lncRNAs actively regulate gene expression. They contribute to chromosomal interactions at close or distant genomic regions, which, in turn, regulate transcription [1]. Ariel et al [2] reveal in a recent study a new molecular mechanism of the Arabidopsis lncRNA APOLO. The authors extend previously reported functions of APOLO in cis‐regulation of chromosomal looping and transcription of its neighbor gene to a set of distant genes involved in auxin‐induced molecular pathways controlling lateral root development. Noteworthy, APOLO recognition of multiple trans‐modulated targets occurs through a novel mechanism involving R‐loop formation.
Subject Categories: Chromatin, Epigenetics, Genomics & Functional Genomics; RNA Biology
Ariel et al reveal in a recent study that the Arabidopsis lncRNA APOLO recognizes and regulates multiple distant target genes through the formation of R‐loops.
LncRNAs can regulate the expression of close or distant genes. The reported mechanisms involved not only include the recruitment, interaction with, and modulation of chromatin‐modifying complexes or DNA‐binding proteins, but also modifications of the epigenetic properties of chromatin and its structure 1. The lncRNA APOLO uses such mechanisms to regulate the transcription of its neighbor gene PID, which encodes a regulator of auxin transport, a phytohormone controlling many facets of plant development. In response to auxin, dynamic changes in the APOLO locus, including DNA de‐methylation, chromatin relaxation, and APOLO transcription, resolve a chromatin loop encompassing the PID promoter facilitating PID transcription. At the same time, DNA remethylation and APOLO‐dependent recruitment of PRC1 restore PID silencing and promote chromatin looping 3. The study by Ariel et al reveals now that the lncRNA APOLO harbors additional trans‐acting regulatory properties. Indeed, the intersection of APOLO ChIRP‐Seq 4 data with RNA‐seq data in APOLO‐overexpressing plants identified a list of genes APOLO binds, and whose transcriptional modulation depends on APOLO, suggesting a trans‐regulatory action of the lncRNA on those targets. Moreover, APOLO de‐regulation not only affects transcript levels of those loci, but also chromosomal looping as shown by chromosome conformation capture (3C). Since an interplay between APOLO and PRC1 had been previously described, the authors searched for gene characteristics in the APOLO targets that could be linked to PRC1. Interestingly, a subset of APOLO targets presented the repressive H3K27me3 histone mark and LHP1 occupancy, with altered transcription responding to LHP1 levels, indicating that APOLO could be trans‐regulating distant genes through a PRC1‐APOLO co‐mechanism. Indeed, overexpression of APOLO reduced LHP1 binding to the studied APOLO targets and impacted local 3D chromatin remodeling at multiple loci, suggesting that the trans‐regulatory mechanism of APOLO involves a PRC1‐dependent mechanism (Fig 1).
Figure 1. The cis‐ and trans‐regulatory mechanisms of the lncRNA APOLO .
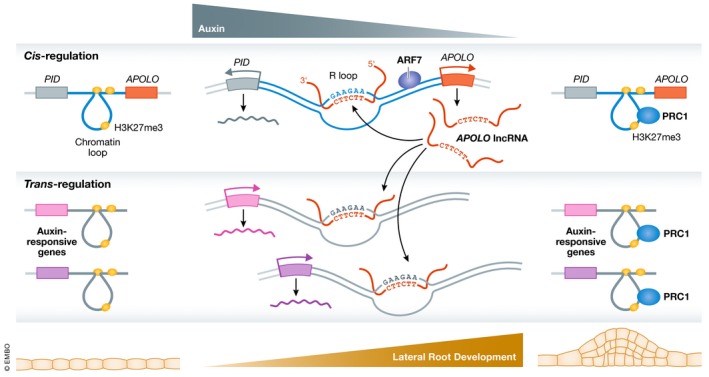
In basal conditions, chromosomal looping and hypermethylation of the APOLO promoter silence its transcription. During lateral root (LR) development, auxin induces ARF7 binding to the PID‐APOLO intergenic region, promoting APOLO transcription. By forming R‐loops, APOLO modulates chromatin loops in cis and in trans, thereby enhancing transcription of target genes. Since APOLO targets are auxin‐responsive genes, their expression contributes to LR development. APOLO‐dependent PRC1 binding and deposition of repressive histone marks restore chromatin loops and transcription silencing of APOLO targets.
Furthermore, the work by Ariel et al unveils that APOLO recognizes multiple distant target genes through the formation of R‐loops. Since APOLO targets were not found in spatially associated genomic regions by Hi‐C, the authors searched for a different mechanism of target recognition. By analyzing putative consensus sequences in the APOLO binding sites by MEME‐ChIP, a short motif was identified, coinciding with the strand‐specific consensus core reported for R‐loops in Arabidopsis 5. This sequence is complementary to APOLO at two positions, suggesting that the interaction between APOLO and its targets could happen by base complementarity in the form of DNA–RNA hybrids. Supporting this idea, the presence of APOLO in R‐loops was experimentally validated by DRIP‐RNA‐qPCR, and APOLO‐dependent formation of R‐loops at its distant targets was demonstrated, since depletion of APOLO abolished the formation of R‐loops at such positions. The authors took advantage of an additional technique to further prove a role for APOLO in the formation of R‐loops at its targets, which is named RNA isolation by DNA purification (RIDP) and is based on the purification of DNA loci from sonicated chromatin with biotinylated DNA probes. The digestion with T5 exonuclease prior to DNA purification degrades ssDNA and dsDNA, leaving DNA–RNA hybrids. The co‐purified RNAs are analyzed by qPCR in comparison with a parallel sample treated with RNase H, to specifically identify RNAs forming hybrids at the pulled‐down loci. Through RIDP‐qPCR, APOLO RNA was shown to recognize its target loci through DNA–RNA duplexes. In line with this observation, the paper provides experimental evidence showing that two complementary TTCTTC boxes in APOLO RNA are essential for the formation of such R‐loops.
