Abstract
Microtubules derived from the Golgi (Golgi MTs) have been implicated to play critical roles in persistent cell migration, but the underlying mechanisms remain elusive, partially due to the lack of direct observation of Golgi MT‐dependent vesicular trafficking. Here, using super‐resolution stochastic optical reconstruction microscopy (STORM), we discovered that post‐Golgi cargos are more enriched on Golgi MTs and also surprisingly move much faster than on non‐Golgi MTs. We found that, compared to non‐Golgi MTs, Golgi MTs are morphologically more polarized toward the cell leading edge with significantly fewer inter‐MT intersections. In addition, Golgi MTs are more stable and contain fewer lattice repair sites than non‐Golgi MTs. Our STORM/live‐cell imaging demonstrates that cargos frequently pause at the sites of both MT intersections and MT defects. Furthermore, by optogenetic maneuvering of cell direction, we demonstrate that Golgi MTs are essential for persistent cell migration but not for cells to change direction. Together, our study unveils the role of Golgi MTs in serving as a group of “fast tracks” for anterograde trafficking of post‐Golgi cargos.
Keywords: cell migration, Golgi microtubules, microtubule defects, post‐Golgi cargo, STORM
Subject Categories: Cell Adhesion, Polarity & Cytoskeleton; Membrane & Intracellular Transport
This study reveals that Golgi MTs are more stable and polarized than non‐Golgi MTs. Golgi MTs support cell migration persistence without affecting directionality of motile cells.
Introduction
Cell migration requires efficient cargo trafficking 1, 2, 3, 4. Previous studies have demonstrated the importance of retrograde trafficking in cell motility 5, 6, 7, 8. Interestingly, disrupting the ER to Golgi trafficking 9 or the TGN budding 10 also hinders cell migration, suggesting that the anterograde trafficking is also important for cells to move. Despite these findings, the specific cellular structures ensuring the post‐Golgi cargo to function in sustaining persistent cell migration have been under debate.
Cargo trafficking is largely dependent on the MT network and its associated regulators and motors. The MT array is composed of centrosome‐anchored MTs which symmetrically distribute in the cell in a radial fashion, as well as asymmetric MTs which originate from or are stabilized at subcellular locations other than the centrosome. The Golgi apparatus has been reported to be a major hub for non‐centrosome MTs to nucleate from. Nucleation and stabilization of the Golgi MTs are under the regulation of A‐kinase‐anchoring protein (AKAP450) 11, 12, CLIP‐associated proteins (CLASPs) 13, 14, 15, and calmodulin‐regulated spectrin‐associated protein 2 (CAMSAP2) 16, 17, 18.
Previous reports claimed that the Golgi MTs functioned to maintain the crescent‐moon‐shaped Golgi ribbon 13, 19, 20, 21. However, a recent study using electron microscopy by Wu et al 16 has argued that loss of Golgi MTs by depletion of AKAP450 and CAMSAP2 did not alter the Golgi stacks. Meanwhile, accumulated evidence suggested that another role of the Golgi MTs was the juxtanuclear positioning of the Golgi apparatus 2, 22, 23, 24. The improper positioning of Golgi caused by loss of Golgi MTs has been reported to be detrimental in wound healing, suggesting that the Golgi MTs may be required in directional cell migration 22. In line with these observations, Golgi MTs have also been implicated to be essential for cargo trafficking based on the observation that loss of these MTs led to vesicle retention around the Golgi 16, 25 and crucial for insulin secretion 25. However, the underlying mechanisms remain elusive, partially due to the lack of direct observation of Golgi MT‐dependent vesicular trafficking and its relevance to cell migration.
Here, taking advantage of super‐resolution stochastic optical reconstruction microscopy (STORM) and live‐cell single‐particle analysis, we were able to track cargo trafficking on individual MTs and trace the originations of these tracks in motile cells. Based on the spatial association with the Golgi apparatus, we classified MTs into Golgi‐associated MTs (GaMTs) and non‐GaMTs. Interestingly, we discovered that post‐Golgi cargo trafficking was much faster on GaMTs than on non‐GaMTs. Compared to non‐GaMTs, GaMTs were morphologically more polarized toward the cell leading edge with significantly fewer inter‐MT intersections and lattice repair sites. Our STORM/live‐cell imaging showed that cargos frequently paused at MT intersections and MT lattice repair sites. Furthermore, using an optogenetic system, we demonstrated that the GaMTs were essential for persistent cell migration but not required for cells to change direction. Taken together, we proved that the Golgi MTs serve as a group of “fast tracks” for anterograde trafficking of post‐Golgi cargos to support persistent cell migration.
Results
Super‐resolution STORM imaging reveals interphase Golgi MTs
To understand how Golgi MTs contribute to cargo trafficking and how that links to cell motility, we applied super‐resolution STORM imaging to reveal the detailed infrastructure of Golgi MTs in an interphase cell (Appendix Fig S1A and B), which were unable to be resolved by conventional fluorescence imaging due to the high density 20. In order to avoid artifacts caused by the 3D projection into the 2D plane, we performed 3D STORM imaging of MTs in 600‐nm‐thick sections (Appendix Fig S1C and D). Our strategy was able to resolve individual MTs and allow the tracing of MT originations (Fig 1, Appendix Fig S1E and F, and Movie EV1). The MTs with clear spatial association with the Golgi apparatus in our STORM images were referred to as Golgi‐associated MTs (GaMTs) hereafter (Fig 1B and Appendix Fig S1E). GaMTs, marked in red, had one or more contact sites with the Golgi membrane (Appendix Fig S1E), while non‐GaMTs, marked in green (Appendix Fig S1E), showed no connections with the Golgi.
Figure 1. Classification of GaMTs and non‐GaMTs based on super‐resolution imaging.
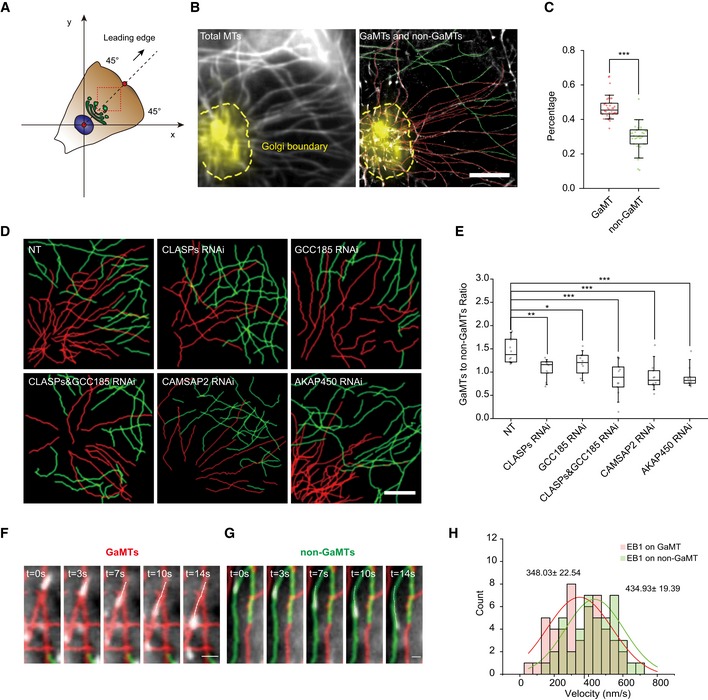
- Cartoon description of the segmentation of a polarized cell into four quadrants. The red dots indicate the center of the nucleus and the cell leading edge membrane, and the black arrow indicates the cell front‐rear direction. The red dashed line box indicates the STORM imaging region.
- Left: representative conventional image of interphase MT network in the 1st quadrant of a human retinal pigment epithelium (HRPE) cell. Gray: α‐tubulin; yellow: TGN‐46; yellow dashed line: Golgi boundary. Right: the classified MT subgroups. Red: GaMTs; green: non‐GaMTs. Scale bar: 5 μm.
- Box–whisker plot presents the proportion of GaMT and non‐GaMTs (data were pooled from three independent experiments and n = 36 cells). The ends of whiskers set as the 10 and 90% of the entire population. ***P < 0.001, two‐tailed Mann–Whitney.
- Six representative images of MT networks extracted from MT STORM images of HRPE cells under combinative KDs. Cells were rotated to orient their leading edges locating in the 1st quadrant. Upper: control cell; CLASPs KD cell; GCC185 KD cell. Lower: CLASPs + GCC185 KD cell; CAMSAP2 KD cell; AKAP450 KD cell. Red: GaMT; green: non‐GaMT. Scale bar: 5 μm.
- Box–whisker plot presents the ratio of GaMT/non‐GaMT under combinative KDs (one representative of three independent experiments and n = 12 cells). The ends of the whiskers are set at 10 and 90% of the entire population. *P < 0.05, **P < 0.01, ***P < 0.001, ns, no significant difference, unpaired t‐test.
- Time series show EB1 movement on individual GaMTs. Red: GaMT; green: non‐GaMT; white: EB1 tracks. Scale bar: 1 μm.
- Time series show EB1 movement on individual non‐GaMTs. Red line: GaMT; green line: non‐GaMT; white line: EB1 tracks. Scale bar: 0.5 μm.
- EB1 velocity distributions of GaMTs and non‐GaMTs. Red curve and green curve represent the Gaussian fittings (data were pooled from three independent experiments and n = 92 tracks). ***P < 0.001, unpaired t‐test.
Source data are available online for this figure.
To spatially characterize individual MTs, we defined a Cartesian coordinates for each cell with the migrating cell leading edge as the 1st quadrant (plus and minus 45° from the dashed line passing the leading edge middle point and the nuclear center) (Fig 1A) 26. The originations of 78% MTs in the leading edge quadrant of the cell were retrieved from STORM images, in which the ratio of GaMTs/non‐GaMTs was 1.5. The remaining 22% MTs with unclear originations were left unclassified (Fig 1C and Appendix Fig S1E). Similar results were observed when we used different Golgi markers‐TGN46, Man‐II, and GM130 to label trans‐, media‐, or cis‐Golgi (Appendix Fig S1G and H). It is worth noticing that such analyses indeed require super‐resolution imaging as conventional imaging showed a much higher ratio of GaMTs/non‐GaMTs than that of STORM imaging (Appendix Fig S1F). Moreover, when we disrupted Golgi with brefeldin A (BFA), the GaMTs disappeared rapidly, indicating their dependence on intact Golgi structure (Appendix Fig S1I–K and Movie EV2).
