Abstract
Grapes are one of the most highly consumed fruits across the world. In ancient Europe the leaves and the sap of grape plants has been used in traditional treatment for ages. Besides being a wellspring for vitamins and fibre, the skin and seeds of grapes are highly rich in Polyphenols specifically proanthocyanidins, which can be used as a functional ingredient to address various health issues by boosting the natural bio-processes of the body. Since, grape seeds are by product of wine making companies therefore can be easily procured. The present review article briefly describes the various pharmacological activities of grape seed extract and different experimental studies were done which supports the beneficial health qualities of the extract. Through different and various studies, it was proved that the proanthocyanidin rich grape seed extract provides benefits against many diseases i.e. inflammation, cardiovascular disease, hypertension, diabetes, cancer, peptic ulcer, microbial infections, etc. Therefore, beside from using it as a nutraceutical or cosmeceutical, as a result they may have a potential to substitute or complement in currently used drugs in the treatment of diseases by developing it into other successful pharmaceutical formulations for better future prospective.
Keywords: Grape seed extract (GSE), Proanthocyanidin, Antioxidant, Oxidative stress, Vascular endothelial growth factor (VEGF)
Introduction
Grape (Vitis vinifera) belongs to family Vitaceae. Grapes itself is widely consumed all over the world. Major producers of grapes worldwide are USA, China, Italy and Europe. In Europe, grapes, its leaves and the sap have been used in traditional treatment for ages. There are many categories of grapes with respect to their uses like wine grapes, table grapes, seedless, edible seed and raisin grapes. Seeds of grapes can be collected as a byproduct from any wine manufacturing industry. The seeds of red wine grapes are usually used to gather Grape Seed Extract (GSE).
Chemical composition of GSE
In order to obtain GSE, Grape seeds were separated from the grapes manually, air-dried (in the shade, 25–30 °C) for 1 week, and grounded to fine powder. The grounded grape seed powder was macerated in 70% ethanol (25% w/v) for 3 days at room temperature and filtered. The filtrate was dried at room temperature (about 25 °C) to evaporate ethanol and powdered GSE was obtained (Badavi et al. 2013). Dried seeds of grapes contain around 35% fiber along with 29% extractable components including Phenolic compounds, proteins (11%), mineral (3%) and water (7%) (Matthaus 2008). GSE is having abundant source of polyphenols. Polyphenols and flavonoids present in the GSE have been shown remarkable interest based on positive reports of their antioxidant properties and ability to serve as free radical scavengers (Georgiev et al. 2014). Grape seed polyphenols have a higher antioxidant activity as compared to other well-known antioxidants (such as vitamin C, vitamin E, and β-carotene). Beside their antioxidant activity, it also contains some enzymes that catalyze the release of histamine during inflammation and allergies (Ali et al. 2010). The amount of oil present in grape seed depends on the variety of the grape (usual range 10–16% of dry weight). Grape seed oil also contains a high amount fatty acids (unsaturated) ranging from 85 to 90% such as α-linolenic acid (ω − 3) and γ-linolenic acid (ω − 6). These fatty acids are related to a reduction of cardiovascular disease, cancer, hypertension, and autoimmune disorders (Shinagawa et al. 2015). An oil containing high amount of linoleic acid results in the reduction of total blood cholesterol and low-density lipoprotein (LDL) cholesterol. LDL cholesterol is responsible for the formation of arteriosclerosis, therefore; the application of grape-seed oil creates positive effect in the reduction of arteriosclerosis (Matthaus 2008). Fatty acid compositions of GSE include linoleic acid, linolenic acid, oleic acid and palmitic acid, and are much important in lipid metabolism. The major fatty acid in GSE is linoleic acid followed by oleic acid and palmitic acid. The seeds and peels of grapes also contains considerable portion of dietary fiber that lowers the risks of colon cancer, heart disease, diabetes and obesity (Anderson et al. 2009).
Pharmacological activity
GSE shows anti-inflammatory, anti-apoptotic, anti-necrotic, cardiovascular and anti-carcinogenic activity and have beneficial effects against several diseases including skin aging (Farzaei et al. 2015). It also shows a positive effect on wound healing. Presence of antioxidant property in GSE has shown to exert a much stronger oxygen free radical-scavenging effect. Compounds those are having antioxidant properties able to protect the cells against oxidative stress (Matthaus 2008). The antioxidant property of proanthocyanidin is because of the availability of the phenolic-hydrogens as singlet oxygen quenchers and hydrogen donating radical scavengers. It can then act as an inflammatory reaction regulator (related to the oxidative stress), hence, controlling the oxidative stress (Gargari et al. 2011). The use of GSE decreases the oxidative stress and helps in the reduction of anti-inflammation (Hosseinzadeh 2017). The obstacle or disturbance that comes in formation of free radicals and antioxidant defenses is called as oxidative stress. Small quantity of free radicals helps in signal transduction process. ROS (reactive oxygen species) and oxidative stress work as a physiological regulator of vascular gene expression of Vascular Endothelial Growth Factor (VEGF) (Kunsch and Medford 1999).
Wound healing activity and antioxidant potential
Wound healing involves three steps which include: inflammation, proliferation and remodeling. In inflammation, the inflammatory cells like monocytes and macrophages start depositing at the injury site and this step starts immediately after the injury occurs. Proliferative phase of wound healing includes several steps like angiogenesis, epithelialization, collagen aggregation, and formation of granulation tissue and wound interaction. Angiogenesis is the process where new blood vessels grows from existing vessels and hence is crucial in wound healing process. If the flow of blood is restored to the damaged tissues, it’ll provide oxygen and nutrients which is a basic requirement for the support of function and growth of reparative cell (Johnson and Wilgus 2014).
