variety belongs to Oryza sativa subspecies Japonica rice, categorised as sticky variety (Chang et al. 2014).
In vitro digestion—starch hydrolysis
Figure 1 represents the profile of total starch hydrolysed during in vitro digestion for Cw, Cb and CAb. For all samples, the action of α-amylase alone during the oral phase was not very pronounced, hydrolyzing less than 3% of total starch from CAb to less than 7% of total starch from Cb. This step of the digestion is very short (only 2 min) and therefore, there is not enough time for a significant action of this enzyme, which is then stopped when in contact with the acidic medium characteristic of the gastric phase (Whitcomb and Lowe 2007). Despite the low enzyme activity in the oral phase, starch hydrolysis rate in this phase is the highest throughout the digestion, for all samples (i.e., 1.64%/min, 2.59%/min and 0.75%/min for Cw, Cb and CAb, respectively).
Fig. 1.
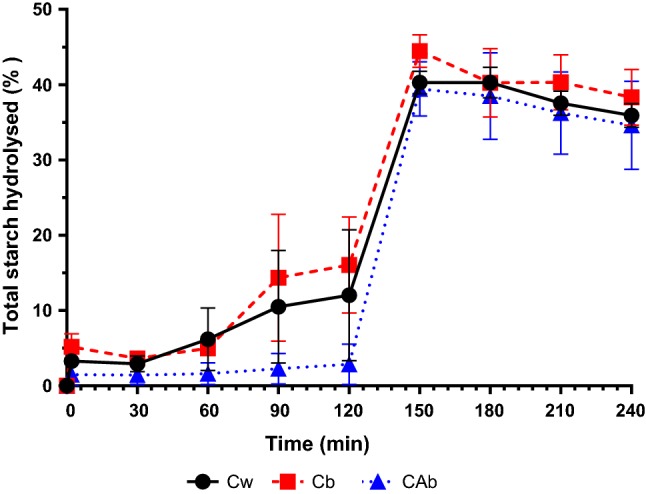
Total starch hydrolysed during in vitro digestion of Cw, Cb, and CAb
In the gastric phase of the in vitro digestion, CAb samples were more resistant to enzymatic digestion when compared to Cb and Cw samples (i.e., % of hydrolyzed starch of 12.02 ± 8.71%, 16.04 ± 6.38% and 2.87 ± 2.68%, for Cw, Cb and CAb, respectively). At this phase, starch hydrolysis is commonly associated to the enzymatic activity of pepsin, which breaks down the protein layer (0.1–0.7% of starch weight) found at the surface and inside the starch (Pérez and Bertoft 2010). The action of this enzyme provoked alterations on starch structure by hydrolyzing protein layers, but was not significant and specific for starch hydrolysis, which is reflected in the low starch hydrolysis rate of the gastric phase (i.e., 0.07%/min, 0.09%/min and 0.01%/min for Cw, Cb and CAb, respectively).
It is also possible to observe that, for all rice samples, there was a rapid starch hydrolysis in the gastric-intestinal transition (i.e., from 120 to 150 min). The starch hydrolysis rate in this transition is comparable to the starch hydrolysis rate of the oral phase (i.e., 0.94%/min, 0.95%/min and 1.22%/min for Cw, Cb and CAb, respectively). For all samples, the highest values of starch hydrolysis were observed in the first point of the intestinal phase, at 150 min. This peak of hydrolysis can be associated with the addition of pancreatin (mixture of amylase, proteases and lipases), which benefits from the neutral environment found in the intestine (i.e., pH 7.0). The results obtained indicate a rapid starch hydrolysis (in less than 30 min) when submitted to pancreatin, which is expected, since it is reported that the enzymatic activity of pancreatic amylase is higher than that of α-amylase (Woolnough et al. 2010). The final values of total starch hydrolysis were 40.28 ± 1.53%, 44.48 ± 2.17% and 39.43 ± 3.59% for Cw, Cb and CAb, respectively. Therefore, only the sample Cb presented significant statistical differences in terms of percentage of total starch hydrolyzed (i.e., p < 0.05, Tukey test). Even though integral rice is known as being more resistant to hydrolysis, which is consequently associated with lower GI, the results obtained can be related to the fact that this sample also presented the lowest amylose content of the three samples, which is more resistant to the enzymatic attack due to its linear structure (Srikaeo and Sangkhiaw 2014). Besides its amylose/amylopectin content and molecular/supra molecular structure, also the intrinsic characteristics of each cultivar (e.g. cell wall intactness, granule/particle size, level of proteins and interactions with starch granules) can be considered key factors in controlling the rate and extent of starch hydrolysis in rice grains (Azizi et al. 2019).
