Abstract
BACKGROUND
Laser ablation (LA) is used as an upfront treatment in patients with deep seated newly diagnosed Glioblastoma (nGBM).
OBJECTIVE
To evaluate the outcomes of LA in patients with nGBM and compare them with a matched biopsy-only cohort.
METHODS
Twenty-four nGBM patients underwent upfront LA at Cleveland clinic, Washington University in St. Louis, and Yale University (6/2011-12/2014) followed by chemo/radiotherapy. Also, 24 out of 171 nGBM patients with biopsy followed by chemo/radiotherapy were matched based on age (< 70 vs ≥ 70), gender, tumor location (deep vs lobar), and volume (<11 cc vs ≥11 cc). Progression-free survival (PFS), overall survival (OS), and disease-specific PFS and OS were outcome measures. Three prognostic groups were identified based on extent of tumor ablation by thermal-damage-threshold (TDT)-lines.
RESULTS
The median tumor volume in LA (n = 24) and biopsy only (n = 24) groups was 9.3 cm3 and 8.2 cm3 respectively. Overall, median estimate of OS and PFS in LA cohort was 14.4 and 4.3 mo compared to 15.8 mo and 5.9 mo for biopsy only cohort. On multivariate analysis, favorable TDT-line prognostic groups were associated with lower incidence of disease specific death (P = .03) and progression (P = .05) compared to other groups including biopsy only cohort. Only age (<70 yr, P = .02) and tumor volume (<11 cc, P = .03) were favorable prognostic factors for OS.
CONCLUSION
The maximum tumor coverage by LA followed by radiation/chemotherapy is an effective treatment modality in patients with nGBM, compared to biopsy only cohort. The TDT-line prognostic groups were independent predictor of disease specific death and progression after LA.
Keywords: GBM, NeuroBlate, LITT, Minimally invasive, Novel treatment, Brain tumor
ABBREVIATIONS
- CRT
concurrent chemo-radiation therapy
- DSOS
disease-specific overall survival
- DSPFS
disease-specific progression-free survival
- EOC
extent of coverage
- GBM
glioblastoma
- KPS
karnofsky performance score
- LA
Laser ablation
- MGMT
06-methylguanine-DNA methyltransferase
- nGBM
newly diagnosed glioblastoma
- OS
overall survival
- PFS
progression-free survival
- TDT
thermal-damage-threshold lines
Glioblastoma (GBM) is an aggressive primary brain tumor with extension of tumor cells into the normal brain tissue beyond the radiographically visible tumor mass, thus implicating the role of systemic (chemotherapy) and loco regional (radiation therapy) therapy in such patients.1-5 Surgical resection followed by concurrent chemo-radiation therapy (CRT) is the standard line of management in patients with newly diagnosed GBM.6 Gross total resection of the tumor has been shown to have an impact on outcome of patients with GBM.7-10 For difficult-to-access tumors, where resection is prohibited by its associated morbidity, biopsy followed by CRT is a reasonable alternative, albeit with less optimal outcomes.11-13
Laser ablation (LA) is a minimally invasive technique that has emerged as a potential surgical tool in patients with deep-seated brain tumors including GBM.14-18 LA has shown promising results in terms of safety and efficacy in different types of brain tumors including high-grade gliomas.15-17,19-21 There are several studies documenting the efficacy of LA in patients with recurrent GBM,16,22-31 however there is a paucity of literature evaluating the role of LA in patients with newly diagnosed Glioblastoma (nGBM, upfront therapy).19,24,25,32 Most of the previously published studies were limited by a fewer number of patients and lack of control group.
In this study, we report the efficacy of LA (followed by standard CRT) as an upfront treatment in patients with nGBM. We have also compared the results of LA therapy with a matched control group who underwent only biopsy (not candidates for standard surgical resection) followed by CRT for nGBM.
METHODS
Study Design and Setting
This multi-institutional retrospective study was carried out at 4 centers (Cleveland clinic, Washington University in St. Louis, Yale and Duke Universities) following Institutional Review Board approval. Written and informed consent were obtained from patients at all centers prior to the standard procedures, no specific consent was needed for the study due to its retrospective nature. Patients who underwent LA as an upfront treatment followed by CRT were included in in LA cohort (Cleveland clinic, Washington University in St. Louis, and Yale University). Moreover, 24 matched biopsy-only patients were selected from a pool of cases from Yale and Duke Universities Universities and were enrolled as a control cohort. Of note, during the study period, there was no case of LA for nGBM at Duke University and only one such case at Yale University (included in our LA group) to minimize selection bias for biopsy only patients.
