Abstract
Objective
The objective of this study was to measure the special expression pattern of lipid metabolism genes and investigate the molecular mechanisms underlying intramuscular fat (IMF) deposition in Longissimus dorsi muscle of Laiwu pigs.
Methods
Thirty-six pigs (Laiwu n = 18; Duroc×Landrace×Yorkshire n = 18) were used for the measurement of the backfat thickness, marbling score, IMF content, and expression of lipid metabolism genes.
Results
Significant correlations were found between IMF content and the mRNA expression of lipid metabolism genes. Of the 14 fat deposition genes measured, fatty acid synthase (FASN) showed the strongest correlation (r = 0.75, p = 0.001) with IMF content, and of the 6 fat removal genes, carnitine palmitoyl transferase 1B (CPT1B) exhibited the greatest negative correlation (r = −0.66, p = 0.003) with IMF content in Laiwu pig. Multiple regression analysis showed that CPT1B, FASN, solute carrier family 27 member 1 (SLC27A1), and fatty acid binding protein 3 (FABP3) contributed 38% of the prediction value for IMF content in Laiwu pigs. Of these four variables, CPT1B had the greatest contribution to IMF content (14%) followed by FASN (11%), SLC27A1 (9%), and FABP3 (4%).
Conclusion
Our results indicate that the combined effects of an upregulation in fat deposition genes and downregulation in fat removal genes promotes IMF deposition in Laiwu pigs.
Keywords: Gene Expression, Intramuscular Fat, Laiwu Pig, Lipid Metabolism, Longissimus dorsi Muscle
INTRODUCTION
Porcine intramuscular fat (IMF) and backfat thickness are essential determinants of fresh meat quality in pig production. It is widely accepted that a higher IMF content has a positive effect on the sensory experience associated with eating better quality meat [1]. The IMF content is typically higher in Chinese indigenous pig breeds than in Western pig breeds and commercial pigs. The Laiwu pig is a Chinese indigenous black pig breed that exhibits excellent meat quality with a particularly high IMF content [2,3].
The IMF is influenced by genetic and other factors, such as age, gender, and nutrition; however, genetic determinants offer the best platform for determining the molecular mechanisms of IMF deposition. Thus, genetic and genomic approaches have been used to identify quantitative trait loci and to evaluate potential candidate genes for IMF deposition in pigs [4,5]. These studies have identified many candidate genes, including some that can be used as biomarkers for the IMF trait [6,7].
The combined effects of lipid metabolism genes on IMF deposition have not yet been reported for the Laiwu pig. The objectives of this research were to measure the expression pattern of lipid metabolism genes in the Longissimus dorsi (LD) muscle of Laiwu pigs, and to use this information to elucidate the molecular mechanisms underlying IMF deposition in this breed.
MATERIALS AND METHODS
This work was approved by the Institutional Animal Care and Use Ethics Committee of Shandong Agricultural University and carried out in accordance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology (Beijing, PR China).
Animals, sample collection and lipid metabolism genes
A total of 36 castrated boars (18 Laiwu pigs and 18 Duroc× Landrace×Yorkshire [DLY] pigs) were selected and managed in two groups at a Laiwu pig breeding farm, and fed the same commercial fattening diet and water was provided ad libitum. The pigs were handled according to the “Regulations on Administration of Hog Slaughter” and “Good manufacturing practice for pig slaughter (GB/T 19479-2004)” of China and slaughtered following standard industry procedures. The initial body weight of the pigs was 30 kg. When the average live weight of the pigs was 80±5 kg, the pigs were slaughtered at a local commercial abattoir following standard industry procedures. Immediately after slaughter, two samples of LD muscle from the left side of the last rib of each pig were collected. One sample was frozen in liquid nitrogen and stored at −80°C for gene expression analysis, and another was stored at 4°C for analysis of IMF content.
The average backfat thickness of each carcass was measured according to the “Technical regulation for testing of carcass traits in lean-type pig (NY/T 825-2004)” of China. Briefly, the backfat thickness at the first rib, last rib and last lumbar vertebra was measured by vernier caliper, and then the average value of the three local was the average backfat thickness. Based on the “Technical regulation for determination of pork quality (NY/T 821-2004)”, marbling scores were evaluated by trained university personnel according to the National Pork Producers Council (1994), and IMF content was evaluated according to the Soxhlet petroleum-ether extraction method. IMF content is expressed as the weight percentage of wet muscle tissue.
