Abstract
The effects of dynamic ultra-high pressure homogenization (UHPH) on the structure and functional properties of whey protein were investigated in this study. Whey protein solution of 10 mg/mL (1% w/w) was prepared and processed by a laboratory scale high pressure homogenizer with different pressures (25, 50, 100, 150, 200, and 250 MPa) at an initial temperature of 25 °C. Then, the solution samples were evaluated in terms of secondary structure, sulfhydryl and disulfide bond contents, surface hydrophobicity, average particle size, solubility, foaming capacity, emulsifying activity, and thermal properties. It was found that the secondary structure of whey protein changed with the dynamic UHPH treatment. The interchange reaction between the disulfide bond and the sulfhydryl group was promoted and the surface hydrophobicity significantly increased. The functional properties of the whey protein accordingly changed. Specifically, after dynamic UHPH treatment, the average particle size of the whey protein and emulsion decreased while the solubility, the foaming capability and the emulsification stability increased significantly. The results also revealed that with the dynamic UHPH at 150 MPa, the best improvement was observed in the whey protein functional properties. The whey protein solubility increased from 63.15 to 71.61% and the emulsification stability improved from 195 to 467 min.
Keywords: Ultra-high pressure homogenization, Whey protein, Structure, Functional properties
Introduction
Proteins are the major structural and functional components of foods such as meat, cheese, egg whites, and most cereals. With the development of the food processing industry, the proteins are increasingly applied in processed foods such as protein drinks and puffed foods (Mao and Hua 2014), to improve their functions and meat processing needs.
Whey protein isolate (WPI, protein content ≥ 90%) and whey protein concentrate (WPC, protein content 60–85%) are the two most commonly used whey proteins due to their high nutritional and excellent processing characteristics (Blayo et al. 2016). The main components of whey protein include β-lactoglobulin (56–60%), α-lactalbumin (18–24%), serum albumin (6–12%), immunoglobulins (6–12%), lactoferrin, growth factors, and peroxidase. Whey protein is a high-quality complete protein and an effective source of active peptides. In order to meet the various needs of processing, whey protein is commonly modified using enzymolysis, fractionation and heat treatment (Lin et al. 2017; Wu et al. 2019).
It has been proposed that the non-thermal processing technology could be used to selectively change the structures and functions of proteins, producing proteins with specific functions and meet the needs for different food processing (Zhang and Haque 2015; Corzomartínez et al. 2015). Dynamic ultra-high pressure homogenization (UHPH) is a new type of physical modification technology offering strong shear, high-speed impact, intense vibration, and sudden release of pressure to change the structure and functional properties of biological macromolecules, such as protein and starch (Hua et al. 2017; Lin et al. 2019a, b). It has been applied in soy protein, peanut globulin, and casein (Zhang and Haque 2015; Corzomartínez et al. 2015). In addition, UHPH has been used for the modification of native whey protein, by inducing protein aggregation or changing proteolysis efficiency (Blayo et al. 2016). Calligaris et al. (2018) reported that the combinations of ultrasound and high-pressure homogenization can improve the emulsification stability of whey protein. Yan et al. (2017) reported that increasing homogenization pressures (25–100 MPa) significantly decreased the particle sizes of whey protein (10% w/w). However, there are no systematic reports on the application of UHPH in changing the conformation structures and improving the functional properties of whey protein. In this study, the UHPH technology was used to process whey proteins and the changes of their structures and functional properties were also investigated. The purpose of this study is to improve the functional properties of whey protein and broaden its application fields, and provide references for the application of dynamic UHPH treatment in the ultra-micro emulsifying and enzymatic hydrolysis of proteins.
Materials and methods
Chemicals
Whey protein isolate (WPI, protein content ≥ 90%) was purchased from Yuanye Biotechnology Co., Ltd (Shanghai, China). Trichloroacetic acid (TCA), glycine, Tris, 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), 8-anilino-1-naphthalene-sulfonate (ANS), and anhydrous ethanol were from Kemiou Chemical Reagent Co., Ltd (Tianjin, China). β-mercaptoethanol was obtained from Best Reagent Co., Ltd (Chengdu, China).
Preparation of whey protein solution
Whey protein solution at the final concentration of 10 mg/mL (1% w/w) was prepared with deionized water and incubated at 30–40 °C in a water bath for 5 min. Then the protein solution was homogenized with a sterile homogenizer (Ningbo Xinzhi Biotech Co., Ltd., China) for 2 min at mid-range, to make the whey proteins completely dissolved. Finally, the fluid was treated with FPG12805 high pressure homogenizer (SFP Inc.UK) for 1 cycle at 25, 50, 100, 150, 200, and 250 MPa, respectively (room temperature, feed volume is 10 mL), and the final temperature of whey protein solution after the cycle was recorded. The operating principles of the homogenizer were described in Floury et al. (2002). Every measurement was carefully sampled for immediately analysis, and each WPI solution treated by UHPH was prepared at least twice (the homogenisation series had been done 3 times in this research) to ensure processing repeatability.
