Abstract
A 40-year-old male visited our institute complaining of transient loss of consciousness. He had been implanted with an implantable cardioverter defibrillator (ICD) due to idiopathic ventricular fibrillation for secondary prevention. His past genetic screening detected a single nucleotide SCN5A mutation (pR18Q), while neither QT prolongation nor ST segment elevation in the right precordial leads was observed. An interrogation of the ICD revealed that a shock therapy successfully terminated ventricular fibrillation at the time syncope occurred. His electrocardiogram revealed ventricular premature contractions (VPCs) with a short coupling interval of 250 ms. Since the spontaneous occurrence of non-sustained polymorphic ventricular tachycardia following the same VPCs was observed after admission, he was diagnosed with a short-coupled variant of Torsades de Pointes (ScTdP). Contact mapping on the basal inferior right ventricular free wall, exhibiting the earliest activation, revealed pre-potentials preceding the QRS by 30 ms during the VPCs. Radiofrequency ablation was performed to reduce the triggering VPCs. To the best of our knowledge, this is the first report describing a case of ScTdP harboring an SCN5A mutation. The present N-terminally mutated SCN5A was originally reported in relation to Brugada syndrome, whereas the detailed mechanism remains to be elucidated.
〈
Learning objective: The fundamental genetic disorders of short-coupled variant of Torsades de Pointes (ScTdP) are not clear. The present case harboring a mutation of SCN5A exhibited no long-QT or Brugada syndrome, which may implicate an unknown mechanism of the development of ScTdP.〉
Keywords: NaV1.5, SCN5A, Sodium channel, Torsade de Pointes, Ventricular fibrillation, Polymorphic ventricular tachycardia
Introduction
A short-coupled variant of Torsades de Pointes (ScTdP) is one of the causes of idiopathic ventricular fibrillation (IVF). Although this condition is considered to account for a significant group of IVF patients, the genetic background remains unclear. We describe a case of ScTdP harboring a mutation of the SCN5A gene, a subunit of the cardiac sodium channels.
Case report
A 40-year-old male visited our institute complaining of transient loss of consciousness. He had been implanted with an implantable cardioverter defibrillator (ICD) 7 years previously due to idiopathic ventricular fibrillation (VF) for secondary prevention. He had no family history of sudden death. His past genetic screening detected a single nucleotide mutation of SCN5A (c58G>A, pR18Q), whereas neither QT prolongation nor ST segment elevation in the right precordial leads was observed even under the administration of catecholamines or sodium channel blockers. Transthoracic echocardiography and contrast multi-detector computed tomography detected no structural or functional abnormalities of the heart. His previous Holter monitor revealed 769 ventricular ectopies without VF on 50 mg of oral amiodarone and 5 mg of carvedilol. An interrogation of the ICD revealed that a single shock therapy with 25 J successfully terminated the VF at the time the syncope occurred. His 12-lead electrocardiogram showed sinus rhythm with frequent monomorphic ventricular premature contractions (VPCs), of which the coupling interval was markedly short at 250 ms (Fig. 1). He was immediately admitted for close observation. Since the spontaneous occurrence of polymorphic ventricular tachycardia following the same VPCs was observed, he was diagnosed with a ScTdP. Because medication including a beta-blocker and verapamil was not effective in suppressing the VPC, he underwent catheter treatment a week after admission. Contact mapping on the basal inferior right ventricular free wall exhibiting the earliest activation revealed pre-potentials during VPCs preceding the QRS by 30 ms (Fig. 2). Radiofrequency ablation was applied to reduce the triggering VPCs. He was discharged without any complications, however, a second ablation procedure was necessary for recurrences of ScTdP 2 years after the first session. The location of the triggering VPC was similar to that in the first session.
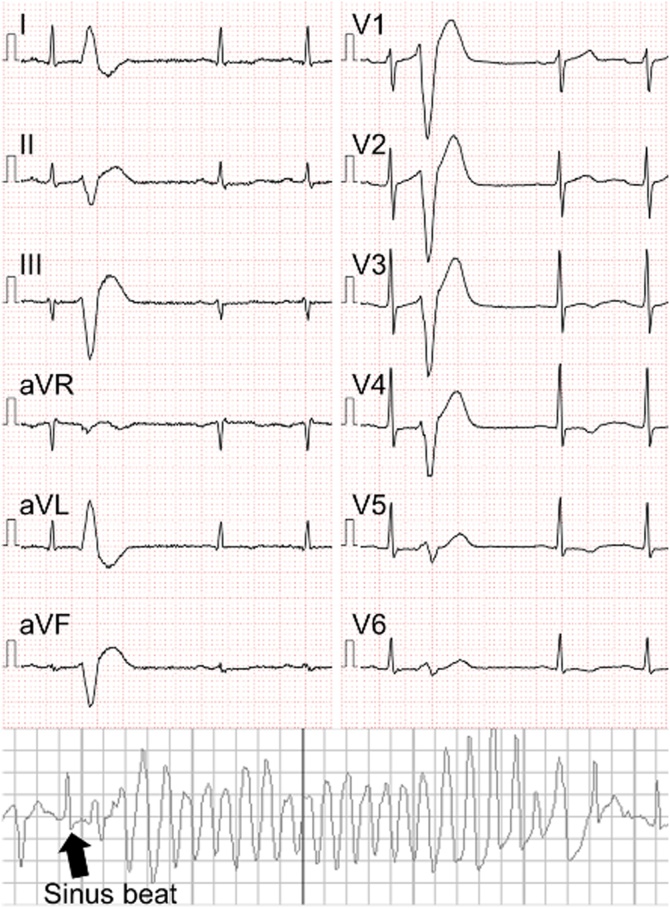
Fig. 1.