Lipid metabolism genes selection, RNA extraction and quantitative real-time polymerase chain reaction
Thirty lipid metabolism genes were chosen from previous studies on pigs and other animals (Table 1) for their involvement in lipogenesis, fat uptake, fatty acid esterification, lipolysis, or fatty acid oxidation. Adipocytokine and transcription factors were also considered as key molecules in the regulation of adipogenesis.
Table 1.
Information of lipid metabolism related genes
| Gene name | Gene symbol | SSC | GenBank ID |
|---|---|---|---|
| Acetyl-CoA carboxylase alpha | ACACA | 12 | NM_001114269 |
| Acyl-CoA oxidase 1 | ACOX1 | 12 | NM_001101028 |
| Acyl-CoA synthetase long-chain family member 3 | ACSL3 | 15 | NM_001143698 |
| Acyl-CoA synthetase short-chain family member 2 | ACSS2 | 17 | NM_001143695 |
| Adiponectin | ADIPOQ | 13 | NM_214370 |
| Adiponectin receptor 1 | ADIPOR1 | 10 | NM_001007193 |
| 1-acylglycerol-3-phosphate O-acyltransferase 1 | AGPAT1 | 7 | NM_001033008 |
| CCAAT/enhancer binding proteins (C/EBP), alpha | CEBPA | 6 | AF103944 |
| CCAAT/enhancer binding proteins (C/EBP), beta | CEBPB | 17 | NM_001199889 |
| Catalase | CAT | 2 | NM_214301 |
| CD36 molecule (thrombospondin receptor) | CD36 | 9 | NM_001044622 |
| Carnitine palmitoyl transferase 1B (muscle) | CPT1B | - | NM_001007191 |
| Diacylglycerol acyltransferase 1 | DGAT1 | 4 | NM_214051 |
| Diacylglycerol acyltransferase 2 | DGAT2 | 9 | NM_001160080 |
| Fatty acid binding protein 3, muscle and heart | FABP3 (H-FABP) | 6 | NM_001099931 |
| Fatty acid binding protein 4, adipocyte | FABP4 (A-FABP) | - | NM_001002817 |
| Fatty acid synthase | FASN | 12 | NM_001099930 |
| Leptin | LEP | 18 | NM_213840 |
| Leptin receptor | LEPR | 6 | NM_001024587 |
| Lipase, hormone-sensitive | LIPE (HSL) | 6 | NM_214315 |
| Lipoprotein lipase | LPL | - | NM_214286 |
| Monoglyceride lipase | MGLL | 13 | NM_001143718 |
| Patatin-like phospholipase domain containing 2 | PNPLA2 (ATGL) | 2 | NM_001098605 |
| Peroxisome proliferator-activated receptor alpha | PPARA | 5 | NM_001044526 |
| Peroxisome proliferator-activated receptor gamma | PPARG | 13 | NM_214379 |
| Retinoid X receptor gamma | RXRG | 4 | NM_001130213 |
| Stearoyl-coenzyme A desaturase | SCD | 14 | NM_213781 |
| Solute carrier family 27 (fatty acid transporter), member 1 | SLC27A1 (FATP1) | 2 | NM_001083931 |
| Solute carrier family 2 (facilitated glucose transporter) member 4 | SLC2A4 (GLUT4) | 12 | NM_001128433 |
| Sterol regulatory element binding transcription factor 1 | SREBF1 (SREBP-1C) | 12 | NM_214157 |
SSC, Sus scrofa chromosome.
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. This total RNA was quantified by measuring the optical density at 260 nm, and its integrity was evaluated by 1% agarose gel electrophoresis. Ratios of absorption (260/280 nm) of all preparations were between 1.8 and 2.0. Total RNA was then reverse transcribed to cDNA using a PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, Dalian, China) according to the manufacturer’s instructions.
Real-time polymerase chain reaction (PCR) was performed using SYBR Premix Ex Taq (Takara, China) and an Mx3000P Real-Time PCR System (Stratagene, La Jolla, CA, USA). Amplifications were performed in a 25 μL reaction volume containing 12.5 μL of 2× SYBR Premix ExTaq, 0.5 μL of each primer, 2 μL of diluted cDNA, 0.5 μL of ROX Reference Dye II, and sterile water. The PCR amplification was carried out as follows: 95°C for 10 s, then 40 cycles of 95°C for 5 s and 58°C for 10 s and 72°C for 15 s, followed by 1 cycle of 95°C for 1 min, 61°C for 30 s, and 95°C for 30 s to calculate the melting curve. To exclude between-run variation, all samples were amplified in triplicates and the mean was used for further analysis. Primer sets used are listed in Supplemental Table S1.