Measurement of secondary structure
The secondary structure of whey protein in the solution was measured using a Chirascan circular dichroic spectrometer (Applied Photophysics Ltd.) (Jambrak et al. 2008). Deionized water served as a blank control. A quartz cuvette with 0.1 cm of pathlength was used. The samples were scanned from 190 to 250 nm at a scanning speed of 50 nm/min, resolution of 0.2 nm, slit width of 1.0 nm, sensitivity of 20 mdeg, and response time of 0.25 s. The contents of secondary structure conformational units such as α-helix, β-sheet, β-turn, and random coil of whey protein were analyzed by the spectrometer software. Each sample was measured in triplicate.
Determination of sulfhydryl group and disulfide contents in whey protein solution
Free sulfhydryl group (SHF) content in whey protein solution was measured as in a previous report (Friedman 1994). Briefly, 0.5 mL of the homogenized whey protein solution was mixed with 2.5 mL of Tris–glycine and 8 M urea solution (0.086 M Tris, 0.09 M glycine, 0.004 M EDTA and 8 M urea) and 0.02 mL of 5,5′-dithiobis-2-nitrobenzoic acid solution (DTNB, 4 mg/mL). The mixture was incubated at 25 °C for 30 min and its absorbance was measured at 412 nm (A412). The mixture without protein solution served as the blank control.
Total sulfhydryl group (SHT) content in whey protein solution was measured by a previously reported method (Friedman 1994). In short, 0.2 mL of protein solution was mixed with 1.0 mL of Tris–glycine and 10 M urea solution (0.086 M Tris, 0.09 M glycine, 0.004 M EDTA and 10 M urea) and 0.02 mL of β-mercaptoethanol and incubated at 25 °C for 1 h. Then 10 mL of 12% TCA was added to the mixture. The mixture was incubated for another 1 h and centrifuged at 3000 rpm for 10 min. The pellet was washed with 12% TCA solution in duplicate and then mixed with 3 mL of Tris–glycine and 8 M urea solution and 0.03 mL of DTNB solution (4 mg/mL) at 25 °C for 30 min. The absorbance at 412 nm (A412) of the mixture was measured. The mixture without protein solution served as the blank control. The sulfhydryl and disulfide bond contents in whey protein solution were calculated using the following equations:
| 1 |
| 2 |
where 73.53 is 106/(1.36 × 104) and 1.36 × 104 is the molar extinction coefficient of Ellman’s reagent; and C is the protein concentration of the sample in mg/mL.
Surface hydrophobicity determination
The surface hydrophobicity of whey protein solution was measured using the fluorescent probe 8-anilino-1-naphthalene-sulfonate (ANS) method (Zhao et al. 2012). Protein solutions at concentrations of 0.005%, 0.01%, 0.02%, 0.05%, 0.1%, and 0.2% and 8.0 mM ANS solution were prepared using deionized water. Protein solution (4 mL) was mixed with 20 μL of ANS solution and then the fluorescence intensity of the mixture (FI’) was rapidly measured on a Cary Eclipse fluorescence spectrophotometer (Agilent, USA). A mixture without ANS solution served as a control, and its fluorescence intensity was recorded as FI0. The excitation wavelength and the emission wavelength used here were 338 nm and 496 nm, respectively. FI = FI′ − FI0. A graph was made with the concentrations of protein as abscissa and FI as ordinate. The slope of the curve is the surface hydrophobicity index H0 of whey protein.
Particle size distribution of whey protein emulsion
The particle size distribution (mean particle size, polydispersity index) was measured using the dynamic laser light scattering (DLS) method (Zhang et al. 2017). The particle size of the homogenized whey protein emulsion solution was measured with a Zetasizer ZS90 nanoparticle size meter (Malvern Instruments Ltd., UK) in triplicate.
Measurement of whey protein solubility
The total protein contents in the treated solutions were measured using the Kjeldahl method (Le et al. 2011). The solutions were centrifuged at 10,000 rpm for 15 min to remove insoluble proteins. The whey protein content in the supernatant was measured by using a Kjeltec TM8400 automatic Kjeldahl apparatus (FOSS, Denmark). The percentage of whey protein to total protein in the supernatant was calculated to evaluate the protein’s solubility in the solution. The formula used was as follows:
| 3 |
Determination of whey protein foaming capability
Whey protein foaming capability was measured according to a previous report (Yin et al. 2008), i.e., 100 mL of the treated whey protein solution was dispersed with a high-speed dispersion homogenizer at 9500 rpm for 2 min. The volume of foam was recorded at 0 min (V0) and again at 30 min (V30) in a graduated cylinder. The foaming capability and foaming stability were calculated using the following formulas.
| 4 |
| 5 |
Determination of whey protein emulsifying activity
The method used in this study was modified from a previous report (Liu et al. 2015). Briefly, 12 mL of dynamic UHPH-treated protein solution was mixed with 4 mL of soybean oil and emulsified with an IKAT25 Basic High Speed Disperser (IKA, Germany) at 13,500 rpm for 2 min. The emulsion (50 μL) was immediately taken from the bottom of the beaker and mixed with 5 mL of SDS solution (0.1 g/100 mL). The absorbance of the mixed solution was measured at 500 nm at 0 min (A0) and again at 30 min (A30). The emulsifying activity could be calculated based on the following equations.
| 6 |
| 7 |
where T = 2.303; EA is emulsifying activity, m2/g; ES is emulsion stability, min; c is the pre-emulsion protein concentration, g/mL; φ is optical path (0.01); and θ is the volume fraction of oil in emulsion (0.25).