Besides the interesting mechanistic features of APOLO, Ariel and colleagues demonstrate the importance of APOLO in regulating auxin‐responsive genes, controlling Lateral Root (LR) development of Arabidopsis. APOLO is transcriptionally regulated by auxin, and its targets overlap with auxin‐responsive genes, including PID. Therefore, the expression of APOLO throughout LR development, governed by auxin, was assessed by analyzing GLU staining and confocal fluorescence in transgenic plants expressing GFP‐GUS reporter genes under the control of the full PID‐APOLO intergenic region. This construct showed that the APOLO promoter is activated during LR development, with differing localizations in the developing root over time. Furthermore, analysis of public databases showed an enrichment of APOLO target genes for pathways related to cell wall composition and organization, and a high overlap with genes involved in ARF‐dependent LR development. The authors not only demonstrate that APOLO participates in the genetic regulatory network governing LR development, but also identify ARF7 as the upstream regulator of APOLO in auxin‐induced physiological contexts, by identifying a cis‐acting element in the intergenic region between PID and APOLO where ARF7 binds.
Mechanistically, APOLO represents a great example of the versatility of lncRNAs in the regulation of gene expression and chromosomal looping by a variety of molecular mechanisms, in interplay with transcriptional activators or repressors, in this case, PRC1. Similarly, the human lncRNA HOTTIP approximates its targets by the formation of a chromatin loop and binds to WDR5 to activate transcription6. However, whereas HOTTIP targets are clustered, and the spatial proximity is necessary for RNA‐dependent trans‐regulation, APOLO uses a novel mechanism to recognize spatially distant targets through the formation of R‐loops. While it had previously been reported that lncRNAs can recognize single distant targets through R‐loop formation 7, APOLO uses R‐loop formation for the recognition of multiple not spatially associated targets, which demonstrates that gene co‐regulation by lncRNAs is not necessarily limited by distance. While the formation of R‐loops by base complementarity appears to be a clear means of APOLO to recognize its targets, it remains to be determined whether the DNA–RNA hybrids contribute themselves to the modulation of chromatin conformation of APOLO targets, given their broad roles as regulatory structures. For instance, also in Arabidopsis, R‐loop stabilization regulates the transcription of the lncRNA COOLAIR, affecting the expression of its antisense gene, FLC 8. The formation of R‐loops in a transcription‐independent manner, as it happens for APOLO, remains intriguing, given that the term R‐loop typically refers to the three‐stranded structure composed by the DNA–RNA hybrid that normally forms during transcription, when the double‐stranded DNA is separated, and the nascent RNA anneals with the template DNA strand, displacing the non‐template DNA strand 9. Further research will be needed to understand in which situations the double‐stranded DNA is sufficiently opened to hybridize with RNA in a transcription‐independent context. This could be happening, for instance, during the replication of DNA.
APOLO contributes to the increasing list of regulatory lncRNAs with roles in essential cellular and developmental processes, in this case, LR development of Arabidopsis, by trans‐regulating a specific signature of genes. Whereas lncRNAs are generally classified as cis‐acting or trans‐acting, APOLO acts through both mechanisms, indicating that lncRNA versatility allows for elegant adaptation to genome complexity and for quick responses to environmental changes.
EMBO Reports (2020) 21: e50107
See also: https://doi.org/10.1016/j.molcel.2019.12.015 (2020)
References
- 1. Marchese FP, Raimondi I, Huarte M (2017) Genome Biol 18: 206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ariel F, Lucero L, Christ A et al (2020) Mol Cell 10.1016/j.molcel.2019.12.015 [DOI] [PubMed] [Google Scholar]
- 3. Ariel F, Jegu T, Latrasse D et al (2014) Mol Cell 55: 383–396 [DOI] [PubMed] [Google Scholar]
- 4. Chu C, Qu K, Zhong FL et al (2011) Mol Cell 44: 667–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu W, Xu H, Li K et al (2017) Nat Plants 3: 704–714 [DOI] [PubMed] [Google Scholar]
- 6. Wang KC, Yang YW, Liu B et al (2011) Nature 472: 120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cloutier SC, Wang S, Ma WK et al (2016) Mol Cell 61: 393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun Q, Csorba T, Skourti‐Stathaki K et al (2013) Science 340: 619–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. García‐Muse T, Aguilera A (2019) Cell 179: 604–618 [DOI] [PubMed] [Google Scholar]