It has been shown that CLASPs, recruited to the Golgi membrane by trans‐Golgi network (TGN) GRIP‐domain‐containing protein GCC185, CAMSAP2, and A‐kinase anchor protein 450 (AKAP450), serve to stabilize MTs nucleated at or derived from the Golgi 12, 16, 18. In order to confirm whether the previously identified genes regulate the MTs that spatially in contact with the Golgi, we examined the GaMTs upon knocking down (KD) of these genes. Reduced MT number in the 1st quadrant was observed (Appendix Fig S1L and M) and the GaMT/non‐GaMT ratio dropped dramatically (Fig 1D and 1E), confirming the critical role of these genes in maintaining the MTs that are in spatial contact with the Golgi. We also observed that CAMSAP2 KD showed stronger effect in reducing the GaMT population than KD of GCC185 or CLASPs (Fig 1E). In a previous study, CAMSAP2 depletion was shown to reduce non‐centrosome MTs without changing the Golgi‐derived MTs 16. In conjunction with this observation, our data indicated that CAMSAP2 may regulate a subpopulation of non‐centrosomal MTs which are derived from cellular origins other than the Golgi, and later captured by the Golgi apparatus. Therefore, in our experiment, CAMSAP2 KD reduced the MTs that were in contact with but did not derive from the Golgi.
Previous findings revealed that Golgi‐derived MTs were more resistant to ice or nocodazole‐induced depolymerization 13, 26. We found that after ice or nocodazole treatment, most of the remaining stable MTs located in the 1st quadrant (Appendix Fig S1N and O), and meanwhile, the GaMT/non‐GaMT ratio increased from 1.5 to above 5 (Appendix Fig S1P and Q). These observations indicated that GaMTs are more stable than non‐GaMTs.
We next evaluated MT dynamics by analyzing the trajectories of the growing end‐binding protein EB1, which indicate the growing MTs 27. EB1 trajectories were polarized and enriched in the 1st quadrant (Appendix Fig S1R–T). EB1 velocity was significantly increased in CAMSAP2 or CLASPs/GCC185 KD cells (Appendix Fig S1U), suggesting that the growth rate of GaMTs was lower than non‐GaMTs. In order to confirm it, we registered EB1 trajectories onto individual MTs via superimposing live‐cell single‐particle trajectories onto STORM images of MTs (Fig 1F and G, Movies EV3 and EV4). We observed that the growth rate of GaMTs (348.33 ± 22.54 nm/s) was significantly slower than that of non‐GaMTs (434.93 ± 19.39 nm/s) (Fig 1H).
Taken together, we have revealed the MT population in spatial contact with the Golgi in a migrating cell during interphase. Using ice/nocodazole treatments, genetic disruptions, and EB1 tracking, we showed that GaMTs are nearly functionally equivalent to other Golgi MTs previously defined based on different basis. Importantly, as the classification of GaMTs is based on the spatial association with Golgi, this will be a valuable addition to genetic manipulation or in vitro reconstitution approaches to dissect the specific roles of centrosomal vs. non‐centrosomal microtubules, linking the subcellular localization of MTs with their functions.
MTs associated with the Golgi are fast tracks for post‐Golgi cargos
We next investigated the role of Golgi MTs in vesicle trafficking. Via the retention using selective hooks (RUSH) system 28, we synchronized and visualized E‐cadherin (Ecad) cargo trafficking in HRPE cells (Fig 2A). Based on analysis of 45 cells and 4,443 cargos, we observed that the 1st quadrant contained significantly more cargos compared to the other quadrants (Fig 2B). Interestingly, when assigning cargo speed into four intervals (0–100 nm/s, 100–200 nm/s, 200–400 nm/s, and > 400 nm/s), we found that, among all four quadrants, 90% of the cargos faster than 400 nm/s appeared in the 1st quadrant (Fig 2C). This result revealed a distinct feature of the leading edge quadrant in dominating fast cargo trafficking.
Figure 2. Cargo velocity on GaMTs and non‐GaMTs.
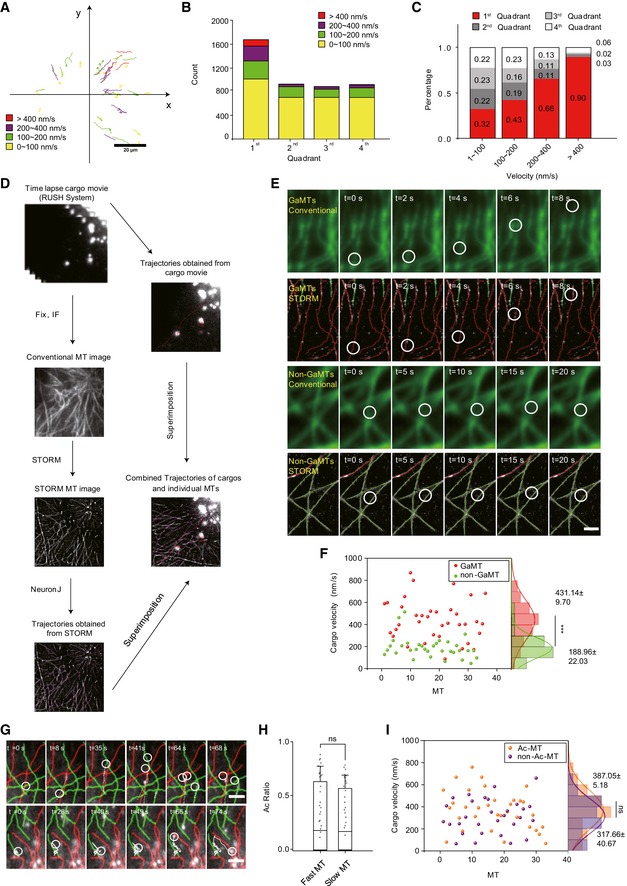
- Representative trajectories of Ecad cargos tracked over 120 s in an HRPE cell. Colors represent different average velocity ranges. Red: > 400 nm/s; purple: 200–400 nm/s; green: 100–200 nm/s; yellow: 0–100 nm/s. Scale bar: 20 μm.
- Total number of cargos within different velocity ranges in the four quadrants, respectively (data were pooled from three independent experiments and n = 45 cells).
- Proportions of cargos with different velocity in the four quadrants. Red: the 1st quadrant; dark gray: the 2nd quadrant; light gray: the 3rd quadrant; white: the 4th quadrant (data were pooled from three independent experiments and n = 45 cells).
- Flow chart shows the method of combining STORM imaging with single‐particle tracking in live cells. Red lines indicate the cargo trajectories. Lower right, MT networks (gray) were extracted and present in purple.
- Representative time series show one fast cargo moving on a GaMT and a slow cargo moving on a non‐GaMT. Upper, conventional imaging; lower, STORM imaging. Red line: GaMT; green line: non‐GaMT; white circle: cargo position. Scale bar: 2 μm.
- Marginal distributions show the velocity on GaMT (red dots) or non‐GaMTs (green dots). Red curve and green curve, Gaussian fitting curves of velocity distribution on GaMT or non‐GaMT, respectively (data were pooled from three independent experiments and n = 69 MTs). Peak of red curve (431.14 ± 9.7 nm/s) and peak of purple curve (188.96 ± 22.03 nm/s). ***P < 0.001, unpaired t‐test.
- Upper panel: Time series show an example of several fast cargos present on GaMT. Lower panel: Time series show one slow cargo on non‐GaMT switching onto a GaMT and becoming faster. Red line: GaMT; green line: non‐GaMT; white circle: cargo position. White line, cargo trajectory. Scale bar: 2 μm.
- Box–whisker plot presents the averaged acetylation level of fast or slow MT tracks (data were pooled from two independent experiments and n = 63 MTs). The ends of the whiskers are set at 10 and 90% of the entire population, ns, no significant difference, unpaired t‐test.
- Marginal distributions of the mean velocity of cargos on segments of Ac‐MTs (orange dots) and non‐Ac‐MTs (purple dots). Orange curve and purple curve represent the Gaussian fittings (data were pooled from two independent experiments and n = 63 MTs). ns, no significant difference, unpaired t‐test.
Source data are available online for this figure.
Following the same strategy, we also examined two other post‐Golgi cargos, the tumor necrosis factor‐α (TNF) and the vesicular stomatitis Indiana virus G protein (VSVG), as well as one recycling cargo marked by Rab5 (Rab5 cargos, associated with early endosomes) (Appendix Fig S2A–I). In contrast to all three post‐Golgi cargos (Ecad, TNF, and VSVG) displaying polarized distribution of cargo velocity, the Rab5 cargos showed no obvious difference among all four quadrants (Appendix Fig S2I). Collectively, these data suggested that certain trafficking‐supporting system exists in the cell leading edge quadrant, affecting post‐Golgi cargo behavior in a specific way.
The spatially biased post‐Golgi cargo trafficking prompted us to pinpoint the role of GaMT tracks. To directly test the hypothesis that efficiently directed post‐Golgi cargo trafficking via specialized MTs was required for cell motility, we systematically analyzed cargo behavior on individual MTs by superimposing live‐cell single‐particle trajectories of trafficking cargos onto STORM images of MTs 29. This allowed us to track cargo motility along individual MT tracks (Fig 2D). We captured events where the cargo on the GaMT transported over 5 μm during 8 s with an average velocity of ~600 nm/s (Fig 2E and Movies EV5). In contrast, we also observed cargos on the non‐GaMT that moved back‐and‐forth and only traveled less than 1 μm during 20 s (Fig 2E and Movie EV6). We analyzed 36 cargos on GaMTs and 33 cargos on non‐GaMTs in 36 cells and concluded that the cargo velocity on GaMTs (431.1 ± 9.7 nm/s) was significantly higher than that on non‐GaMTs (188.9 ± 22.03 nm/s; Fig 2F). This difference could be caused by certain intrinsic properties distinct for the GaMTs, or due to differed cargo properties. Interestingly, we observed events in which a slowly transporting cargo on a non‐GaMT switched track onto a GaMT and immediately reached a high speed (Fig 2G, Movies EV7 and EV8). These observations strongly suggested that biased cargo velocity between GaMTs and non‐GaMTs was mainly due to certain intrinsic properties of the tracks rather than cargo properties such as associated motor types and numbers.