Thermal properties assessment
The thermal properties of whey protein were analyzed with a Q200 differential scanning calorimeter (TA Company, USA) (Lin et al. 2019a, b). The instrument was calibrated with indium. The treated whey protein solutions were lyophilized and stocked into aluminum sample trays with sealed cap. An empty aluminum sample tray served as a blank control. The samples were scanned with a differential scanning calorimeter at the range of 30–100 °C, scanning speed of 10 °C/min, and nitrogen was used as a carrier gas. Their DSC curves were obtained. The denaturation temperature and changes in enthalpy were calculated to determine the thermal stability and degree of aggregation of whey protein samples.
Statistics analysis
All the treatments were carried out with three independent repetitions. Statistics analysis was performed using IBM SPSS 17.0 (Armonk, NY, USA) and Origin 8.0 (Northampton, MA, USA). The data were expressed as mean ± standard deviation.
Results and discussion
Effect of homogenization pressure on the temperature of whey protein
When UHPH is used, the temperatures of whey protein solution rise because of high shear. There is a relationship between the rising temperature and the homogeneous pressure level. The temperature of whey protein solution increased linearly from 32 to 52 °C with the increase of pressure from 25 to 200 MPa. A large amount of heat could be generated when the protein emulsion was forced to pass through the homogenization valve under ultra high pressure produced by the pump (Blayo et al. 2016). However, with the further increase of homogeneous pressure, the change of temperature tends to be gentle, and the difference between 200 and 250 MPa was not significant, which indicated that the increase of pressure could lead to higher material temperature within a certain range, which revealed that the higher pressure could not result in the greater change of temperature. It was also reported that the temperature of casein solution could be controlled below 55 °C by dynamic UHPH treatment (< 250 MPa) (Wang et al. 2019). Compared with the traditional thermal processing, it is more moderate and energy-saving (Dissanayake et al. 2013).
Effect of homogenization pressure on the secondary structure of whey protein
α-helix is the most common and stable secondary structure in proteins. The results shown in Table 1 suggested that the α-helix content in whey protein first increased and then decreased significantly (P < 0.05) with the rise of homogenization pressure. This slight increase of α-helix content is probably due to the complete solubilisation of whey protein under the UHPH treatment (< 50 MPa), and its reduction probably because of the protein denaturation. The dynamic UHPH (≥ 50 MPa) affects the stability of intramolecular hydrogen bonds of protein, and the H-bond is important for α-helix stabilization (Sano et al. 2010). It is suggested that α-helix is sensitive to pressure and the UHPH treatment can change the protein structure. β-sheet is typical structure of conversion from globule to fibers and probably accompanies the occur of protein denaturation (Zhao et al. 2012). In this study, the total contents of β-sheet in whey protein first decreased (P > 0.05) and then increased significantly (P < 0.05), while a significant (P < 0.05) reduction was found in β-turn contents, as the homogenization pressure increased, suggesting that the protein was dissociated and re-polymerized. However, the percentage of random coil in whey protein showed no significant differences (P > 0.05) between different UHPH treatment groups. The whole results suggested that the dynamic high-pressure homogenization disrupted the secondary structure of whey protein and then changed the conformation of the protein molecule, which consequently affects the protein’s tertiary and quaternary structure and function.
Table 1.
Influence of pressure level on secondary structure content in whey protein
| Sample (MPa) | α-helix (%) | β-sheet (%) | β-turn (%) | Random coil (%) |
|---|---|---|---|---|
| 0 | 14.13 ± 0.31ab | 26.12 ± 0.33ef | 17.31 ± 0.51a | 43.22 ± 0.30a |
| 25 | 14.75 ± 0.14a | 25.83 ± 0.41f | 16.83 ± 0.46b | 43.12 ± 0.27a |
| 50 | 14.44 ± 0.22ab | 26.87 ± 0.25e | 16.70 ± 0.39b | 43.43 ± 0.43a |
| 100 | 14.01 ± 0.17b | 28.41 ± 0.41d | 15.65 ± 0.42c | 43.61 ± 0.10a |
| 150 | 13.76 ± 0.29bc | 30.19 ± 0.31c | 14.3 ± 0.35d | 43.33 ± 0.23a |
| 200 | 13.52 ± 0.18bc | 31.92 ± 0.19b | 13.13 ± 0.45e | 43.59 ± 0.19a |
| 250 | 13.11 ± 0.26c | 32.95 ± 0.25a | 12.81 ± 0.36f | 43.64 ± 0.07a |
a–f Different letters indicate statistically significant differences (P < 0.05) within columns
Effect of homogenization pressure on whey protein sulfhydryl groups and disulfide bonds
Sulfhydryl group (–SH) and disulfide (–S–S) are important functional groups in proteins and play an important role in protein functions, such as gel formation and protein film formation. The results in Fig. 1 showed that sulfhydryl (–SH) content in whey protein first increased and then decreased, while the disulfide (–S–S) content first decreased and then increased, as the homogenization pressure increased. Some studies also reported that certain pressure treatments on protein can cause mutual conversion between sulfhydryl groups and disulfide bonds, which loosens protein structure and promotes protein denaturation (Friedman 1994). The –SH and –S–S content in whey protein changed after UHPH treatment, which was mainly due to intramolecular non-covalent bonds cleavage (Sen et al. 2002). The spatial structure of the homogenized protein was destroyed under high pressure, causing peptide chains to unfold and disulfide bonds to break. And then, the broken disulfide bonds were reduced to sulfhydryl groups, which increases the sulfhydryl content in the protein solution. While, this value (–SH content) declined above 100 MP (Fig. 1). Excessively high pressure can over-expose sulfhydryl groups, which can further oxidize some sulfhydryl groups to disulfide, and inappropriate disulfide bond formation will consolidate protein aggregation at high pressure. These results suggested that UHPH treatment may denature the whey protein and trigger the –SH–S–S interchange reaction and the variations in –SH and –S–S content may also cause variations in the properties (unfold, aggregation and so on) of whey protein. As reported by Xi and He (2018), a reduction of –SH content indicated cross-linking effect on protein, whereas an increase usually indicates protein unfolding and backbone fragmentation.