Beta-2 microglobulin, eukaryotic translation elongation factor 1 alpha 1 (EEF1A1), glyceraldehyde-3-phosphate dehydrogenase, peptidylprolyl isomerase A (PPIA), and TATA box-binding protein were amplified as endogenous control genes. The stability of the candidate reference genes was evaluated with geNorm (v3.5) [8]. The most stably expressed reference genes and their optimal number for normalization were determined. Standard curves were generated using pooled cDNA from the samples being assayed, and the ΔCq method was used to quantify the mRNA expression levels of lipid metabolism genes.
Statistical analysis
The means procedure was used to calculate the mean and standard deviation values for the measured parameters (SAS Institute, v8.2, Inc., Cary, NC, USA). One-way analysis of variance followed by t-test were used to compare data between the two pig groups. Pearson’s correlation coefficients between carcass characteristics or lipid metabolism gene expression in LD muscle and IMF content were calculated using the CORR procedure. Principal component (PC) analysis was performed to analyze the correlations of all variables (transcription of 30 genes). Stepwise regression of SAS was used to develop equations predicting IMF content using mRNA abundance of lipid metabolism genes in LD muscle. The IMF content was the dependent variable and mRNA abundance of the 30 lipid metabolism genes was the independent variables. Data in the tables and text are presented as mean± standard error of the mean.
RESULTS
Backfat thickness, marbling score and intramuscular fat content
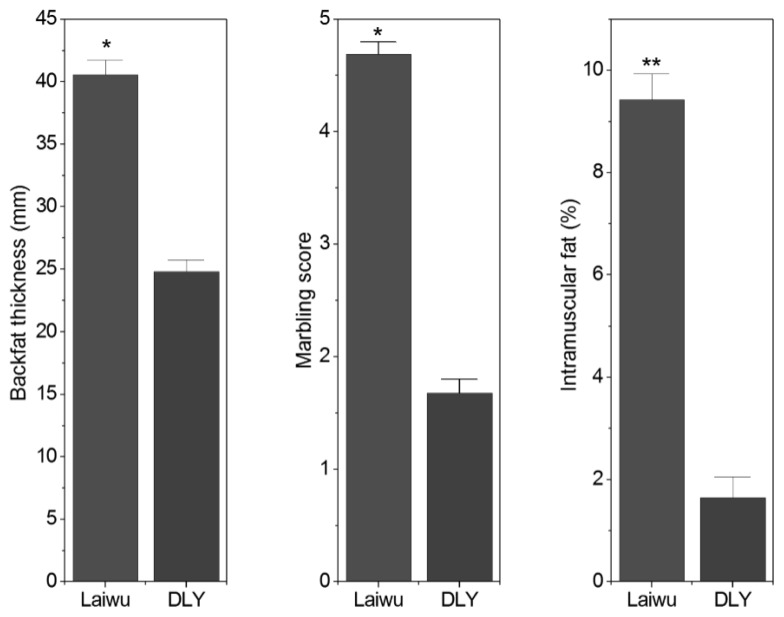
The average backfat thickness, marbling score and IMF content were significantly higher in Laiwu than DLY pigs (Figure 1). The IMF content was 9.43% and 1.64% for Laiwu and DLY pigs, respectively (p<0.05). Marbling score, a measure of the amount and distribution of IMF in LD muscle, was positively correlated with IMF content in Laiwu (r = 0.48, p = 0.039) and DLY pigs (r = 0.43, p = 0.046). Likewise, average backfat thickness was positively correlated with IMF content in Laiwu (r = 0.72, p = 0.008) and DLY pig (r = 0.72, p = 0.007).
Figure 1.
