Abstract
Granulomatosis with polyangiitis is a rare systemic inflammatory disorder mainly affecting the small vessels. Cardiac involvement is rare, conduction delay being the most rare one. This case reports on a middle-aged male patient with heart failure symptoms due to a 1st degree atrioventricular block with a marked PR prolongation of 480 ms on electrocardiography (ECG). Because of heart failure symptoms as well as elevated N-terminal pro-B-type natriuretic peptide and no other relevant findings in the blood test it was initially planned to treat the conduction disorder with a pacemaker. During further investigations a certain diagnosis of granulomatosis with polyangiitis was determined. After administration of high-dose steroids a complete clinical remission of heart failure symptoms and normal conduction on ECG were demonstrated, so that no pacemaker therapy was needed.
<Learning objective: Although atrioventricular (AV) conduction disorders have been described previously in patients with granulomatosis with polyangiitis, this is the first case reporting of vasculitis causing heart failure symptoms due to non-severe conduction disorder such as 1st degree AV-block. Even in the case of non-severe AV conduction delay, which causes symptoms, it is essential to investigate possible rare causes of the conduction disorder before considering pacemaker treatment.
Keywords: Vasculitis, Conduction delay, Heart failure, Pacemaker, Electrocardiogram
Introduction
Granulomatosis with polyangiitis, GPA (previously known as Wegener’s granulomatosis), is a systemic inflammatory disorder mainly affecting the small vessels. The classic diagnostic criteria for GPA include a triad of necrotizing granulomas of upper and lower respiratory system, systemic vasculitis, and necrotizing glomerulonephritis [1]. GPA is a complex and potentially lethal disease with high mortality rate if left untreated. Early detection of the disease and the introduction of immunosuppressive therapy has resulted in improved prognosis and decreased mortality rate [2].
The American College of Rheumatology has established the following criteria for the diagnosis of GPA in order to distinguish the disease from other vasculitides: (a) a urinary sediment containing red blood cell casts or more than five red blood cells per high-power field, (b) abnormal findings on the chest radiograph, (c) oral ulcers or nasal discharge, (d) granulomatous inflammation on biopsy [3]. The presence of two or more of these four criteria was associated with an 88% sensitivity and 92% specificity. DeRemee and colleagues utilized cytoplasmic staining antineutrophil cytoplasmic antibodies (cANCA) results if an organ manifestation has been detected [4]. Typically the cANCA in immunofluorescence with proteinase 3-specifity (in enzyme-linked immunosorbent assay) are detected if GPA is present. Cardiac involvement is rare; the most common cardiac manifestation is pericarditis (n = 6.35%) while the least common one is severe conduction disorder (n = 1.6%) [5].
Case report
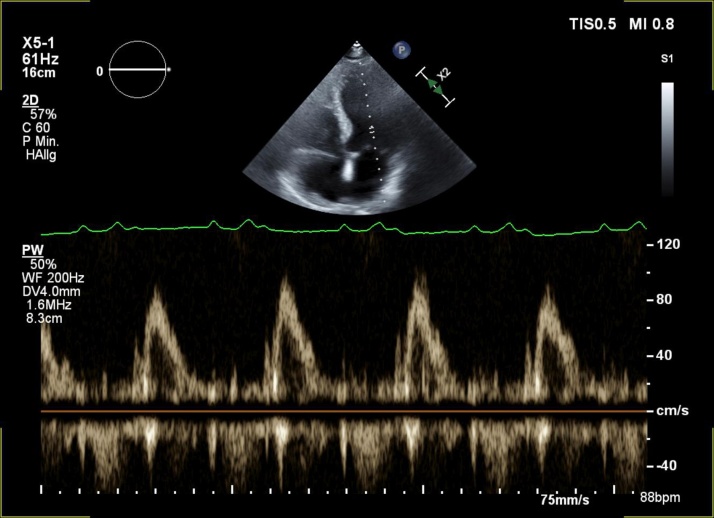
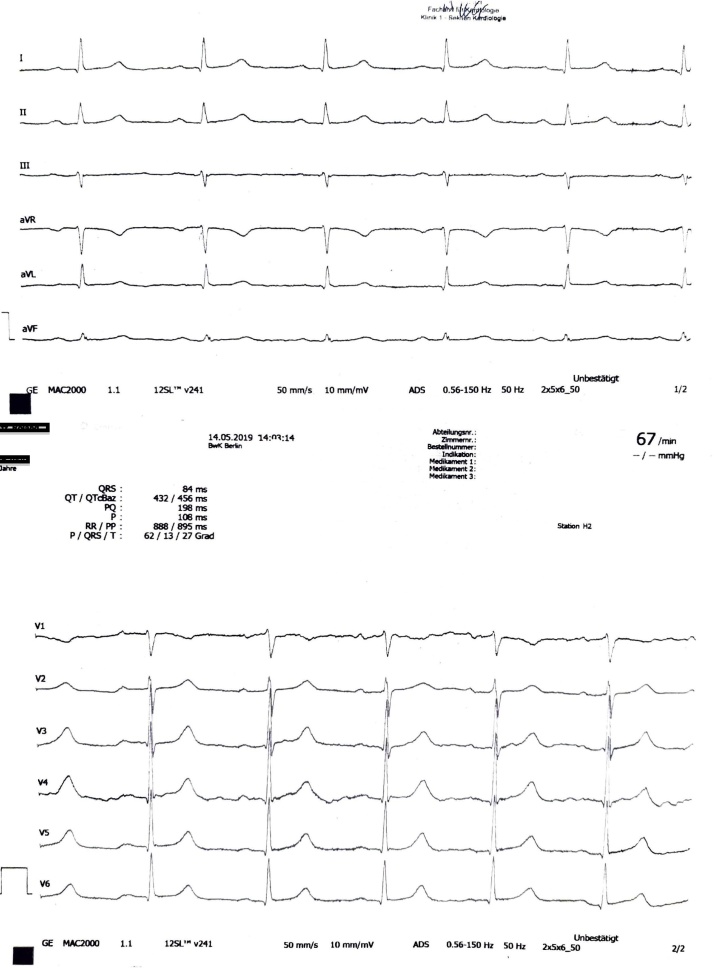