Variables and Participants
Twenty-four out of 28 patients, who underwent LA for nGBM at Cleveland clinic, Washington University in St. Louis, and Yale University (6/2011-12/2014) were included in the LA cohort. Patients with pathologically confirmed nGBM, Karnofsky performance score(KPS) of ≥60 who underwent post-op CRT were included in our study. Infratentorial and multifocal nGBM were excluded from our study. In addition, 171 patients from a pool of 325 patients who underwent biopsy for nGBM at Yale and Duke Universities (2010 to 2015) were selected based on the inclusion/exclusion criteria. Median follow up in the LA group was 9.3 (2-43) mo and in the control arm was 14.7 (2-41) mo.
Data Sources/Measurement, Study Size, and Bias
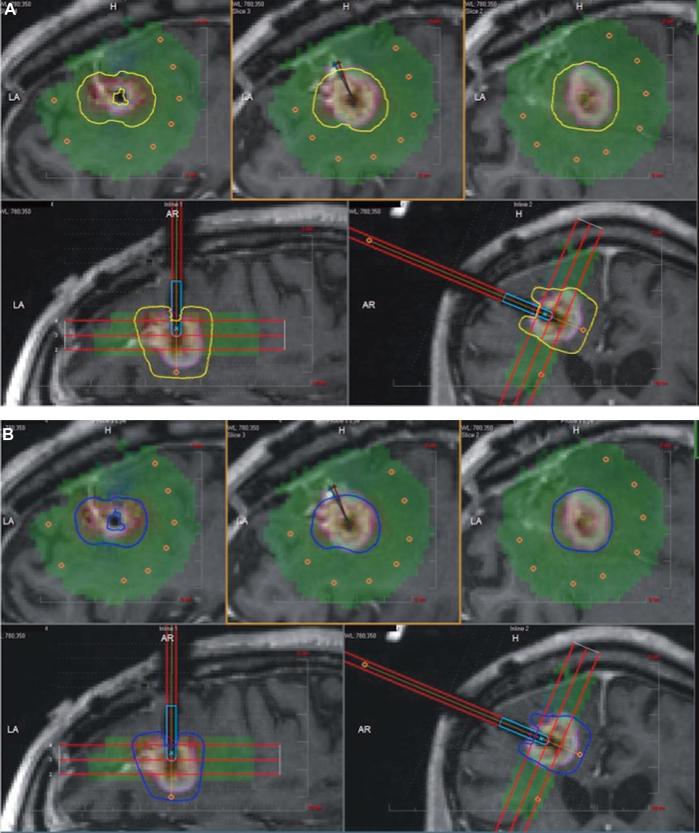
Electronic medical records were reviewed for demographics, patient characteristics, intraoperative findings, imaging data, follow-up, and outcomes. Of 28 consecutive patients with nGBM who underwent LA in the study arm, two patients were excluded, as they did not pursue standard CRT following LA (low preoperative KPS). In addition, one patient was excluded due to lack of follow-up data (she was from out of state) and another one due to mortality 4 d after the LA procedure due to septicemia not related to the intracranial surgical site. Therefore, 24 patients were considered for the outcome analysis in this study. For the biopsy cohort, 171/325 patients met the inclusion criteria. Thereafter, a propensity score-matched control group biopsy-only patients (n = 24) was created based upon age (<70 yr vs ≥70 yr), gender, tumor location (deep vs lobar), and tumor volume (<11 cc vs ≥11 cc). Extent of tumor coverage was performed after importing pre- and intra-operative magnetic resonance imaging scans as well as treatment data including thermal-damage-threshold (TDT)-lines into iPlan software® (Brainlab, Munich, Germany). Yellow TDT-line was defined as the tumor tissue that has been heated to 43°C for 2 min, the blue TDT-line as tumor heated to 43°C for 10 min and white TDT-line as tumor heated to 43°C for 60 min. The LA procedure was performed using the NeuroBlate System (Monteris Medical Corporation, Plymouth, MN) and has been explained in our previous publications in detail (Figure 1).16,19
FIGURE 1.
Treatment of right frontal tumor using LA. A, yellow TDT-line (defined as the tumor tissue that has been heated to 43°C for 2 min) and B, blue TDT-line (defined as tumor heated to 43°C for 10 min).
Quantitative Variables and End Points
Progression-free survival (PFS), overall survival (OS), and disease-specific PFS and OS (DSPFS and DSOS) were the outcome measures. Tumor progression was defined as per the response assessment in neuro-oncology (RANO) criterion for high-grade glioma.33,34 PFS was defined as the time interval between LA and tumor progression or death from any cause, whichever was earlier. OS was defined as the time interval between surgery and death from any cause. DSPFS and DSOS were analyzed using competing risks methods where death from reasons other than tumor progression was considered a competing risk.