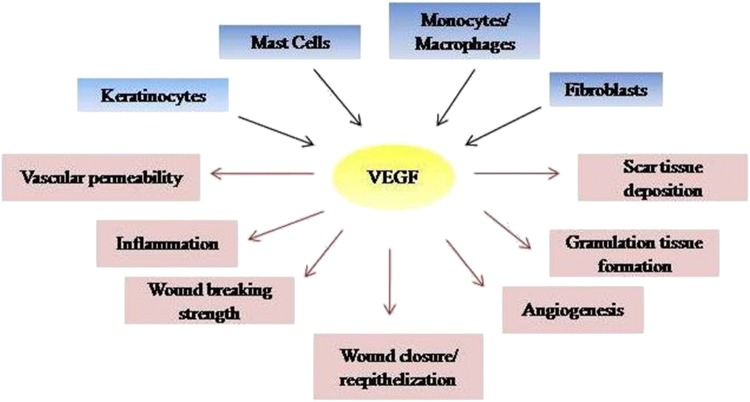
Angiogenesis process is stimulated by Vascular Endothelial Growth Factor (VEGF; Fig. 1 ) which is the most effective growth factor involved in the process of healing of wounds. VEGF is a signal glycoprotein produced by the cells, also known as Vascular Permeability Factor. In angiogenesis VEGF stimulates the migration and reproduction of epithelial cell. Johnson and Wilgus 2014 stated that VEGF and angiogenesis are two most important factors for the cutaneous wound repair regulation. GSE contains polyphenyl phenolic bioflavonoids, proanthocyanidins which accelerates the process of wound healing (Soto et al. 2015). Different studies of GSE for the management of wound healing and their benefits where mentioned in Table 1. Proanthocyanidins specifically induces the expression of VEGF in human keratinocytes cells responsible for wound healing (Sen et al. 2002). Some epidemiologic studies demonstrated that the phenolic compound present in GSE decreases the endothelial contraction of vessels, activate nitric oxide synthesis, control platelet aggregation and prevent LDL cholesterol oxidation (Sandoo et al. 2010).
Fig. 1.
Sources and actions of VEGF during wound healing. During wound healing, production of VEGF is done by a variety of cell types such as: keratinocytes, mast cells, macrophages, and fibroblasts. During wound healing process, VEGF stimulates vascular permeability, angiogenesis, granulation tissue formation, scar tissue deposition; strengthen of wound, closure of wound and re-epithelization
Table 1.
Various studies on GSE for wound healing
| SI no. | Methods | Benefits | References |
|---|---|---|---|
| 1 | Excision wound model in rats | Reduction of wound area, Faster wound healing | Nayak et al. (2010, 2011) |
| 2 | Animal models of surgical wounds, skin abrasions, burns, lacerations, excisional wounds and open fractures | Initiation of gene expression that reasons for the formation of VEGF | Dai et al. (2011) |
| 4 | Tissue glutathione oxidation and 4-hydroxynonenal immunostaining | Faster wound contraction, increased the synthesis of VEGF and greater amount of connective tissue deposition | Khanna et al. (2002) |
| 5 | Chemical reactions involving anthocyanins and/or flavanols | Preventing the oxidation of LDL lipoproteins, platelet aggregation, and the damage of RBCs through radical scavenging | Cheynier (2005) |
| 6 | Fatty acid (FA) composition, tocopherol content, carotenoid profile, total phenolic content (TPC), oxidative stability index (OSI), color, physical properties, and radical-scavenging capacities against peroxyl (oxygen radical-scavenging capacity) and stable DPPH (diphenylpicrylhydrazyl) radicals | Prevention of degenerative diseases associated with oxidative stress in different tissues and organic systems | Parry et al. (2006) |
| 7 | CP-induced nephrotoxicity | Protection from cisplatin-induced nephrotoxicity | Gao et al. (2014) |
| 8 | Oxygen radical absorbance capacity assay | Inhibition of lipid oxidation and ROS scavenging | Xia et al. (2010) |
| 9 |
Oxidative stress and subsequent liver injury studies Liver histology; Western blot and reverse transcriptase; TUNEL staining |
Improves antioxidant defenses, such as preventing ischemia/reperfusion-induced and carbon tetrachloride- induced liver injury | Dai et al. (2014); Xu et al. (2015) |
| 11 | Antioxidative potential of blood plasma; intragastric intubation | Increases the resistance of plasma against oxidative stress | Koga et al. (1999) |
| 12 | Markers of oxidative stress; total antioxidant activity and the plasma concentrations of alpha-tocopherol | Increases the level of α-tocopherol in RBCs membranes and antioxidant protection | Simonetti et al. (2002) |
| 13 | Protective effect of DMGS on toxicity induced by Adriamycin (ADR) in isolated rat hepatocytes; lactate dehydrogenase (LDH) release to estimate cytotoxicity; thiobarbituric acid reactant substances (TBARS) and carbonyl group levels were measured as biomarkers of oxidative stress, ATP and GSH levels as estimation of intra-cellular effect | Provide cell membrane protection against oxidative damage and consequently prevents lipid and protein oxidation | Valls-Belles et al. (2006) |
Researchers of Ohio University’s heart and lung Research institute reported that the GSE helps in wound healing by two ways: (1) GSE helps body in the regeneration of the damaged blood vessels and (2) GSE increases the quantity of free radicals present at the wound site (Ohio State University 2002). Free radical helps in killing and clearing of pathogenic bacteria and endotoxin from the site and helps in curing of wounds.
The property of wound healing of GSE was also evaluated using an excision wound model in rats. The wound healing assessment of GSE was done by the estimation of rate of wound contraction and level of hydroxyl proline content at the wound site. The dose of 100 mg kg−1 was given as a treatment. At the end of the study it was summarized that the level of hydroxyl proline on the wound site was higher and the reduction of wound area was greater as compare to control and standard (Nayak et al. 2011). In another study, the wound healing effect was investigated on nine mice. Two small wounds were induced on each mice by puncturing. GSE was applied in one wound and the other one was treated with normal saline solution as a control. After 5 days, tiny area of mice skin from the edge was excised and treated with GSE and normal saline. The sign of patterns of healing of the GSE treated samples and saline treated tissues were compared. It was found the wound which was treated with GSE healed faster as compared to control and the significant generation of new skin was observed. In addition, it was also reported that the quantity of tenascin was increased when the granulated wound tissue was treated with GSE. Moreover, increased of tenascin, researchers also found that the GSE also helps in the initiation of gene that reasons for the formation of VEGF (Dai et al. 2011).
The wound healing effect of GSE on rat palatal mucosa was investigated in DARC (Drug Applied Research Centre) in Tabriz University of Medical Sciences. In this study, 20 rats were taken and divided into test group and control group and each group contained equal numbers of rats. It was found the rate of wound healing was slower in the placebo sample as compared to experimental sample. On day 11th there is complete healing of epithelium in sample containing GSE was observed. In addition, they also found more organized granulation tissue and the rate of collagen fiber deposition also higher in the test sample. This study concluded that the GSE is effective and plays a crucial part in wound healing process. Hemmati et al. (2014) conducted a double-blind clinical trial to examine the effect of topical GSE 2% cream on surgery wound healing. In this study two groups were involved i.e. Treatment and placebo and the surgery was done on skin fage and moles to produce surgery wounds except on face by surgical scissors. The treatment group was treated with 2% GSE cream. The result shows a complete wound repair on an average of 8 days for the treatment-group and 14 days for placebo. On the basis of this study, it was opined that the proanthocyanidin present in GSE triggered the release of VEGF, thereby, led to wound contraction and increased the amount of connective tissue at the wound site, resulted in improve the cellular structure of wound.