This report presents the case of a 60-year-old male patient who presented to our emergency department with heart failure symptoms [New York Heart Association (NYHA) II/III], migrating arthralgia of the major joints of upper and lower extremities, and elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in serum. The symptoms had been present for six weeks and had been progressing. An electrocardiogram (ECG) showed a 1st degree atrioventricular (AV)-block with 480 ms PR prolongation, where the P-wave appeared prior to T-wave (Fig. 1). Echocardiography showed a regular left ventricular ejection fraction and patent valves. The transmitral Doppler signal demonstrated a singular diastolic signal with no A-wave due to atrial activation during systole against the closed AV valves (Fig. 2). There was no significant diastolic dysfunction (left atrial size normal, no relevant TR-velocity, mean E/E' in normal range), and no other abnormalities.
Fig. 1.
Electrocardiogram at admission (50 mm/s): Marked PR prolongation of 480 ms, P-wave is followed by the T-wave. No other abnormalities.
Fig. 2.
Pulsed wave Doppler signal of mitral inflow. No E/A due to atrial contraction against the closed atrioventricular valves during systole.
In the absence of reversible causes for AV conduction disorder, a symptomatic 1st degree AV-block with a clear correlation between symptoms and conduction delay earns a Class IIa indication for permanent pacemaker therapy [6]. In the presence of a PR prolongation of >300 ms it is presumed that pacemaker therapy with standard AV-delay parameters would result in up to 100% ventricular stimulation burden. To avoid a pacemaker-induced cardiomyopathy we planned to implant a His-pacemaker. Furthermore, we performed a 24-h Holter ECG, where episodes of 2nd degree AV-block Type I as well as Type II were seen, however, without any relevant pause. The standard blood test showed an elevated C-reactive protein level of 65 mg/l. Leucocytes, as well as creatinine and other blood biochemical parameters were in the normal range, urinary test at admission showed no pathologies. In order to exclude rheumatic disorders and other systemic diseases as well as infections such as Lyme borreliosis – known for being a frequent cause of severe conduction disorders – a large spectrum of blood samples, as well as serology for cardiotropic pathogens, blood culture, and rheumatic factors was assessed. The rheumatoid factor as well as anti-cyclic citrullinated peptide antibody were negative. We could also exclude cardiotopic pathogens such as cytomegalovirus, coxsackie, Epstein-Barr virus, parvovirus B19, Chlamydia, and Coxiella. Borreliosis-serology was negative, however we found a positive serology for Mycoplasma pneumoniae. As this could be a cause for conduction disorder as well as arthralgia [7], [8], we decided to treat the infection, postpone the pacemaker-implantation and follow up on ECG and symptoms.
We also assessed blood samples for vasculitis diagnostic such as cANCA-antibodies, however the results were not available at discharge.
We initiated a symptomatic therapy (diuretics and beta-blocker) as well as doxycycline 100 mg twice a day for three weeks and discharged the patient with a re-hospitalization appointment in about three weeks.