Statistical Analysis
SAS version 9.2 (SAS Institute Inc, Cary, North Carolina) and R (version 3.3.3) was used for all data analyses. Fisher's exact test, Cochran-Armitage trend test, and the Wilcoxon rank sum test were used for comparison between the two patient groups. PFS and OS were summarized using the Kaplan–Meier method and compared using log-rank tests or proportional hazards models. DSPFS and DSOS35 were summarized using cumulative incidence and analyzed using Fine and Gray model. Cumulative incidence measures the frequency of death/progression during a time interval and a lower cumulative incidence indicates a favorable outcome. Recursive partitioning algorithm was used to find binary cut-off points for age and tumor volume. A propensity score matching was done based on the multivariable logistic regression using co-variables. Pairs of patients were derived using 1:1 nonreplacement matching from the biopsy and LA group. Two- tailed p values <.05 were considered significant.
RESULTS
Participants and Descriptive Data
Overall 50% (12/24) of patients in LA group were male, median age at surgery was 54 and 29% (7/24) were ≥70 yr of age. In the biopsy-only cohort, 58.3% of patients (14/24) were males, median age was 64 yr (range 32-85 yr) with 25% (6/24) of patients were ≥70 yr of age (Table 1).
TABLE 1.
Patient and Disease Characteristics in NeuroBlate (Monteris Medical) vs Biopsy Group (n [%]) or Median (Range)
| NeuroBlate | Biopsy | ||
|---|---|---|---|
| Factor | (n = 24) | only (n = 24) | P value |
| Gender | .72 | ||
| Female | 12 (50.0%) | 10 (41.7%) | |
| Male | 12 (50.0%) | 14 (58.3%) | |
| Agea | 54 (34-75) | 64(32, 85) | 1.00 |
| <70 yr | 17 (70.8%) | 18 (75.0%) | |
| ≥70 yr | 7 (29.2%) | 6 (25.0%) | |
| Location | 1.00 | ||
| Lobar | 11 (45.8%) | 11 (45.8%) | |
| Deep | 13 (54.2%) | 13 (54.2%) | |
| Volume (cc) | 9.3 (1.3-62.8) | 8.2 (0.9-187) | 1.00 |
| < 11 cc | 16 (66.7%) | 16 (66.7%) | |
| ≥ 11 cc | 8 (33.3%) | 8 (33.3%) | |
| Ki-67 | .57 | ||
| <20% | 8 (42%) | 5 (28%) | |
| >20% | 11 (58%) | 13 (72%) |
aAt time of LITT for NeuroBlate patients; at diagnosis for biopsy only patients
Thalamus was the common site in patients with nonlobar tumors who underwent LA (29%, 7/24 vs 32%, 11/24), whereas corpus callosum was the common nonlobar location in the control group (54% 6/24 vs 8%, 2/24). Ki-67 was available for 18 patients (LA group) and 19 patients (biopsy-only group). Isocitrate dehydrogenase (IDH1) was performed in 14 patients of LA group (negative in all patients) and 20 patients of control group (positive in 2 patients). Similarly, 06-methylguanine-DNA methyltransferase (MGMT) was also performed in 10 patients of LA group and 20 patients of control group and it was methylated in 3 and 10 patients respectively. The median tumor volume in study group was 9.33 cm3 (range 1.31-62.78) and in biopsy group was 8.2 cm3 (0.9-187). Tumor volume was more than 11 cm3 in 33% (8/24) of patients in the study group and 85% (8/24) of patients in the biopsy only group (Table 1).