Some post‐translational modifications (PTMs) of MT are known to affect cargo trafficking. For instance, acetylated MTs (Ac‐MTs) and detyrosinated MTs (Detyr‐MTs) have been reported to promote binding and motility of kinesin‐1 motors 30. We stained tyrosinated (Tyr‐MTs), detyrosinated, and acetylated tubulin for GaMTs and non‐GaMTs. We found that both Ac‐MTs and Detyr‐MTs were enriched in the first quadrant, while Tyr‐MTs were evenly distributed in all quadrants (Appendix Fig S2J and K). As GaMTs are enriched in the first quadrant, we then focused on whether acetylation or detyrosination contributes to the difference between GaMTs and non‐GaMTs. After STORM imaging and MT grouping, we found both Ac‐MTs and Detyr‐MTs were slightly enriched on GaMTs (32.9% vs. 15.9% for Ac‐MTs; 26.2% vs. 13.7% for Detyr‐MTs; Appendix Fig S2L–O). We then interrogated the role of MT PTMs in differed cargo velocity. Ac‐MTs were specifically labeled, and two‐color STORM imaging was applied to monitor MT acetylation level between fast and slow cargo trafficking tracks. We were able to detect only very marginal difference in the acetylation level between the GaMT tracks (26.8%) and the non‐GaMT tracks (23.1%) (Fig 2H). As acetylation often appeared segmented along MTs, we then carefully examined cargo velocity on Ac‐MT segments and non‐Ac‐MT segments. Likewise, we were only able to detect minor difference in cargo velocity between Ac‐MT segments (387.05 ± 15.18 nm/s) and non‐Ac‐MT segments (317.66 ± 40.67 nm/s) (Fig 2I). In addition, we performed orthogonal analysis by carefully comparing cargo speed on Ac‐GaMT, non‐Ac‐GaMT, Ac‐non‐GaMT, and non‐Ac‐non‐GaMT. The results showed that the average cargo velocities of Ac‐GaMTs and non‐Ac‐GaMTs were far faster than that on non‐GaMTs, regardless of their PTMs (Appendix Fig S2R and [Link], [Link], [Link], [Link]). We also carefully examined the detailed motility behavior of cargos on different MTs. The results showed that cargos on non‐GaMTs underwent more frequent pausing and reversing events than GaMTs, also independent of their PTMs (Appendix Fig S2T and U). Similar with acetylation, the average cargo velocities of Detyr‐GaMTs and non‐Detyr‐GaMTs were faster than that on non‐GaMTs, regardless of their PTMs (Appendix Fig S2S and [Link], [Link], [Link], [Link]). Thus, we conclude that acetylation and detyrosination have limited contribution to the differential cargo speed on GaMTs and non‐GaMTs.
GaMTs contain less MT repair sites that benefit fast cargo trafficking
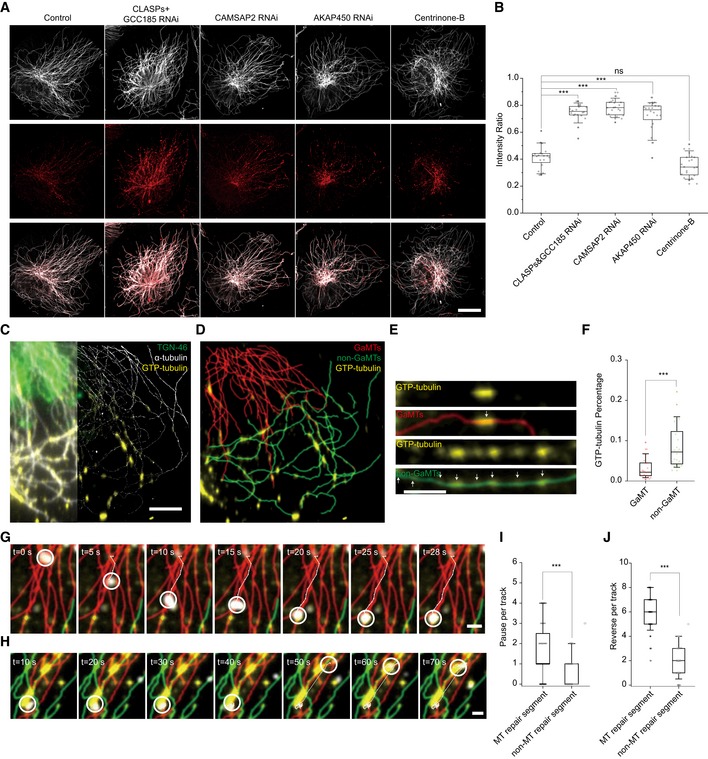
The MT defects have been shown in vitro to affect kinesin‐based cargo transporting 31, 32, 33. To evaluate the MT defects level in GaMTs vs. non‐GaMTs, we applied the established GTP‐tubulin perfusion assay to identify MT defects (MT lattice repair sites) in live cells 34. Interestingly, upon KD of CLASPs, CAMSAP2, or AKAP450, MT repair ratio was significantly increased (Fig 3A and B). Meanwhile, after Centrinone‐B treatment to deplete centrosomal MTs 35, MT repair level remained unchanged compared with control cells (Fig 3B). To investigate the repair sites in more detail, we then used STORM imaging to analyze the MT repair level on individual GaMTs and non‐GaMTs (Fig 3C and D). We discovered that GaMTs harbored much fewer MT repair sites compared to non‐GaMTs (Fig 3E and F). We then interrogated whether MT repair sites would halt cargo trafficking in vivo by applying live‐cell imaging combined with STORM super‐resolution imaging. We captured events where cargos paused at the GTP‐tubulin‐labeled MT repair sites (Fig 3G and H). Quantification of cargo motility revealed that cargos paused and reversed more frequently on MT repair segments than non‐repair segments (Fig 3I and J, Movies EV17 and EV18), lending an explanation to the observation that cargos on non‐GaMTs underwent more frequent pausing and reversing events than GaMTs (Appendix Fig S2T and U). This is the first evidence, to our knowledge, supporting that MT defects affect cargo transporting in cells. These observations suggest that the difference in MT defects between GaMTs and non‐GaMTs serves as the major mechanism for fast and slow cargo transport.
Figure 3. Non‐GaMT harbors more MT repair sites where cargos frequently pause and reverse.
-
AFive representative images of MT repair sites in HRPE cells under combinative KDs. Cells were rotated to orient their leading edges locating in the 1st quadrant. From left to right, control cell, CLASPs&GCC185 KD cell, CAMSAP2 KD cell, AKAP450 KD cell, and centrinone‐B‐treated cell. Gray: α‐tubulin; red: GTP‐tubulin. Scale bar: 20 μm.
-
BBox–whisker plot presents intensity analysis of MT repair sites in HRPE cells under combinative KDs (one representative of three independent experiments and n = 20 cells). ***P < 0.001, unpaired t‐test.
-
CConventional image (left) and STORM image (right) of MTs and MT repair sites. Gray: α‐tubulin; yellow: GTP‐tubulin; green: Golgi. Scale bar: 5 μm.
-
DMT repair sites on GaMTs and non‐GaMTs extracted from (C). Red: GaMTs; green: non‐GaMTs; yellow: GTP‐tubulin.
-
EMT repair sites on GaMTs and non‐GaMTs separately presented in detail. White arrows indicate GTP‐tubulin‐labeling sites. Scale bar: 5 μm.
-
FBox–whisker plot presents the ratio of MT repair sites on GaMTs and non‐GaMTs separately (one representative of three independent experiments and n = 8 cells). ***P < 0.001, unpaired t‐test.
-
GRepresentative time series show one fast cargo moving on a GaMT and slowing down at the MT repair site. Red: GaMT; green: non‐GaMT; yellow: GTP‐tubulin; white circle: cargo position. Scale bar: 2 μm.
-
HRepresentative time series show one cargo moving fast between two MT repair sites and slowing down at the MT repair sites. Red: GaMT; green: non‐GaMT; yellow: GTP‐tubulin; white circle: cargo position. Scale bar: 2 μm.
-
L, JPause (I) and reverse (J) events of cargos on MT repair segment and non‐MTs repair segment, respectively (data were pooled from two independent experiments and n = 22 cells). ***P < 0.001, ns, no significant difference, unpaired t‐test.
GaMTs are polarized MTs with few intersections
The MT morphology has been implicated to affect vesicle trafficking. Previous studies have reported MT intersections affect motor pausing and reversing frequency 29, 36 and that MT curvature is relevant to MT terminal stability and motor behavior 37. We then examined the geometrical characteristics of GaMTs containing directionality, curvature, and intersections. We applied Single‐Molecule Localization Microscopy Image Filament Network Extractor (SIFNE) 38 to analyze the morphological characteristics of GaMTs and non‐GaMTs (Fig 4 and Appendix Fig S3). Briefly, MT images were extracted and replotted (Fig 4A). The directionality of MTs was presented in a rose‐plot (Fig 4B), which indicates that the direction of GaMTs was more converged than that of non‐GaMTs. We defined the MT polarity as the angle between a MT and the major axis of all MTs in a cell (Materials and Methods). The analysis showed that GaMTs were more directed toward the cell leading edge, and contrarily, non‐GaMTs appeared more randomly orientated (Fig 4C). In contrast, the curvature of GaMTs and non‐GaMTs did not show significant difference (Appendix Fig S3). We also analyzed the intersections of the MT network. The results indicated that GaMTs had fewer intersections than non‐GaMTs (Fig 4D), in line with the observation that fewer cargo pausing and switching events were on GaMTs than non‐GaMTs (Appendix Fig S2T and U).