The patient presented to our emergency department with different symptoms five days after discharge. In addition to his dyspnea (being now NYHA III) he complained about sensibility disorder in both legs. The ECG was unchanged showing the same AV conduction delay of 480 ms; the NT-proBNP level was 2250 pg/ml - twice as high as at the first admission. The CRP had gone up to 120 mg/l, the urinary test showed now a nephritic syndrome with mild proteinuria and hematuria, creatinine was in the upper normal range. All the other parameters did not change, staying in the normal range. A neurological check-up found a severe neuropathy of lower extremities. During the following neurological diagnostic process several factors as well as cANCA-antibodies we had assessed earlier came out in favor of vasculitis, with cANCA-antibody of 1:200 (normal value <1:10), and a proteinase 3-antibody of 140 IE/ml (normal value <5 IE/ml). At the end of diagnostic investigation a GPA was diagnosed. This was also proven histologically, a Gömöri trichrome stain from the biopsy of N. suralis and M. gastrocnemius showed a vessel with wall-destructing inflammation and necrosis, typical for GPA. A high-dose steroid therapy with methylprednisolone (1000 mg/day) was initiated. During the next three days a dramatic improvement in all symptoms and of the laboratory findings was observed, on the fifth day of treatment an ECG with complete normal PR interval was documented (Fig. 3). The patient was transferred to the Clinical Immunology and Rheumatology Department of the Charite Universitätsmedizin Berlin, a pauci-immune glomerulonephritis was additionally diagnosed and cyclophosphamide was added to the treatment until a full remission was established. Because of the significant toxicity associated with cyclophosphamide therapy, a remission maintenance therapy with rituximab has been suggested for the further course of treatment. The patient was transferred to a post-acute care clinic (rehabilitation clinic) with no symptoms and improved laboratory findings.
Fig. 3.
Electrocardiogram after three days of steroid therapy. Completely normal PR interval of 180 ms. No other abnormalities.
Discussion
Since the treatment with doxycycline was continued after diagnosing GPA, it remains controversial whether the vasculitis was the only cause for conduction disorder or whether the M. pneumoniae infection also contributed to the conduction disorder. However, since we saw no changes during the first week of antibiotic therapy and in contrast a dramatic improvement in just a few days after starting the steroid therapy, we strongly believe that the conduction disorder and the heart failure symptoms were present completely due to GPA. The antibiotic therapy has been continued though for the full three weeks to be sure the M. pneumoniae infection has been fully treated as well.
There have been several case reports showing a severe conduction disorder in GPA [9], however we found no mention of persisting heart failure symptoms under this condition in the literature. The patient was admitted to the hospital initially with an NT-proBNP serum level of 1108 pg/ml, at the second admission it was measured as high as 2250 pg/ml, whereas two weeks after treatment initiation and clinical improvement a 480 pg/ml NT-proBNP level has been measured and the patient did not have any shortness of breath or other complaints resembling the clinical symptoms of heart failure.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Fahey J., Leonard E., Churg H., Godman G. Wegener’s granulomatosis. Am J Med. 1954;17:168–179. doi: 10.1016/0002-9343(54)90255-7. [DOI] [PubMed] [Google Scholar]
- 2.Kubaisi B., Abu Samra K., Foster C.S. Granulomatosis with polyangiitis (Wegener’s disease): an updated review of ocular disease manifestations. Intractable Rare Dis Res. 2016;5:61–69. doi: 10.5582/irdr.2016.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leavitt R., Fauci A., Bloch D., Michel B., Hunder G., Arend W. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum. 1990;33:1101–1107. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 4.DeRemee R.A. The nosology of Wegener’s granulomatosis utilizing the ELK format augmented by c-ANCA. Adv Exp Med Biol. 1993;336:209–215. doi: 10.1007/978-1-4757-9182-2_31. [DOI] [PubMed] [Google Scholar]
- 5.McGeoch L., Carette S., Cuthbertson D., Hoffman G.S., Khalidi N., Koening C.L. Cardiac involvement in granulomatosis with polyangiitis. J Rheumatol. 2015;42:1209–1212. doi: 10.3899/jrheum.141513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brignole M., Auricchio A., Baron-Esquivias G., Bordachar P., Boriani G., Breithardt O.A. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA) Eur Heart J. 2013;34:2281–2329. doi: 10.1093/eurheartj/eht150. [DOI] [PubMed] [Google Scholar]
- 7.Agarwala B.N., Ruschhaupt D.G. Complete heart block from mycoplasma pneumonia infection. Pediatr Cardiol. 1991;12:233–236. doi: 10.1007/BF02310573. [DOI] [PubMed] [Google Scholar]
- 8.Jones M.C. Arthritis and arthralgia in infection with Mycoplasma pneumoniae. Thorax. 1970;25:748–750. doi: 10.1136/thx.25.6.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos L.P.S., Bomfim V.G., Bezerra C.F., Costa N.V., Carvalho R.B.P., Carvalho R.S. Heart conduction system defects and sustained ventricular tachycardia complications in a patient with granulomatosis with polyangiitis. A case report and literature review. Rev Bras Ter Intensiva. 2017;29:386–390. doi: 10.5935/0103-507X.20170052. [DOI] [PMC free article] [PubMed] [Google Scholar]