Some results previously described in the literature (Tamura et al. 2017) are not in agreement with the hydrolysis profiles obtained here. The differences found are presumably due to the use of different in vitro digestion protocols: many of those found in the literature do not simulate each digestion phase separately, thus resulting in a continuously increasing starch hydrolysis and use different enzymes with different activities. In fact, a wide range of in vitro digestion protocols with differences in digestive enzyme activities have been applied to rice and other starchy foods (Tables 2 and 3), which difficult the comparison of results and causes discrepancy of results among laboratories. The results of this study, obtained using the INFOGEST harmonized protocol, should represent a more realistic starch hydrolysis profile and the corresponding fate of food digesta, besides contributing to the increase of comparability of experimental results between laboratories. Generally, the results obtained here are in agreement with reports of hydrolysis profiles for other starchy foods when applying the same methodology (Bustos et al. 2017).
Table 2.
Comparison of in vitro digestion protocols applied for starch hydrolysis studies
| Oral phase | Gastric phase | Intestinal phase | Food | % Starch hydrolysis | % RDS | % SDS | % RS | GI | Reference |
|---|---|---|---|---|---|---|---|---|---|
| INFOGEST harmonized digestion protocol | Three “Carolino” rice varieties | 39.43–44.48 | 1.50–5.19 | 37.00–39.29 | 55.53–60.53 | 62.8–69.6 | This work | ||
| INFOGEST harmonized digestion protocol | Three Iranian rice varieties (Hashemi, Domsiyah and Gohar) | 100.56–56.88 | – | – | 0.39–0.86 | – | Azizi et al. (2019) | ||
| – | Pepsin (1 h at 40 °C) | α-amylase (3 h at 37 °C); amyloglucosidase (45 min at 60 °C) | Rice varieties “Njavara”, “Jyothi” and “IR 64” | 62.7–67.3 | (Digestible starch) 79.16–88.66 | 0.64–1.10 | 73.1–74.8 | Deepa et al. (2010) | |
| Artificial saliva with pancreatic α-amylase (250 U/mL) (1 min at 37 °C) | Pepsin (3200 U/mL) (30 min at 37 °C) | Pancreatin (4x U.S.P.) and amyloglucosidase (140 U/mL) (180 min at 37 °C) | Rice varieties “Xing 2#”, “Jinongsimiao”, “Yuxiangyouzhan” and “Beihan 1#” | 78.05–85.94 | – | – | – | – | Kuang et al. (2016) |
| SSF containing α-amylase (0.3–1.7 U/mL) (2 min) | SGF containing pepsin (30 min) | SIF containing pancreatin, invertase and amyloglucosidade (270 min) | Rice variety “Koshihikari” | 84.2–95.9 | – | – | 0.6 | – | Tamura et al. (2017) |
| – | – | α-amylase (120 U/mL) and amyloglucosidase (80 U/mL) (120 min at 37 °C) | Rice starch; Rice starch-pullulan complex | – | 61.89–78.76 | 15.35–30.20 | 5.12–13.06 | – | Chen et al. (2017) |
| Artificial saliva containing α-amylase | Pepsin (30 min at 37 °C) | Pancreatin and amyloglucosidase (4 h) | Rice noodles | – | (Non-resistant starch) 67.27–81.08 | 0.41–16.41 | 71.72–99.00 | Srikaeo and Sangkhiaw (2014) | |
| – | – | Pancreatic α-amylase (290 U/mL) and amyloglucosidase (15 U/mL) (120 min at 37 °C) | Waxy maze starch | – | 32.4–44.2 | 45.7–49.2 | 9.8–18.4 | – | Miao et al. (2011) |
| – | Pepsin (30 min at 37 °C) | Pancreatin, amyloglucosidase (1200 U/mL) and invertase (3000 U/mL) (2 h with shaking at 37 °C) | Millet flour; Millet starch; Wheat flour | – | 36.9–50.7 | 38.3–46.3 | 8.8–24.9 | 64.4–93.6 | Ren et al. (2016) |
