Abstract
Brain metastases are frequent in patients with lung cancer and a major cause of morbidity and mortality. Finding a biomarker predicting brain metastases could facilitate early start of treatment and thereby reduce morbidity and possibly improve overall survival. Previous studies suggest S100B as a possible biomarker for this purpose. This prospective study enrolled 185 patients with newly diagnosed stage IV non-small cell lung cancer (NSCLC). A total of 22 patients had brain metastases verified by magnetic resonance imaging or computed tomography at the time of enrollment. Serum S100B levels were measured in blood samples collected prior to any treatment from 22 patients who had brain metastases at enrollment and from 50 patients randomly selected from the remaining 163 patients without brain metastases at enrollment. No statistically significant difference was found in the levels of serum S100B between patients with and without brain metastases [range 0.018-0.209 µg/l, mean 0.049 µg/l, 95% confidence interval (CI), 0.032-0.061 µg/l] and (range 0.016-0.130 µg/i, mean 0.044 µg/l, 95% CI, 0.037-0.051 µg/l), respectively, (P=0.852). Univariate analysis of prognostic factors for S100B indicated a correlation (P<0.2) with sex (P=0.088) and histology (adenocarcinoma vs. squamous cell carcinoma/others) (P=0.028). In the multivariate analysis only histology (P=0.029) remained statistically significant. Conclusion: The present study found no significant correlation between the level of serum S100B and the presence of brain metastases in patients with advanced NSCLC. The clear cut-off of S100B in patients with and without brain metastases reported in other studies could not be verified in this study. Further studies investigating the role of S100B as a biomarker for brain metastases in non-small cell lung cancer are warranted.
Keywords: S100B, non-small cell lung cancer, brain metastases, biomarker, prognostic marker
Introduction
The rate of non-small cell lung cancer (NSCLC) patients presenting with brain metastases at the time of diagnosis is 10-20 and 30-50% develop brain metastases during the course of the disease (1-3). Brain metastases may have a severe impact on morbidity thus affecting quality of life due to a variety of symptoms such as nausea, vomiting, dizziness, headaches, vision problems and neurological difficulties and are a major cause of mortality. In previous studies S100B has been suggested as a biomarker for detection of early brain metastases before symptoms arise and may therefore be of great clinical importance enabling early initiation of treatment and possibly both improved quality of life and improved overall survival (OS) (2,4).
S100B is a calcium binding protein. It is found as a homo- or hetero-dimers of two different subunits, A and B. S100AB and S100BB are described as S100B and both are specific for astrocytes and Schwann cells. The protein is expressed mainly in glial cells with the highest expression in astrocytes. Lower expression is found in Schwann cells in the peripheral nervous system, melanocytes, adipocytes and chondrocytes (2,5).
It is well known that S100B can downregulate the tumor suppressor gene p53 in melanocytes and inhibit apoptosis (6). It has been suggested that S100B promotes brain metastases of lung adenocarcinoma by promoting cell proliferation, preventing apoptosis and increasing cell migration and invasion (3,7). Low concentrations of S100B stimulate neurite outgrowth and survival of neurons whereas high concentrations stimulate the expression of inflammatory cytokines and induce apoptosis (5).
Elevated serum S100B is primarily found under pathological conditions that also compromises the blood-brain barrier as seen in traumatic brain injury, intracerebral vascular disease, malignancy, epilepsy and schizophrenia (2). One exception is patients with malignant melanoma (without brain metastases) where serum values increase with the clinical stage due to the expression of S100B in melanocytes (8).
The clinical utility of S100B has been evaluated in several studies demonstrating that elevated levels are correlated with a poorer outcome in patients with traumatic brain injury (9-11). Also, S100B has been shown to be a predictive biomarker of OS before first line treatment of disseminated malignant melanoma with high levels of S100B correlating with poorer outcome (12).
In a prospective study including 38 patients with newly diagnosed NSCLC and no symptoms of brain metastases, it was investigated if serum S100B level could be used as a biomarker to detect subclinical brain metastases. Magnetic resonance imagining showed brain metastases in seven patients, all presenting with elevated S100B level (>0.10 µg/l). A total of nine patients also presenting with elevated levels of S100B had vascular brain damage with no evidence of brain metastases. However no patients without brain metastases or vascular disease in the brain were found to have elevated levels of S100B, thus suggesting that serum S100B may be used as a screening tool to select patients in need of further examination for brain metastases (10).