Fig. 1.

Effect of pressure level on the content of sulfhydryl group (SH) and disulfide bond (S–S) of whey protein
Effect of homogenization pressure on surface hydrophobicity of whey protein
Hydrophobicity is important to maintain protein tertiary structure, which determines the protein stability, conformation, and functions. In aqueous media non-polar amino acid residues tend to be oriented toward the inside of a protein molecule because of the hydrophobic interactions. This minimizes the direct contact with water. However, hydrophobic interactions can still occur on the surface of protein molecules, rendering the protein surface hydrophobic. The results in Fig. 2a showed that the surface hydrophobicity of homogenized whey protein solution tended to decrease and then significantly (P < 0.05) increase with the rise of pressure. Before the whey protein is homogenized (in natural state), most of the non-polar side chains of amino acid residues become embedded in the inner region of protein molecules and form a stable hydrophobic core, while most of the polar side chains of amino acid residues distribute on the surface of proteins to ensure the stability of water molecule interaction in hydrophilic environment (Riebroy et al. 2009). However, the UHPH treatment with pressure over 150 MPa made whey protein solutions to be homogeneous with smaller particle sizes (refer to Fig. 2b), leading to larger surface area, exposing more hydrophilic regions, and then reduced the surface hydrophobicity. In addition, the UHPH treatment with pressure less than 150 MPa made the spatial structure of whey protein become less compact. The protein molecules were dissociated and unfolded, hydrophobic side chains were redistributed, so that more amino acid residues of non-polar were exposed to the surface of protein, and the surface hydrophobicity of protein was increased.
Fig. 2.
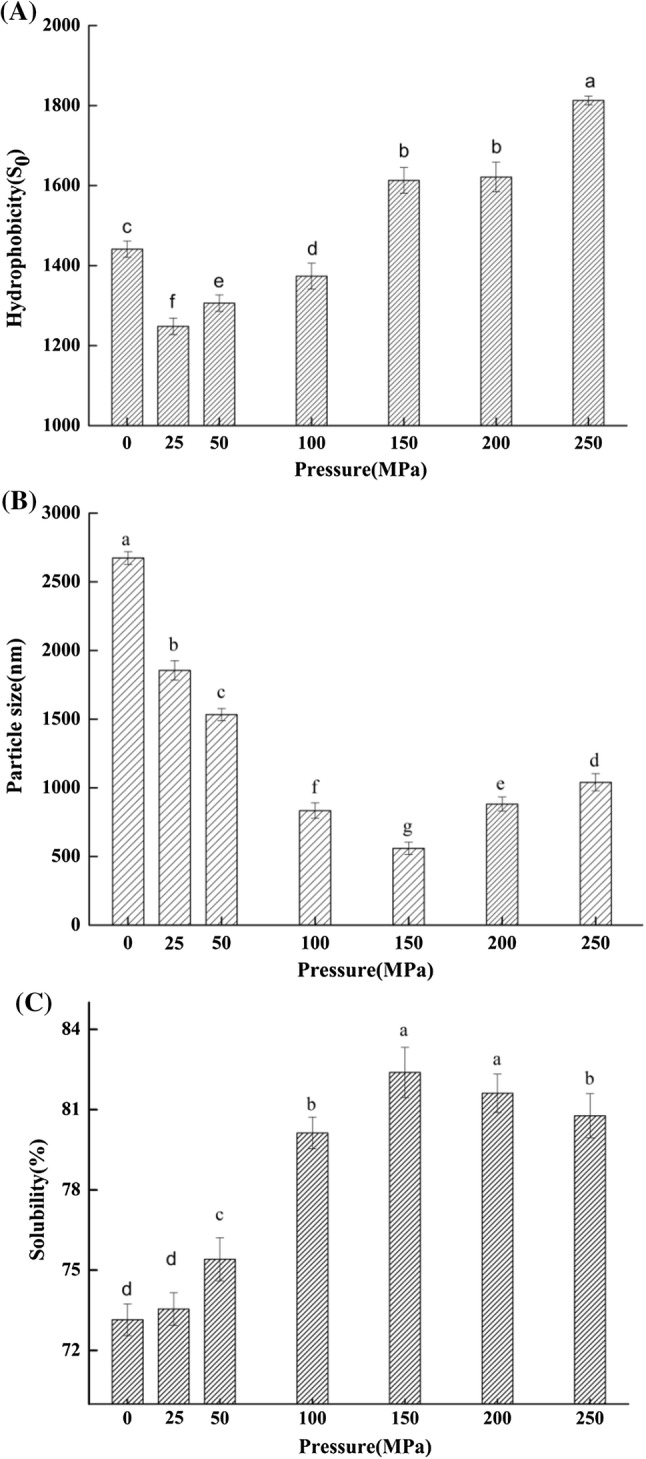
Effect of pressure level on the hydrophobicity (a), average particle size (b) and solubility (c) of whey protein [a–f Different letters indicate statistically significant differences (P < 0.05)]
Effect of homogenization pressure on particle size of whey protein emulsion
The results shown in Fig. 2b indicated that the average particle size of emulsion declined and then raised significantly (P < 0.05) as the pressure increased. The emulsion had the lowest particle size when it was treated at 150 MPa. The results suggested that high homogenization pressure (less than 150 MPa) can greatly reduce the average particle size of the whey protein emulsion. Under high-pressure homogenization, the average particle size of whey protein solution decreased significantly (P < 0.05) after dynamic UHPH treatment, because whey protein was subjected to shear, high-speed impact, violent vibration, pressure instantaneous release and other dynamic effects in the high pressure reaction chamber. As a result, the protein particles were smashed and its aggregates were destroyed, thus reducing the particle size, rendering the solution more homogeneous (Ding and Kan 2017). The whey protein particles are disrupted more severely as the homogenization pressure increases. However, due to the thermal effect (when higher homogeneous pressure is used, temperatures rise because of high shear), the broken protein particles become agglomerated again to form some large particles, resulting in the significant (P < 0.05) increase of the average particle size of the emulsion (Sørensen et al. 2015).