| Samples were dispersed in pepsin solution for 30 min at 37 °C. Then was added | Pepsin (30 min at 37 °C) | Pancreatin and invertase (20 min at 37 °C); amyloglucosidase (40 U/mL) (30 min at 70 °C) | Corn flakes; White bread; Spaghetti; Pearled barley | – | (RAG) 9.4–75.7 | (SAG) 0.7–12.0 | 1.2–40.0 | – | Englyst et al. (1999) |
| INFOGEST harmonized digestion protocol. | White bread (WB); Gluten-free bread (GFB); Sheeted pasta (SP); Whole-grain pasta (WP); Gluten-free pasta (GFP); Whole cookies (WC); Peach cookies (PC) |
WB: 87.0; GFB: 76.5; SP: 72.6; WP: 92; GFP: 54.3; WC: 50,7; PC: 43.6 |
(ca.) WB: 79; GFB: 76; SP: 46; WP: 59; GFP: 39; PC: 37; WC: 32 |
(ca.) WB: 8; GFB: 2; SP: 20; WP: 25; GFP: 16; PC: 8; WC: 17 |
(ca.) WB: 12; GFB: 24; SP: 33; WP: 15; GFP: 45; PC: 50; WC: 55 |
– | Bustos et al. (2017) | ||
Table 3.
Comparison of in vitro digestion protocols applied for starch morphological studies
| Oral phase | Gastric phase | Intestinal phase | Food | Initial dimension | Morphological changes | Dimensions after digestion | Reference |
|---|---|---|---|---|---|---|---|
| INFOGEST harmonized digestion protocol | Three “Carolino” rice varieties |
(After cooking) Area (µm2): 1331.85–1405.21; Perimeter (µm): 141.50–145.02; Diameter (µm): 33.49–54.95 |
After 60 min (gastric phase) into digestion rice starch granules begin to become undefined; After 150 min (intestinal phase) rice starch granules were disrupted | Area (µm2): 461.21–540.70; Perimeter (µm): 81.70–88.20; Diameter (µm): 20.30–32.57 | This work | ||
| – | SGF containing pepsin (30 min) | SIF containing pancreatin (210 min) | Polished rice grain |
(Homogenized cooked rice) Diameter (µm): ca. 5–50 |
After 30 min (gastric phase) there was not changes to granules dimensions; Dimensions after 5 min and 120 (intestinal phase) were in the same range |
(Homogenized cooked rice) Diameter (µm): ca. 1–5 |
Tamura et al. (2016) |
| – | – | α-amylase (120 U/mL) and amyloglucosidase (80 U/mL) (120 min at 37 °C) | Rice starch; Rice starch-pullulan complex | – | Small particles were significantly positively correlated with RS (Pearson correlation); Medium and large particles were significantly negatively correlated with RS |
(Small particles) Diameter (µm): < 62.2 (Medium particles) Diameter (µm): 62.2–209.3 (Large particles) Diameter (µm): > 209.3 |
Chen et al. (2017) |
| – | – | Pancreatic α-amylase (290 U/mL) and amyloglucosidase (15 U/mL) (120 min at 37 °C) | Waxy maze starch |
(Granules size) Diameter (µm): 5–25; Molecular weight (107 g mol−1): 21.9 |
Increasing enlargement of holes in granules and increasing hydrolysis of internal regions as a result of longer time hydrolysis |
(Granules size) Molecular weight after 20 min (107 g mol−1): 16.5; Molecular weight after 120 min (107 g mol−1): 7.9 |
Miao et al. (2011) |
Rapidly digestible starch, slowly digestible starch, resistant starch and glycemic index
Through the analysis of starch hydrolysis rate and extension, rapidly digestible starch (RDS), slowly digestible starch (SDS), resistant starch (RS) and glycemic index (GI) were determined and results are presented in Table 1.
