Abstract
Background
Atraumatic hip pain in children is one of the most common symptoms with which pediatricians, orthopedists, and general practitioners are confronted, with an incidence of 148 cases per 100 000 persons per year.
Methods
This article is based on publications up to April 2019 that were retrieved by a selective search in the PubMed database, including case reports and reviews.
Results
Infants with fever often have purulent coxitis, which can be diagnosed by blood tests and ultrasonography. Toddlers and older children may suffer from painful restriction of motion of the hip joint, associated with limping (antalgic gait) or even the inability to walk. The main elements of the differential diagnosis in children aged 2–10 are coxitis fugax and idiopathic necrosis of the femoral head (Perthes disease). In children aged 10 and up, and in adolescents, slipped capital femoral epiphysis (SCFE) is typical. Bone tumors and rheumatic diseases must always be considered as well. The initial diagnostic steps on presentation of a child with restricted hip movement should be plain x-rays and joint ultrasonography for the detection of an effusion. Suspicion of a tumor is the main indication for tomographic imaging (computed tomography or magnetic resonance imaging).
Conclusion
The underlying cause of hip pain in children should be diagnosed early to avoid adverse sequelae.
Joint pain is one of the commonest symptoms routinely seen by pediatricians, orthopedists, and general practitioners in children and adolescents. Most often, the painful joint is a hip joint, with an annual incidence 148.1/100 000 (1). Hip pain in children is often accompanied by a protective limp and reduced mobility of the hip. To reach a provisional diagnosis, the possible differential diagnoses for the patient’s age should be considered; since most of these conditions and most of the medical knowledge about them are age-related, this usually leads to success (2, 3).
Targeted treatment and prognosis depend on the underlying disease. A delay in diagnosis, especially in the case of septic coxitis, can lead to irreversible destruction of the hip joint.
Learning goals
Incidence.
The commonest cause of joint pain in children, with an annual incidence of 148.1/100 000, is a painful hip joint.
After reading this article, the reader will have acquired:
An overview of the basic diagnostic procedure for hip pain in children;
An understanding of the conditions that cause hip pain in children, and of their pathophysiology;
A practical algorithm for reliable diagnosis and treatment, taking account of the possible differential diagnoses for hip pain in children.
Diagnosis
The diagnosis of pediatric hip pain should always follow a standard algorithm. This starts with targeted history taking and clinical examination; imaging and/or blood tests may follow if necessary.
History taking
Clinical features.
Hip pain in children is often accompanied by a protective limp and reduced mobility of the hip.
In addition to the duration and intensity of the pain, a structured history should include whether the symptoms are related to movement and to time of day. Generalized symptoms such as fever, night sweating, and weight loss (B symptoms) may be an indication of a systemic illness or systemic involvement. In addition to general diseases, questions should be asked about the child’s neuromotor development. The Graf hip ultrasound examination technique is part of the general hip sonography screening in Germany and is therefore included in the so-called “U3 examination” (one of a series of screening examinations for children in Germany). With the results recorded in the child’s examination document, it can give an indication of any maturation deficit. If repeated fractures have occurred, or visible deformities are present, the possibility not only of child abuse, but also of skeletal disease (e.g., a form of osteogenesis imperfecta) should be considered.
Clinical examination
In a child old enough to walk and stand, gait should be observed. Often protective limping is seen; in some cases this may be the only identifiable symptom. Knee pain should also prompt examination of the hip joint, as knee pain can be an expression of projection of the posterior ramus of the obturator nerve and often masks the actual hip complaint. The patient should always be undressed for the examination, and the hip joint is examined in comparison with the contralateral side, to allow any incorrect posture, muscle atrophy, or differences between joint excursions on the two sides to be identified. Comparison between right-side and left-side internal rotation in supine and in prone positions is particularly important, as this is often the first and sometimes the only joint excursion to be restricted.
Imaging
In infants and toddlers, ultrasound is the imaging method of choice. In addition to good soft-tissue diagnostic imaging and showing any joint effusion, it also enables identification of fractures. Bilateral examination is mandatory, as any difference between the two sides is often pathological in origin. For musculoskeletal pain, the gold standard is plain radiography in two planes as the first imaging method (4). If the cause of the pain cannot be identified on radiography or ultrasonography, axial imaging should be carried out—normally magnetic resonance imaging (MRI), more rarely computed tomography (CT). These procedures are particularly valuable for tumor diagnosis. Because they are elaborate to perform (the patients may require sedation or anesthesia), they should only be used when clearly indicated, and only when the results will affect management decisions.
Common causes of hip pain
History taking.
The Graf hip ultrasound examination technique is part of the general hip sonography screening (“U3”) in Germany, with its results recorded in the child’s examination document. It can give an indication of any maturation deficit.
In children, hip pain as a symptom and the nature and earliest manifestation of the commonest hip conditions are strongly age-related. The clinician who is aware of this will be able to narrow down the possible differential diagnoses and speedily progress the diagnostic search in the right direction. The Table shows the commonest diseases of the hip joint that can cause hip pain in children together with their prevalence by age group. Below, the commonest causes of hip pain in children are described in order of age at first manifestation, together with their pathophysiology, diagnosis, and treatment. Of note is the low level of evidence, which is due to the lack of prospective randomized studies in pediatric patients.