Effect of homogenization pressure on the aqueous solubility of whey protein
Aqueous solubility is the first necessary functional property of the protein, which is the basis for the protein’s other functions and an important manifestation of protein hydration. The effect of dynamic UHPH treatment on the aqueous solubility of whey protein is shown in Fig. 2c. The solubility first increased significantly (P < 0.05) then decreased as the pressure increased. The possible reason (refer to Fig. 2b) is that when the whey protein is treated by UHPH in the reaction chamber with various kinetic actions, smaller protein particles are generated, which increases the contact area between the protein and water. Also, when the protein is homogenized at high pressure, polymerized chains of whey protein are disrupted, which consequently increases the solubility of whey protein (Cheng et al. 2014). However, when a certain pressure is applied, not only the polymerized chains of whey protein are disrupted, but also most of the hydrophobic structures and a few hydrophilic structures that have been hidden in the interior of the protein are exposed (refer to Fig. 2a). Then, the hydrophobicity of whey protein was enhanced, and its binding with water was weakened. As a result, the aqueous solubility of whey protein decreased at the high homogenization pressure. The similar phenomenon was also found by the previous report (Oliveira et al. 2015).
Effect of homogenization pressure on whey protein foaming capacity
The foaming capacity of a component is its ability to form a thin film at the gas–liquid interface to incorporate air and stabilize the foam, including foaming capacity and foaming stability. The changes in whey protein foaming capacity and foaming stability at different pressure levels are shown in Fig. 3. The results indicated that the foaming capacity of whey protein first increased and then decreased as the pressure increased, while the foaming stability showed a downward-upward-downward trend as homogenization pressure increased, which is consistent with the results reported by Plancken et al. (2007). Protein molecules are typical amphiphilic structures, and they exhibit strong interfacial activity at the gas–liquid interface. This helps to decrease the interfacial tension of protein solution and promote foaming. Because dynamic UHPH treatment increases the solubility of whey protein and reduces the interfacial tension, the processing method consequently increases the foaming of whey protein. High-pressure homogenization also opens protein structures, enhancing foam formation. However, homogenization at extremely high pressure destroys the balance of the hydrophobic and hydrophilic groups and the charged polar groups in the whey protein solutions, which destroys the balance of the protein membrane at the gas–water interface and suppresses protein foam formation and maintenance (Ding and Kan 2017). So the foaming stability of whey protein decreased sharply from 200 MPa.
Fig. 3.