These values differ from those obtained using in vitro digestion methodologies for starch hydrolysis that do no simulate each digestive phase separately (where samples were solubilized and catalyzed with pepsin, and digestion was performed using pancreatin and amyloglucosidase) (Deepa et al. 2010; Kuang et al. 2016; Srikaeo and Sangkhiaw 2014). Although RS content depends on the rice variety and can inclusively be modulated by post-harvest processes (Azizi et al. 2019), the different methodologies used for quantification had very different output values. For instance, the studies presented in Table 2 indicate that RDS, the portion of starch hydrolysed until 30 min of digestion, constitutes more than 30% of the total starch. In our study, Cw, Cb and CAb samples presented less than 5% of RDS, which can be explained by the inhibition of α-amylase action in acid conditions when passing from the oral to gastric phase in INFOGEST in vitro digestion protocol, as opposed to the continuous action of pancreatin and amyloglucosidase during their incubation period with samples in optimal enzymatic conditions. In terms of RS, Cw, Cb and CAb samples present between 55 and 60%, respectively, which also differs from the results presented by other authors (see Table 2). A higher percentage of RS is desired, because of its physiologic effects in the intestine (similar to fibres) (Topping, Fukushima, and Bird 2003), being also described as having health benefits in non-transmissible diet-related diseases (Ludwig 2002). Higher amounts of RDS and SDS are representative of foods with high GI, caused by the rapid release of glucose in digestion.
In terms of GI, all samples can be characterized as medium GI foods, since they present a GI between 55 and 70 (Brand-Miller et al. 2002), which is in agreement with the values reported in carbohydrate GI tables (Atkinson, Foster-Powell, and Brand-Miller 2008). Despite the differences between them in hydrolysis percentages and starch fractions, the GI of the samples did not present statistically significant differences (p > 0.05).
These results support that the in vitro digestion protocol applied will influence GI and other important nutritional parameters used for food characterization, producing different outcomes that cannot be compared. For this reason, it is important to apply harmonized in vitro digestion protocols.
Morphological study of rice starch granules
RSG are described as the smaller starch granules found in staple foods in their native state, with a diameter from 2 to 7 µm, reaching up to 100 µm in diameter after cooking (Patindol, Siebenmorgen, and Wang 2015). FITC was the selected staining agent for the assays because of its reactivity towards sulfhydryl groups of proteins and slower fluorescence decay observed on the tested samples (van de Velde et al. 2002).
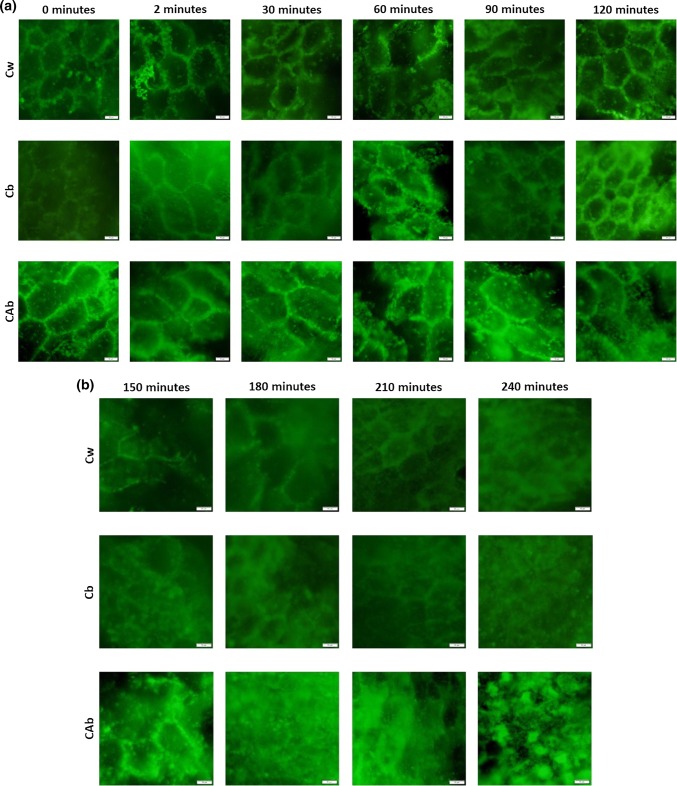
Figure 2a represents the RSG images of the initial samples until the end of the gastric phase (0–120 min).