Also, subsequent studies suggested that serum S100B might serve as biomarker to detect brain metastases in patients with newly diagnosed NSCLC. Thus, a study by Choi et al (2) including 128 patients with newly diagnosed NSCLC found a sensitivity of 89% regarding the ability of serum S100B to detect brain metastases, but a rather low specificity which was found to be 43%. In addition, another study by Pang et al (4) including 30 NSCLC patients showed significantly increased levels of S100B in 15 patients with brain metastases compared to 15 patients without brain metastases with a very clear cut-off at 0.031 µg/l. Surprisingly, Liu et al (7) showed a correlation between S100B and longer OS in NSCLC patients by analyzing 20 members of the S100 calcium binding protein family.
Moreover, normal levels of S100B were found in patients with glioma at the time of diagnosis and during treatment with no influence on OS, whereas in patients with recurrent glioma an elevated level of S100B at the time of recurrence was associated with shorter median survival compared to patients without elevated S100B (13).
In our previous study, including 101 breast cancer patients, 46 of which had brain metastases, serum levels of S100B were not able to identify those with brain metastases (14).
The clinical value of S100B to detect brain metastases or predict outcome in patients with NSCLC remains unclear. The aim of this study was to compare baseline level of serum S100B in NSCLC patients with and without brain metastases.
Materials and methods
Study population
The cohort of patients were obtained from a prospective biomarker trial of advanced NSCLC (S-20070014) conducted between March 2007 and February 2010 at the Department of Oncology, Vejle Hospital, University Hospital of Southern Denmark (Vejle, Denmark) (15). The Declaration of Helsinki was observed and signed informed consent was obtained from all patients. A total of 185 patients with newly diagnosed stage IV NSCLC were enrolled and pre-treatment blood samples were drawn at the time of diagnosis. A total of 22 patients had verified brain metastases at the time of enrollment. Patient characteristics are shown in Table I.
Table I.
Patient characteristics.
| Characteristics | n | % | n | % | P-value |
|---|---|---|---|---|---|
| Age | 0.016 | ||||
| ≤65 | 16 | 72.7 | 21 | 42 | |
| ≥65 | 6 | 27.3 | 29 | 58 | |
| Sex | 0.332 | ||||
| Male | 15 | 68.2 | 28 | 56 | |
| Female | 7 | 31.8 | 22 | 44 | |
| Performance status | 0.206 | ||||
| 0 | 9 | 40.9 | 13 | 26 | |
| 1-2 | 13 | 59.1 | 37 | 74 | |
| Smoking | 0.285 | ||||
| Non-smoker + | |||||
| Former smoker | 14 | 63.6 | 25 | 50 | |
| Smoking | 8 | 36.4 | 25 | 50 | |
| Histology | 0.054 | ||||
| Adenocarcinoma | 19 | 86.4 | 32 | 64 | |
| Squamous carcinoma/other | 3 | 13.6 | 18 | 36 | |
| Liver metastases | 0.647 | ||||
| Yes | 3 | 13.6 | 9 | 18 | |
| No | 19 | 86.4 | 41 | 82 | |
| Bone metastases | 0.945 | ||||
| Yes | 5 | 22.7 | 11 | 22 | |
| No | 17 | 77.3 | 39 | 78 |
The present study was approved by The Regional Committees on Health Research Ethics for Southern Denmark (S-20110005). Serum S100B levels from the 22 patients with brain metastases were compared with a control group of 50 patients randomly selected from the remaining 163 patients without known brain metastases as outlined in the flow chart (Fig. 1).
Figure 1.
Flowchart outlining 185 pts with NSCLC. A total of 22 had brain metastases. 163 had no brain metastases. From the latter group 50 pts was selected as the ‘control group’. NSCLC, non-small cell lung cancer; pts, patients.
Clinical and histopathological data
Histopathological data were obtained from the local database at the Department of Pathology, Vejle Hospital. Clinical data such as age, sex, Eastern Cooperative Oncology Group performance status (PS), smoking history, histology and time of death were collected from the patient record.
Sample storage and handling
Baseline venous blood (22 ml) was drawn in the non-fasting state. Samples were collected with minimal stasis in evacuated blood collection tubes. Blood was allowed to clot at room temperature before the samples were centrifuged at 2,000 x g for 10 min at 20̊C and immediately after centrifugation the serum phase was carefully transferred into cryo-tubes and stored at minus 80̊C until use.