Average backfat thickness, marbling score and IMF content of Laiwu and Duroc×Landrace×Yorkshire (DLY) pigs. * p<0.05, ** p<0.01.
mRNA expression of lipid metabolism genes
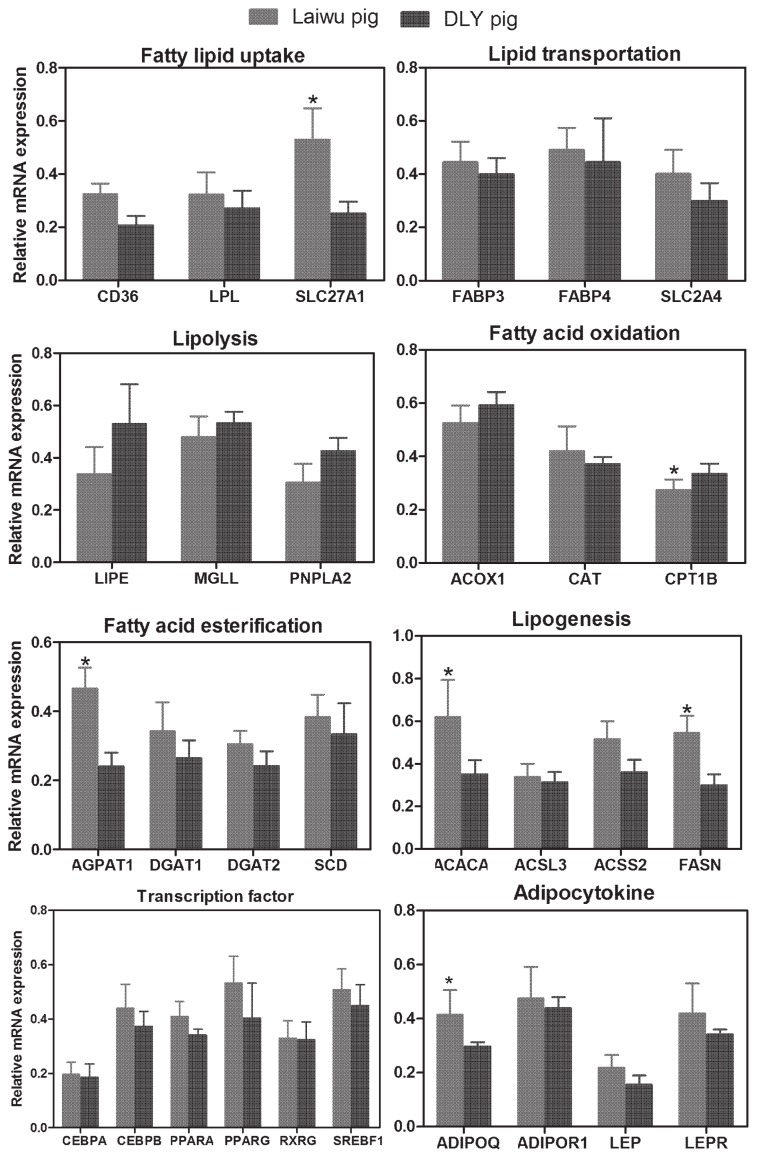
Evaluation of candidate reference genes by geNorm showed that EEF1A1 and PPIA were the most stably expressed genes. These two genes were used to normalize the expression of lipid metabolism genes. The normalized mRNA abundance of lipid metabolism genes is reported in Figure 2. The expression of acetyl-CoA carboxylase alpha (ACACA), fatty acid synthase (FASN), solute carrier family 27 member 1 (SLC27A1), adiponectin (ADIPOQ), and 1-acylglycerol-3-phosphate O-acyltransferase 1 in muscle tissue was significantly higher in Laiwu than DLY pigs. Conversely, of the fatty acid oxidation genes, only carnitine palmitoyl transferase 1B (CPT1B) showed a significant difference between groups, being significantly higher in DLY than Laiwu pigs (p<0.05). The expression of fatty acid binding protein 4, FASN, peroxisome proliferator-activated receptor gamma (PPARG), and ADIPOQ in backfat tissue was significantly higher in Laiwu than DLY pigs (p<0.05; Supplementary Figure S1). Expression of the remaining genes showed no significant differences between Laiwu and DLY pigs.
Figure 2.
mRNA abundance of lipid metabolism genes in Laiwu and Duroc×Landrace×Yorkshire (DLY) pigs. * p<0.05.