Table. Commonest diseases of the hip in children.
| Age | Prevalence | Etiology | Clinical features | Diagnosis | Therapy | |
| Septic coxitis | 0–4 years | 1–4/ 100 000 | Hematogenous bacterial infection of the hip joint | Guarding and protective behavior, general unwellness, fever | Laboratory tests (blood count, CRP, ESR), ultrasound, arthrocentesis (cell count), blood cultures | Joint irrigation, antibiotics, mobilization |
| Transient synovitis | 2–10 years | 76/100 000 | Transient hip joint effusion, often virus-related | Protective limp, limited range of motion in the hip joint | Ultrasound, laboratory tests as indicated | Physical rest, pain relief if required |
| Perthes disease | 5–7 years | 0.4–29.0/ 100 000 | Aseptic femoral head necrosis | Protective limp, limited range of motion | Positive „figure 4“ sign, ultrasound, pelvic and axial hip radiographs | Physiotherapy, rest during periods of acute pain, avoidance of axial compressive loading |
| Juvenile rheumatoid arthritis | 2–18 years | 14.8/ 100 000 | Rheumatoid oligo- or polyarthritis of the hip joint | Protective limp, limited range of motion | Laboratory tests (blood count, CRP, ESR, ANA, rheumatoid factors, HLA-B27), ultrasound, AP radiograph, MRI as required | Pain relief (NSAIDs), DMARDs (e.g., MTX), biologics (e.g., etanercept), physiotherapy |
| Benign and malignant tumors | 2–18 years | 5% of all tumors | Space-occupying lesion with chondral, osseous or connective tissue components | Uninterrupted pain or nocturnal pain | Radiography in two planes, MRI, CT, bone scintigraphy | No-touch lesion, chemo-/radiotherapy, surgical resection |
| Slipped capital femoral epiphysis | Adolescence | 0.33–24.58/ 100 000 | Nontraumatic epiphyseal slippage | Protective limp, limited range of motion, positive Drehmann sign | Ultrasound, pelvic and axial (“true lateral” as described by Imhäuser) hip radiographs | In situ screw fixation, osteotomy, Ganz/modified Dunn osteotomy, contralateral prophylactic screw placement |
| Hip dysplasia, hip displacement | Adolescence | 2–5% | Underdevelopment of the hip joint, impaired ossification of the acetabulum | Pain on movement of the hip | Pelvic radiograph, axial hip radiograph | Triple osteotomy, hip replacement |
ANA, antinuclear antibodies; AP, anteroposterior; CRP, C-reactive protein; DMARDs, disease-modifying antirheumatic drugs; ESR, erythrocyte sedimentation rate; MRI, magnetic resonance imaging; NSAIDs, nonsteroidal anti-inflammatory drugs; MTX, methotrexate; OT, osteotomy
Septic coxitis
Septic coxitis.
Septic coxitis is a hematogenous bacterial infection of the hip joint that can in principle occur at any age, but is seen predominantly in infants and toddlers (below the age of four).
Septic coxitis is a hematogenous bacterial infection of the hip joint that can in principle occur at any age, but is seen predominantly in infants and toddlers (below the age of four). As the epiphyseal growth plate does not yet function as a protective barrier at this age, the pathogens spread into the epiphysis. The commonest pathogen is Staphylococcus aureus. Septic coxitis can also occur as a sequela of acute or subacute osteomyelitis. Clinically, in over 90% of cases generalized symptoms such as fever and general unwellness are seen (5, 6). Movement is restricted by pain, with protective limping and guarding. The parents of infants with septic coxitis often report poor feeding and loud crying, especially when the hip is abducted (e.g., during diaper changing). Septic coxitis represents an absolute emergency and must therefore be rapidly diagnosed and immediately treated to avoid risking destruction of the femoral head and epiphysiolysis. Only if appropriate treatment is instigated within 3 days of disease onset can irreparable damage to the cartilage be prevented (7). After the general clinical examination, therefore, if septic coxitis is suspected, ultrasonography of both hips should be carried out at once. In addition, blood is taken to determine inflammation markers (C-reactive protein value, leukocyte count, and erythrocyte sedimentation rate), which in infants may be increased only slightly or not at all even in the presence of massive joint empyema. If evidence of joint effusion is shown and blood tests show elevated inflammation markers, arthrocentesis must be carried out immediately, and if macroscopic pus is present in the aspirate, surgical irrigation must follow the aspiration (8). For this reason, in infants and toddlers it is advisable to perform the arthrocentesis under anesthesia, so as to be in a position to irrigate the joint in the same session, without further delay. If the aspirate is grossly normal, the leukocyte count should be ascertained and native samples sent for microbiologic and pathologic analysis. To complete the diagnostic tests, before antibiotic treatment is started, blood cultures should also be obtained. Postoperatively, empiric antibiotic treatment should be started with a second-generation cephalosporin (8); if necessary, this can be changed once the antibiogram is available. The decision as to whether “second-look surgery” is indicated is made on the basis of the clinical course and/or the lab-determined inflammation markers (9, 10). Besides antibiotic treatment for a total of 4 to 6 weeks (11), follow-up care includes immediate postoperative hip joint mobilization, as otherwise the risk is that the joint will stiffen. Parents should always be told of the risk that changes in growth may occur after septic coxitis, possibly necessitating orthopedic treatment later on for axis correction.
Joint effusion.