Biochemical analysis
Serum S-100B was analyzed using the CE marked Elecsys S100 Immunoassay (Roche Diagnostics GmbH). The assay is an automated sandwich immunoassay using two monoclonal antibodies against S100B forming a complex to be measured by direct chemiluminescent technology. Serum specimens were measured according to the manufacturer's protocol, and each sample was analyzed twice to ensure the results were valid. The lower detection limit was 0.005 µg/l and the assay was controlled by commercial controls at two levels: 0.176 and 2.280 µg/l with an inter-assay coefficient of variation of 1.3 and 3.6%, respectively. The reference interval in healthy individuals without NSCLC is 0.02-0.13 µg/l. A cut-off at 0.120 µg/l was used which is in accordance with the results by Yoon et al (11).
Statistical analysis
Statistical analysis was performed using STATA 11 (StataCorp LP). Using qq-plots and a Shapiro-Wilk test it was found that S100B was not normally distributed. Box-Cox analysis determined the need for log transformation with -0.4. Kendall's tau was used to determine associations between high levels of correlated blood samples. A Wilcoxon rank sum test was used to test for differences in S100B levels between the two groups. The levels of S100B are presented as the range, mean and 95% confidence intervals.
A univariate analysis was used to determine which exposure variables might be associated with the outcome (Table II). Variables with P<0.2 were included in a multivariate logistic regression analysis to further explore their relation to the levels of S100B. A receiver operating characteristics (ROC) curve analysis was used to set a cut-off value for S100B in patients with and without brain metastases, respectively. P<0.05 was considered to indicate a statistically significant difference.
Table II.
Univariate analysis of prognostic factors of brain metastases.
| Factors | P-value |
|---|---|
| Sex | |
| Male: female | 0.088 |
| Age | |
| ≤65; >65 | 0.972 |
| Performance status | |
| 0; 1-2 | 0.691 |
| Smoking | |
| Never/former; recent/unknown | 0.952 |
| Histology | |
| Adenocarcinoma; squamous cell carcinoma/others | 0.028 |
| Brain metastases | |
| No; yes | 0.852 |
| Distant metastases | |
| No; yes | 0.244 |
Results
Patient characteristics
The final analysis included a total of 72 patients. A total of 22 of the patients had brain metastases and 50 did not have brain metastases. The two groups were comparable only differing in age (P=0.016). In the group of patients with brain metastases patients were younger and with a better PS compared to the other group. The most common histological subtype was adenocarcinoma (Table I).
S100B
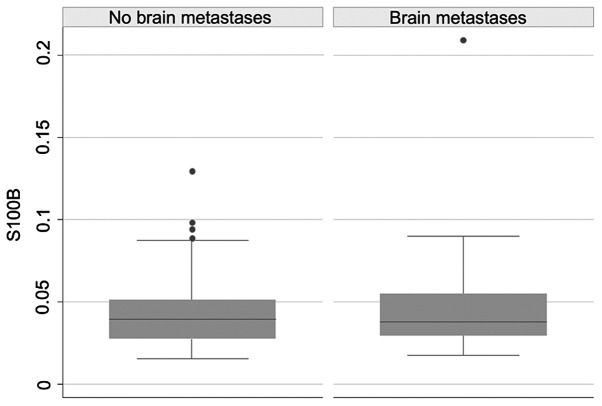
No statistically significant difference in the level of S100B was found between the group with and without brain metastases [range 0.018-0.209 µg/l, mean 0.049 µg/l, 95% confidence interval (CI), 0.032-0.067 µg/l and range 0.016-0.130 µg/l, mean 0.044 µg/l, 95% CI, 0.037-0.051 µg/l], respectively (P=0.852). One patient was considered an outlier and excluded due to S100B levels >1.0 µg/l in both measurements, which is far higher than in any of the other patients (Fig. 2).
Figure 2.
Boxplot of S100B levels in patients with NSCLC with and without brain metastases. There was no significant difference in the S100B levels between the two groups.
Univariate and multivariate analysis
A univariate analysis was performed for the following variables: Sex (male; female), age (≤65;>65), PS (0;1-2), smoking history (never/former; current/unknown), histology (adenocarcinoma; squamous cell carcinoma/others), brain metastases (no; yes), distant metastases other than in the brain (no; yes), including metastases in the liver, bone, cutis and kidney. Only sex (P=0.088) and histology (P=0.028) from the candidate variables for multivariate modelling had a P<0.2. Table I presents the results of the univariate analysis. In the multivariate analysis only histology (P=0.029) remained statistically significant while sex (P=0.132) was not statistically significant. Only three patients had S100B levels exceeding the cut-off value of 0.120 µg/l. The ROC curve analysis did not reveal a cut-off value for S100B in patients with and without brain metastases (Fig. S1).