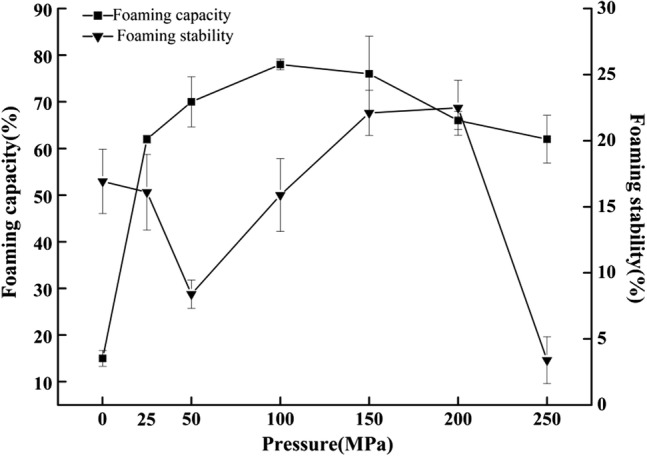
Effect of pressure level on foaming capacity and foaming stability of whey protein
Effect of homogenization pressure on the whey protein emulsifying activity
Protein emulsifying activity (EA) is the ability of a protein to reduce the interfacial tension of the oil–water surface and combine the oil and water into an emulsion (Fasinu et al. 2015). Emulsion stability (ES) is the ability of a protein to maintain the emulsion without allowing the oil and water phrases to separate (Perreault et al. 2017). EA and ES of the homogenized whey protein were evaluated at different pressures. The results shown in Fig. 4 indicated that whey protein EA first decreased and then increased as homogenization pressure increased. Whey protein showed the highest EA under normal homogenization pressure. While the ES of homogenized whey protein decreased at the beginning, increased later, and then decreased again. The whey protein homogenized at 150 MPa exhibited the best ES. Because of the interactions between the mechanical and thermal effects during homogenization, the flexibility of protein molecular chain decreased and proteins were partially denatured. As a result, it is not easy to combine the protein and oil, which would decrease the protein’s EA. ES is related to time and particle size of the emulsion. The smaller the size of the particles in the emulsion, the greater stability of the emulsion could be obtained. High-pressure homogenization reduces the particle size of the emulsion (refer to Fig. 2b) and renders the solution homogeneous, which increases the ES. However, the aggregation of large particles in the solution under much higher pressure disrupts the inter-molecular forces in the protein emulsion, rendering it less stable.
Fig. 4.
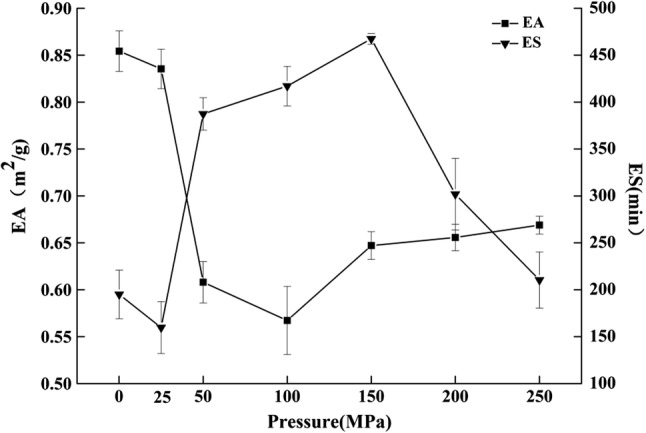
Effect of pressure level on emulsifying ability and emulsion stability of whey protein
Effect of homogenization pressure on the thermal properties of whey protein
Heat denaturation is a coordinated process, in which heat is absorbed when protein is denatured. The initial denaturation temperature and the peak temperature indicate the thermal stability of the protein, and the endothermic enthalpy is the amount of heat needed for protein denaturation (Zhang et al. 2009). The results shown in Table 2 indicated that all of these three evaluation parameters first decreased (P < 0.05) and then increased significantly (P < 0.05) with the increase of homogenization pressure. When the homogenization pressure was 150 MPa, all three parameters reached the minimum, i.e., the initial denaturation temperature was 48.13 °C, the peak temperature was 69.16 °C, and the endothermic enthalpy was 103.12 J g−1. The three parameters of whey protein began to decline with the increase of homogenization pressure to some extent, indicating that the dynamic high-pressure homogenization reduced the thermal stability of whey protein and the heat needed for whey protein denaturation. The possible reasons for this may be that high-pressure homogenization loosens protein structure, changes protein conformation, and disrupts some interaction forces between molecules (Kitabatake and Doi 2015). However, the thermal stability and endothermic enthalpy of homogenized whey protein increased when the pressure increased further, but still relatively lower than the untreated ones. This is likely due to the inappropriate formation of –S–S bond which stabilizes protein aggregates (refer to Fig. 1). In summary, moderate dynamic high-pressure (150 MPa) homogenization reduces the thermal stability of whey protein and the energy required for protein denaturation.
Table 2.