If evidence of joint effusion is shown and blood tests show elevated inflammation markers, arthrocentesis must be carried out immediately.
„Second-look“surgery.
The decision as to whether “second-look surgery” is indicated is made on the basis of the clinical course and/or the lab-determined inflammation markers.
Transient synovitis
Transient synovitis (also called coxitis fugax, and in everyday clinical practice “irritable hip”), is not strictly a disease but a symptom. Pathophysiologically, this is a transient, painful joint effusion that occurs after a recent viral infection. In many cases, movement of the affected joint is restricted by pain; in clinical terms this may range from a slight limp to complete immobilization of the patient. However, unlike with septic coxitis, there are no generalized signs of illness (12, 13). Transient synovitis is self-limiting, with an average duration of symptoms of 5 days, or up to 14 days if the course is protracted. The differential diagnosis should rule out septic coxitis. Ultrasonography shows an effusion, but without elevated inflammatory markers (14). In most cases only pain relief as needed (adjusted for weight) and resting of the joint are required. Therapeutic arthrocentesis should be considered only if the joint effusion is massive and the pain severe. The question of whether transient synovitis predisposes to the later development of Perthes disease has occasionally been raised, but has been answered in the negative (15). If pain is persistent or recurrent, the patient should re-attend so that other causes can be excluded.
Perthes disease (Legg–Calvé-Perthes disease)
Transient synovitis.
Transient synovitis is self-limiting, with an average duration of symptoms of 5 days, or up to 14 days if the course is protracted. The differential diagnosis should rule out septic coxitis.
Perthes disease.
Perthes disease is aseptic femoral head necrosis in a toddler. It is caused by disruption of the blood flow, but the cause of this disruption is unclear.
Perthes disease is an aseptic femoral head necrosis in a toddler. It is caused by disruption of the blood flow, but the cause of this disruption is unclear (16). The exact pathological mechanism remains unknown, but predisposing factors have been identified and include genetic factors, vascularization, coagulation disorders, growth factors, and social conditions. Boys are affected four times as often as girls (17). In clinical practice, the classifications introduced by Catterall and Herring are widely used. Catterall’s classification records the extent of the necrotic area, while Herring’s focuses on the significance of the height of the lateral pillar (18, 19). Of the “head-at-risk” signs described by Catterall, which include lateral calcification, subluxation/lateralization, metaphyseal involvement, horizontalization of the growth plate, and Gage’s sign, only lateral calcification and subluxation/lateralization have been proven to have prognostic significance (20). Collapse of the femoral head is a self-limiting process and typically passes through the stages described by Waldenström (21) within an average of 2 years, with reorganization occurring in the final stage (figure 1). Stulberg’s classification, based on the shape of the repaired femoral head, is used to describe the congruence of the two parts of the joint (femoral head and acetabulum) (22). Clinically, limping is seen in affected children, together with reduced but often pain-free mobility. Typically, the “figure 4 sign” on the affected side is positive. For this, with the child supine, the foot of the leg being assessed is placed against the contralateral knee such that flexion of about 45° at the hip and 90° at the knee occurs. In patients with a normal hip joint, this position when viewed from above produces the shape of a figure “4.” If Perthes’ disease is suspected, after bilateral hip ultrasonography, radiography in two planes is carried out: an anteroposterior (AP) pelvic view and a Lauenstein axial view of the affected side. In the early stage, joint effusion is seen only on ultrasound, whereas radiography shows no abnormality. To confirm the diagnosis at this early stage, MRI may be performed (17). The goal of treatment is always to maintain joint mobility through regular intensive physiotherapeutic exercise. Both radiologically and clinically, joint congruence—containment of the two parts of the joint—plays the key role. Loss of containment must be prevented by every possible means, so if lateralization occurs, surgical centralization of the joint with pelvic and/or intertrochanteric osteotomy may become necessary. Only during periods of severe pain should weightbearing be avoided. The most important prognostic factors for the course of this disease are the patient’s age at diagnosis (age at onset <6 years is favorable), the initial classification of severity (Catterall and/or Herring), and the recorded Catterall “head-at-risk signs” (23– 25).
Figure 1.
Radiographic staging of Perthes disease (Waldenström classification)
a) Early stage; b) condensation; c) fragmentation; d) reossification; e) healed
Slipped capital femoral epiphysis
Treatment of Perthes disease.
The goal of treatment is always to preserve joint congruence (containment).
Important prognostic features are:
The patient’s age at diagnosis (age at onset < 6 years is favorable), the initial classification of severity (Catterall and/or Herring), and two of Catterall‘s “head-at-risk signs”: lateral calcification and subluxation/lateralization.