Figure 4. GaMTs are more polarized and contain less intersections.
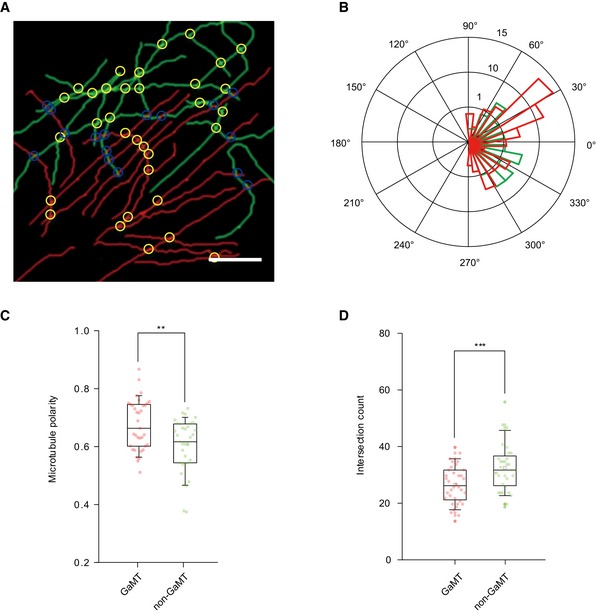
- A representative image of MT networks extracted from MT STORM image of an HRPE cell. Red line: GaMT; green line: non‐GaMT. Yellow circle: intersection. Blue circle: intersections formed by GaMTs and non‐GaMTs. Scale bar: 5 μm.
- Rose‐plot presents the orientation of GaMTs and non‐GaMTs. Red: GaMTs; green: non‐GaMTs.
- Box–whisker plot presents the projections of GaMTs and non‐GaMTs to the leading edge direction (data were pooled from three independent experiments and n = 36 cells). The ends of the whiskers are set at 10 and 90% of the entire population, **P < 0.01, unpaired t‐test.
- Box–whisker plot presents the intersection counts of GaMTs and non‐GaMTs (data are pooled from three independent experiments and n = 36 cells). The ends of the whiskers are set at 10 and 90% of the entire population, ***P < 0.001, unpaired t‐test.
Source data are available online for this figure.
The above results indicated that the GaMTs were more stable with fewer defects, less‐crossed and more oriented toward the cell leading edge. These properties seem to support GaMTs to serve as reliable and efficient post‐Golgi cargo tracks.
GaMTs are required for cell migration persistence but are not essential for the direction changes
Polarized vesicle delivery is required for cell migration 23, 39, 40. In light of the findings that GaMTs were more orientated to the leading edge (Fig 4B and C) and that they mediated fast cargo trafficking (Fig 2), we further interrogated the role of GaMTs in cell migration. In random migration, we tracked cell trajectories and quantified the migration persistence as the ratio of the Euclidian distance and the accumulated distance 6, 41. Compared to control cells, CLASPs&GCC185 KD or CAMSAP2 KD cells failed to maintain their direction and moved more randomly (Fig 5A and Movie EV19). Quantitative analysis indicated that cell migration persistence was indeed significantly reduced in CLASPs&GCC185 KD or CAMSAP2 KD cells (Fig 5B–E). Similar results were observed with wound‐healing assays (Appendix Fig S4A–C and Movie EV20). These data suggested that GaMTs were necessary for persistent cell migration.
Figure 5. GaMTs are required for persistent cell migration but not for cell to change directions.
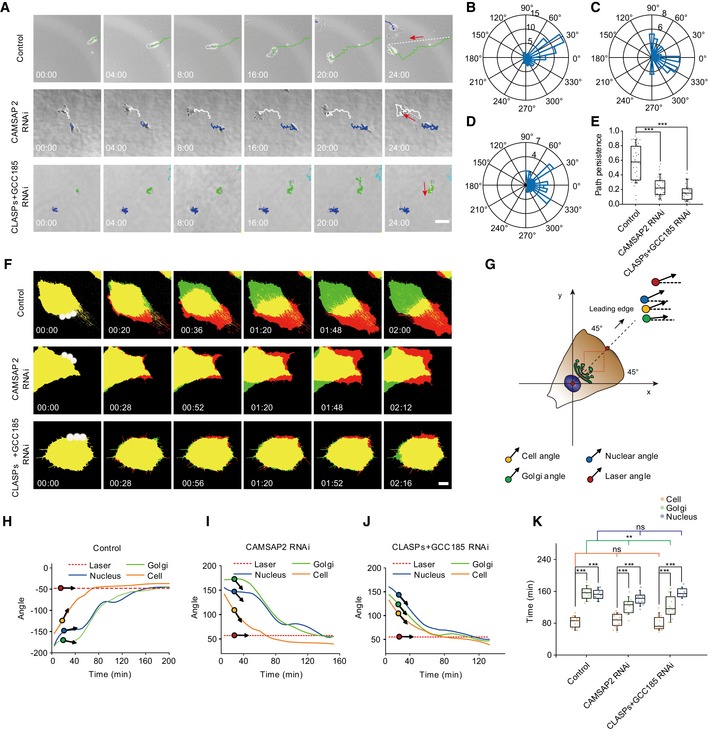
-
ARandom migration of HRPE cells during 24 h. Upper: control cell; middle: CAMSAP2 KD cell; and lower: CLASPs&GCC185 KD cell. Red arrow and white dashed lines indicate the migration direction. Different migration trajectories are distinguished with different colors. Scale bar: 50 μm.
-
B–DRose‐plot presents the representative directionality of an HRPE cell. (B) Control cell; (C) CAMSAP2 KD cell; and (D) CLASPs&GCC185 KD cell.
-
EBox–whisker plot presents the migration persistence of control cell, CAMSAP2 KD cell, and CLASPs&GCC185 KD cell (one representative of three independent experiments and n = 34 cells). The ends of the whiskers are set at 10 and 90% of the entire population, ***P < 0.001, unpaired t‐test.
-
FControlled cell migration of three representative cells in PA‐Rac1 assay. White spot: 440 nm laser spot; green area: the original position of the cell; red area: the current position of the cell; yellow area: the overlapped area. Scale bar: 10 μm.
-
GCartoon presents a controlled migrating HRPE cell with Golgi, cell, and nucleus. Black arrow with red dot: laser direction; black arrow with orange dot: cell body direction; black arrow with blue dot: nuclear direction; black arrow and green dot: Golgi direction.
-
H–JAngle change curve of cell, nucleus, and Golgi. Red dashed line: laser angle; blue line: nucleus angle; orange line: cell angle; green line: Golgi angle. H: control cell; I: CAMSAP2 KD cell; and J: CLASPs&GCC185 KD cell.
-
KBox–whisker plot shows the total time required for the nucleus, Golgi, and cell body reaching the given laser angle. Blue: time required for nucleus; green: time required for Golgi; orange: time required for cell body (one representative of three independent experiments and n = 14 cells). The ends of the whiskers are set at 10 and 90% of the entire population, ***P < 0.001, ns, no significant difference, unpaired t‐test.
Source data are available online for this figure.
To further dissect the role of GaMTs in the recognition and maintenance of cell migration direction, we employed an optogenetic tool to steer cell movement (Appendix Fig S4D–F) 42. First, we locally excited PA‐Rac1 at the non‐leading edge cell periphery to switch on the “turn” signal. Then, we imaged the following cellular responses in control and CLASPs&GCC185 or CAMSAP2 KD cells (Fig 5F and G). We found that, upon optical stimulation, a new leading edge formed followed by directional turning of the Golgi apparatus and the nucleus (Fig 5F and K and Movie EV21). Eventually, the whole cell body aligned toward the new cell direction and started to crawl (Fig 5H and Movie EV21). Surprisingly, cells lacking GaMTs were still able to complete this whole direction‐turning process (Fig 5I and J), albeit the Golgi turning speed was increased compared to the control cell (Fig 5K), supporting the previous reports that Golgi MTs function in Golgi positioning 2, 22, 23, 24. These observations indicated that GaMTs were not required for cells to change direction.
GaMTs maintain cell migration persistence via efficient post‐Golgi vesicle transport to the cell leading edge
A number of studies have shown that in migration cells, post‐Golgi vesicles are preferentially transported toward the cell leading edge in an MT‐dependent manner 1, 23, 39. As a post‐Golgi cargo, the cadherin–catenin complex was found to promote directional cell migration 43, 44. Indeed, KD of Ecad or MMP cargo significantly reduced cell migration persistence (Appendix Fig S5A and Movie EV22). To examine whether delivery of Ecad or MMP cargo to the plasma membrane through vesicular trafficking contributed to efficient cell motility, we used a SNARE toxin BONT/C to inhibit plasma fusion 3. Under this treatment, cell migration persistence was dramatically reduced (Appendix Fig S5A and B, and Movie EV22).
Our findings that GaMTs served as fast tracks to support Ecad cargo trafficking toward the cell leading edge (Fig 2) prompted us to investigate the role of GaMTs in Ecad regulated cell migration persistence (Fig 6A–F). A more careful analysis of Ecad cargo distribution revealed more Ecad cargos moving along GaMTs than non‐GaMTs (Fig 6E). When we disrupted GaMTs via KD of CLASPs&GCC185 and CAMSAP2, the number of Ecad cargos delivered to the membrane of cell leading edge was significantly reduced (Fig 6B–D and F, and Movie EV23). The GaMT‐dependent biased cargo delivery to the cell leading edge also held for other post‐Golgi vesicles such as TNF, VSVG, and MMP (Appendix Fig S4C–I). In contrast, the recycling Rab5 cargos did not show GaMT‐dependent polarized trafficking (Fig 6B–D and G, and Movie EV24). Collectively, these observations indicated that GaMTs played an essential role in persistent cell migration by supporting efficient anterograde trafficking of post‐Golgi vesicles to the cell leading edge.
Figure 6. GaMTs efficiently deliver post‐Golgi cargos to the cell leading edge.