Tumor Coverage by Thermal Damage Threshold Lines
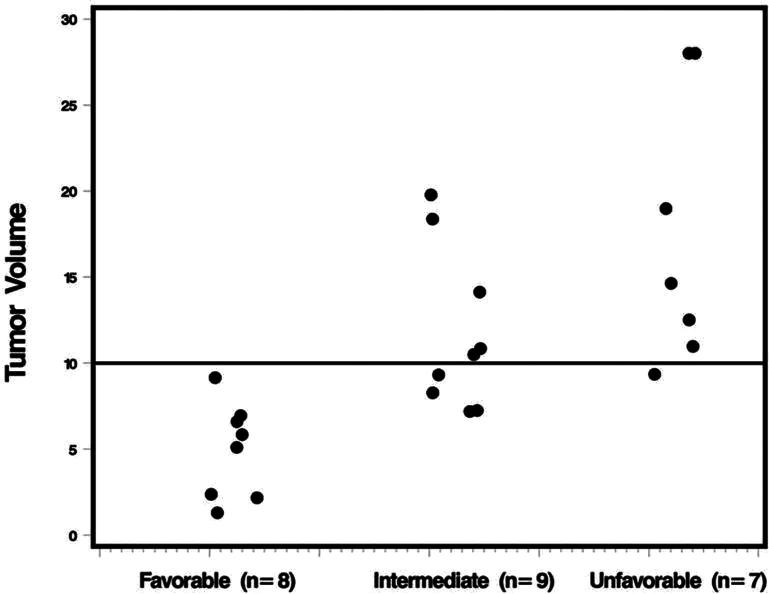
The median proportion of tumor contained within the yellow and blue TDT-line was 99% (range 59%-100%) and 95% (range 46%-100%) respectively. In general, this corresponded to a relatively small volume of disease not being treated: median 0.09 cm3 (range 0-6.86 cm3) outside the yellow line; median 0.47 cm3 (range 0-13.18 cm3) outside the blue line; and a median 0.38 cm3 (range 0-6.32 cm3) in the blue-yellow transition zone. In 67% (16/24) of patients, >90% of tumor coverage with blue TDT-line was observed and 58% (14/24) of patients had >98% tumor coverage with yellow TDT-line (Table 2). Using time to progression, three prognostic groups were identified: favorable: ≤0.025 cm3 of tumor volume within the yellow-blue transition zone (n = 8 patients); intermediate >0.025 cm3 in the transition zone and >90% tumor coverage by the blue line (n = 9 patients); and unfavorable >0.025 cm3 in the transition zone and <90% coverage by blue line (n = 7 patients).
TABLE 2.
NeuroBlate (Monteris Medical) Parameters and Their Relationship to Different Outcomes in Univariate Analysis
| Factor | PFS | DSPFS | OS | DSOS | |||||
|---|---|---|---|---|---|---|---|---|---|
| No (%) or median (range) | Median (mo) | P*a | Subhazard ratio (95% C.I) | P*b | Median (mo) | P*a | Subhazard ratio (95% C.I) | P*b | |
| Residual volume by blue TDT-line (cont.) | 0.47 cm3 (0-13.18) | .007 | 1.25 (1.1-1.3) | .0001 | .97 | 1.15 (0.9-1.3) | .06 | ||
| ≤ 0.025 cm3 | 7 (29%) | 10.7 | .01 | 4.37 (1.3-13.7) | .01 | 11.8 | .54 | 2.19 (0.6-7.5) | .21 |
| > 0.025 cm3 | 17 (71%) | 3.8 | 15.2 | ||||||
| Extent of coverage by blue TDT-line (cont.) | 95% (46-100%) | .002 | <0.01 (0.01-0.07) | .0001 | .96 | .371 (0.04-3.8) | .41 | ||
| ≤ 90% | 8 (33%) | 3.3 | .003 | 0.37 (0.1-1.1) | .07 | 14.4 | .37 | 0.52 (0.1-1.6) | .25 |
| > 90% | 16 (67%) | 5.5 | 15.2 | ||||||
| Residual volume by yellow TDT-line (cont.) | 0.09 cm3 (0-6.86) | .01 | 1.50 (1.2-1.8) | .0001 | .84 | 1.15 (0.9-1.5) | .31 | ||
| < 0.05 cm3 | 12 (50%) | 6.0 | .005 | 2.28 (0.9-5.7) | .08 | 15.2 | .45 | 1.26 (0.4-3.5) | .66 |
| ≥ 0.05 cm3 | 12 (50%) | 3.3 | 14.4 | ||||||
| Extent of coverage by yellow TDT-line (cont.) | 99% (59-100%) | .004 | 0.01 (0.01-0.4) | .02 | .94 | 0.55 (0.04-8.7) | .67 | ||
| ≤ 98% | 10 (42%) | 3.3 | .008 | 0.33 (0.1-0.8) | .02 | 14.4 | .74 | 0.63 (0.2-1.9) | .40 |
| > 98% | 14 (58%) | 5.5 | 15.2 | ||||||
| Tumor volume in blue- yellow transitional zone (cont.) | 0.38 cm3 (0-6.32) | .008 | 1.56 (1.2-1.9) | .0002 | .75 | 1.32 (1.1-1.6) | .01 | ||
| ≤ 0.025 cm3 | 8 (33%) | 8.2 | .04 | 6.52 (1.9-21.8) | .002 | 10.1 | .93 | 3.08 (0.8-11.3) | .09 |
| > 0.025 cm3 | 16 (67%) | 4.0 | 15.2 | ||||||
| TDT-line risk group (cont.) | |||||||||
| Unfavorable | 7 (29%) | 3.3 | .008 | 4.15 (2.1-8.1) | .0001 | 14.4 | .81 | 0.45 (0.2-0.9) | .04 |
| Intermediate | 9 (38%) | 4.5 | 21.4 | ||||||
| Favorable | 8 (33%) | 8.2 | 10.1 | ||||||
PFS: progression-free survival, DSPFS: disease specific progression-free survival, OS: overall survival, DSOS: disease specific overall survival, C.I: confidence interval, TDT-line: thermal damage threshold line
aLog rank test if 2 categories, Wald test from proportional hazards model if >2 categories or if the factor is measured on a continuum
bGray's test. The first group listed is the reference; ratios >1 indicate the risk of death is greater in the second group; ratios <1 that it is less. For factors measured factors or factors with >2 categories the hazard ratio represents the change in risk for a one unit (category) increment
*P value in front of major heading represents comparison across continuous variable and p value in front of subheadings represent comparison across the subgroups as categorical variable.