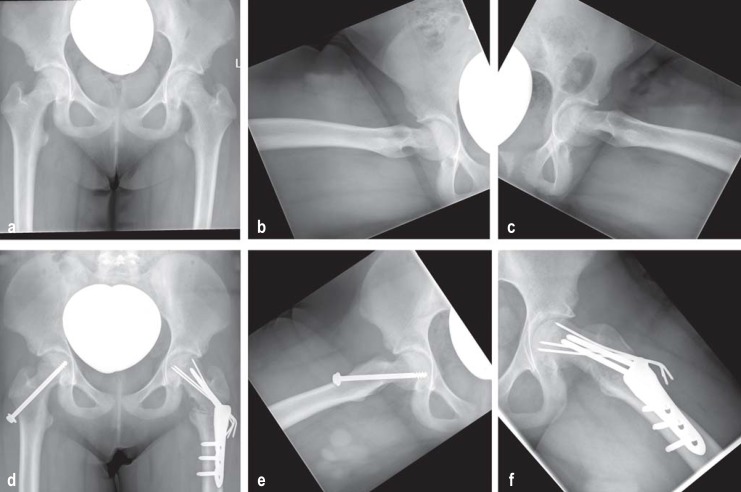
The pathophysiology of this nontraumatic epiphysiolysis in adolescents is multifactorial. Boys are somewhat more frequently affected than girls. There appears to be a correlation between hormones, obesity, and high mechanical load. Clinically, an acute form (symptoms <2 weeks) and a chronic form of the disease (symptoms >2 weeks) are distinguished. A special form is the “acute on chronic” form, in which a sudden increase in pain occurs in a patient with a longstanding positive history. Often, the patient reports localized pain in the knee but examination reveals painful reduced mobility of the hip (especially in internal rotation) in a patient in otherwise good health (26). A positive Drehmann sign on the affected side (where the examiner notes unavoidable passive external rotation of the hip—i.e., internal rotation of the lower leg—when the hip joint is flexed) is pathognomonic. The natural course of the disease is for the symptoms to gradually disappear spontaneously, and as a result diagnosis is often delayed (27). In cases where slipped capital femoral epiphysis (SCFE) is suspected, the next diagnostic step should be radiography in two planes (AP pelvic view and Lauenstein axial view of the affected side). MRI does not have a role in the diagnostic procedure in the early stage of SCFE (4). Surgical treatment depends on the angle of slip of the femoral head from the femoral neck. In German-speaking countries, where the angle of slip is up to 30°, “in situ screw fixation” using one screw or three K wires is carried out to prevent further slipping of the growth plate. Where the angle of slip is greater than 30°, a pre-arthritic deformity is present, so reshaping osteotomies are performed in addition (figure 2). Finally, surgical hip dislocation with open reduction and fixation of the epiphysis (periacetabular osteotomy) and modified Dunn osteotomy are becoming more widely practiced. Complication rates of up to 37% are reported for these procedures; the rate of femoral head necrosis is reported at 23%, and there is a significant correlation between complication rate and surgical expertise (28). SCFE should always be treated surgically; the acute form should be treated emergently and the chronic form within a short time. Since in 50% of cases slipping will at some time occur in the normal hip as well, prophylactic screw placement on the contralateral side is recommended.
Figure 2.
Radiographic diagnosis of slipped capital femoral epiphysis and its surgical management, showing the time course
a) Preoperative anteroposterior (AP) pelvic radiograph; b) Preoperative true lateral view of the right hip;
c) Preoperative true lateral view of the left hip; (pathological: slippage >50°);
d) Postoperative AP pelvic radiograph after prophylactic in situ screw placement (right hip) and valgization–flexion–derotation osteotomy (Weber–Imhäuser osteotomy) (left hip);
e) Postoperative axial view of the right hip with prophylactic in situ screw placement
f) Postoperative axial view of the left hip with valgization–flexion–derotation osteotomy (Weber–Imhäuser osteotomy)
Hip dysplasia and hip dislocation
Slipped capital femoral epiphysis.
The pathophysiology of this nontraumatic epiphysiolysis of the adolescent is multifactorial.
Symptoms of slipped capital femoral epiphysis.
Often, the patient reports localized pain in the knee but examination reveals painful reduced mobility of the hip (especially in internal rotation) in a patient in otherwise good health.
These clinical entities represent a congenital or acquired malformation of the acetabulum, which fails to provide a firm seating for the as yet soft, cartilaginous femoral head. The most extreme form is hip dislocation. At present, the question whether introducing general ultrasound hip screening for neonates could bring an improvement in terms of sensitivity and specificity, or any advantage compared to targeted screening by clinical examination, is still under debate (29). A retrospective analysis showed that the introduction of general ultrasound screening as part of the U3 examination led to a marked reduction in the number of surgical interventions in young children (toddlers), by 46% (30). However, it remains unclear whether only unstable and dislocated hips should be diagnosed and treated, or dysplastic hips as well.
Hip dysplasia and hip displacement.
Worldwide, abnormalities of hip development together with the resulting degenerative changes are among the commonest skeletal disorders seen in adolescents.
Children with spastic cerebral palsy.
It is known that the affected children are born with normal hips, and only gradually, as a result of the spastic muscle imbalance, does hip lateralization and, later, hip displacement develop.