Fig. 2.
Images from fluorescence microscopy (40 × magnification) of the rice starch granules a prior (0 min) and during the oral (2 min) and gastric phases of in vitro digestion; b during intestinal phase of in vitro digestion. Scale bars correspond to 20 µm
After cooking, the RSG of the three samples presented a circular/hexagonal shape, which is in accordance with previous morphological studies (Tamura et al. 2016). RSG did not present visible arrangement or structural differences after 2 min of digestion (mouth simulation). RSG structure starts to become more undefined after 60 min. Until the end of the gastric phase (i.e., 120 min), RSG present a decrease in dimensions, particularly visible on Cb, which was also the sample that had a higher total starch hydrolysis described previously.
As observed for the hydrolysis profile of the three samples, the peak of degradation was observed at 150 min, which corresponds to the first image of the intestinal phase presented in Fig. 2b. At this point, the observation of complete RSG was not possible, and only disrupted portions of the exterior membrane and the microstructures of starch were visible. Until the end of the digestion, RSG with smaller dimensions were visible, presumably due to a rearrangement of starch granules after the peak of hydrolysis as suggested before. Similar outcomes, presented in Table 3, were observed using other in vitro digestion methodologies, although, it can be concluded that the description of RDS and SDS morphology is lacking. On the other hand, RSG observed at the end of the digestion process can be a representation of RS morphology, since it corresponds to starch that was not hydrolyzed, thus having resisted to the digestion process.
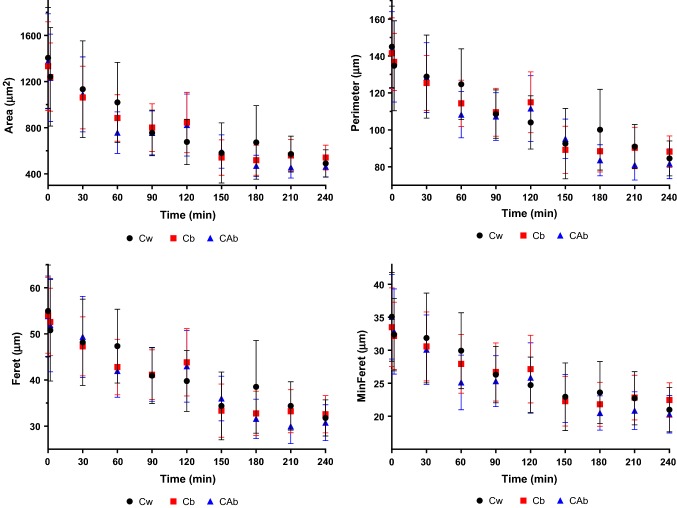
The variation of the RSG dimensions during the digestion process are presented in Fig. 3. All samples presented a similar behavior, with a continuous decrease in all dimensional parameters. RSG of the sample Cw presented higher initial dimensions and the most significant decrease in dimensions. With the milling process, and consequent bran removal, Cw endosperm surface area becomes more available to enzymatic degradation (Boers et al. 2015). When comparing the two brown rice samples, despite Cb having higher GI, the RSG of the sample CAb exhibited higher dimensional decrease, presenting the smallest dimensions at the end of the digestion process. In the case of Cw and CAb, although RSG had similar dimensional decrease, samples presented different hydrolysis percentages and rates, which can be associated to their kernels.
Fig. 3.
RSG measurements of Cw, Cb and CAb during in vitro digestion. Measurements were made in Imagej software, allowing the determination of area, perimeter, Feret diameter and minimum Feret diameter. Dots represent the mean and bars represent the standard deviation
With these results, it can be concluded that RSG morphological changes can be important for the study and determination of starch fractions, starch hydrolysis and hydrolysis rates, which are determinant factors for GI prediction.