It was reported that faster wound contraction, increased the synthesis of VEGF and greater amount of connective tissue deposition was observed when mice treated topically with GSE and this was due to presence of resveratrol in GSE (Khanna et al. 2002). The most cited bioactive property attributed to the phenolic compounds is their antioxidative capacity, preventing the oxidation of LDL lipoproteins, platelet aggregation, and the damage of RBCs through radical scavenging (Cheynier 2005). Removal of free radicals; mainly hydroxyl radical and chelation of metals are the biological mechanism associated with the antioxidant property, influences functioning of the immune system and cell signaling (Soobrattee et al. 2005). The radical scavenging activities of phenolic compounds are supported by their reducing power through hydrogen donating and single oxygen quenching. Due to these activities compounds interact with biological systems and prevent degenerative diseases associated with oxidative stress in different tissues and organic systems (Parry et al. 2006).
In many data it has been shown that GSE is able to improve antioxidant defenses, such as preventing ischemia/reperfusion-induced and carbon tetrachloride- induced liver injury (Dai et al. 2014; Xu et al. 2015), alleviates oxidative reproductive toxicity induced by arsenic, and provides protection from cisplatin-induced nephrotoxicity (Gao et al. 2014).
The antioxidant capacity of GSE has been widely studied; compounds are capable of inhibiting lipid oxidation and ROS scavenging. Xia et al. 2010 measured the antioxidant capability with the help of oxygen radical absorbance capacity assay in which the antioxidant capacity of grape and its by-products was compared including leaves, skin, wine, and seeds. 42.18 mmol of Trolox equivalent/g was the highest antioxidant capacity observed in grape seeds (Xia et al. 2010). This high antioxidant capacity in grapes is probably due to the presence of high amount of catechin, gallic acid, epicatechin, proanthocyanidin, and procyanidins (Hernández-Jiménez et al. 2009). The antioxidant activity of phenolic compounds mainly involves two mechanisms i.e. metal chelating properties along with their effect on signal pathways and on gene expression and free radical scavenging (Soobrattee et al. 2005).
Apart from anti-oxidant properties of proanthocyanidin, the concentration of free radicals on the wound site also increases which helps in the proliferation of cells, formation of connective tissue and new blood vessels (Nutra Ingredients 2002). Another experiment was performed in which researchers measured the amount of hydroxyproline of the granulation tissue for the evaluation of the wound healing property of GSE, period of epithelialization and wound contraction in male rats which was treated with GSE with respect to control (Petroleum jelly) and standard (Mupirocin). Excision wound model of rats were considered for the study. Three groups were made i.e. Test, standard and control in which animals divide randomly in groups of six each. GSE was given to test group whereas the animals of standard and control were treated with mupirocin ointment and petroleum jelly. The study revealed that the test group showed faster healing as compare to control and standard. In addition, it was also reported that the wound contraction and hydroxyproline content at the wound site was significantly more than control and standard along with the less period of epithelialization (Nayak et al. 2010).
In another study, it was observed that the intake of proanthocyanidins increases the resistance of plasma against oxidative stress and may contribute to physiological functions through their in vivo antioxidant properties (Koga et al. 1999). It was reported that the GSE ingestion increases the level of α-tocopherol in RBCs membranes and antioxidant protection of GSE exerted by sparing lipid soluble vitamin E (Simonetti et al. 2002). It was also observed that when milled grape seed was defatted, as a by-product wine was obtained from the oil extraction of grape seed having ability of providing protection to cell membrane against oxidative damage and consequently prevents lipid and protein oxidation (Valls-Belles et al. 2006).
Anti-inflammation
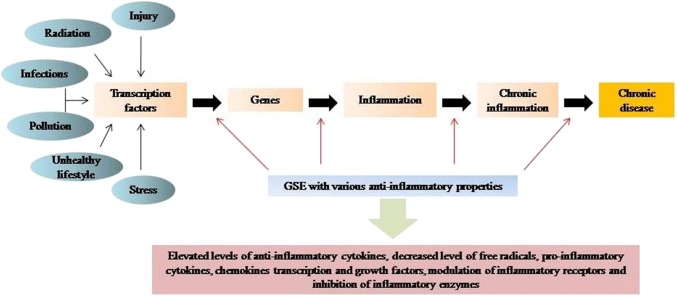
Chronic diseases, which may be associated with increased mortality and morbidity rates worldwide, are generally accompanied by inflammation processes, which are often difficult to control with available therapies and interventions. Inflammation can be defined as the responses generated by the body with respect to the injuries for example- swelling, redness, generation of heat at the injury site etc. These inflammatory responses results in the movement of serum proteins and leukocytes to the injury site. The events of inflammation are mediated by inflammatory mediator’s prostaglandins and leukotrienes (Fig. 2). In this context, the consumption of nutrients having anti-inflammatory properties would be beneficial in the treatment of chronic diseases.
Fig. 2.
Schematic diagram depicting the consequence in inflammation and role of GSE in inflammation. GSE attenuate oxidative stress (Cetin et al. 2008) and decrease the Low-Density Lipoprotein (LDL) levels, and thereby reduce the inflammatory process related to some diseases. GSE rich in polyphenolic compounds causes anti-inflammatory effect. Polyphenols present in GSE are capable of inhibiting arachidonic acid release, responsible for the synthesis of leukotrienes and prostaglandins, which further activates the inflammatory response (Santangelo et al. 2007)
Cardiovascular activity
Cardiovascular disorders (CVD) are among one of the major problems that arises due to the modern and unhealthy lifestyle which results in the primary cause of death mortality worldwide. CVD refers in general to the condition in which the functions of the heart and the blood vessels alter that can lead to cardiac arrest, heart stroke, chest pain etc. Lifestyle plays a crucial part in the prevention of CVD. Proper diet rich in natural fruits and vegetables, regular exercise etc. reduces the risk of CVD. Diabetes mellitus, high BP (blood pressure) and dyslipidemias are some examples of the risk factors for progression of atherosclerosis and CVD and can be reducing by changing the pattern of lifestyle (Masana et al. 2017). Deposition of lipoprotein in the intimal layer of the arteries leads to the formation of atherosclerotic plaque. In addition, changes of plasma lipid profile induce formation of hydroxyperoxide and lysis of phospholipids, oxysterol, and other lipids (Bagchi et al. 2003). Oxidized low density lipoprotein (Ox-LDL) particles play a key role in atherosclerosis’s pathogenesis. Exposure of endothelial cells to Ox-LDL results in migration of monocytes to subendothelial layers, which transform into macrophages and releases growth factors. In addition, smooth muscle cell and fibroblast proliferation, platelet aggregation, and angiotensin II-like effects also trigger on exposure to Ox-LDL (Mitra et al. 2011). In this context, the free radical scavenger plays an important role in the pathophysiology of CVD (Bagchi et al. 2003; Lopez Farré and Casado 2001). Promising results of lipid-lowering properties of GSE has been studied and investigated in many animals and some human studies; however, some investigators have debated their effect on lipid profiles (Caimari et al. 2013).