Bold and italics: significant
Outcome Data, Main Results, and Other Analyses
Multivariate Analysis of Factors That Affected Outcome in LA Group
Clinical and demographic factors associated with outcome in LA were: tumor volume that correlated with all 4 outcomes (PFS, OS, DSPFS, and DSOS); pre-op KPS that correlated with PFS; and age, which correlated with OS (Table 3). The amount of tumor contained in the LA blue-yellow transition zone and the new extent of coverage (EOC) prognostics groups (favorable, intermediate, and unfavorable) were significantly associated with all outcome variables other than OS (Table 2). It is worth noting that the new EOC prognostic groups (favorable, intermediate, and unfavorable) correlated with the tumor volume (Figure 2). The individual blue and yellow line parameters correlated with both the PFS outcome variables (PFS and DSPFS) and did not correlate with either OS or DSOS outcome variables. Multivariate analyses of each outcome were summarized in (Table 4). The only prognostic factor for PFS in multivariate analysis was EOC prognostic groups (P = .008). Age (with a cut point of 70 yr; P = .03) and tumor volume (with a cut point of 11cm;3P = .04) were significant prognostic factors for OS. For DSPFS, EOC prognostic groups (P = .0002) and location (lobar location vs deep location; P = .0009) were significant predictive factors. Whereas for DSOS, EOC prognostic groups (P = .0001), age (with a cut point of 70; P = .03), and gender (P = .04) were significant prognostic factors (Table 4).
TABLE 3.
Patient Characteristics and Their Relationship to Different Outcomes Following LA in Univariate Analysis
| Factor | PFS | DSPFS | OS | DSOS | |||||
|---|---|---|---|---|---|---|---|---|---|
| No (%) or median (range) | Median (mo) | Pa | Sub-hazard ratio (95% C.I) | Pb | Median (mo) | Pa | Sub-hazard ratio (95% C.I) | Pb | |
| Gender | |||||||||
| Female | 10 (42%) | 4.7 | .59 | 1.38 (0.56-3.41) | .48 | 24.9 | .76 | 3.10 (0.95-10.2) | .06 |
| Male | 14 (58%) | 4.2 | 14.4 | ||||||
| Age (yr) | 54 (34-75) | .61 | 0.98 (0.95-1.02) | .30 | .39 | 1.00 (0.96-1.04) | .98 | ||
| < 70 | 18 (75%) | 4.3 | .63 | 0.43 (0.13-1.40) | .16 | 21.4 | .03 | 1.31 (0.35-4.96) | .69 |
| ≥ 70 | 6 (25%) | 4.8 | 8.4 | ||||||
| Pre-op KPSc | |||||||||
| 60-70 | 7 (29%) | 3.3 | .008 | 0.89 (0.60-1.32) | .16 | 7.4 | .15 | 0.86 (0.44-1.70) | .67 |
| 80 | 8 (33%) | 3.5 | 14.4 | ||||||
| 90-100 | 9 (38%) | 9.0 | 15.2 | ||||||
| Tumor location | |||||||||
| Lobar | 13 (54%) | 4.5 | .31 | 0.79 (0.33-1.92) | .61 | 14.4 | .65 | 0.47 (0.15-1.41) | .17 |
| Othersd | 11 (46%) | 4.1 | 22.4 | ||||||
| KI 67 (%)e | 20% (3-80%) | .87 | 1.01 (0.99-1.02) | .31 | .42 | 1.02 (1.00-1.04) | .03 | ||
| < 20% | 5 (28%) | 5.7 | .08 | 3.09 (0.74-12.9) | .12 | 15.2 | .26 | 4.05 (0.57-28.8) | .16 |
| ≥ 20% | 13 (72%) | 4.1 | 8.5 | ||||||
| Tumor volume (cm3) | 9.33 (1.3-62.8) | .04 | 1.04 (1.02-1.07) | .0006 | .61 | 1.04 (1.01-1.08) | .02 | ||
| < 11 | 16 (67%) | 5.3 | .06 | 2.26 (0.84-6.08) | .11 | 21.4 | .04 | 3.32 (0.85-12.9) | .08 |
| ≥ 11 | 8 (33%) | 3.6 | 8.1 | ||||||
PFS: progression-free survival, DSPFS: disease specific progression-free survival, OS: overall survival, DSOS: disease specific overall survival, C.I: confidence interval, KPS: Karnofsky performance status
aLog rank test if 2 categories, Wald test from proportional hazards model if > 2 categories or if the factor is measured on a continuum
bGray's test. The first group listed is the reference; ratios > 1 indicate the risk of death is greater in the second group; ratios < 1 that it is less. For factors measured factors or factors with > 2 categories the hazard ratio represents the change in risk for a 1 unit (category) increment
c3 patients were KPS 60; 1 was KPS 100
dThalamus (n = 7), callosum (n = 2), insula (n = 2)
emissing for 6 patients
FIGURE 2.