Correlations between the expression of lipid metabolism genes and intramuscular fat content
There were many significant Pearson’s correlations between IMF content and the mRNA abundance of lipid metabolism genes (Table 2). In muscle tissue of both pig groups, IMF content was positively correlated (p<0.05) with the expression of lipid transportation and adipocytokine genes. The IMF content was also correlated (p<0.05) with mRNA abundance of diacylglycerol acyltransferase 1 (DGAT1), stearoyl-coenzyme A desaturase, lipase (LIPE), CCAAT/enhancer binding proteins (C/EBP), beta (CEBPB), peroxisome proliferator-activated receptor alpha (PPARA), PPARG, and sterol regulatory element binding transcription factor 1 (SREBF1). Monoglyceride lipase (MGLL) expression was negatively correlated and LIPE expression positively correlated with IMF content. In Laiwu pig, IMF content was positively correlated with lipoprotein lipase, SLC27A1, ACACA, acyl-CoA synthetase short-chain family member 2 (ACSS2), FASN, and CCAAT/enhancer binding proteins (C/EBP), alpha (CEBPA) expression, and negatively correlated with patatin-like phospholipase domain containing 2 (PNPLA2), acyl-CoA oxidase 1 (ACOX1), and CPT1B expression.
Table 2.
Correlation coefficients between gene expression and IMF content and backfat thickness
| Genes | IMF content | Backfat thickness | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Laiwu pig | DLY pig | Laiwu pig | DLY pig | |||||
|
|
|
|
|
|||||
| r | p | r | p | r | p | r | p | |
| Fatty lipid uptake | ||||||||
| CD36 | 0.47 | 0.051 | 0.21 | 0.404 | 0.27 | 0.275 | 0.36 | 0.067 |
| LPL | 0.71 | 0.039 | 0.39 | 0.051 | 0.39 | 0.071 | 0.27 | 0.058 |
| SLC27A1 | 0.48 | 0.043 | 0.38 | 0.067 | 0.65 | 0.097 | 0.51 | 0.074 |
| Lipid transportation | ||||||||
| FABP3 | 0.39 | 0.003 | 0.32 | 0.025 | 0.26 | 0.098 | 0.38 | 0.117 |
| FABP4 | 0.66 | 0.000 | 0.41 | 0.008 | 0.46 | 0.085 | 0.54 | 0.082 |
| SLC2A4 | 0.39 | 0.046 | 0.30 | 0.023 | 0.36 | 0.147 | 0.29 | 0.053 |
| Lipogenesis | ||||||||
| ACACA | 0.43 | 0.015 | 0.49 | 0.171 | 0.32 | 0.200 | 0.47 | 0.054 |
| ACSL3 | 0.46 | 0.082 | 0.64 | 0.118 | 0.31 | 0.079 | 0.36 | 0.065 |
| ACSS2 | 0.48 | 0.042 | 0.42 | 0.083 | 0.61 | 0.089 | 0.57 | 0.078 |
| FASN | 0.75 | 0.001 | 0.07 | 0.092 | 0.25 | 0.061 | 0.31 | 0.054 |
| Fatty acid esterification | ||||||||
| AGPAT1 | 0.38 | 0.119 | 0.23 | 0.065 | 0.22 | 0.062 | 0.27 | 0.083 |
| DGAT1 | 0.51 | 0.033 | 0.34 | 0.037 | 0.13 | 0.084 | 0.17 | 0.061 |
| DGAT2 | 0.37 | 0.049 | 0.49 | 0.171 | 0.24 | 0.053 | 0.32 | 0.053 |
| SCD | 0.48 | 0.042 | 0.29 | 0.045 | 0.39 | 0.109 | 0.45 | 0.085 |
| Lipolysis | ||||||||
| LIPE | 0.65 | 0.003 | 0.39 | 0.011 | 0.30 | 0.224 | 0.42 | 0.082 |