Influence of pressure level on thermal property of whey protein
| Pressure (MPa) | Denaturation initial temperature (°C) | Peak temperature (°C) | Endothermic enthalpy ΔH (J g−1) |
|---|---|---|---|
| 0 | 59.72 ± 1.35a | 92.03 ± 2.61a | 231.71 ± 7.32a |
| 25 | 54.62 ± 2.68b | 81.93 ± 2.26b | 162.73 ± 5.57c |
| 50 | 51.27 ± 1.97c | 75.79 ± 1.77c | 141.68 ± 4.68d |
| 100 | 50.46 ± 1.15c | 72.66 ± 2.48d | 129.46 ± 5.41e |
| 150 | 48.13 ± 1.53d | 69.16 ± 2.07e | 103.12 ± 3.36f |
| 200 | 50.93 ± 2.29c | 76.56 ± 2.93c | 129.55 ± 4.15e |
| 250 | 53.46 ± 3.97b | 81.54 ± 1.17b | 169.81 ± 5.56b |
a–f Different letters indicate statistically significant differences (P < 0.05) within columns
Conclusion
In summary, the dynamic UHPH treatment had significant effects on the structure of whey protein. The secondary structure of whey protein changed with the dynamic UHPH treatment. The interchange reaction between the disulfide bond and the sulfhydryl group was also promoted and the surface hydrophobicity significantly increased. As a result, the solubility, foaming capacity, emulsifying activity, and thermal properties of whey protein was obviously affected. It was found that that whey protein homogenized at 150 MPa had the best solubility, foaming capacity, emulsifying activity, and thermal properties. Its solubility increased from 63.15 to 71.61%, emulsification stability improved from 195 to 467 min, initial denaturation temperature decreased from 59.72 to 48.13 °C, peak temperature decreased from 92.03 to 69.16 °C, and endothermic enthalpy decreased from 231.71 to 103.12 J g−1. In these ways, dynamic high-pressure homogenization can affect whey protein’s structure and improve its functional properties. The obtained results can promote the applications of dynamic UHPH treatment in the ultra-micro emulsifying and enzymatic hydrolysis of proteins.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 31571912) and the Major Science and Technology Project in Henan (No. 161100110600).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Blayo C, Vidcoqauthor O, Dumayauthor E. Effects of high pressure processing (hydrostatic high pressure and ultra-high pressure homogenisation) on whey protein native state and susceptibility to tryptic hydrolysis at atmospheric pressure. Food Res Int. 2016;79:40–53. doi: 10.1016/j.foodres.2015.11.024. [DOI] [Google Scholar]
- Calligaris S, Plazzotta S, Valoppi F, Anese M. Combined high-power ultrasound and high-pressure homogenization nanoemulsification: the effect of energy density, oil content and emulsifier type and content. Food Res Int. 2018;107:700–707. doi: 10.1016/j.foodres.2018.03.017. [DOI] [PubMed] [Google Scholar]
- Cheng J, Ren W, Wang S, Zhang Z, Zhao W, College F. Effect of physical treatments on soluble protein of highly denatured defatted soy flakes. J Food Sci Technol. 2014;45:1253–1260. [Google Scholar]
- Corzomartínez M, Mohan M, Dunlap J, Harte F. Effect of ultra-high pressure homogenization on the interaction between bovine casein micelles and ritonavir. Pharm Res. 2015;32:1055–1071. doi: 10.1007/s11095-014-1518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Kan J. Optimization and characterization of high pressure homogenization produced chemically modified starch nanoparticles. J Food Sci Technol. 2017;54:4501–4509. doi: 10.1007/s13197-017-2934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake M, Ramchandran L, Piyadasa C, Vasiljevic T. Influence of heat and pH on structure and conformation of whey proteins. Int Dairy J. 2013;28:56–61. doi: 10.1016/j.idairyj.2012.08.014. [DOI] [Google Scholar]
- Fasinu EG, Ikhu-Omoregbe DIO, Jideani VA. Influence of selected physicochemical factors on the stability of emulsions stabilized by bambara groundnut flour and starch. J Food Sci Technol. 2015;52:7048–7058. doi: 10.1007/s13197-015-1818-z. [DOI] [Google Scholar]
- Floury J, Desrumaux A, Axelos MAV, Legrand J. Degradation of methylcellulose during ultra-high-pressure homogenization. J Food Hydrocol. 2002;16:47–53. doi: 10.1016/S0268-005X(01)00039-X. [DOI] [Google Scholar]
- Friedman M. Improvement in the safety of foods by sulfhydryl-containing amino acids and peptides. A review. J Agric Food Chem. 1994;42:3–20. doi: 10.1021/jf00037a002. [DOI] [Google Scholar]
- Hua X, Xu S, Wang M, Chen Y, Yang H, Yang R. Effects of high-speed homogenization and high-pressure homogenization on structure of tomato residue fibers. Food Chem. 2017;232:443–449. doi: 10.1016/j.foodchem.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Jambrak AR, Mason TJ, Lelas V, Herceg Z, Herceg IL. Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J Food Eng. 2008;86:281–287. doi: 10.1016/j.jfoodeng.2007.10.004. [DOI] [Google Scholar]
- Kitabatake N, Doi E. Surface tension and foaming of protein solutions. J Food Sci. 2015;47:1218–1221. doi: 10.1111/j.1365-2621.1982.tb07651.x. [DOI] [Google Scholar]
- Le TT, Bhandari B, Holland JW, Deeth HC. Maillard reaction and protein cross-linking in relation to the solubility of milk powders. J Agric Food Chem. 2011;59:12473–12479. doi: 10.1021/jf203460z. [DOI] [PubMed] [Google Scholar]
- Lin D, Zhou W, Zhao J, Lan W, Chen R, Li Y. Study on the synthesis and physicochemical properties of starch acetate with low substitution under microwave assistance. Int J Biol Macromol. 2017;103:316–326. doi: 10.1016/j.ijbiomac.2017.05.056. [DOI] [PubMed] [Google Scholar]