TDT-line risk groups and tumor volume. Two patients with tumor volumes >20 cm3 (42.33 and 62.78) were recoded to 28 for ease of presentation.
TABLE 4.
Multivariate Results for 4 Different Outcomes Following LA
| Factor | Hazard ratio (95% C.I.)a | P |
|---|---|---|
| PFSb | ||
| TDT-line risk group (favorable vs intermediate vs unfavorable) | 2.41 (1.26-4.59) | .008 |
| DSPFSc | ||
| TDT-line risk group (favorable vs intermediate vs unfavorable) | 18.49 (3.96-86.24) | .0002 |
| Tumor location (lobes vs other) | 0.07 (0.01-0.33) | .0009 |
| OSb | ||
| Age (<70 vs ≥70) | 4.89 (1.27-18.80) | .02 |
| Tumor volume (<11 vs ≥11 cm3) | 4.32 (1.16-16.02) | .03 |
| DSOSc | ||
| TDT-line risk group (favorable vs intermediate vs unfavorable) | 6.30 (2.45-16.23) | .0001 |
| Age (<70 vs ≥70) | 10.99 (1.32-91.24) | .03 |
| Gender (Female vs Male) | 9.45 (1.10-80.89) | .04 |
PFS: progression-free survival, DSPFS: disease specific progression-free survival, OS: overall survival, DSOS: disease specific overall survival, C.I: confidence interval, TDT-line: thermal damage threshold line
aThe first group listed is the reference; ratios > 1 indicate the risk of death is greater in the second group; ratios < 1 that it is less. For NeuroBlate risk group the (sub) hazard ratio represents the increase in risk for a one category increment
bWald test
cGray test
OS of LA vs Control Group
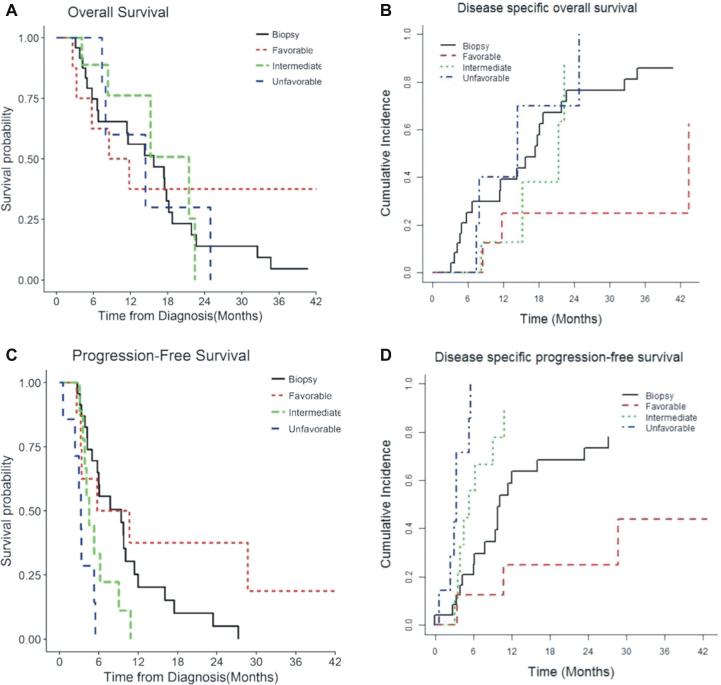
Overall 18 (75%) patients progressed and 15 patients (62%) died during the follow-up in the study group. Of 15 patients with mortality during follow-up, 4 (17%) died due to medical causes (such as pulmonary embolism) with no evidence of intracranial progression at mortality. In the biopsy only cohort, 18/24 patients (75%) had recurrence during follow up and 19/24 patients (79.2%) died during extended follow-up. Of these patients with mortality, 3 (12.5%) patients died due to medical causes without tumor progression. Median OS was estimated to be 14.4 mo in the LA group compared to 15.8 mo in the matched biopsy group (P = .78). DSOS cumulative incidence in the LA group with favorable EOC prognostic factors was 25% ± 3% that was better (lower) than unfavorable EOC group 40% ± 6% (P = .021), and biopsy only group 31% ± 1% (P = .033). Compared to biopsy only group, the difference in OS between 2 groups (favorable and unfavorable EOC) did not show statistical significance P = .64 and .78 respectively, Table 5. However, patients with favorable EOC prognostic factors in the study arm showed improved DSOS (P = .03) compared to those in the control arm (Table 5 and Figure 3).