There are some studies and evidences which show that cardio protective benefits (Table 2) are exhibited by certain fruits and vegetables, such as grapes and intake of food items that are rich in polyphenols to reduces the risk of CVD. Many epidemiological studies were performed which suggest that the consumption of grapes rich in polyphenols decreases the cardiovascular mortality. There are many mechanisms by which GSE prevent the atherosclerosis, including inhibition or limit the oxidation of low-density lipoprotein (LDL), lowering the blood pressure, reducing inflammation, inhibition of the platelet aggregation, and activating some proteins which prevents the cell senescence (Dohadwala and Vita 2009). The in vitro and in vivo study provides evidence that there is a relation between presence of antioxidant phenolics and the reduction of oxidized LDL particles and consumption of diets rich in phenolic antioxidant reduced the rate of occurrence of cardiac disease (Kinsella et al. 1993). The capability of the (poly)phenols present in GSE avert radical oxidation of the polyunsaturated fatty acids of low-density lipoproteins (LDL), which occurs frequently through oxidative modification of the apoprotein toward an atherogenic form, has a direct follow up on the prevention of CVD (Rimm et al. 1993). A study was performed in which the anti-atherogenic activity of GSE was investigated. This study was conducted on 287 patients who are diagnosed with abnormal plaque Carotid Intima-Media Thickness (CIMT) or asymptomatic carotid plaques. During this study, it was observed that GSE inhibits the progression of mean maximum CIMT and decreases the size of carotid plaque in patients. Thus, it was concluded that the GSE loaded with proanthocyanidin has atherogenic effects (Cao et al. 2015).
Table 2.
Various studies on GSE for Cardiovascular disorder
| Sl. no. | Methods | Benefits | References |
|---|---|---|---|
| 1 | Carotid B-mode ultrasound | Inhibits the progression of plaque in CIMT | Cao et al. (2015) |
| 2 | Cytochrome c reduction assay, and correlated the enhanced production of superoxide anion with increased lipid peroxidation, DNA fragmentation and membrane micro viscosity | Defends against reperfusion-induced injury | Bagchi et al. (2003) |
| 3 | Antioxidant properties, Endothelial function (cultured endothelial cells are used), anti-platelet effects (used a coronary artery platelet aggregation model) | Inhibits the oxidation of LDL, improving endothelial function, inhibition of platelet aggregation, reducing inflammation, lowering blood pressure and prevent cell senescence by activating novel proteins | Dohadwala and Vita (2009) |
| 4 | Measurement of inhibitory effects on pancreatic lipase and lipoprotein lipase activities and on lipolysis of 3T3-L1 adipocytes was assayed using grape seed extract | Minimize the accumulation of fat in adipose tissue and dietary fat absorption by inhibiting fat-metabolizing enzymes; pancreatic lipase and lipoprotein lipase | Moreno et al. (2003) |
| 5 | Anti-thrombotic effect of proanthocyanidin was assessed by a shear-induced thrombosis test in vitro and by a laser-induced thrombosis test in the mouse carotid artery, in vivo | Inhibits the thrombus formation | Sano et al. (2005) |
| 6 | Anti-platelet action of extracts was compared with action of well characterized antioxidative and anti-platelet commercial monomeric polyphenol-resveratrol | Protects against myocardial injury, myocardial ischaemia–reperfusion, reduction in platelet adhesion, aggregation and generation of superoxide anion | Olas et al. (2008) |
| 7 | Dose dependent effect was checked directly by adding tomato and grape pomace to the cholesterol diet of male Wistar rat | Prevent LDL oxidation | Bobek (1999) |
It was also reported antioxidants contained in the red GSE are able to inactivate superoxide anions and prevent lipid peroxidation. Red GSE has effective cardio protective against reperfusion-induced injury by free radicals after ischemia (Bagchi et al. 2003). During some preclinical in vivo studies it was revealed that winery residues prevent LDL oxidation and 15% grape pomace in a cholesterol diet (0.3%) is capable of reducing the rat liver and serum levels of cholesterol by half, while elevating high-density lipoproteins (HDL) by 26% (Bobek1999). Thus, GSE is capable to reduce the risk of CVD by inhibiting the oxidation of LDL, improving endothelial function, inhibition of platelet aggregation, reducing inflammation, lowering blood pressure and prevent cell senescence by activating novel proteins (Dohadwala and Vita 2009). In addition, GSE can also use to minimize the accumulation of fat in adipose tissue and dietary fat absorption by inhibiting fat-metabolizing enzymes; pancreatic lipase and lipoprotein lipase (Moreno et al. 2003). It was reported that oral and intravenous administration of procyanidins obtained from grape seeds significantly inhibits the formation of thrombus in mice models (Sano et al. 2005). It was also established GSE provides protection against myocardial injury and myocardial ischaemia–reperfusion, reduction in platelet adhesion, aggregation and generation of superoxide anion in rats (Olas et al. 2008).
Antihypertensive activity
Hypertension refers to the condition in which the forces of blood against the wall of arteries are too high. Normal blood-pressure is near 120/80. If the blood pressure rises above 140/90 then it is said to be hypertension and when it is above 180/120 then causes severe issues. In India per year around 10 million peoples suffer in hypertension. If hypertension left untreated then it causes severe health issues like- stroke. It was proposed that before 2026, the cases of hypertension will increase up to 24%. Fruits and vegetables rich in flavonoids and polyphenols can be used as a natural therapy for controlling hypertension (Quinones et al. 2013). The antihypertensive activity of GSE was investigated and was found that GSE rich in proanthocyanidin is capable of controlling hypertension. GSE exerts antihypertensive action by suppression of oxidative stress, inhibiting the angiotensin converting enzyme (ACE) and nitric oxide mediated vasodilation (Gonzalez et al. 2014).
It is well recognized that the oxidative stress is well implicated in pathogenesis of hypertension. In this context, agents that can suppress oxidative stress are an effective therapeutic option for the management and treatment of hypertension. The in vitro experiment suggests that GSE can protect cells from ROS-mediated DNA damage because of its antioxidant property (Li et al. 2010).