| MGLL | −0.61 | 0.009 | −0.33 | 0.024 | −0.62 | 0.065 | −0.57 | 0.821 |
| PNPLA2 | −0.58 | 0.011 | −0.29 | 0.065 | −0.46 | 0.078 | −0.49 | 0.056 |
| Fatty acid oxidation | ||||||||
| ACOX1 | −0.56 | 0.015 | 0.42 | 0.085 | −0.56 | 0.016 | −0.39 | 0.071 |
| CAT | 0.52 | 0.063 | 0.36 | 0.086 | 0.19 | 0.068 | 0.29 | 0.044 |
| CPT1B | −0.66 | 0.003 | −0.43 | 0.062 | −0.49 | 0.087 | −0.36 | 0.079 |
| Adipocytokine | ||||||||
| ADIPOQ | 0.47 | 0.047 | 0.37 | 0.012 | 0.44 | 0.086 | 0.43 | 0.063 |
| ADIPOR1 | 0.67 | 0.002 | 0.45 | 0.019 | 0.57 | 0.082 | 0.39 | 0.104 |
| LEP | 0.41 | 0.044 | 0.36 | 0.008 | 0.32 | 0.091 | 0.46 | 0.148 |
| LEPR | 0.58 | 0.012 | 0.29 | 0.025 | 0.28 | 0.065 | 0.28 | 0.057 |
| Transcription factors | ||||||||
| CEBPA | 0.66 | 0.003 | 0.67 | 0.068 | 0.41 | 0.087 | 0.37 | 0.052 |
| CEBPB | 0.74 | 0.000 | 0.43 | 0.024 | 0.31 | 0.066 | 0.29 | 0.083 |
| PPARA | 0.65 | 0.003 | 0.36 | 0.014 | 0.48 | 0.029 | 0.51 | 0.039 |
| PPARG | 0.75 | 0.001 | 0.29 | 0.027 | 0.38 | 0.063 | 0.39 | 0.075 |
| RXRG | 0.39 | 0.112 | 0.32 | 0.065 | 0.21 | 0.218 | 0.59 | 0.175 |
| SREBF1 | 0.67 | 0.002 | 0.49 | 0.038 | 0.39 | 0.065 | 0.41 | 0.052 |
IMF, intramuscular fat; DLY, Duroc×Landrace×Yorkshire.
However, the mRNA abundance of ACOX1 and PPARA in backfat tissue was correlated with the IMF content in Laiwu pig, and catalase and PPARA was correlated with the IMF content in DLY pig.
Principal component analysis and stepwise multiple regression analysis
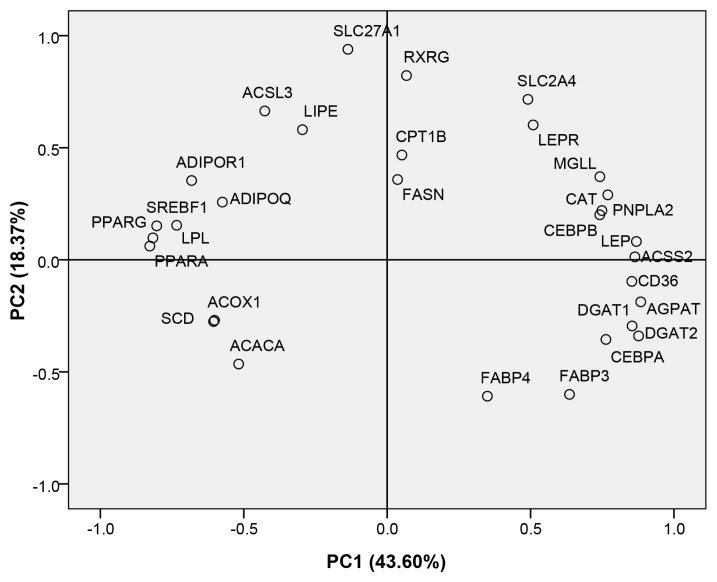
The PC analysis was used to determine whether any of the 30 genes analyzed significantly predicted IMF content in Laiwu pigs. The PC analysis extracted two factors, PC1 and PC2, that explained 61.97% of total data variation (43.60% and 18.37% respectively) (Figure 3). A stepwise multiple regression analysis was also performed, with four independent variables showing significant results (p<0.05, Table 3). The total prediction value for IMF content from these variables, i.e. CPT1B, FASN, SLC27A1, and FABP3 expression, was 38%. Of the four variables, CPT1B represented the greatest contribution to IMF content (14%), followed by FASN (11%), SLC27A1 (9%), and FABP3 (4%). These results indicate that CPT1B may be an important factor for predicting IMF content, and thus the decrease in fatty acid oxidation caused by reduced CPT1B expression may be important for IMF deposition in Laiwu pigs.
Figure 3.
Variable scores for principal component (PC) 1 and 2 of the PC analysis. PC 1 and 2 explained 43.60% and 18.37% of the total variance, respectively. The PC plot representing variables in the rotated plan after PC analysis. The rotation method used was Varimax with Kaiser Normalization.
Table 3.