- Lin D, Zhou W, He Q, Xing B, Wu Z, Chen H. Study on preparation and physicochemical properties of hydroxypropylated starch with different degree of substitution under microwave assistance. Int J Biol Macromol. 2019;125:290–299. doi: 10.1016/j.ijbiomac.2018.12.031. [DOI] [PubMed] [Google Scholar]
- Lin D, Zhou W, Yang Z. Study on physicochemical properties, digestive properties and application of acetylated starch in noodles. Int J Biol Macromol. 2019;128:948–956. doi: 10.1016/j.ijbiomac.2019.01.176. [DOI] [PubMed] [Google Scholar]
- Liu LL, Wang H, Ren GY, Xu D, Dan L, Yin GJ. Effects of freeze-drying and spray drying processes on functional properties of phosphorylation of egg white protein. Int J Agric Biol Eng. 2015;8:116–123. [Google Scholar]
- Mao XY, Hua YF. Chemical composition, molecular weight distribution, secondary structure and effect of nacl on functional properties of walnut (Juglans regia L.) protein isolates and concentrates. J Food Sci Technol. 2014;51:1473–1482. doi: 10.1007/s13197-012-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CF, Corrêa AP, Coletto D, Daroit DJ, Cladera-Olivera F, Brandelli A. Soy protein hydrolysis with microbial protease to improve antioxidant and functional properties. J Food Sci Technol. 2015;52:2668–2678. doi: 10.1007/s13197-014-1317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault V, Hénaux L, Bazinet L, Doyen A. Pretreatment of flaxseed protein isolate by high hydrostatic pressure: impacts on protein structure, enzymatic hydrolysis and final hydrolysate antioxidant capacities. Food Chem. 2017;221:1805–1812. doi: 10.1016/j.foodchem.2016.10.100. [DOI] [PubMed] [Google Scholar]
- Plancken IVD, Loey AV, Hendrickx ME. Foaming properties of egg white proteins affected by heat or high pressure treatment. J Food Eng. 2007;78:1410–1426. doi: 10.1016/j.jfoodeng.2006.01.013. [DOI] [Google Scholar]
- Riebroy S, Benjakul S, Visessanguan W, Erikson U, Rustad T. Acid-induced gelation of natural actomyosin from atlantic cod (Gadus morhua) and burbot (Lota lota) J Food Hydrocoll. 2009;23:26–39. doi: 10.1016/j.foodhyd.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Sano T, Ohno T, Otsukafuchino H, Matsumoto JJ, Tsuchiya T. Carp natural actomyosin: thermal denaturation mechanism. J Food Sci. 2010;59:1002–1008. doi: 10.1111/j.1365-2621.1994.tb08177.x. [DOI] [Google Scholar]
- Sen M, Kopper R, Pons L, Abraham EC, Burks AW, Bannon GA. Protein structure plays a critical role in peanut allergen stability and may determine immunodominant IgE-binding epitopes. J Immunol. 2002;169:882–887. doi: 10.4049/jimmunol.169.2.882. [DOI] [PubMed] [Google Scholar]
- Sørensen H, Mortensen K, Sørland GH, Larsen FH, Paulsson M, Ipsen R. Dynamic ultra-high pressure homogenisation of whey protein-depleted milk concentrate. J Int Dairy J. 2015;46:12–21. doi: 10.1016/j.idairyj.2014.09.012. [DOI] [Google Scholar]
- Wang CY, Ma YP, Liu BG, Kang ZL, Geng S, Wang JN. Effects of dynamic ultra-high pressure homogenization on the structure and functional properties of casein. Int J Agric Biol Eng. 2019;12:229–234. [Google Scholar]
- Wu Z, Huang Y, Xiao L, Lin D, Yang Y, Wang H. Physical properties and structural characterization of starch/polyvinyl alcohol/graphene oxide composite films. Int J Biol Macromol. 2019;123:569–575. doi: 10.1016/j.ijbiomac.2018.11.071. [DOI] [PubMed] [Google Scholar]
- Xi J, He M. High hydrostatic pressure (hhp) effects on antigenicity and structural properties of soybean β-conglycinin. J Food Sci Technol. 2018;55:630–637. doi: 10.1007/s13197-017-2972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Park SH, Balasubramaniam VM. Influence of high pressure homogenization with and without lecithin on particle size and physicochemical properties of whey protein-based emulsions. J Food Process Eng. 2017;40:e12578. doi: 10.1111/jfpe.12578. [DOI] [Google Scholar]
- Yin SW, Tang CH, Cao JS, Hu EK, Wen QB, Yang XQ. Effects of limited enzymatic hydrolysis with trypsin on the functional properties of hemp (Cannabis sativa L.) protein isolate. Food Chem. 2008;106:1004–1013. doi: 10.1016/j.foodchem.2007.07.030. [DOI] [Google Scholar]
- Zhang X, Haque ZZ. Generation and stabilization of whey based monodispered nanoemulsions using ultra-high pressure homogenization and small amphipathic co-emulsifier combinations. J Agric Food Chem. 2015;63:10070–10077. doi: 10.1021/acs.jafc.5b03889. [DOI] [PubMed] [Google Scholar]
- Zhang J, Rui D, Tian Y, Konno K. Characterisation of acid-soluble collagen from skin of silver carp (Hypophthalmichthys molitrix) Food Chem. 2009;116:318–322. doi: 10.1016/j.foodchem.2009.02.053. [DOI] [Google Scholar]
- Zhang Z, Regenstein JM, Zhou P, Yang Y. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrasonics Sonochem. 2017;34:960–967. doi: 10.1016/j.ultsonch.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Selomulya C, Xiong H, Chen XD, Xia R, Wang S, Xie J, Peng H, Sun W, Zhou Q. Comparison of functional and structural properties of native and industrial process-modified proteins from long-grain indica rice. J Cereal Sci. 2012;56:568–575. doi: 10.1016/j.jcs.2012.08.012. [DOI] [Google Scholar]