TABLE 5.
NeuroBlate (Monteris Medical) Prognostic Groups Compared With Biopsy Control
| Groupc | n | Median survival or cumulative incidence in 12 mo | P valuec (compared to biopsy after adjustment) |
|---|---|---|---|
| OSa | |||
| Biopsy | 24 | 15.8 | |
| Favorable | 8 | 10.1 | .64 |
| Intermediate | 9 | 21.4 | .80 |
| Unfavorable | 7 | 14.4 | .78 |
| DSOSb | |||
| Biopsy | 24 | 31% ± 1% | |
| Favorable | 8 | 25% ± 3% | .033 (in favor of LA) |
| Intermediate | 9 | 13% ± 2% | .31 |
| Unfavorable | 7 | 40% ± 6% | .43 |
| PFSa | |||
| Biopsy | 24 | 5.97 | |
| Favorable | 8 | 8.20 | .21 |
| Intermediate | 9 | 4.52 | .12 |
| Unfavorable | 7 | 3.28 | .001 (in favor of biopsy) |
| DSPFSb | |||
| Biopsy | 24 | 63% ± 1% | |
| Favorable | 8 | 25% ± 3% | .05 |
| Intermediate | 9 | 88% ± 3% | .17 |
| Unfavorable | 7 | 100% ± 2% | <.001 (in favor of biopsy) |
LA: laser ablation
aLog rank test comparisons to biopsy only
bGray's test for comparisons to biopsy only
cAdjusted for age (≤ 70 yr vs > 70 yr), gender, tumor location and tumor volume (≤ 11 cc vs > 11 cc)
FIGURE 3.
TDT-line prognostic groups and its effect on 4 different outcomes in comparison with biopsy only patients. A , OS; B , DSOS; C , PFS; D , DSPFS.
Local Control and PFS of LA vs Control Group
Median PFS in the LA group and matched biopsy group was 4.3 mo and 5.9 mo respectively. DS-PFS in the LA group favorable and unfavorable prognostic groups was 25% ± 3% and 100% ± 2%; compared to 63% ± 1% in the control group. PFS and DSPFS did not show statistically significant differences between the LA and biopsy group, in both univariate and multivariate analysis (Median PFS: LA - 4.3 mo [95% CI 3.3-5.7]; biopsy—5.97 [95% CI 3.87-9.05], P = .94; DSPFS Cumulative Incidence at 12 mo: LA - 70% ± 11% vs biopsy—63% ± 1%, P = .84). Compared to the patients in the control arm (biopsy only), patients with favorable EOC prognostic factors in the LA arm had longer DSPFS (P = .05; Table 5 and Figure 3).
Short-Term Outcome of LA
Median post-op hospital stay in LA group was 3 d (range: 1-26 d). Median estimate blood loss in LA group was 10cc (range: 5-100cc). Post-op complications occurred in 9 patients after LA including 6 cases with worsening of neurological deficit after LA (4 patients had worsening of pre motor deficits; 1 patient had new onset right upper extremity weakness and another one had new onset mild hemiparesis following LA), 20 cases of moderate to severe intratumoral bleeding (treated conservatively), and 1 patient with deep vein thrombosis after LA. Four patients (2 frontal, 1 thalamus, and 1 with lentiform nucleus GBM) with worsening of preoperative motor deficits had permanent weakness, which did not improve during follow-up.