Conclusions
In conclusion, the three Carolino rice varieties tested can be classified as long grain rice, with medium GI. The percentage of total starch was higher in the sample that was milled (i.e., Cw), supporting that post-harvesting processes have high impact on sample characteristics. The importance of amylose during digestion was also confirmed, since the sample with lower amylose content (i.e., Cb) presented the highest percentage of total starch hydrolyzed at the end of the digestion and lower percentage of RS. Also, this work highlights the importance of the application of a harmonized in vitro digestion protocol, once it allows interlaboratorial comparisons. Its application to understand rice starch hydrolysis along with morphological changes of RSG during the process can be fundamental to provide a better insight of the effects caused by post-harvest processing and cooking procedures of rice. Applying uniform methodologies will facilitate the information flow and allow a better correlation between food nutritional properties and starch characteristics, which can be important in the preparation of rice-based food products (or, more generally, carbohydrates-based foods), presumably resulting in the formulation of lower GI foods. In that sense, this work provides a possible interlaboratory comparison basis for future works.
Acknowledgements
Daniel A. Madalena acknowledge the Foundation for Science and Technology (FCT) for his fellowship (SFRH/BD/129127/2017). This work was supported by Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UID/BIO/04469/2019 unit and BioTecNorte operation (NORTE-01-0145-FEDER-000004) funded by the European Regional Development Fund under the scope of Norte2020 - Programa Operacional Regional do Norte. The authors would also like to thank the investment project nº 017931 – Development of rice products with low glycemic index- co-funded by Fundo Europeu de Desenvolvimento Regional (FEDER) through Programa Operacional Competitividade e Internacionalização (COMPETE 2020) (POCI-01-0247-FEDER-017931).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31(12):2281–2283. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi R, Capuano E, Nasirpour A, Pellegrini N, Golmakani MT, Hosseini SMH, Farahnaky A. Varietal differences in the effect of rice ageing on starch digestion. Food Hydrocoll. 2019;95(March):358–366. doi: 10.1016/j.foodhyd.2019.04.057. [DOI] [Google Scholar]
- Björck I, Granfeldt Y, Liljeberg H, Tovar J, Georg Asp N. Food properties affecting the digestion and absorption of carbohydrates. Am J Clin Nutr. 1994;59:699S–705S. doi: 10.1093/ajcn/59.3.699S. [DOI] [PubMed] [Google Scholar]
- Boers HM, Hoorn JST, Mela DJ. A systematic review of the influence of rice characteristics and processing methods on postprandial glycaemic and insulinaemic responses. Br J Nutr. 2015 doi: 10.1017/S0007114515001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Miller JC, Holt SH, Pawlak DB, McMillan J. Glycemic index and obesity. Am J Clin Nutr. 2002;76(1):281S–285S. doi: 10.1093/ajcn/76/1.281s. [DOI] [PubMed] [Google Scholar]
- Bustos MC, Vignola MB, Pérez GT, León AE. In Vitro digestion kinetics and bioaccessibility of starch in cereal food products. J Cereal Sci. 2017;77:243–250. doi: 10.1016/j.jcs.2017.08.018. [DOI] [Google Scholar]
- Chang UJ, Hong YH, Jung EY, Suh HJ. Rice and the glycemic index. Wheat Rice Dis Prev Health. 2014 doi: 10.1016/B978-0-12-401716-0.00027-1. [DOI] [Google Scholar]