-
AProjections of 120 frame movies present leading edge delivery of two different types of cargos. Left: Ecad cargo; right: Rab5 cargo. Yellow line marks the cell boundary. Scale bar: 20 μm.
-
B–DExamples of cargo delivery toward the leading edge, time series present at 40, 80, and 120 s, respectively. (B) control cell; (C) CAMSAP2 KD cell; and (D) CLASPs&GCC185 KD cell. Red arrow heads indicate cargo current position. Size of cropped regions: 30 μm × 5 μm.
-
EBox–whisker plot presents the Ecad cargo number on GaMTs and non‐GaMTs (data were pooled from three independent experiments and n = 36 cells). The ends of the whiskers are set at 10 and 90% of the entire population, **P < 0.01, unpaired t‐test.
-
FBox–whisker plot presents the number of Ecad cargos finally reached the leading edge membrane during 120 s (one representative of three independent experiments and n = 24 cells). The ends of the whiskers are set at 10 and 90% of the entire population, **P < 0.01, two‐tailed Mann–Whitney.
-
GBox–whisker plot shows the number of Rab5 cargos that finally reached the leading edge membrane during 120 s (one representative of three independent experiments and n = 23 cells). The ends of the whiskers are set at 10 and 90% of the entire population, ns, no significant difference, two‐tailed Mann–Whitney.
Source data are available online for this figure.
Discussion
Golgi MTs have been implicated to play critical roles in cell migration, but the underlying mechanisms remain elusive, partially due to the lack of direct observation of Golgi MT‐dependent vesicular trafficking and its relevance to cell migration. In this study, we applied STORM super‐resolution imaging to visualize Golgi MTs (Fig 1), providing the first description of interphase Golgi MT architecture based on their association with Golgi in spite of the origination of the MTs. Previous studies have defined Golgi MTs according to different assays such as EB tracking (Golgi‐originated MT) 12, nocodazole treatment (Golgi‐nucleated MT) 15, or MTs derived from newly formed Golgi stacks after mitotic exit (Golgi‐derived MT) 26. It is worthwhile noting that MTs associated with the Golgi are not necessarily originated/nucleated from the Golgi. In fact, MTs docking at the Golgi membrane may have nucleated at other sites instead of the anchoring sites for several reasons. For instance, Golgi and centrosome share the MT nucleation machinery 45 and MT minus‐end stabilizing factors such as CAMSAP2 16, which could shuttle MT nucleating seeds between Golgi and centrosome. Therefore, classification of GaMTs solely based on the spatial association with Golgi includes both Golgi‐originated and Golgi‐captured MTs, regardless of their originations and linking the subcellular localization of MTs with their functions. Importantly, the features we observed with GaMTs that (i) more resistant to ice or nocodazole treatments, (ii) regulated by GCC185, CLASPs, and CAMSAP2, and (iii) less dynamic in plus end tracking strongly suggest that GaMTs are nearly functionally equivalent to other Golgi MTs defined on different basis. Moreover, in our study, we frequently observed MTs that pass through the Golgi showed similar properties in defect level, crossover, and cargo velocity with the MTs originating/derived from the Golgi. This observation implicates that some molecular machineries on Golgi membrane such as CLASPs and CAMSAPs might decorate and stabilize the MTs that pass through the Golgi apparatus.
Using correlative live‐cell imaging and STORM imaging, we discovered that GaMTs served as “fast tracks” for post‐Golgi cargos (Fig 2) to support persistent cell migration (Fig 5). The underlying mechanisms for GaMTs being fast tracks may be a collective consequence of different cargo and MT properties. For instance, the cargo velocity can be regulated by the type and number of different motors on the cargo via “tug‐of‐war” 46, 47, 48, 49. We have excluded this possibility based on the observation of immediate sped‐up of cargos when switching from a non‐GaMT track onto a GaMT track (Fig 2G). Instead, this phenomenon strongly suggested that GaMTs are intrinsically different from non‐GaMTs in properties of the MT per se.
A number of MT properties can affect cargo behavior, including post‐translational modifications (PTMs) 30, 50, MT‐associated protein (MAPs) 51, 52, MT defects 31, and morphology of MTs and MT networks 29, 36. MT acetylation and detyrosination have been reported to promote kinesin‐1 motility 30. However, we found that the acetylation and detyrosination showed minimal contribution to cargo velocity on GaMTs and non‐GaMTs (Appendix Fig S2R and S), but whether any other PTMs discriminate GaMTs and non‐GaMTs remains to be investigated. MAPs can also affect cargo transport in a variety of manners, such as roadblocks 53 and stabilization of MTs with less lattice defects 54, 55. In fact, MT lattice integrity is known to promote the efficiency of kinesin‐based transport 31, while lattice defects allow katanin‐based MT severing 56 and α‐TAT enzyme entry into MT lumen 57. Here, we discovered that GaMTs contain much fewer MT repair sites compared to non‐GaMTs (Fig 3E and F), and cargos pause and reverse more frequently on defect segments than non‐defect segments (Fig 3I and J). We have provided extensive evidence to show that the difference in MT defects between GaMTs and non‐GaMTs serves as the major mechanism for fast and slow cargo transport. Lastly, regarding the contribution of MT morphology, our STORM data showed that GaMTs were more polarized toward the cell leading edge than non‐GaMTs (Fig 4A–C). Moreover, the intersections of GaMTs were significantly fewer than that of non‐GaMTs. In summary, we reason that the lower level in both intersections and defects on GaMTs is likely the mechanisms that make GaMTs fast tracks compared to non‐GaMTs. The entire mechanisms, including many different types of MT PTMs, remain to be a critical subject for future studies.
The role that actin plays in cell migration has been widely studied and the dynamic remodeling of F‐actin is suggested to be a key process 1, 58. In contrast, MTs have been understudied in the context of cell migration. Two previous studies have demonstrated that Golgi MTs are important for cell motility 26 and wound healing 22, but the detailed mechanisms are lacking. Our data indicated that GaMTs function as specialized fast tracks for transport of the post‐Golgi cargos required for persistent cell migration (Fig 5A–E). In addition, the PA‐Rac1 assay (Fig 5H–K) showed that for cells to change direction, GaMTs are dispensable while actin remodeling is necessary 59. The data depicted an upstream–downstream coordination between actin and MTs in cell migration, in line with the observation that the direction change of Golgi apparatus was always after that of the cell body (Fig 5K) as well as the report that the Golgi direction is not correlated with the cell migration direction in the freely migrating cell 60. Moreover, we noticed that disruption of GaMTs by CLASPs&GCC185 or CAMSAP2 KD markedly increased the turning speed of Golgi apparatus (Fig 5K), supporting the previous reports that Golgi MTs function in Golgi positioning 2, 22, 23, 24. Therefore, besides serving as fast tracks to maintain persistent cell migration, GaMTs might also function as a check‐point to ensure the cell to take the right migration direction, while it senses the environment with multiple filopodia/lamellipodia protrusions. Indeed, CAMSAP2‐deficient endothelial cells exhibited multiple protrusions instead of one major protrusion 61. More recently, MT disruption has been reported to cause cell fragmentation due to failure in retracting pathfinding protrusions in migrating neutrophils 62.
In summary, our work shed light on the role of Golgi MTs, as a special subgroup of MT cytoskeleton, in migration of isolated cells. In the future, it would be interesting to investigate how Golgi MTs function in collective cell migration as well as pathological processes, such as cancer cell invasion, immune cell infiltration, and in vivo wound‐healing response.
Materials and Methods
Antibodies
Antibodies used in this study were used as follows:
CLASP1 (rabbit, Abcam, 1:1,000 for WB), CLASP2 (rat, Abcam, 1:1,000 for WB), α‐tubulin (rabbit, Abcam, 1:100 for conventional imaging, 1:50 for STORM, 1:1,000 for WB), acetylated tubulin (mouse, Abcam, 1:100 for conventional imaging, 1:50 for STORM, 1:1,000 for WB), CAMSAP2 (rabbit, Proteintech, 1:1,000 for WB), GCC185 (rabbit, Bethyl, 1:1,000 for WB), Ecad (rabbit, Abclonal, 1:1,000 for WB), GM130 (mouse, BD, 1:100 for conventional imaging, 1:50 for STORM), TGN‐46 (sheep, Serotec, 1:100 for conventional imaging, 1:50 for STORM), beta‐tubulin E7 (mouse, kind gift from Prof.Jian Ju, Peking university, 1:100 for conventional imaging, 1:50 for STORM, 1:1,000 for WB), anti‐Syntaxin (mouse, kind gift from Prof. Liangyi Chen, Peking university, 1:1,000 for WB), and anti‐SNAP‐25 (mouse, kind gift from Prof. Liangyi Chen, 1:1,000 for WB),
Cell culture
The human retinal pigment epithelium (HRPE) cell line was a kind gift from Prof. Wei Guo, University of Pennsylvania. HRPE cells were maintained in DMEM/Ham's F‐12 (Invitrogen, #11320033) with 10% fetal bovine serum (FBS) (Gibco, #16010‐159) and 100 μg/ml penicillin and streptomycin (Invitrogen). Cells were grown under standard cell culture conditions (5% CO2, humidified atmosphere at 37°C) and were plated on DMEM/Ham's F‐12 or DMEM pre‐incubated glass coverslips 24 h before experiments. For cell passage, cells were washed with PBS (Life Technologies, #14190500BT) and digested with trypsin (Gibco, #25200‐056). All cell lines were routinely tested for potential mycoplasma contamination (MycoAlert, Lonza), and all tests were negative.