A study was carried out by Quinones et al. (2013) in which the antihypertensive effect of GSE was assessed in male spontaneously hypertensive rats. The outcome was that the GSE produces a significant decrease in systolic-diastolic blood pressure. The study opined GSE exerts antihypertensive activity by increasing the antioxidant endogen system (Quinones et al. 2013).
GSE also shows positive impacts on the blood pressure and it is more obvious to obese and the patients with metabolic syndrome or disorders (Zhang et al. 2016). The effects of white grape pomace flour on thirty-eight male human subject of age between 30 and 65 years having metabolic syndrome was performed. At the end of study, it was seen that the systolic-diastolic blood pressure was significantly decreased. This indicates that the consumption of grapes rich in polyphenols at regular basis improves the blood pressure (Urquiaga et al. 2015). A double-blind placebo crossover study was performed on sixty mildly hypertensive subjects, in which the anti-hypertensive activity of two extracts i.e. grape-red wine extract and grape alone was carried out for around 4 weeks. It was found that the foods, rich in catechin, procyanidins and polyphenols help into maintain a healthy blood pressure and grape alone is not capable of providing sufficient quantity of procyanidins and catechins (Draijer et al. 2015).
Treatment of peptic ulcer
It was reported that GSE rich in proanthocyanidin protects and provides immunity against chronic and acute gastric and intestinal oxidative injury through membrane microviscosity in gastric and duodenal membrane and inhibition of lipid peroxidation (Bagchi et al. 1999). It exhibited antioxidant activity and higher gastro protective as compared to vitamin C and E (Cuevas et al. 2011). This gastro protective effect of GSE might be due to protein binding ability of procyanidins covering the stomach surface (Saito et al. 1998).
Resveratrol is a polyphenol found in GSE, suppresses the growth of Helicobacter pylori, -interleukin-8 secretion induced by H. pylori, generation of reactive oxygen species, and changes in human gastric epithelial cells morphology (Zaidi et al. 2009). It was found that low dose of resveratrol (2 mg kg−1) exhibits ulcer healing activity, whereas, in high dose (10 mg kg−1) it becomes ulcerogenic. The mechanism behind low dose ulcer healing activity is attributed to stimulation of COX1, inhibition of neutrophil aggregation, PGE2&eNOS and improvement of angiogenesis (Dey et al. 2009).
The inflammatory protective role of GSE on bowel disease results in modulation of gut microflora, decreasing Faecalibacterium prausnitzii within the intestinal lumen and blocking gut inflammatory response. Saito et al., 1998 showed GSE containing proanthocyanidin possess a preventive effect on hydrochloride/ethanol-induced gastric injury (Saito et al. 1998). This study indicated that GSE rich in proanthocyanidins play a key role for observed beneficial effects in inflammatory bowel disease.
Anticancer activities
Phenolic compounds present in GSE exhibits anticancer and cell cycle modulation activity (Huang et al. 2012). These phenolic compounds show cytotoxic activity against tumor cells without affecting the normal healthy cells (Engelbrecht et al. 2007). The suggested mechanisms of anticancer activity attributed to expression of pro-angiogenic factors like-angiopoietins and the vascular endothelial growth factor (Huang et al. 2012), and the inactivation of phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB) signaling pathway which leads to induction of apoptosis of cancer cells (Engelbrecht et al. 2007).
Cheah et al. (2014) reported that the low molecular weight procyanidins, present in GSE, increased the toxicity of the chemotherapeutic agent 5-fluorouracil on cancer cells (Cheah et al. 2014). This study opined that GSE can be used as a supplement in treatment of cancer.
In 2015 a study was conducted by Lacatusu et al. in which the lipid nanocarriers of Grape seed oil and laurel leaf oil (natural oils) was developed and the effectiveness of nanocarriers in combating certain tumor cells and counteracting free radicals were evaluated between tumor cells of MDA-MB 231 and HeLa cell lines and normal cells of L929 and B16 cell lines. A significant decrease in proliferation of tumor cell was observed. Thus, lipid nanocarriers based on natural oils like- Grape seed oil and laurel leaf oil may significantly improve the therapeutic efficacy of antitumor drugs in clinical applications (Lacatusu et al. 2015).
Antimicrobial activity
The GSE have shown an effective antimicrobial property; they are efficiently used against Gram-positive bacteria (Bacillus cereus, Staphylococcus aureus, Bacillus coagulans and Bacillus subtilis), but are more effective against Gram-negative bacteria like-Pseudomonas aeruginosa or Escherichia coli (Jayaprakasha et al. 2003). Resveratrol shows a positive antimicrobial activity against many pathogens, such as Staphylococcal aureus, Psedomonas aeruginosa and Enterococcus faecalis (Chan 2002). In normal skin, when resveratrol applied topically increases production of cathelicidin, which inhibits the growth of Staphylococcal aureus and induces antimicrobial peptides (Park et al. 2013). The antimicrobial activity showed by resveratrol, involves the induction of oxidative damage to bacterial membrane without affecting the host cells, especially in E. coli. These findings gave an idea about the use of resveratrol that would aid traditional therapies in which antibiotics failed or were ineffective (Subramanian et al. 2014). Anastasiadi et al. (2009) performed an experiment on the evaluation of antimicrobial activity of GSE, rich in flavan-3-ols. In this context, he exhibited that the higher amount of flavonoids and their derivatives in GSE were responsible for the antimicrobial-activity (Anastasiadi et al. 2009). In another study, Vaquero et al. (2007), suggested that caffeic acid, quercetin, and quercetin-3-O-rutinoside of GSE are responsible for inhibit Listeria monocytogenes (Vaquero et al. 2007). Similarly, it has been brought to notice that the polymeric phenolic fractions of GSE exhibited the highest specific inhibition activity for almost all Listeria species (Rhodes et al. 2006). The study of Paulo et al. (2010) reported that the antimicrobial effect of GSE is attributed by changes in cell morphology and DNA content (Paulo et al. 2010).
Other activities
Teeth continuously expose to food, germs, bacteria etc., leads to different chemical reactions and the formation of harmful acids, results in demineralization of teeth. GSE is a well rich source of proanthocyanidin, which strengthen the collagen-based tissues (by increasing the collagen cross links). Additionally, GSE also increases the synthesis of collagen and the conversion of collagen from soluble into insoluble one. To confirm the re-mineralization process on teeth by proanthocyanidins, a study was performed in which the effect of GSE on the artificial enamel caries in the primary human-teeth was done which showed that the GSE enhances the re-mineralization process (Mirkarimi et al. 2013).