Stepwise multiple regression analysis for predicting IMF content using the mRNA abundance of lipid metabolism genes in Laiwu pig
| Variables | Regression coefficient | SE | Partial R2 | Pr>F |
|---|---|---|---|---|
| Intercept | 3.19 | 0.21 | - | <0.0001 |
| CPT1B | −0.57 | 0.20 | 0.14 | 0.017 |
| FASN | 2.00 | 0.32 | 0.11 | 0.014 |
| SLC27A1 | 0.37 | 0.11 | 0.09 | 0.006 |
| FABP3 | 0.50 | 0.21 | 0.04 | 0.031 |
| Total R2 | - | - | 0.38 | - |
IMF, intramuscular fat; SE, standard error; CPT1B, carnitine palmitoyl transferase 1B; FASN, fatty acid synthase; SLC27A1, solute carrier family 27 member 1; FABP3, fatty acid binding protein 3, muscle and heart.
DISCUSSION
Lipogenesis is the process by which acetyl coenzyme A is converted to fatty acids [9]. Ponsuksili et al [10] reported that key genes involved in lipogenesis were upregulated in the fatter German Landrace pigs. In our study, IMF content was correlated with the expression of ACACA, ACSS2, and FASN in Laiwu pigs. The most significant of these was between FASN expression and IMF content, where the mRNA abundance of FASN accounted for 11% of the variability in IMF content explained by the 30 genes analyzed. Taken together, our results suggest that the upregulation in gene expression increased the capacity for fatty acid synthesis and increased the fat accumulation in skeletal muscle.
Previous research shows that SLC27A1 promotes long-chain fatty acids uptake into differentiating adipocytes [11]. In the present study, SLC27A1 expression was correlated with IMF content in Laiwu pigs. Importantly, the mRNA abundance of SLC27A1 accounted for 9% of the variability in IMF content explained by the 30 candidate genes we analyzed, suggesting that increased SLC27A1 expression may promote IMF deposition in Laiwu pigs.
Diacylgycerol acyltransferase (DGAT) catalyzes the final step in triacylglycerol biosynthesis by converting diacylgycerol and fatty acyl-coenzyme A to triacylglycerol [12]. In our study, DGAT1 and DGAT2 expression were correlated with IMF deposition in Laiwu pigs. Therefore, DGAT1 and DGAT2 may play a key role in modulating fat deposition in Laiwu pigs.
Fat removal by lipolysis is an important factor in IMF de position in muscle in pigs. In this study, LIPE expression was positively correlated and MGLL and PNPLA2 expression negatively correlated with IMF content in Laiwu pigs. In agreement with our results, a previous study reported that MGLL and PNPLA2 mRNA abundance were negatively correlated with IMF content in Korean cattle steers [13]. These results suggest that decreased lipolysis may enhance fat deposition in Laiwu pigs.
Fatty acid oxidative potential may also be important for IMF deposition in skeletal muscle in pigs. A recent study reported that decreased CPT1B expression contributes to fat accumulation in obesity [14]. In our study, CPT1B expression was negatively correlated with IMF content in Laiwu pigs, with the mRNA abundance of CPT1B accounting for 14% of the variability in IMF content explained by the 30 genes analyzed. The CPT1B gene may thus be used as a genetic marker for IMF deposition in Laiwu pigs, suggesting that decreased fatty acid oxidation in muscle contributes to increasing fat deposition in this breed.
ADIPOQ can inhibit the synthesis of malonyl-coenzyme A via the cell surface receptor ADIPOR1, resulting in an increase in mitochondrial import and fatty acid oxidation [15]. We found ADIPOQ expression to be positively correlated with IMF content in muscle of Laiwu pigs, suggesting that ADIPOQ may promote lipid deposition in this breed.
CEBPA, CEBPB, PPARA, and PPARG expression were also correlated with IMF content in the Laiwu pigs of our study, with PPARG showing the strongest correlation with IMF content. These results indicate that an increase in CEBPA, CEBPB, PPARA, and PPARG expression leads to enhanced lipogenesis in intramuscular adipose tissue. Moreover, SREBF1 showed a strong correlation with IMF content, suggesting that SREBF1 may be a candidate gene for determination of lipogenic capacity. Together, these results indicate that increased expression of transcription factor genes promotes fat deposition in Laiwu pigs.