- Chen L, Tian Y, Zhang Z, Tong Q, Sun B, Rashed Marwan MA, Jin Z. Effect of pullulan on the digestible, crystalline and morphological characteristics of rice starch. Food Hydrocoll. 2017;63:383–390. doi: 10.1016/j.foodhyd.2016.09.021. [DOI] [Google Scholar]
- Deepa G, Singh V, Akhilender Naidu K. A comparative study on starch digestibility, glycemic index and resistant starch of pigmented (‘Njavara’ and’Jyothi’) and a non-pigmented (‘IR 64’) rice varieties. J Food Sci Technol. 2010;47(6):644–649. doi: 10.1007/s13197-010-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger L, Ménard O, Delgado-Andrade C, Alvito P, Assunção R, Balance S, Barberá R, et al. The harmonized INFOGEST in Vitro digestion method: from knowledge to action. Food Res Int. 2016;88(October):217–225. doi: 10.1016/j.foodres.2015.12.006. [DOI] [Google Scholar]
- Englyst KN, Englyst HN, Hudson GJ, Cole TJ, Cummings JH. Rapidly available glucose in foods: an in vitro measurement that reflects the glycemic response. Am J Clin Nutr. 1999;69(3):448–454. doi: 10.1093/ajcn/69.3.448. [DOI] [PubMed] [Google Scholar]
- Goñi I, Garcia-Alonso A, Saura-Calixto F. A starch hydrolysis procedure to estimate glycemic index. Nutr Res. 1997;17(3):427–437. doi: 10.1016/S0271-5317(97)00010-9. [DOI] [Google Scholar]
- Jenkins DJA, Kendall CWC, Augustin LSA, Franceschi S, Hamidi M, Marchie A, Jenkins AL, Axelsen M. Glycemic index: overview of implications in health and disease. Am J Clin Nutr. 2002;76(1):266S–273S. doi: 10.1093/ajcn/76/1.266S. [DOI] [PubMed] [Google Scholar]
- Joint FAO/WHO Codex Alimentarius International Food Standards (2019) Codex alimentarius: Standard for rice CXS 198-1995. http://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/. Accessed 25 Nov 2018
- Juliano BO (1993) FAO Food and Nutrition Series No. 26 RICE in human nutrition prepared in collaboration with FAO By. http://books.irri.org/9251031495_content.pdf. Accessed 13 Oct 2018
- Kuang Q, Jinchuan X, Wang K, Zhou S, Liu X. Structure and digestion of hybrid indica rice starch and its biosynthesis. Int J Biol Macromol. 2016;93:402–407. doi: 10.1016/j.ijbiomac.2016.08.023. [DOI] [PubMed] [Google Scholar]
- Ludwig DS. The glycemic index. JAMA. 2002;287(18):2414. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- Miao M, Zhang T, Wanmeng M, Jiang B. Structural characterizations of waxy maize starch residue following in vitro pancreatin and amyloglucosidase synergistic hydrolysis. Food Hydrocoll. 2011;25(2):214–220. doi: 10.1016/j.foodhyd.2009.12.004. [DOI] [Google Scholar]
- Minekus M, Alminger M, Alvito P, Ballance S, Bohn T, Bourlieu C, Carrière F, et al. A standardised static in vitro digestion method suitable for food—an international consensus. Food Funct. 2014;5(6):1113–1124. doi: 10.1039/C3FO60702J. [DOI] [PubMed] [Google Scholar]
- Muthayya S, Sugimoto JD, Montgomery S, Maberly GF. An overview of global rice production, supply, trade, and consumption. Ann NY Acad Sci. 2014;1324(1):7–14. doi: 10.1111/nyas.12540. [DOI] [PubMed] [Google Scholar]
- Nobre C, Sousa SC, Silva SP, Pinheiro AC, Coelho E, Vicente AA, Gomes AMP, Coimbra MA, Teixeira JA, Rodrigues LR. In Vitro digestibility and fermentability of fructo-oligosaccharides produced by Aspergillus ibericus. J Funct Foods. 2018;46(July):278–287. doi: 10.1016/J.JFF.2018.05.004. [DOI] [Google Scholar]
- Patindol JA, Siebenmorgen TJ, Wang Y-J. Impact of environmental factors on rice starch structure: a review. Starch Stärke. 2015;67(1–2):42–54. doi: 10.1002/star.201400174. [DOI] [Google Scholar]