Discussion
The current study found no difference in serum levels of S100B between NSCLC patients with and without brain metastases. Thus, the present study was not able to confirm the results of previous studies which suggested that S100B may be used as a diagnostic biomarker to detect early brain metastases in patients with advanced NSCLC (2,4,16). The levels of S100B were surprisingly equal in the two groups, suggesting that S100B cannot be used as a biomarker to detect brain metastases. This correlates with the results from our previous study showing no difference between breast cancer patients with and without brain metastases (14).
Both Choi et al (2) and Pang et al (4) found significantly elevated levels of S100B in patients with brain metastases compared to patients without brain metastases, although the measured levels of S100B still remained within the normal range. The clinical value of the very clear cutoff of S100B in patients with and without brain metastases demonstrated by Choi et al (2) could not be verified in the present study. Chen et al (16) found that S100B is a sensitive and specific biomarker of brain metastasis in Chinese patients and can be used as a prognostic tool in daily clinics.
Trauma to the brain caused by traumatic brain injury, intracerebral vascular disease, malignancy, epilepsy and schizophrenia is known to cause high levels of serum S100B. It is obvious to conclude that the number, size and location of brain metastases might be of importance to S100B serum levels (2). Large metastases are likely to cause more damage and thereby release higher levels of S100B into the blood stream, making this information relevant. Patients in the current study had no imaging of the brain performed prior to entering the study, which poses a risk of patients with subclinical metastases falsely having been categorized as ‘without brain metastases’ and furthermore the present study has no supplementary data on other conditions potentially affecting the serum levels of S100B. However, based on the very similar levels of S100B between the two groups and among the patients in each group this is unlikely to have influenced the results.
The univariate analysis was preplanned and to investigate the subject further multivariate logistic regression analysis was chosen too, knowing that the sample size was small, and this might be a limitation in the present study.
Mu et al (17) looked at the levels of serum S100B in 138 patients with small cell lung cancer (SCLC) and compared with a healthy control group. The results showed higher levels of S100B in the group with SCLC. Furthermore, the subgroup with brain metastases had the highest level of serum S100B and higher levels than the other subgroups. They concluded that S100B can serve as a serological marker for brain metastases from SCLC. Maybe these results are partly explained by the high invasion ability seen in this type of lung cancer and thereby more severe brain damage resulting in higher levels of S100B.
In conclusion, the present results did not indicate a significant correlation between the level of S100B and the presence of brain metastases in patients with advanced NSCLC. The current study could not verify a clear cut-off of S100B in patients with and without brain metastases. Therefore, S100B was not found to be useful as a biomarker in advanced NSCLC for detection of brain metastases at the time of diagnosis. Since previous studies have suggested otherwise, further investigation is warranted to determine the clinical role of S100B as a biomarker for brain metastases in NSCLC.
Supplementary Material
Acknowledgements
The authors would like to thank the medical laboratory technologists Mrs Birgitte Knudsen and Mrs Lone Frischknecht for their excellent laboratory work.
Funding
The present study was funded by the Research Council at Vejle Hospital, University Hospital of Southern Denmark (Vejle, Denmark).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MK analyzed and interpreted the data regarding s100B, and was a major contributor in writing the manuscript. TB performed the statistical analysis, analyzed the data regarding S100B, and was a major contributor in writing the manuscript. JSM analyzed the blood samples and contributed to writing and proofreading the manuscript. ADN collected the data and assisted in proofreading the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Declaration of Helsinki was observed and signed informed consent was obtained from all patients. The present study was approved by The Regional Committees on Health Research Ethics for Southern Denmark (approval no. S-20110005).
Patient consent for publication
All patients provided signed informed consent for publication in an international peer-reviewed journal with authorship according to the Vancouver rules.
Competing interests
The authors declare that they have no conflict of interest.