Worldwide, abnormalities of hip development together with the resulting degenerative changes are among the commonest skeletal disorders seen in adolescents (31– 34), although given the lack of prospective studies, it is not possible to tell the correlation between the dysplasia grade shown on ultrasound and the degenerative changes resulting from it. The course and prognosis in children with spastic cerebral palsy should be considered separately. It is known that the affected children are born with normal hips, and only later, as a result of the spastic muscle imbalance, does hip lateralization and, later, hip displacement develop (35). The higher an affected child’s Gross Motor Function Classification System (GMFCS) score (the more severe the affection, the higher the score), the greater the probability of secondary hip dysplasia and/or displacement; it is highest in nonambulant children with a high GMFCS score. One goal in the management of patients with cerebral palsy is, therefore, prevention of hip displacement, and for this reason regular radiographic checkups should be carried out in the outpatient setting (36). Clinically, stress-related pain and markedly reduced mobility are not seen until the advanced stage of hip dysplasia/displacement. In patients with higher-grade findings, limping and a positive Trendelenburg sign are observed. The method of choice for diagnosing hip dysplasia/displacement in a child over the age of 1 year is a pelvic radiograph to assess the acetabular roof. The angular relationships in the acetabulum are first recorded as the acetabular (AC) angle and, in the older child, the center–edge angle (CE angle after closure of the Y cartilage. During growth, the AC angle should continuously diminish and the acetabular roof flatten off, so that by the time the child is 5 years old the AC angle ideally be <20° (37). The CE angle primarily describes the relationship between the femoral head and the acetabulum, and along with a number of other indicators mainly described the coverage of the femoral head, which in adults is a mean of about 35° (<20° is pathological) (38). The Gocht–Shenton–Ménard line is a curved line on the pelvic radiograph, running from the superior border of the obturator foramen and along the inferomedial border of the proximal part of the femur. A break in the continuity of this line is evidence of hip dysplasia or dislocation (figure 3). In patients with high-grade dysplasia, MRI can yield information about the condition of the cartilage. The treatment of choice is surgery at a more advanced age, and so long as the cartilage is in good condition, should consist of joint-preserving osteotomy (figure 4). However, where defects are extensive, joint replacement may be necessary (38, 39).
Figure 3.
Measurements on a pelvic radiograph, showing the reference lines and angular measurements (AC and CE) in an assessment of hip dysplasia/displacement
Figure 4.
Radiographic diagnosis of bilateral hip dysplasia and its surgical treatment with bilateral triple osteotomy
a) Preoperative AP pelvic radiograph;
b) postoperative pelvic radiograph showing right-side triple osteotomy with persistent left hip dysplasia;
c) postoperative pelvic radiograph with additional left-side triple osteotomy (i.e., now bilateral osteotomies after initial bilateral dysplasia; in the time elapsed between the two procedures, some metal parts have been removed from the right acetabular osteotomy and bone remodeling has begun.
Important entities in the differential diagnosis of hip pain in children
Once the above conditions have been ruled out as the cause of hip pain in children, diseases that are not predominantly age-related should be considered.
Of particular importance in the differential diagnosis, therefore, are juvenile rheumatoid arthritis and tumors.
Juvenile rheumatoid arthritis
Management of patients with cerebral palsy.
One goal in the management of patients with cerebral palsy is, therefore, prevention of hip displacement, and for this reason regular radiographic checkups should be carried out in the outpatient setting.
In rheumatic disease, the hip joint can be affected either as the sole joint (monoarthritis) or as one of multiple joints (oligo- or polyarthritis) (40). The causes of juvenile rheumatoid arthritis (JRA) are multifarious, and in most cases no specific cause can be identified. Genetic factors and environmental factors can both contribute to the development of the disease. Various immunological patterns (HLA types) can increase the probability of development of a rheumatic disease (e1). A change in gait is symptomatic, and may be as extreme as a refusal to walk. Usually, the pain that causes this change is due to joint effusion (identifiable on ultrasound), synovial thickening, and reduced mobility. Destruction of the cartilage and bone structures is rare in children and adolescents, and only to be anticipated in long-term cases that are refractory to treatment. The diagnosis of JRA can sometimes be difficult, because the clinical features of these diseases vary so widely. The key element is the history, with an exact record of the circadian timing of symptoms and whether they relate to any sports activities and/or infections (reactive arthritis). In terms of imaging, ultrasound usually suffices, but occasionally MRI is required for to distinguish between inflammation and neoplastic disease (4). Arthrocentesis should always be carried out when an infectious etiology of a joint effusion seems possible, and in rare cases to relieve the joint. In addition to determining inflammation markers, laboratory tests should rule out infectious pathogens, including Borrelia and viruses, that can cause reactive arthritis. To confirm the diagnosis, antinuclear antibodies, rheumatic factors, and HLA-B27 should also be determined (e2). The treatment of JRA follows the general staged scheme for the treatment of rheumatic diseases of childhood, starting with non-steroidal antiinflammatory drugs (e.g., naproxen). Depending on the further symptoms and response to treatment, another disease-modifying antirheumatic drug (DMARD; e.g., methotrexate) and a biologic (e.g., etanercept) may be started (e3). The goal should be treatment escalation up to the point where children can do everything that their peers do. To achieve this, concomitant intensive physiotherapy is useful, both to strengthen the musculature and to prevent the contraction of tendons and ligaments and of the joint capsules (e3). When treatment is escalated in this way, surgery is less often required (e4).
Tumors
Juvenile rheumatoid arthritis.
In juvenile rheumatoid arthritis, arthrocentesis should always be carried out when an infectious etiology of the joint effusion seems possible, and in rare cases to relieve the joint.