Twelve-lead electrocardiography showing a short-coupled ventricular premature contraction during sinus rhythm. The coupling interval was 250 ms from the preceding sinus beat, while a similar premature contraction triggered spontaneous Torsades de Pointes.
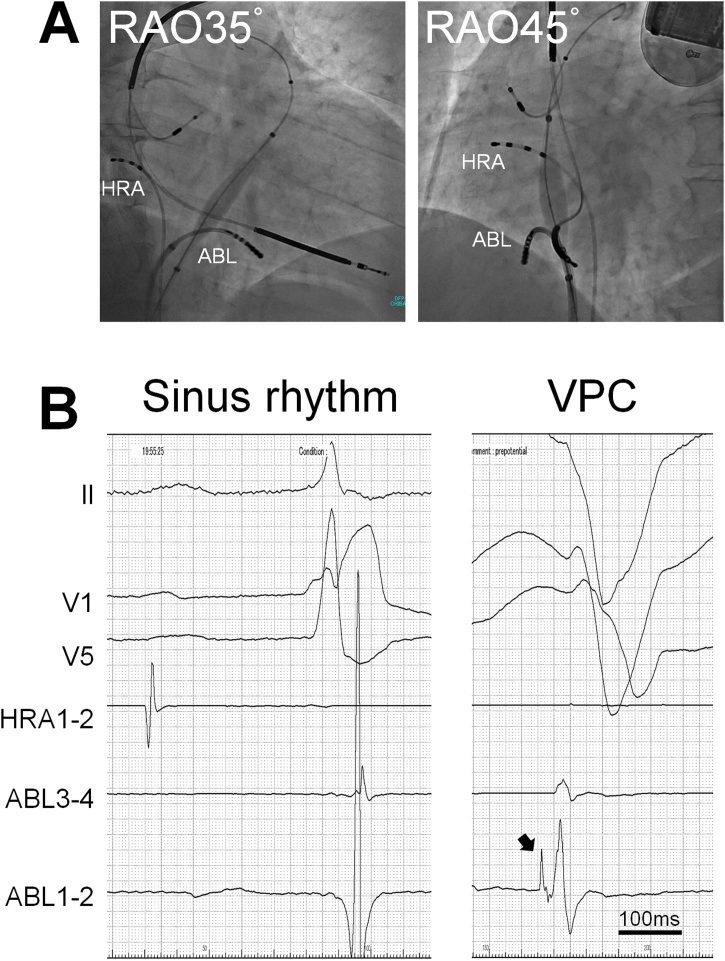
Fig. 2.
(A) Fluoroscopic images during the first session of catheter ablation. The tip of the catheter was located on the site of earliest activation during the trigger ventricular premature contractions. (B) Intracardiac electrocardiogram during sinus rhythm and a ventricular premature contraction. A prepotential indicated by the black arrow was observed at the earliest activation site, which preceded the QRS by 30 ms.
To the best of our knowledge, this is the first report describing an ScTdP case harboring an SCN5A mutation. The present N-terminally mutated SCN5A was originally reported in relation to Brugada syndrome [1]. However, in this case, the morphology of the ST-T segment was not compatible with Brugada syndrome. It is reported that pR18Q does not exhibit a loss-of-function in HEK293 cells [2]. Therefore, in human cardiomyocytes, some unelucidated mechanism leading to IVF may exist. In addition, some mutations of type-2 ryanodine receptors (RyR2) have been reported regarding ScTdP [3], however, we did not investigate RyR2.
Conclusion
This case may implicate the phenotypic heterogeneity of the present mutation. The detailed pathogenicity leading to ScTdP remains to be elucidated.
Conflict of interest
None declared.
Acknowledgment
We thank Mr John Martin for his linguistic assistance in drafting this manuscript.
References
- 1.Kapplinger J.D., Tester D.J., Alders M., Benito B., Berthet M., Brugada J. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. doi: 10.1016/j.hrthm.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutter C., Benndorf K., Zimmer T. Characterization of N-terminally mutated cardiac Na(+) channels associated with long QT syndrome 3 and Brugada syndrome. Front Physiol. 2013;4:153. doi: 10.3389/fphys.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujii Y., Itoh H., Ohno S., Murayama T., Kurebayashi N., Aoki H. A type 2 ryanodine receptor variant associated with reduced Ca(2+) release and short-coupled Torsades de Pointes ventricular arrhythmia. Heart Rhythm. 2017;14:98–107. doi: 10.1016/j.hrthm.2016.10.015. [DOI] [PubMed] [Google Scholar]