References
- 1.Preusser M, Winkler F, Valiente M, Manegold C, Moyal E, Widhalm Tonn JC, Zielinski C. Recent advances in the biology and treatment of brain metastases of non-small cell lung cancer: Summary of a multidisciplinary roundtable discussion. ESMO Open. 2018;3(e000262) doi: 10.1136/esmoopen-2017-000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi H, Puvenna V, Brennan C, Mahmoud S, Wang XF, Phillips M, Janigro D, Mazzone P. S100B and S100B autoantibody as biomarkers for early detection of brain metastases in lung cancer. Transl Lung Cancer Res. 2016;5:413–419. doi: 10.21037/tlcr.2016.07.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang W, Jia Q, Liu L, Zhao X, Tan A, Ma N, Zhang H. S100B promotes the proliferation, migration and invasion of specific brain metastatic lung adenocarcinoma cell line. Cell Biochem Funct. 2011;29:582–588. doi: 10.1002/cbf.1791. [DOI] [PubMed] [Google Scholar]
- 4.Pang X, Min J, Liu L, Liu Y, Ma N, Zhang H. S100B protein as a possible participant in the brain metastasis of NSCLC. Med Oncol. 2012;29:2626–2632. doi: 10.1007/s12032-012-0169-0. [DOI] [PubMed] [Google Scholar]
- 5.Korfias S, Stranjalis G, Papadimitriou A, Psachoulia C, Daskalakis G, Antsaklis A, Sakas DE. Serum S-100B protein as a biochemical marker of brain injury: A review of current concepts. Curr Med Chem. 2006;13:3719–3731. doi: 10.2174/092986706779026129. [DOI] [PubMed] [Google Scholar]
- 6.Lin J, Yang Q, Wilder PT, Carrier F, Weber DJ. The calcium-binding protein S100B down-regulates p53 and apoptosis in malignant melanoma. J Biol Chem. 2010;285:27487–27498. doi: 10.1074/jbc.M110.155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Cui J, Tang YL, Huang L, Zhou CY, Xu JX. Prognostic roles of mRNA expression of S100 in non-small-cell lung cancer. Biomed Res Int. 2018;2018(9815806) doi: 10.1155/2018/9815806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandru A Voinea SV, Bolovan M, Cinca S, Bordea C, Blidaru A. The significance of serum S100, MIA and LDH in cutaneus malignant melanoma. Rom J Biolchem. 2011;48:75–88. doi: 10.2741/e170. [DOI] [PubMed] [Google Scholar]
- 9.Vos PE, Jacobs B, Andriessen TM, Lamers KJ, Borm GF, Beems T, Edwards M, Rosmalen CF, Vissers JL. GFAP and S100B are biomarkers of traumatic brain injury: An observational cohort study. Neurology. 2010;75:1786–1793. doi: 10.1212/WNL.0b013e3181fd62d2. [DOI] [PubMed] [Google Scholar]
- 10.Vogelbaum MA, Masaryk T, Mazzone P, Mekhail T, Fazio V, McCartney S, Marchi N, Kanner A, Janigro D. S100beta as a predictor of brain metastases: Brain versus cerebrovascular damage. Cancer. 2005;104:817–824. doi: 10.1002/cncr.21220. [DOI] [PubMed] [Google Scholar]
- 11.Yoon SM, Choi YJ, Kim HJ, Shim JJ, Bae HG, Yun IG. Prognostic value of serum s100 protein by elecsys s100 immunoassay in patients with spontaneous subarachnoid and intracerebral hemorrhages. J Korean Neurosurg Soc. 2008;44:308–13. doi: 10.3340/jkns.2008.44.5.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weide B, Richter S, Büttner P, Leiter U, Forschner A, Bauer J, Held L, Eigentler TK, Meier F, Garbe C. Serum S100B, lactate dehydrogenase and brain metastasis are prognostic factors in patients with distant melanoma metastasis and systemic therapy. PLoS One. 2013;8(e81624) doi: 10.1371/journal.pone.0081624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holla FK, Postma TJ, Blankenstein MA, van Mierlo TJM, Vos MJ, Sizoo EM, de Groot M, Uitdehaag BMJ, Buter J, Klein M, et al. Prognostic value of the S100B protein in newly diagnosed and recurrent glioma patients: A serial analysis. J Neurooncol. 2016;129:525–352. doi: 10.1007/s11060-016-2204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bechmann T, Madsen JS, Brandslund I, Lund ED, Ormstrup T, Jakobsen EH, Jylling AM, Steffensen KD, Jakobsen A. Predicting brain metastases of breast cancer based on serum S100B and serum HER2. Oncol Lett. 2013;6:1265–1270. doi: 10.3892/ol.2013.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nygaard AD, Garm Spindler KL, Pallisgaard N, Andersen RF, Jakobsen A. The prognostic value of KRAS mutated plasma DNA in advanced non-small cell lung cancer. Lung Cancer. 2013;79:312–317. doi: 10.1016/j.lungcan.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Hu X, Wu H, Jia Y, Liu J, Mu X, Wu H, Zhao Y. Over-expression of S100B protein as a serum marker of brain metastasis in non-small cell lung cancer and its prognostic value. Pathol Res Pract. 2019;215:427–432. doi: 10.1016/j.prp.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Mu S, Ma H, Shi J, Zhen D. The expression of S100B protein in serum of patients with brain metastases from small-cell lung cancer and its clinical significance. Oncol Lett. 2017;14:7107–7110. doi: 10.3892/ol.2017.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.