In terms of differential diagnosis, the presence of any benign or malignant tumor that originates in the pelvis, proximal femur, or their surrounding soft tissue, should always be ruled out. Musculoskeletal tumors can be divided into primary bone tumors and primary soft tissue tumors. Most space-occupying lesions, such as osteochondroma, enchondroma, osteoid osteoma, cartilaginous exostoses, and cysts, are benign. Osteosarcoma and Ewing sarcoma are comparatively rare: only about 5% of all malignant tumors in children are bone sarcomas, which have a peak incidence between the ages of 10 and 15 years (e5). The borderline between benign and malignant tumors is not always sharply defined: there are semi-malignant tumors which fulfill all the criteria of the malignant tumor but do not metastasize. A particular entity is the “tumor-like lesion.” Tumor-like lesions show clinical and radiological features of a bone tumor without fulfilling the criteria of a true tumor. Clinically, these various tumors and tumor-like lesions vary greatly in their behavior; benign lesions are frequently unnoticed until they are discovered as an incidental finding. In general, symptoms depend on the rate of tumor growth: a faster rate of growth is more often painful. The pain is usually less during stress than at night or at rest. About twos-third of bone tumors occur during the pubertal growth period. For diagnosis, radiography and one other imaging technique are required, preferably contrast MRI, otherwise CT and/or two-phase bone scintigraphy. If the presence of a malignant lesion cannot be definitely ruled out on imaging, a biopsy sample is taken through the route of any surgical approach that may be required later, to allow the tumor to be classified precisely. Subsequent management is according to the nature and stage of the tumor.
Tumors as a cause of hip pain.
Musculoskeletal tumors can be divided into primary bone tumors and primary soft tissue tumors. Most space-occupying lesions are benign. Only about 5% of all malignant tumors in children are bone sarcomas.
Further information on CME.
Participation in the CME certification program is possible only over the Internet: cme.aerzteblatt.de. This unit can be accessed until 26 April 2020. Submissions by letter, e-mail or fax cannot be considered.
Vitamin Substitution Beyond Childhood—Requirements and Risks” (issues 1–2/2020) until 5 April 2020,
Acute Kidney Injury—A Frequently Underestimated Problem in Perioperative Medicine” (issue 49/2019) until 1 March 2020.
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN), which is found on the CME card (8027XXXXXXXXXXX). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or else entered in “Meine Daten,” and the participant must agree to communication of the results.
CME credit for this unit can be obtained via cme.aerzteblatt.de until 26 April 2020. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Which of the following conditions causing pain in the hip joint is mainly seen in infants?
Perthes disease
Slipped capital femoral epiphysis
Juvenile rheumatoid arthritis
Ewing sarcoma
Septic coxitis
Question 2
What should be the next diagnostic investigation in a child in whom the general clinical examination suggests septic coxitis?
Radiography and hip spica cast
Magnetic resonance imaging and arthrocentesis
Ultrasonography and blood tests
Bone scintigraphy and physiotherapy
Computed tomography and Bobath therapy
Question 3
In terms of the differential diagnosis, what distinguishes transient synovitis from septic coxitis?
Transient synovitis has a genetic origin.
Transient synovitis is caused by an abnormal fetal position
Transient synovitis more often occurs in children with trisomy 21
Transient synovitis is a sequela of subacute osteomyelitis
In transient synovitis, ultrasonography of the joint effusion does not show any inflammatory markers
Question 4
How soon must treatment for septic coxitis start if irreversible damage is to be prevented?
Within 3 days of disease onset
Within 7 days of disease onset
Within 11 days of disease onset
Within 15 days of disease onset
Within 19 days of disease onset
Question 5
Which of the following diagnostic factors is widely used for classification of the severity of Perthes disease?
Patrick test
Tap test for trochanteric pressure pain
Computed tomography of the hip
Catterall‘s „head-at-risk“ signs
Bone scintigraphy
Question 6
Which of the following treatments is most commonly indicated for transient synovitis?
Physical rest and weight-adjusted pain relief as needed
Biologics and microwave therapy
Antibiotics and cold therapy
Hip replacement and rehabilitation
Spica cast followed by physiotherapy
Question 7
For which disease is the Drehmann sign pathognomonic?
Juvenile rheumatoid arthritis
Transient synovitis
Hip dysplasia
Slipped capital femoral epiphysis
Perthes disease
Question 8
What is the treatment of choice for septic coxitis?
Joint irrigation and antibiotics
Mobilization and heat
Rest and pain relief
Joint replacement and physiotherapy
Avoidance of axial compressive forces and administration of biologics
Question 9
In adolescents with hip dysplasia but with cartilage in good condition, what is the treatment of choice?
Heat treatment and physiotherapy
Intra-articular injection of hyaluronic acid
Joint-preserving osteotomy
Joint replacement
Cartilage transplant surgery
Question 10
What is characteristic of pain that is related to a fast-growing pelvic tumor?
The pain only occurs when the tumor is a bone sarcoma.
Generally, the pain is less intense during weightbearing than at night or when at rest.
The pain occurs on weightbearing.
The pain is cyclical.
The pain only starts after the initiation of medical treatment.