GSE as dietary supplement improves the brain memory, decreased protein carbonyl level, reduced reactive oxygen species production, reduced hypoxic ischemic brain injury, and increased thiol level in the central nervous systems. In this context, studies have shown that the resveratrol could exert neuroprotection against neurodegenerative diseases, ischemia, and seizure (Balu et al. 2006).
GSE in cosmetic and nutraceuticals
In today’s era people want to live young and attractive. They use as many products to protect their skin from harmful effects of environmental stress, UV radiation etc. The facial skin is more susceptible to UV radiation which results in the photo aging. GSE can be used in various cosmetic products. Cosmetics rich in antioxidant provide protection against harmful UV radiations. Skin aging is a natural process which occurs due to the external and internal factors involving genetic, hormonal and environmental factors. Decrease of collagen and its precursor, inflammation and disordered Melanocytes are some changes occur in photo-aging. GSE rich in Proanthocyanidin reduces the lipid oxidation of cellular structure of the skin and inhibits the production of free radicals (Markus and Morris 2008). There are many products available in market having GSE as a core ingredient. These products include tablet, capsules, lotions, creams etc. A study was carried out in which the anti-aging and redox state regulation effects of GSE and cranberry concentrate (CBC) was investigated. After the study it was concluded that proanthocyanidin rich in GSE and CBC extract have promising role as anti-aging and in redox state regulation (Costa et al. 2015). Phenolic acids, phytosterols, flavonoids, tocopherols, carotenoids, tocopherols and tocotrienols, those are having high antioxidant activity and can maintain the skin health are examples of other active compounds of GSE (Devi and Singh 2017).
Conclusion
In conclusion this review article demonstrated that the grape seed extract exerts a potential health benefits against numerous diseases specifically cardiovascular disorders and wound healing effect. Therefore, in future, with intense and insightful studies the grape seed extract can be processed to isolate pure form of proanthocyanidin responsible for many pharmacological activities. The GSE rich in compounds may provide a safe, natural, and cost-effective treatment and apart from cosmetic and nutraceutical products of grape seed extract, it can also be use in the treatment of diseases by developing it into other successful pharmaceuticals formulation for better future prospective.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ali K, Maltese F, Choi YH, et al. Metabolic constituents of grapevine and grape-derived products. Phytochem Rev. 2010;9(3):357–378. doi: 10.1007/s11101-009-9158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadi M, Chorianopoulos NG, Nychas GJE, et al. Antilisterial activities of polyphenol-rich extracts of grapes and vinification byproducts. J Agric Food Chem. 2009;57(2):457–463. doi: 10.1021/jf8024979. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Baird P, Jr, Davis RH, et al. Health benefits of dietary fiber. Nutr Rev. 2009;67(4):188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- Badavi M, Abedi HA, Dianat M, et al. Exercise training and Grape seed extract Co-Administration improves lipid profile, weight loss, Bradycardia, and hypotension of STZ-induced Diabetic rats. Int Cardiovasc Res. 2013;J7(4):111–117. [PMC free article] [PubMed] [Google Scholar]
- Bagchi M, Milnes M, Williams C, et al. Acute and chronic stress-induced oxidative gastrointestinal injury in rats, and the protective ability of a novel grape seed proanthocyanidin extract. Nutr Res. 1999;19(8):1189–1199. doi: 10.1016/S0271-5317(99)00080-9. [DOI] [Google Scholar]
- Bagchi D, Sen CK, Ray SD, et al. Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat Res. 2003;523–524:87–97. doi: 10.1016/S0027-5107(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Balu M, Sangeetha P, Murali G, et al. Modulatory role of grape seed extract on age-related oxidative DNA damage in central nervous system of rats. Brain Res Bull. 2006;68(6):469–473. doi: 10.1016/j.brainresbull.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Bobek P. Dietary tomato and grape pomace in rats: effect on lipids in serum and liver, and on antioxidant status. Br J Biomed Sci. 1999;56(2):109–113. [PubMed] [Google Scholar]
- Caimari A, Del Bas JM, Crescenti A, et al. Low doses of grape seed procyanidins reduce adiposity and improve the plasma lipid profile in hamsters. Int J Obes. 2013;37(4):576–583. doi: 10.1038/ijo.2012.75. [DOI] [PubMed] [Google Scholar]
- Cao A, Wang J, Cao H, et al. Beneficial clinical effects of grape seed proanthocyanidin extract on the progression of carotid atherosclerotic plaques. J Geriatr Cardiol. 2015;12(4):417–423. doi: 10.11909/j.issn.1671-5411.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin A, Kaynar L, Koçyiğit I, et al. The effect of grape seed extract on radiation-induced oxidative stress in the rat liver. Turk J Gastroenterol. 2008;19(2):92–98. [PubMed] [Google Scholar]
- Chan MM. Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogens of the skin. Biochem Pharmacol. 2002;63(2):99–104. doi: 10.1016/S0006-2952(01)00886-3. [DOI] [PubMed] [Google Scholar]
- Cheah KY, Howarth GS, Bindon KA, et al. Low molecular weight procyanidins from grape seeds enhance the impact of 5-fluorouracil che-motherapy on caco-2 human colon cancer cells. PLoS ONE. 2014;9(6):e98921. doi: 10.1371/journal.pone.0098921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheynier V. Polyphenols in foods are more complex than often thought. Am J Clin Nutr. 2005;81(1 Suppl):223S–229S. doi: 10.1093/ajcn/81.1.223S. [DOI] [PubMed] [Google Scholar]