Electroporation and RNA interference
The siRNA or DNA was transfected to the cell using a 2D Nucleofector Device (Lonza). On the day of electroporation, trypsinized cells were transiently transfected with electroporation cup (Bio‐Rad, #165‐2086) using program X‐001. Electroporation buffer contains: Solution I and Solution II. Solution I contains 2 g ATP‐disodium salt (Sigma‐Aldrich, A2383), 1.2 g MgCl2‐6H2O (Sigma‐Aldrich, M2393), with ddH2O up to 10 ml; Solution II contains 6 g KH2PO4 (Sigma‐Aldrich, P5655), 0.6 g NaHCO3 (Sigma‐Aldrich, S‐5761), 0.2 g glucose (Sigma‐Aldrich, G‐6152), adjust pH to 7.4, and add ddH2O to 500 ml. After filter sterilization (0.22 μm), we mixed 80 μl Solution I with 4 ml Solution II and stored them at 4°C for up to 1 month. Experiments were conducted 24 h after DNA transfection and 72 h after siRNA transfection.
All siRNAs were purchased from GenePharma:
CLASP1 siRNA‐targeted sequence: 5′‐GGATGATTTACAAGACTGG‐3′;
CLASP2 siRNA‐targeted sequence: 5′‐GACATACATGGGTCTTAGA‐3′.
GCC185 siRNA: 5′‐GGCTAAT TCTCAGCATTACC‐3′;
CAMSAP2 siRNA: 5′‐UCUCGAAUCUGUUUCUGUGGAGAGG‐3′
Ecad shRNA plasmid (for Ecad RNAi) were provided by Prof. Chen Zhang, Peking University.
Non‐targeting siRNA (GenePharma) was used for controls. Empty pEGFP‑C1 vector (Clontech) served as a control for transfection efficiency.
MT defects labeling
GTP‐tubulin‐labeling method was optimized from previous study 34, and HRPE cells were permeabilized at 37°C for 3 min in PEM buffer (80 mM PIPES, 2 mM EGTA, 1 mM MgCl2, pH 6.9), supplemented with 10% glycerol and 0.1% Triton X‐100. Samples were incubated with hMB11 (1:10,000–1:50,000, Adipogen, #AG‐27B‐0009‐C100) for 10 min at 37°C in PEM buffer containing 10% glycerol and 0.2% BSA. After washing with PEM buffer containing 10% glycerol and 0.2% BSA for five times, HRPE cells were incubated with Alexa 555‐anti‐human antibodies for 10 min at 37°C in PEM buffer containing 10% glycerol and 0.2% BSA.
For co‐labeling, after GTP‐tubulin labeling, cells were fixed in cold methanol (4 min, −20°C) followed by α‐tubulin immunofluorescence staining.
Plasmids
Cis‐Golgi marker GalT‐GFP (#11929), trans‐Golgi marker TGN‐38‐GFP (#128148), SBP‐mCherry‐Ecadherin_puromycin (Ecadherin reporter only, no hook, RUSH system) (#65293), TNF‐SBP‐mCherry_puromycin (TNF only, no hook, RUSH system) (#65285), Ii‐Str_puromycin (ER hook only, RUSH system) (#65309), and Str‐STIM1‐NN_neomycin (ER hook only, RUSH system) (#65311) were all kind gifts from Prof. Xiaowei Chen, Peking University. pFasBac + GFP‐CAMSAP2 (#59039), Str‐Ii_SBP‐EGFP‐Golgin84 (synchronizing trafficking of Golgin‐84 from the ER, RUSH system) (#65303), and Str‐Golgin84_VSVG‐SBP‐EGFP (synchronizing trafficking of VSVG from the Golgi apparatus, RUSH system) (#65305) were purchased from Addgene. VSVG‐GFP (#11912) was a kind gift from Prof. Yusong Guo (The Hong Kong University of Science and Technology). BONT/C plasmid was provided by Professor Liangyi Chen, Peking University. pTriEx‐mCherry‐PA‐Rac1 (#22027) was purchased from Addgene. Α‐tubulin‐mCherry (pEGFP‐N1 backbone), MMP‐EGFP, and actin‐pEYFP‐C1 were constructed in our laboratory.
RUSH system for cargo trafficking synchronization
Cells were transfected with 0.5 μg E‐cadherin reporter and 2 μg hook plasmid. After 16‐h expression, cells were put on the imaging system and D‐biotin (Sigma‐Aldrich #47868) was added to a final concentration 40 μM. After 30 min, when E‐cadherin cargos were entering post‐Golgi trafficking stage 28, we recorded cargo trafficking movies for 120 s. The TNF cargo tracking was done similarly with the Ecad cargo. For Golgi hooked VSVG (Addgene, #65305), we recorded the cargo trafficking movie immediately after adding D‐biotin. For cargo tracking, the frame rate is 1 frame per second.
Immunofluorescence staining
Cells were grown on 35‐mm‐wide, #1 glass coverslips (Shengyou Biotechnology, #043520B). On the day of staining, cell density should be about 70%. Cells were fixed with 37°C warmed fixation buffer (4% paraformaldehyde and 0.1% glutaraldehyde in PBS) for 10 min and then washed two times with 1,000 μl PBS. Then, we incubate the cells with 500 μl 0.1% NaBH4 solution in PBS for 7 min for quenching the background fluorescence of glutaraldehyde. Coverslips were washed three times with PBS and then incubated for 30 min at room temperature with 5% BSA (Jackson, #001‐000‐162) and 0.5% Triton X‐100 (Fisher Scientific) in PBS. All antibodies were diluted in the BSA buffer with 0.5% Triton X‐100. Next, we incubate the cells for 40 min with the appropriate dilution of primary antibody in room temperature. The primary antibodies were then removed, and the cells were washed 5 min with PBS for three times. Secondary antibodies were performed for 60 min with the appropriate dilutions of dye‐labeled secondary antibodies (protected from light) in room temperature. After washed five times with PBS, cells were fixed with fixation buffer for 10 min. The samples were stored in 4°C in PSB for 1 week. For longer storage, the samples need to be washed in ddH2O for three times and air‐dried.
MT disassembly assay
For MT disassembly assay, cells were treated with 8 μM nocodazole (Sigma‐Aldrich #B5936) for 2 h at 37°C. Then, the cells were washed with PBS containing the same concentration of nocodazole until fixation. In ice treatment, cells were incubated with ice for 5 min. Ice‐treated cells were washed with cold PBS and immediately fixed with cold fixation buffer for 15 min.
Western blotting
Human retinal pigment epithelium Cells were grown in 60‐mm petri dishes. Cells were trypsinized and collected and lysed with 2 × SDS loading buffer, boiled for 5 min, and then cleared by centrifugation at 14,000 g for 10 min. Equal amounts of proteins were loaded and were separated by SDS–PAGE on 8% or 15% gels and transferred onto a PVDF membrane. PVDF membrane was then blocked for 1 h in TBST (containing 5% skim milk). Primary and secondary antibodies were diluted in TBST (containing 2.5% skim milk). Primary antibodies were incubated overnight at 4°C overnight. After washed 5 min for three times with PBST, secondary antibodies were incubated at room temperature for 1 h. Primary antibodies were used as follows: anti‐CLASP1 (Abcam), anti‐CLASP2 (Abcam), anti‐CAMSAP2 (Proteintech), anti‐GCC185 (Bethyl), anti‐Syntaxin (kind gift from Liangyi Chen), anti‐SNAP‐25 (kind gift from Liangyi Chen), anti‐Ac‐tubulin (Sigma‐Aldrich), and anti‐E‐cadherin (Abclonal Cell Signaling). Horseradish peroxidase (HRP)‐linked goat anti‐mouse IgG (GE Healthcare) and HRP‐linked goat anti‐rabbit were used as secondary antibodies. Blots were acquired and analyzed by Odyssey imaging system (LI‐COR biosciences) and enhanced chemiluminescence substrates (Thermo Fisher Scientific). Uncropped blot images with molecular weight markers are shown in Appendix Fig S6.
Tracking and fixation assay
The tracking and fixation assay was performed as described previously 29. Briefly, cells plated on glass‐bottomed petri dish (Shengyou Biotechnology, #043520B) were transfected with cargo plasmids and imaged for 120 s to record cargo trafficking in each cell. Immediately after the imaging, we fixed the cells on site without taking the sample off the microscope. To reduce the spatial fluctuation of MTs, the cells were pretreated with low concentration MT dynamics inhibitors: 100 nM paclitaxel (cytoskeleton, #TXD01) and 100 nM nocodazole (Sigma‐Aldrich #m1404) solution in DMEM/F‐12 for 10 min at 37°C, and the cells were maintained with these inhibitors for all steps until cells were fixed. After fixation, we labeled the MTs and performed 3D STORM imaging of the same cell on site.
PA‐Rac‐assay
The optogenetic assay was described in detail previously 42. Briefly, photoreactive LOV (light oxygen voltage) was fused with Rac and this protein (Pa‐Rac) could be activated using 458‐ or 473‐nm lasers to generate localized cell protrusions needed for migration. 35‐mm glass‐bottom dish (Shengyou Biotechnology, #043520B) was pre‐incubated with 10 μg/ml fibronectin (Sigma‐Aldrich, #F2006) for 1 h at 37°C. Cells were transfected with 2 μg PA‐Rac1 plasmid and 1 μg actin‐YFP plasmid for detecting laser response, or 0.5 μg Golgi marker in the migration angle change assay. After 24‐h expression, cells were imaged with spinning disk confocal microscopy. For laser activation, a row (3–5) of 440‐nm laser spots with 7 μm diameter were used to activate the Rac signal. This was achieved with a FRAP system motorized filter wheels under computer control (30% power, 10 cycle). Activation interval was 2 min for actin assembly or 4 min for live‐cell migration. Actin protrusions were recorded every 5 s with 100 ms exposure time, while cell migration movies were recorded for 300 ms every 4 min.
Spinning disk confocal microscopy
Long‐term live‐cell imaging was done by spinning disk confocal microscopy (PerkinElmer, UltraVIEW VoX, America), equipped with a 60 × 1.4 NA oil objective and an Ultra‐888 EMCCD (Andor Technology). A 440‐nm laser (PerkinElmer) was used for activation with an automated FRAP system controlled with Volocity software. The live‐cell incubation system (Chamlide TC‐W) was purchased from LCI, Korea.