CONCLUSION
The expression of most of the lipid metabolism genes selected for this study was significantly associated with IMF content, affirming the role of these genes in lipid deposition in muscle. Our results indicate that the combined effects of increases in fat deposition and decreases in fat removal contribute to increasing the IMF content, and that CPT1B, FASN, SLC27A1, and FABP3 are predictors of IMF content in the LD muscle of Laiwu pigs.
Supplementary Data
ACKNOWLEDGMENTS
This study was supported by the Shandong Provincial Natural Science Foundation (ZR2018BC046), the Shandong Provincial Modern Pig Technology and Industry System Project (SDAIT-08-02), and Funds of Shandong “Double Tops” Program (SYL2017YSTD12).
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Font-i-Furnols M, Tous N, Esteve-Garcia E, Gispert M. Do all the consumers accept marbling in the same way? The relationship between eating and visual acceptability of pork with different intramuscular fat content. Meat Sci. 2012;91:448–53. doi: 10.1016/j.meatsci.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zeng Y, Cui J, et al. Effects of phospholipid hydroperoxide glutathione peroxidase mRNA expression on meat quality of M. Longissimus dorsi in pigs. Eur Food Res Technol. 2011;232:433–40. doi: 10.1007/s00217-010-1407-3. [DOI] [Google Scholar]
- 3.Chen Q-M, Wang H, Zeng Y-Q, Chen W. Developmental changes and effect on intramuscular fat content of H-FABP and A-FABP mRNA expression in pigs. J Appl Genet. 2013;54:119–23. doi: 10.1007/s13353-012-0122-0. [DOI] [PubMed] [Google Scholar]
- 4.Hamill RM, Aslan O, Mullen AM, et al. Transcriptome analysis of porcine M. semimembranosus divergent in intramuscular fat as a consequence of dietary protein restriction. BMC Genomics. 2013;14:453. doi: 10.1186/1471-2164-14-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Switonski M, Stachowiak M, Cieslak J, Bartz M, Grzes M. Genetics of fat tissue accumulation in pigs: a comparative approach. J Appl Genet. 2010;51:153–68. doi: 10.1007/BF03195724. [DOI] [PubMed] [Google Scholar]
- 6.Serão NVL, Veroneze R, Ribeiro AMF, et al. Candidate gene expression and intramuscular fat content in pigs. J Anim Breed Genet. 2011;128:28–34. doi: 10.1111/j.1439-0388.2010.00887.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Xue W, Jin B, Zhang X, Ma F, Xu X. Candidate gene expression affects intramuscular fat content and fatty acid composition in pigs. J Appl Genet. 2013;54:113–8. doi: 10.1007/s13353-012-0131-z. [DOI] [PubMed] [Google Scholar]
- 8.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. research0034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith S, Witkowski A, Joshi AK. Structural and functional organization of the animal fatty acid synthase. Prog Lipid Res. 2003;42:289–317. doi: 10.1016/S0163-7827(02)00067-X. [DOI] [PubMed] [Google Scholar]
- 10.Ponsuksili S, Murani E, Walz C, Schwerin M, Wimmers K. Pre- and postnatal hepatic gene expression profiles of two pig breeds differing in body composition: insight into pathways of metabolic regulation. Physiol Genomics. 2007;29:267–79. doi: 10.1152/physiolgenomics.00178.2006. [DOI] [PubMed] [Google Scholar]
- 11.Pohl J, Ring A, Hermann T, Stremmel W. Role of FATP in parenchymal cell fatty acid uptake. Biochim Biophys Acta Mol Cell Biol L. 2004;1686:1–6. doi: 10.1016/j.bbalip.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Yen CLE, Stone SJ, Koliwad S, Harris C, Farese RV. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283–301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong J, Kwon EG, Im SK, Seo KS, Baik M. Expression of fat deposition and fat removal genes is associated with intramuscular fat content in longissimus dorsi muscle of Korean cattle steers. J Anim Sci. 2012;90:2044–53. doi: 10.2527/jas.2011-4753. [DOI] [PubMed] [Google Scholar]
- 14.Ratner C, Madsen AN, Kristensen LV, et al. Impaired oxidative capacity due to decreased CPT1b levels as a contributing factor to fat accumulation in obesity. Am J Physiol Regul Integr Comp Physiol. 2015;308:R973–82. doi: 10.1152/ajpregu.00219.2014. [DOI] [PubMed] [Google Scholar]
- 15.Yamauchi T, Nio Y, Maki T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.