- Costa A, Pegas PES, Assumpcao EC, et al. Assessment of clinical effects and safety of an oral supplement based on Marine protein, vitamin C, grape seed extract, Zinc & tomato extract in the improvement of visible signs of skin aging in men. Clin Cosmet Investig Dermatol. 2015;8:319–328. doi: 10.2147/CCID.S79447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas VM, Calzado YR, Guerra YP, et al. Effects of grape seed extract, vitamin C, and Vitamin E on ethanol- and aspirin induced ulcers. Adv Pharmacol Sci Article ID. 2011;740687:6. doi: 10.1155/2011/740687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Kharkwal GB, Tanaka M, et al. Animal models of external traumatic wound infections. Virulence. 2011;2(4):296–315. doi: 10.4161/viru.2.4.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai N, Zou Y, Zhu L, et al. Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (CCl4)–induced steatosis and liver injury in rats via CYP2E1 regulation. J Med Food. 2014;17(6):663–669. doi: 10.1089/jmf.2013.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi S, Singh R. Antioxidant and anti-hypercholesterolemic potential of Vitis vinifera leaves. Pharmacogn J. 2017;9(4):565–572. doi: 10.5530/pj.2017.4.90. [DOI] [Google Scholar]
- Dey P, Guha S, Chattopadhyay S, Bandyopadhyay SK. Biphasic activity of resveratrol on indomethacin-induced gastric ulcers. Biochem Biophys Res Commun. 2009;381(1):90–95. doi: 10.1016/j.bbrc.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Dohadwala MM, Vita JA. Grapes and cardiovascular disease. J Nutr. 2009;139(9):1788S–1793S. doi: 10.3945/jn.109.107474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draijer R, Graaf Y, Slettenaar M, et al. Consumption of a polyphenol-rich grape-wine extract lowers ambulatory blood pressure in mildly hypertensive subjects. Nutrients. 2015;7(5):3138–3153. doi: 10.3390/nu7053138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht AM, Mattheyse M, Ellis B, et al. Proanthocyanidin from grape seeds inactivates the PI3-kinase/PKB pathway and induces apoptosis in a colon cancer cell line. Cancer Lett. 2007;258(1):144–153. doi: 10.1016/j.canlet.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Farzaei MH, Abdollahi M, Rahimi R. Role of dietary polyphenols in the management of peptic ulcer. World J Gastroenterol. 2015;21(21):6499–6517. doi: 10.3748/wjg.v21.i21.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Liu G, Hu Z, et al. Grape seed proanthocyanidin extract protects from cisplatin-induced nephrotoxicity by inhibiting endoplasmic reticulum stress-induced apoptosis. Mol Med Rep. 2014;9(3):801–807. doi: 10.3892/mmr.2014.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargari BH, Abedini S, Babaei H, et al. Effect of supplementation with grape seed (Vitis vinifera) extract on antioxidant status and lipid peroxidation in patient with type II diabetes. J Med Plant Res. 2011;5(10):2029–2034. [Google Scholar]
- Georgiev V, Ananga A, Tsolova V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients. 2014;6(1):391–415. doi: 10.3390/nu6010391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J, Valls N, Brito R, et al. Essential hypertension and oxidative stress: new insights. World J Cardiol. 2014;6(6):353–366. doi: 10.4330/wjc.v6.i6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Jiménez A, Gómez-Plaza E, Martínez-Cutillas A, et al. Grape skin and seed proanthocyanidins from Monastrell x Syrah grapes. J Agric Food Chem. 2009;57(22):10798–10803. doi: 10.1021/jf903465p. [DOI] [PubMed] [Google Scholar]
- Hemmati AA, Foroozan M, Houshmand G, Moosavi ZB, et al. The topical effect of grape seed extract 2% cream on surgery wound healing. Glob J Health Sci. 2014;7(3):52–58. doi: 10.5539/gjhs.v7n3p52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh F. The healing effect of Grape seed oil enema with or without sesame oil in acetic acid induced ulcerative colitis of rats. World J Plast Surg. 2017;6(2):176–182. [PMC free article] [PubMed] [Google Scholar]
- https://news.osu.edu/grape-seed-extract-help-speed-up-wound-recovery-study-suggests. Accessed 31 Jan 2019
- https://www.nutraingredients.com/Article/2002/12/03/Grape-seed-extract-boosts-wound-healing Accessed 31 Jan 2019
- Huang S, Yang N, Liu Y, et al. Grape seed proanthocyanidins inhibit colon cancer-induced angiogenesis through suppressing the expression of VEGF and Ang1. Int J Mol Med. 2012;30(6):1410–1416. doi: 10.3892/ijmm.2012.1147. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha GK, Selvi T, Sakaria KK. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res Int. 2003;36(2):117–122. doi: 10.1016/S0963-9969(02)00116-3. [DOI] [Google Scholar]
- Johnson KE, Wilgus TA. Vascular Endothelial growth factor and Angiogenesis in the regulation of cutaneous wound repair. Adv wound care. 2014;3(10):647–661. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Venojarvi M, Roy S, et al. Dermal wound healing properties of redox-active grape seed proanthocyanidins. Free Radic Bio Med. 2002;33(8):1089–1096. doi: 10.1016/S0891-5849(02)00999-1. [DOI] [PubMed] [Google Scholar]
- Kinsella JE, Frankel E, German B, et al. Possible mechanism for the protective role of the antioxidant in wine and plant foods. Food Technol. 1993;47:85–89. [Google Scholar]
- Koga T, Moro K, Nakamori K, et al. Increase of antioxidative potential of rat plasma by oral administration of proanthocyanidin-rich extract from grape seed. J Agric Food Chem. 1999;47(5):1892–1897. doi: 10.1021/jf9810517. [DOI] [PubMed] [Google Scholar]
- Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85(8):753–766. doi: 10.1161/01.RES.85.8.753. [DOI] [PubMed] [Google Scholar]
- Lacatusu I, Badea N, Badea G, et al. Lipid nanocarriers based on natural oils with high activity against oxygen free radicals and tumor cell proliferation. Mater Sci Eng C Mater Biol Appl. 2015;56:88–94. doi: 10.1016/j.msec.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Li J, Liu H, Ramachandran S, et al. Grape seed proanthocyanidins ameliorate doxorubicin-induced cardiotoxicity. Am J Chin Med. 2010;38(3):569–584. doi: 10.1142/S0192415X10008068. [DOI] [PubMed] [Google Scholar]
- Lopez Farré A, Casado S. Heart failure, redox alterations, and endothelial dysfunction. Hypertension. 2001;38:1400–1405. doi: 10.1161/hy1201.099612. [DOI] [PubMed] [Google Scholar]
- Markus MA, Morris BJ. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin Interv Aging. 2008;3(2):331–339. [PMC free article] [PubMed] [Google Scholar]
- Masana L, Ros E, Sudano I, et al. Is there a role for lifestyle changes in cardiovascular prevention? What, when and how. Atheroscler Suppl. 2017;26:2–15. doi: 10.1016/S1567-5688(17)30020-X. [DOI] [PubMed] [Google Scholar]
- Matthaus B. Virgin grape seed oil: is it really a nutritional highlight? Eur J Lipid Sci Technol. 2008;110(7):645–650. doi: 10.1002/ejlt.200700276. [DOI] [Google Scholar]