Dual‐color 2D STORM and 3D STORM
Stochastic optical reconstruction microscopy images were collected in the HILO mode using a home‐built microscopy system based on an Olympus IX83 inverted microscopy equipped with a 100 × 1.4 NA oil immersion objective, and 405‐nm (100 mW), 488‐nm (300 mW), 561 nm (1,000 mW), and 647‐nm (1,000 mW) lasers, three emission filters (ET525/50, ET605/52, and ET705/72 m, Chroma Technology), and an Andor‐897 EMCCD (Andor Technology) (frame rate of 1,000 ms per frame for live‐cell tracking and 10 ms per frame for STORM imaging). Focus was maintained by a custom‐built focus lock system as previously described 63. 10% GLOX was added to protect Cy5 and Cy3b from photo‐bleaching, and 10% β‐mercaptoethanol to promote photo‐blinking 64. Imaging processing was described in detail previously 64, 65. Dual‐color STORM imaging was sequentially performed in an order of Cy5 and Cy3b. 2‐μm SiO2 beads (Invitrogen) were used for drift correction. Cylindrical lens‐based astigmatism was used for 3D STORM imaging 63.
Single‐particle tracking
Trajectories of Ecad, TNF, MMP, VSVG, and Rab5 cargos were tracked by an automated particle tracking software: Fluorescence Image Evaluation Software for Tracking and Analysis (FIESTA) 66. Fitting and statistics were performed with Origin analysis software (OriginLab).
STORM image reconstruction
Image processing of STORM data was performed as described previously 64, 65. Briefly, STORM images were analyzed by Insight 3 (a kind gift of Bo Huang, University of California, San Francisco, CA), and rendered in Vaa3D and ImageJ. Customized MATLAB scripts were used for batch processing.
Quantification of the MT geometry
Quantification of the MT asymmetry was previously described 26. The central cell area was excluded from analysis. Each cell was divided into four quadrants (all Golgi quadrants were rotated to the upper right quadrant). The MT number in each quadrant was quantified.
For GaMTs vs non‐GaMTs analysis, all STORM images were collected in the first quadrant of the cell. The ratio of GaMTs/non‐GaMTs refers to the ratio of pixel numbers. Data were normalized with MT length.
For PTM (acetylation and detyrosination) ratio analysis, MT defect ratio analysis, and MT intersection analysis, the ratio is obtained by dividing the ratio of GaMT PTM by that of non‐GaMTs. Data were normalized with MT length.
The MT tracks were extracted with NeuronJ 67, 68 and plotted with MATLAB. MT orientation and curvature were analyzed with SIFNE 38.
Statistics
For box–whisker plot, the box contains 50% of the entire population, and the ends of the whiskers are set at 10 and 90% of the entire population. Peak values in Fig 2, Appendix Fig S2, and Appendix Fig S3 are expressed as mean ± s.e.m. Normality of data distribution was tested using the Shapiro–Wilk test. Experimental groups (normally distributed data) were compared using unpaired t‐tests. While data were not normally distributed, we performed the non‐parametric two‐tailed Mann–Whitney test. Experiments were randomized. The researchers were not blinded to assignment during experiments and data analysis, except for the analysis of optogenetic experiments. Statistical significance was described in *P < 0.05, **P < 0.01, ***P < 0.001, ns, no significant difference. The times of independent experiments and cells assessed are stated in the Figure legends. Statistics raw data are provided as Source Data.
Author contributions
YS, CW, and HH conceived the project and designed the experiments. HH performed the imaging experiments and data analysis. JN, ML, JY, and HH performed the plasmid construction and Western blotting. BX and QPS performed the data analysis. JQ and SZ performed cell culturing and migration data collection. YS, CW, and HH wrote the manuscript. All authors participated in the discussion of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Movie EV5
Movie EV6
Movie EV7
Movie EV8
Movie EV9
Movie EV10
Movie EV11
Movie EV12
Movie EV13
Movie EV14
Movie EV15
Movie EV16
Movie EV17
Movie EV18
Movie EV19
Movie EV20
Movie EV21
Movie EV22
Movie EV23
Movie EV24
Source Data for Appendix
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Acknowledgements
We thank Prof. Xiaowei Chen (Peking University) for the RUSH system construct, reagents, and discussion. We thank Prof. Li Yu (Tsinghua University), Prof. Guangshuo Ou (Tsinghua University), Prof. Xin Liang (Tsinghua University), and Prof. Pingyong Xu (Institute of Biophysics, Chinese Academy of Sciences) for helpful discussion. We thank Prof. Liangyi Chen (Peking University) for the BONT plasmids. We thank Dr. Sheng Wang (Peking University) for the actin‐YFP construct. We thank Peng Shi (Peking University Health Science Center) for the effort to map the differences in PTMs between GaMTs and non‐GaMTs. We thank Prof. Ming Lei and Tianyu Zhao (Xi'an Institute of Optics and Precision Mechanics, Chinese Academy of Sciences) for SIM imaging of tyrosinated MTs and detyrosinated MTs. We thank the Core Facilities of the School of Life Sciences at Peking University for the confocal imaging support. This work was supported by funding from the National Key R&D Program of China, No. 2017YFA0505300, and the National Science Foundation of China 21573013 and 21825401 for Y.S; and the National Key R&D Program of China 2017YFA0506500 for C.W.
EMBO Reports (2020) 21: e48385
Contributor Information
Congying Wu, Email: congyingwu@hsc.pku.edu.cn.
Yujie Sun, Email: sun_yujie@pku.edu.cn.
References
- 1. Etienne‐Manneville S (2013) Microtubules in cell migration. Annu Rev Cell Dev Biol 29: 471–499 [DOI] [PubMed] [Google Scholar]
- 2. Yadav S, Linstedt AD (2011) Golgi positioning. Cold Spring Harb Perspect Biol 3: a005322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tayeb MA, Skalski M, Cha MC, Kean MJ, Scaife M, Coppolino MG (2005) Inhibition of SNARE‐mediated membrane traffic impairs cell migration. Exp Cell Res 305: 63–73 [DOI] [PubMed] [Google Scholar]
- 4. Orlando K, Guo W (2009) Membrane organization and dynamics in cell polarity. Cold Spring Harb Perspect Biol 1: a001321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones MC, Caswell PT, Norman JC (2006) Endocytic recycling pathways: emerging regulators of cell migration. Curr Opin Cell Biol 18: 549–557 [DOI] [PubMed] [Google Scholar]
- 6. Shafaq‐Zadah M, Gomes‐Santos CS, Bardin S, Maiuri P, Maurin M, Iranzo J, Gautreau A, Lamaze C, Caswell P, Goud B et al (2016) Persistent cell migration and adhesion rely on retrograde transport of beta(1) integrin. Nat Cell Biol 18: 54–64 [DOI] [PubMed] [Google Scholar]
- 7. Caswell PT, Norman JC (2006) Integrin trafficking and the control of cell migration. Traffic 7: 14–21 [DOI] [PubMed] [Google Scholar]
- 8. Tang BL, Ng EL (2009) Rabs and cancer cell motility. Cell Motil Cytoskeleton 66: 365–370 [DOI] [PubMed] [Google Scholar]
- 9. Bershadsky AD, Futerman AH (1994) Disruption of the Golgi apparatus by brefeldin A blocks cell polarization and inhibits directed cell migration. Proc Natl Acad Sci USA 91: 5686–5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prigozhina NL, Waterman‐Storer CM (2004) Protein kinase D‐mediated anterograde membrane trafficking is required for fibroblast motility. Curr Biol 14: 88–98 [DOI] [PubMed] [Google Scholar]
- 11. Rivero S, Cardenas J, Bornens M, Rios RM (2009) Microtubule nucleation at the cis‐side of the Golgi apparatus requires AKAP450 and GM130. EMBO J 28: 1016–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia AR, McLeod IX et al (2007) Asymmetric CLASP‐dependent nucleation of noncentrosomal microtubules at the trans‐Golgi network. Dev Cell 12: 917–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller PM, Folkmann AW, Maia AR, Efimova N, Efimov A, Kaverina I (2009) Golgi‐derived CLASP‐dependent microtubules control Golgi organization and polarized trafficking in motile cells. Nat Cell Biol 11: 1069–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mimori‐Kiyosue Y, Grigoriev I, Lansbergen G, Sasaki H, Matsui C, Severin F, Galjart N, Grosveld F, Vorobjev I, Tsukita S et al (2005) CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus‐end dynamics at the cell cortex. J Cell Biol 168: 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akhmanova A, Hoogenraad CC, Drabek K, Stepanova T, Dortland B, Verkerk T, Vermeulen W, Burgering BM, De Zeeuw CI, Grosveld F et al (2001) Clasps are CLIP‐115 and ‐170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell 104: 923–935 [DOI] [PubMed] [Google Scholar]
- 16. Wu J, de Heus C, Liu Q, Bouchet BP, Noordstra I, Jiang K, Hua S, Martin M, Yang C, Grigoriev I et al (2016) Molecular pathway of microtubule organization at the Golgi apparatus. Dev Cell 39: 44–60 [DOI] [PubMed] [Google Scholar]
- 17. Jiang K, Hua S, Mohan R, Grigoriev I, Yau KW, Liu Q, Katrukha EA, Altelaar AF, Heck AJ, Hoogenraad CC et al (2014) Microtubule minus‐end stabilization by polymerization‐driven CAMSAP deposition. Dev Cell 28: 295–309 [DOI] [PubMed] [Google Scholar]
- 18. Tanaka N, Meng W, Nagae S, Takeichi M (2012) Nezha/CAMSAP3 and CAMSAP2 cooperate in epithelial‐specific organization of noncentrosomal microtubules. Proc Natl Acad Sci USA 109: 20029–20034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arnette C, Efimova N, Zhu X, Clark GJ, Kaverina I (2014) Microtubule segment stabilization by RASSF1A is required for proper microtubule dynamics and Golgi integrity. Mol Biol Cell 25: 800–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu X, Kaverina I (2013) Golgi as an MTOC: making microtubules for its own good. Histochem Cell Biol 140: 361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rios RM, Sanchis A, Tassin AM, Fedriani C, Bornens M (2004) GMAP‐210 recruits gamma‐tubulin complexes to cis‐Golgi membranes and is required for Golgi ribbon formation. Cell 118: 323–335 [DOI] [PubMed] [Google Scholar]