►Participation is possible only via the Internet: cme.aerzteblatt.de
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- 1.Krul M, van der Wouden JC, Schellevis FG, van Suijlekom-Smit LW, Koes BW. Acute non-traumatic hip pathology in children: incidence and presentation in family practice. Fam Pract. 2010;27:166–170. doi: 10.1093/fampra/cmp092. [DOI] [PubMed] [Google Scholar]
- 2.Konermann W, De Pellegrin M. Die Differenzialdiagnose der kindlichen Hüftschmerzen im Sonogramm. Der Orthopäde. 1993;22:280–287. [PubMed] [Google Scholar]
- 3.Konermann W, Gruber G. Hüftgelenkerkrankungen im Kindes- und Jugendalter - sonographische Differenzialdiagnosen. Der Orthopäde. 2002;31:288–292. doi: 10.1007/s00132-001-0255-z. [DOI] [PubMed] [Google Scholar]
- 4.Tallen G, Bielack S, Henze G, et al. Musculoskeletal pain: a new algorithm for differential diagnosis of a cardinal symptom in pediatrics. Klin Padiatr. 2014;226:86–98. doi: 10.1055/s-0034-1366989. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Aggarwal AN. Bone and joint infections in children: Septic Arthritis. Indian J Pediatr. 2016;83:825–833. doi: 10.1007/s12098-015-1816-1. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal A, Aggarwal AN. Acute hematogenous osteomyelitis. Indian J Pediatr. 2016;83:817–824. doi: 10.1007/s12098-015-1806-3. [DOI] [PubMed] [Google Scholar]
- 7.Grill T, Rustler T. Spätfolgen der Säuglingskoxitis. Orthopäde. 1997;26:848–857. doi: 10.1007/s001320050164. [DOI] [PubMed] [Google Scholar]
- 8.Leitlinie Bakterielle Gelenkinfektionen. www.awmf.org/uploads/tx_szleitlinien/012-010l_S1_ Bakterielle_Gelenkinfektionen_ 2014-06-abgelaufen.pdf. 2014 [Google Scholar]
- 9.Rutz E, Brunner R. Septic arthritis of the hip—current concepts. Hip Int. 2009;19:S9–S12. doi: 10.1177/112070000901906s03. [DOI] [PubMed] [Google Scholar]
- 10.Stutz G, Gächter A. Diagnostik und stadiengerechte Therapie von Gelenkinfekten. Unfallchirurg. 2001;104:682–686. doi: 10.1007/s001130170068. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Arias M, Balsa A, Mola EM. Septic arthritis. Best Pract Res Clin Rheumatol. 2011;25:407–421. doi: 10.1016/j.berh.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Caird MS, Flynn JM, Leung YL, Millman JE, D`Italia JG, Dormans JP. Factors distinguishing septic arthritis from transient synovitis of the hip in children A prospective study. J Bone Joint Surg Am. 2006;88:1251–1257. doi: 10.2106/JBJS.E.00216. [DOI] [PubMed] [Google Scholar]
- 13.Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81:1662–1670. doi: 10.2106/00004623-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Bernd L, Niethard FU, Graf J, Kaps H-P. Die flüchtige Hüftgelenksentzündung. Z Orthop Unfall. 1992;130:529–535. doi: 10.1055/s-2008-1039665. [DOI] [PubMed] [Google Scholar]
- 15.Stobbe S, Pennekamp PH, Filler T, Gödecke S, Lieb A, Placzek R. Does coxitis fugax predispose for later Perthes` disease?—first results of an insurance data-based study. Z Orthop Unfall. 2015;153:80–84. doi: 10.1055/s-0034-1383347. [DOI] [PubMed] [Google Scholar]
- 16.Catterall A. The natural history of Perthes` disease. J Bone Joint Surg Br. 1971;53:37–53. [PubMed] [Google Scholar]
- 17.Nelitz M, Lippacher S, Krauspe R, Reichel H. Perthes disease—current principles of diagnosis and treatment. Dtsch Arztebl Int. 2009;106:517–523. doi: 10.3238/arztebl.2009.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catterall A. Natural history, classification, and x-ray signs in Legg-Calvé-Perthes’ disease. Acta Orthop Belg. 1980;46:346–351. [PubMed] [Google Scholar]
- 19.Herring JA, Neustadt JB, Williams JJ, Early JS, Browne RH. The lateral pillar classification of Legg-Calvé-Perthes Disease. J Pediatr Orthop. 1992;12:143–150. doi: 10.1097/01241398-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Westhoff B, Martiny F, Krauspe R. Perthes Disease. Z Orthop Unfall. 2014;152:617–633. doi: 10.1055/s-0034-1382865. [DOI] [PubMed] [Google Scholar]
- 21.Waldenström H. The Definite form of the coxa plana. Acta Radiol. 2016;57:e79–e94. doi: 10.1177/0284185116642923. [DOI] [PubMed] [Google Scholar]
- 22.Herring JA, Kim HAT, Browne R. Legg-Calve-Perthes disease. Part I: Classification of radiographs with use of the modified lateral pillar and Stulberg classifications. J Bone Joint Surg Am. 2004;86-A:2103–2120. [PubMed] [Google Scholar]
- 23.Farsetti P, Tudisco C, Caterini R, Potenza V, Ippolito E. The Herring lateral pillar classification for prognosis for Perthes Disease Late results in 49 patients treated conservatively. J Bone Joint Surg Br. 1995;77:739–742. [PubMed] [Google Scholar]
- 24.Herring JA, Kim HT, Browne R. Legg-Calvé-Perthes Disease Part II: Prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am. 2004;86-A:2121–3134. [PubMed] [Google Scholar]