- Mirkarimi M, Eskandarion S, Bargrizan M, et al. Remineralisation of Artificial caries in Primary teeth by grape seed extract: an In vitro study. J Dent Res Dent Clin Dent Prospects. 2013;7(4):206–210. doi: 10.5681/joddd.2013.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Goyal T, Mehta JL. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovasc Drugs Ther. 2011;25(5):419–429. doi: 10.1007/s10557-011-6341-5. [DOI] [PubMed] [Google Scholar]
- Moreno DA, Ilic N, Poulev A, et al. Inhibitory effects of grape seed extract on lipases. Nutrition. 2003;19(10):876–879. doi: 10.1016/S0899-9007(03)00167-9. [DOI] [PubMed] [Google Scholar]
- Nayak BS, Ramdath DD, Marshall JR, et al. Wound healing activity of the skin of the common grape (Vitis vinifera) variant, Cabernet sauvignon. Phytother Res. 2010;24(8):1151–1157. doi: 10.1002/ptr.2999. [DOI] [PubMed] [Google Scholar]
- Nayak BS, Ramdath DD, Marshall JR, et al. Wound healing properties of the oils of Vitis vinifera and Vaccinium macrocarpon. Phytother Res. 2011;25(8):1201–1208. doi: 10.1002/ptr.3363. [DOI] [PubMed] [Google Scholar]
- Olas B, Wachowicz B, Tomczak A, et al. Comparative antiplatelet and antioxidant properties of polyphenol-rich extracts from: berries of Aronia melanocarpa, seeds of grape and bark of Yucca schidigerain vitro. Platelets. 2008;19(1):70–77. doi: 10.1080/09537100701708506. [DOI] [PubMed] [Google Scholar]
- Park K, Elias PM, Hupe M. Resveratrol stimulates sphingosine-1-phosphate signaling of cathelicidin production. J Invest Dermatol. 2013;133(8):1942–1949. doi: 10.1038/jid.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry J, Hao Z, Luther M, et al. Characterization of cold-pressed onion, parsley, cardamom, mullein, roasted pumpkin, and milk thistle seed oils. J Am Oil Chem Soc. 2006;83(10):847–854. doi: 10.1007/s11746-006-5036-8. [DOI] [Google Scholar]
- Paulo L, Ferreira S, Gallardo E, et al. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J Microbiol Biotechnol. 2010;26(8):1533–1538. doi: 10.1007/s11274-010-0325-7. [DOI] [Google Scholar]
- Quinones M, Guerrero L, Suarez M, et al. Low molecular procyanidin rich grape seed extract exerts antihypertensive effect in males spontaneously hypertensive rats. Food Res Int. 2013;51(2):587–595. doi: 10.1016/j.foodres.2013.01.023. [DOI] [Google Scholar]
- Rhodes PL, Mitchell JW, Wilson MW, et al. Antilisterial activity of grape juice and grape extracts derived from Vitis vinifera variety Ribier. Int J Food Microbiol. 2006;107(3):281–286. doi: 10.1016/j.ijfoodmicro.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Stampfer MJ, Asherio A, et al. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 1993;328(20):1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- Saito M, Hosoyama H, Ariga T, et al. Antiulcer activity of grape seed extract and Procyanidins. J Agri Food Chem. 1998;46(4):1460–1464. doi: 10.1021/jf9709156. [DOI] [Google Scholar]
- Sandoo A, van Zanten JJCSV, Metsios GS, et al. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 2010;4:302–312. doi: 10.2174/1874192401004010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T, Oda E, Yamashita T, et al. Anti-thrombotic effect of proanthocyanidin, a purified ingredient of grape seed. Thromb Res. 2005;115(1–2):115–121. doi: 10.1016/j.thromres.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Santangelo C, Varì R, Scazzocchio B, et al. Poly-phenols, intracellular signalling and inflammation. Ann Ist Super Sanita. 2007;43(4):394–405. [PubMed] [Google Scholar]
- Sen CK, Khanna S, Gordillo G, et al. Oxygen, oxidants and antioxidants in wound healing an emerging paradigm. Ann N Y Acad Sci. 2002;957:239–249. doi: 10.1111/j.1749-6632.2002.tb02920.x. [DOI] [PubMed] [Google Scholar]
- Shinagawa FB, de Santana FC, Torres LRO, et al. Grape seed oil: a potential functional food? Food Sci Technol. 2015;35(3):399–406. doi: 10.1590/1678-457X.6826. [DOI] [Google Scholar]
- Simonetti P, Ciappellano S, Gardana C, et al. Procyanidins from Vitis vinifera seed: in vivo effects on oxidative stress. J Agric Food Chem. 2002;50(21):6217–6221. doi: 10.1021/jf011412+. [DOI] [PubMed] [Google Scholar]
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, et al. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res. 2005;579(1–2):200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Soto ML, Falqué E, Dominguez H. Relevance of natural phenolics from grape and derivative products in the formulation of cosmetics. Cosmetics. 2015;2:259–276. doi: 10.3390/cosmetics2030259. [DOI] [Google Scholar]
- Subramanian M, Goswami M, Chakraborty S, et al. Resveratrol induced inhibition of Escherichia coliproceeds via membrane oxidation and independent of diffusible reactive oxygen species generation. Redox Biol. 2014;2:865–872. doi: 10.1016/j.redox.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquiaga I, D’A cuna S, Dicenta S, et al. Wine grape Pomace flour improves blood pressure, fasting glucose and protein damage in Humans: a randomized control trial. Biol Res. 2015;48:49. doi: 10.1186/s40659-015-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Belles V, Torres MC, Muñiz P, Beltran S, et al. Defatted milled grape seed protects adriamycin-treated hepatocytes against oxidative damage. Eur J Nutr. 2006;45(5):251–258. doi: 10.1007/s00394-006-0591-1. [DOI] [PubMed] [Google Scholar]
- Vaquero MJR, Alberto MR, Manca de Nadra MC. Antibacterial effect of phenolic compounds from different wines. Food Control. 2007;18(2):93–101. doi: 10.1016/j.foodcont.2005.08.010. [DOI] [Google Scholar]
- Xia EQ, Deng GF, Guo YJ, et al. Biological activities of polyphenols from grapes. Int J Mol Sci. 2010;11(2):622–646. doi: 10.3390/ijms11020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZC, Yin J, Zhou B, et al. Grape seed proanthocyanidin protects liver against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress. World J Gastroenterol. 2015;21(24):7468–7477. doi: 10.3748/wjg.v21.i24.7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi SFH, Ahmed K, Yamamoto T, et al. Effect of resveratrol on Helicobacter pylori-induced interleukin-8 secretion, reactive oxygen species generation and morphological changes in human gastric epithelial cells. Biol Pharm Bull. 2009;32(11):1931–1935. doi: 10.1248/bpb.32.1931. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu S, Li L, et al. The impact of grape seed extract treatment on blood pressure changes: a meta-analysis of 16 randomized controlled trials. Medicine. 2016;95(33):e4247. doi: 10.1097/md.0000000000004247. [DOI] [PMC free article] [PubMed] [Google Scholar]