- 22. Hurtado L, Caballero C, Gavilan MP, Cardenas J, Bornens M, Rios RM (2011) Disconnecting the Golgi ribbon from the centrosome prevents directional cell migration and ciliogenesis. J Cell Biol 193: 917–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yadav S, Puri S, Linstedt AD (2009) A primary role for Golgi positioning in directed secretion, cell polarity, and wound healing. Mol Biol Cell 20: 1728–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bisel B, Wang Y, Wei JH, Xiang Y, Tang D, Miron‐Mendoza M, Yoshimura S, Nakamura N, Seemann J (2008) ERK regulates Golgi and centrosome orientation towards the leading edge through GRASP65. J Cell Biol 182: 837–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trogden KP, Zhu X, Lee JS, Wright CVE, Gu G, Kaverina I (2019) Regulation of glucose‐dependent Golgi‐derived microtubules by cAMP/EPAC2 promotes secretory vesicle biogenesis in pancreatic beta cells. Curr Biol 29: 2339–2350.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vinogradova T, Miller PM, Kaverina I (2009) Microtubule network asymmetry in motile cells: role of Golgi‐derived array. Cell Cycle 8: 2168–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matov A, Applegate K, Kumar P, Thoma C, Krek W, Danuser G, Wittmann T (2010) Analysis of microtubule dynamic instability using a plus‐end growth marker. Nat Methods 7: 761–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boncompain G, Divoux S, Gareil N, de Forges H, Lescure A, Latreche L, Mercanti V, Jollivet F, Raposo G, Perez F (2012) Synchronization of secretory protein traffic in populations of cells. Nat Methods 9: 493–498 [DOI] [PubMed] [Google Scholar]
- 29. Balint S, Verdeny Vilanova I, Sandoval Alvarez A, Lakadamyali M (2013) Correlative live‐cell and superresolution microscopy reveals cargo transport dynamics at microtubule intersections. Proc Natl Acad Sci USA 110: 3375–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cai D, McEwen DP, Martens JR, Meyhofer E, Verhey KJ (2009) Single molecule imaging reveals differences in microtubule track selection between Kinesin motors. PLoS Biol 7: e1000216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang WH, Li Q, Rifat Faysal KM, King SJ, Gopinathan A, Xu J (2016) Microtubule defects influence kinesin‐based transport in vitro . Biophys J 110: 2229–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gramlich MW, Conway L, Liang WH, Labastide JA, King SJ, Xu J, Ross JL (2017) Single molecule investigation of kinesin‐1 motility using engineered microtubule defects. Sci Rep 7: 44290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vale RD, Coppin CM, Malik F, Kull FJ, Milligan RA (1994) Tubulin GTP hydrolysis influences the structure, mechanical properties, and kinesin‐driven transport of microtubules. J Biol Chem 269: 23769–23775 [PubMed] [Google Scholar]
- 34. de Forges H, Pilon A, Cantaloube I, Pallandre A, Haghiri‐Gosnet AM, Perez F, Pous C (2016) Localized mechanical stress promotes microtubule rescue. Curr Biol 26: 3399–3406 [DOI] [PubMed] [Google Scholar]
- 35. Wong YL, Anzola JV, Davis RL, Yoon M, Motamedi A, Kroll A, Seo CP, Hsia JE, Kim SK, Mitchell JW et al (2015) Cell biology. Reversible centriole depletion with an inhibitor of Polo‐like kinase 4. Science 348: 1155–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ross JL, Shuman H, Holzbaur EL, Goldman YE (2008) Kinesin and dynein‐dynactin at intersecting microtubules: motor density affects dynein function. Biophys J 94: 3115–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brouhard GJ, Rice LM (2014) The contribution of alphabeta‐tubulin curvature to microtubule dynamics. J Cell Biol 207: 323–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Z, Nishimura Y, Kanchanawong P (2017) Extracting microtubule networks from superresolution single‐molecule localization microscopy data. Mol Biol Cell 28: 333–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schmoranzer J, Kreitzer G, Simon SM (2003) Migrating fibroblasts perform polarized, microtubule‐dependent exocytosis towards the leading edge. J Cell Sci 116: 4513–4519 [DOI] [PubMed] [Google Scholar]
- 40. Stehbens SJ, Paszek M, Pemble H, Ettinger A, Gierke S, Wittmann T (2014) CLASPs link focal‐adhesion‐associated microtubule capture to localized exocytosis and adhesion site turnover. Nat Cell Biol 16: 561–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maiuri P, Terriac E, Paul‐Gilloteaux P, Vignaud T, McNally K, Onuffer J, Thorn K, Nguyen PA, Georgoulia N, Soong D et al (2012) The first world cell race. Curr Biol 22: R673–R675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM (2009) A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461: 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mizoguchi T, Ikeda S, Watanabe S, Sugawara M, Itoh M (2017) Mib1 contributes to persistent directional cell migration by regulating the Ctnnd1‐Rac1 pathway. Proc Natl Acad Sci USA 114: E9280–E9289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vassilev V, Platek A, Hiver S, Enomoto H, Takeichi M (2017) Catenins steer cell migration via stabilization of front‐rear polarity. Dev Cell 43: 463–479.e5 [DOI] [PubMed] [Google Scholar]
- 45. Sanders AA, Kaverina I (2015) Nucleation and dynamics of Golgi‐derived microtubules. Front Neurosci 9: 431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hancock WO (2014) Bidirectional cargo transport: moving beyond tug of war. Nat Rev Mol Cell Biol 15: 615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hendricks AG, Perlson E, Ross JL, Schroeder HW III, Tokito M, Holzbaur EL (2010) Motor coordination via a tug‐of‐war mechanism drives bidirectional vesicle transport. Curr Biol 20: 697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ehrenberg M, McGrath JL (2004) Actin motility: staying on track takes a little more effort. Curr Biol 14: R931–R932 [DOI] [PubMed] [Google Scholar]
- 49. Holzbaur EL, Goldman YE (2010) Coordination of molecular motors: from in vitro assays to intracellular dynamics. Curr Opin Cell Biol 22: 4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sirajuddin M, Rice LM, Vale RD (2014) Regulation of microtubule motors by tubulin isotypes and post‐translational modifications. Nat Cell Biol 16: 335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Monroy BY, Sawyer DL, Ackermann BE, Borden MM, Tan TC, Ori‐McKenney KM (2018) Competition between microtubule‐associated proteins directs motor transport. Nat Commun 9: 1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baas PW, Qiang L (2005) Neuronal microtubules: when the MAP is the roadblock. Trends Cell Biol 15: 183–187 [DOI] [PubMed] [Google Scholar]
- 53. Dixit R, Ross JL, Goldman YE, Holzbaur EL (2008) Differential regulation of dynein and kinesin motor proteins by tau. Science 319: 1086–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bechstedt S, Brouhard GJ (2012) Doublecortin recognizes the 13‐protofilament microtubule cooperatively and tracks microtubule ends. Dev Cell 23: 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vitre B, Coquelle FM, Heichette C, Garnier C, Chretien D, Arnal I (2008) EB1 regulates microtubule dynamics and tubulin sheet closure in vitro . Nat Cell Biol 10: 415–421 [DOI] [PubMed] [Google Scholar]
- 56. Davis LJ, Odde DJ, Block SM, Gross SP (2002) The importance of lattice defects in katanin‐mediated microtubule severing in vitro . Biophys J 82: 2916–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coombes C, Yamamoto A, McClellan M, Reid TA, Plooster M, Luxton GW, Alper J, Howard J, Gardner MK (2016) Mechanism of microtubule lumen entry for the alpha‐tubulin acetyltransferase enzyme alphaTAT1. Proc Natl Acad Sci USA 113: E7176–E7184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carlier MF, Pantaloni D (2007) Control of actin assembly dynamics in cell motility. J Biol Chem 282: 23005–23009 [DOI] [PubMed] [Google Scholar]
- 59. Etienne‐Manneville S (2004) Actin and microtubules in cell motility: which one is in control? Traffic 5: 470–477 [DOI] [PubMed] [Google Scholar]
- 60. Uetrecht AC, Bear JE (2009) Golgi polarity does not correlate with speed or persistence of freely migrating fibroblasts. Eur J Cell Biol 88: 711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martin M, Veloso A, Wu J, Katrukha EA, Akhmanova A (2018) Control of endothelial cell polarity and sprouting angiogenesis by non‐centrosomal microtubules. eLife 7: e33864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Renkawitz J, Kopf A, Stopp J, de Vries I, Driscoll MK, Merrin J, Hauschild R, Welf ES, Danuser G, Fiolka R et al (2019) Nuclear positioning facilitates amoeboid migration along the path of least resistance. Nature 568: 546–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huang B, Jones SA, Brandenburg B, Zhuang X (2008) Whole‐cell 3D STORM reveals interactions between cellular structures with nanometer‐scale resolution. Nat Methods 5: 1047–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang B, Wang W, Bates M, Zhuang X (2008) Three‐dimensional super‐resolution imaging by stochastic optical reconstruction microscopy. Science 319: 810–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bates M, Huang B, Dempsey GT, Zhuang X (2007) Multicolor super‐resolution imaging with photo‐switchable fluorescent probes. Science 317: 1749–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ruhnow F, Zwicker D, Diez S (2011) Tracking single particles and elongated filaments with nanometer precision. Biophys J 100: 2820–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Meijering E (2010) Neuron tracing in perspective. Cytometry A 77: 693–704 [DOI] [PubMed] [Google Scholar]
- 68. Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M (2004) Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 58: 167–176 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Movie EV5
Movie EV6
Movie EV7
Movie EV8
Movie EV9
Movie EV10
Movie EV11
Movie EV12
Movie EV13
Movie EV14
Movie EV15
Movie EV16
Movie EV17
Movie EV18
Movie EV19
Movie EV20
Movie EV21
Movie EV22
Movie EV23
Movie EV24
Source Data for Appendix
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6