- 25.Wiig O, Terjesen T, Svenningsen S. Prognostic factors and outcome of treatment in Perthes` disease: a prospective study of 368 patients with five-year follow-up. J Bone Joint Surg Br. 2008;90:1364–1371. doi: 10.1302/0301-620X.90B10.20649. [DOI] [PubMed] [Google Scholar]
- 26.Bollmann C, Wirth T. Verzögerte Diagnosestellung bei Epiphyseolysis capitis femoris. Orthop Praxis. 2009;45:108–112. [Google Scholar]
- 27.Kocher MS, Bishop JA, Weed B, et al. Delay in diagnosis of slipped capital femoral epiphysis. Pediatrics. 2004;113:e322–e325. doi: 10.1542/peds.113.4.e322. [DOI] [PubMed] [Google Scholar]
- 28.Upasani VV, Matheney TH, Spencer SA, Kim YJ, Millis MB, Kasser JR. Complications after modified Dunn osteotomy for the treatment of adolescent slipped capital femoral epiphysis. J Pediatr Orthop. 2014;34:661–667. doi: 10.1097/BPO.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 29.Shorter D, Hong T, Osborn DA. Cochrane Review: Screening programmes for developmental dysplasia of the hip in newborn infants. Evid Based Child Health. 2013;8:11–54. doi: 10.1002/ebch.1891. [DOI] [PubMed] [Google Scholar]
- 30.Thallinger C, Pospischill R, Ganger R, Radler C, Krall C, Grill F. Long- term results of nationwide general ultrasound screening system for developmental disorders of the hip: the Austrian hip screening program. J Child Orthop. 2014;8:3–10. doi: 10.1007/s11832-014-0555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitz MR, Blumberg TJ, Nelson SE, Sees JP, Sankar WN. What`s new in pediatric hip? J Pediatr Orthop. 2018;38:e300–e304. doi: 10.1097/BPO.0000000000001166. [DOI] [PubMed] [Google Scholar]
- 32.Wyles CC, Heidenreich MJ, Jeng J, Larson DR, Trousdale RT, Sierra RJ. The John Charnley Award: Redefining the natural history of osteoarthritis in patients with hip dysplasia and impingement. Clin Orthop Relat Res. 2017;475:336–350. doi: 10.1007/s11999-016-4815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Kries R, Ihme N, Altenhofer L, Niethard FU, Krauspe R, Rückinger S. General ultrasound screening reduces the rate of first operative procedures for developmental dysplasia of the hip: a case-control study. J Pediatr. 2012;160:271–275. doi: 10.1016/j.jpeds.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 34.Placzek R. Hüftgelenkdysplasie und Hüftgelenkluxation. In: Wirtz DC, Stöckle U, editors. Hüfte: Expertise Orthopädie und Unfallchirurgie. Stuttgart Thieme: 2018. pp. 95–98. [Google Scholar]
- 35.Hägglund G1, Alriksson-Schmidt A1, Lauge-Pedersen H1, Rodby-Bousquet E2, Wagner P2, Westbom L1. Prevention of dislocation of the hip in children with cerebral palsy: 20-year results of a population-based prevention programme. Bone Joint J. 2014;96-B:1546–1552. doi: 10.1302/0301-620X.96B11.34385. [DOI] [PubMed] [Google Scholar]
- 36.Thielemann F, Ziegler J, Günther KP. Diagnostik und Therapie der Hüftdysplasie. Kinder und Jugendmedizin. 2007;7:371–378. [Google Scholar]
- 37.Tönnis D. Die angeborene Hüftdysplasie und Hüftluxation im Kindes- und Erwachsenenalter. Berlin: Springer. 1984:129–134. [Google Scholar]
- 38.Wiberg G. Studies on dysplastic acetabula and congenital subluxation of the hip joint: with special reference to the complication of osteoarthritis. Acta Chir Can. 1939;58:7–38. [Google Scholar]
- 39.Tönnis D. Hüftdysplasie - Was ist bei der dreifachen Beckenosteotomie zu beachten? Z Orthop Unfallchir. 2008;146:564–569. [Google Scholar]
- 40.Rietschel C, Latta K. Rheumatische Gelenkerkrankungen im Kindes- und Jugendalter. Der Orthopäde. 2012;41:227–240. doi: 10.1007/s00132-011-1879-2. [DOI] [PubMed] [Google Scholar]
- E1.Prahalad S, Shear ES, Thompson SD, Giannini EH, Glass DN. Increased prevalence of familial autoimmunity in simplex and multiplex families with juvenile rheumatoid arthritis. Arthritis Rheum. 2002;46:1851–1856. doi: 10.1002/art.10370. [DOI] [PubMed] [Google Scholar]
- E2.Wallace CA, Giannini EH, Huang B, et al. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011;63:929–936. doi: 10.1002/acr.20497. [DOI] [PubMed] [Google Scholar]
- E3.Leitlinie Juvenile Idiopathische Arthritis 2011. www.awmf.org/ uploads/tx_szleitlinien/027-020l_S2K_Juvenile_Idiopathische_Arthritis_2011-10_abgelaufen.pdf (last accessed on 17 October 2019) [Google Scholar]
- E4.Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69:868–871. doi: 10.1136/ard.2009.112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Kinderkrebsregister. Jahresbericht. www.kinderkrebsregister.de/typo3temp/secure_downloads/22605/0/2df4719687ba2596d4216218a4f4632763b64847/jb2018_WEB_oor_s.pdf (last accessed on 17 October 2019) 2018 [Google Scholar]