- Pérez S, Bertoft E. The molecular structures of starch components and their contribution to the architecture of starch granules: a comprehensive review. Starch Stärke. 2010;62(8):389–420. doi: 10.1002/star.201000013. [DOI] [Google Scholar]
- Ramos PE, Abrunhosa L, Pinheiro A, Cerqueira MA, Motta C, Castanheira I, Chandra-Hioe MV, Arcot J, Teixeira JA, Vicente AA. Probiotic-loaded microcapsule system for human in situ folate production: encapsulation and system validation. Food Res Int. 2016;90(December):25–32. doi: 10.1016/J.FOODRES.2016.10.036. [DOI] [PubMed] [Google Scholar]
- Ren X, Chen J, Molla MM, Wang C, Diao X, Shen Q. In Vitro starch digestibility and in vivo glycemic response of foxtail millet and its products. Food Function. 2016;7(1):372–379. doi: 10.1039/c5fo01074h. [DOI] [PubMed] [Google Scholar]
- Rodrigues DB, Chitchumroonchokchai C, Mariutti Lilian R B, Mercadante AZ, Failla ML. Comparison of two static in vitro digestion methods for screening the bioaccessibility of carotenoids in fruits, vegetables, and animal products. J Agric Food Chem. 2017;65(51):11220–11228. doi: 10.1021/acs.jafc.7b04854. [DOI] [PubMed] [Google Scholar]
- Roy P, Ijiri T, Okadome H, Nei D, Orikasa T, Nakamura N, Shiina T. Effect of processing conditions on overall energy consumption and quality of rice (Oryza Sativa L.) J Food Eng. 2008;89(3):343–348. doi: 10.1016/j.jfoodeng.2008.05.015. [DOI] [Google Scholar]
- Santos T, Lemos D, Guiné RPF, Correia PR (2014) Biometric characteristics of rice cultivars. In VII Congreso Ibérico de Agroingenieria y Ciencias Hortícolas: Innovar y Producir para el Futuro. Madrid. F. G. UPM, ed.:174-179. ISBN:84-695-9055-3
- Srikaeo K, Sangkhiaw J. Effects of amylose and resistant starch on glycaemic index of rice noodles. LWT Food Sci Technol. 2014;59(2):1129–1135. doi: 10.1016/j.lwt.2014.06.012. [DOI] [Google Scholar]
- Tamura M, Singh J, Kaur L, Ogawa Y. Impact of structural characteristics on starch digestibility of cooked rice. Food Chem. 2016;191:91–97. doi: 10.1016/j.foodchem.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Tamura M, Okazaki Y, Kumagai C, Ogawa Y. The importance of an oral digestion step in evaluating simulated in vitro digestibility of starch from cooked rice grain. Food Res Int. 2017;94(April):6–12. doi: 10.1016/j.foodres.2017.01.019. [DOI] [PubMed] [Google Scholar]
- Tester RF, Qi X, Karkalas J. Hydrolysis of native starches with amylases. Anim Feed Sci Technol. 2006;130(1–2):39–54. doi: 10.1016/J.ANIFEEDSCI.2006.01.016. [DOI] [Google Scholar]
- Topping DL, Fukushima M, Bird AR. Resistant starch as a prebiotic and synbiotic: state of the art. Proc Nutr Soc. 2003;62(01):171–176. doi: 10.1079/PNS2002224. [DOI] [PubMed] [Google Scholar]
- van de Velde F, van Riel J, Tromp RH. Visualisation of starch granule morphologies using confocal scanning laser microscopy (CSLM) J Sci Food Agric. 2002;82(13):1528–1536. doi: 10.1002/jsfa.1165. [DOI] [Google Scholar]
- Whitcomb DC, Lowe ME. Human pancreatic digestive enzymes. Dig Dis Sci. 2007;52(1):1–17. doi: 10.1007/s10620-006-9589-z. [DOI] [PubMed] [Google Scholar]
- Woolnough JW, Bird AR, Monro JA, Brennan CS. The effect of a brief salivary α-amylase exposure during chewing on subsequent in vitro starch digestion curve profiles. Int J Mol Sci. 2010;11(8):2780–2790. doi: 10.3390/ijms11082780. [DOI] [PMC free article] [PubMed] [Google Scholar]