DISCUSSION
Key Results and Interpretation
In our study, median OS following LA and concurrent chemo/radiotherapy was estimated to be 14.4 mo, which is similar to 15.8 mo following surgical resection (complete/partial) and concurrent chemo/radiotherapy, as reported by Stupp et al.2 Similarly, the median OS in our matched biopsy only group (n = 24) was 15.8 mo, which is better than 9.4 mo reported in the literature.2 Median PFS following LA and chemo radiotherapy was 4.3 mo in our study, which is slightly lower than that following surgery (resection and biopsy) and CRT (6.9 mo).2 This difference in PFS may be attributed to the inclusion of biopsy only group with the resection group in the published study,2 compared to LA only patients in our study. As LA is an alternative to surgical resection to achieve tumor cytoreduction, it is preferable to compare 2 modalities upfront. Interestingly, DSPFS (cumulative Incidence at 12 mo) following LA was estimated to be 70% ± 11% in our study, compared to 12 mo PFS of 26.9% ± 5.2% in surgery (resection and biopsy) followed by CRT.2 Overall, PFS and OS following either LA or surgical resection followed by CRT are comparable. Moreover, patients who underwent LA had comparable OS (14.4 mo vs 15.8 mo; LA vs matched biopsy cohort) and improved DSOS (25% ± 3% vs 31% ± 1%, LA vs biopsy) than the biopsy only (control) group in our study, which is comparable to that reported in the literature as mentioned above. Hawasli and colleagues32 in a recent literature review concluded that based on level IV evidence, upfront LA can be considered as a reasonable alternative in patients with high grade glioma, who are otherwise not suitable candidates for standard surgical resection, our results reiterate these findings.
Extent of tumor coverage by TDT-lines was a significant prognostic factor for PFS (P = .008), disease specific PFS (P = .0002) and disease specific OS (or death due to GBM progression; P = .0001). Similarly, favorable tumor coverage had a significant impact on DSPFS, and DSOS compared to those with unfavorable tumor coverage or those with biopsy only group, after adjustment for age gender, tumor location, and tumor volume. In concordance to these findings, our prior multicenter study had shown improvement in PFS with near total TDT-line coverage in patients with high-grade glioma (mix of newly diagnosed and recurrent WHO grade 3 and 4 glioma) following LA.19
Generalizability
Cytoreduction using microsurgical resection with the aim of achieving gross total resection has been shown to have a significant impact on OS and PFS in patients with GBM.7,9,12,36-42 Stereotactic biopsy followed by standard CRT in patients with deep-seated or difficult to access tumors has been shown to have inferior outcomes compared to those who underwent gross total resection for GBM.1,6,12,43 Median OS following biopsy with CRT and biopsy with postoperative radiation therapy (RT) only in patients with nGBM has been reported to be 9.4 mo and 7.9 mo respectively.6 In contrast, median OS in patients who underwent microsurgical resection (complete/partial) for GBM followed by CRT and RT only was reported to be 12.9 mo and 15.8 mo respectively.6 LA was initially introduced as an alternative therapeutic option in patients with either deep-seated GBMs or those who are not good candidates for standard microsurgical resection (medical comorbidities).15,23 There are limited case reports and small case series addressing the efficacy of LA as an upfront therapy in patients with high-grade glioma.19,24-27,29,32,44 The current study is the first and largest study evaluating the efficacy of LA as an upfront therapy in such patients. Since the majority of the patients in our study had difficult to access tumors and were not good candidates for surgery, we compared these patients with matched biopsy only group who received standard CRT following biopsy.
Future Directions
Preliminary animal studies had shown disruption of blood brain barrier following hyperthermia with potentially enhanced beneficial effects of radiation and chemotherapy on glioma stem cells.45-50 Similarly, Leuthardt and colleagues showed that blood brain barrier disruption occurs within 4 wk following LA, which subsequently resolved by 6 wk.51 In addition, a smaller surgical incision used during LA also offers an opportunity to start radiation/chemotherapy earlier as compared to standard surgical resection.
Limitations
There are inherent limitations of our study due to its retrospective nature and lack of randomization. Also, the smaller sample size limits the statistical power of our study. Patients with short median follow-up were included in this study, with differences in median follow-up of 5 mo between the cohorts, which could potentially skew the data and therefore results need to be carefully interpreted. Molecular analysis such as IDH and MGMT status was not included in our analysis. Since our LA group included patients as early as 5 yr ago and control group included patients up to 10 yr ago, many of these patients were devoid of data related to MGMT status or molecular analysis and hence were not included in this analysis. Short-term outcome data such as rate of complication, length of hospital stay and effect on KPS was not analyzed in this study and shall be focused in our future studies. Nevertheless, our study is the first largest study contributing to the efficacy of LA as an upfront therapy in patients with nGBM and we hope to resolve our limitations in our recently lunched prospective multicenter study of LA in nGBM, which is expected to be completed in the next 2 yr.
CONCLUSION
Maximum or favorable tumor coverage by LA followed by CRT is an effective treatment modality in patients with nGBM as compared to biopsy alone followed by CRT. Minimally invasive nature of LA makes it a reasonable alternative option in patients who are either not suitable for aggressive surgery or choose not to undergo standard resection. The extent of tumor coverage by hyperthermic lines (TDT-lines) was independent predictor of DSOS and DSPFS, which can be equated to the impact of the extent of surgical resection on outcome in patients with nGBM.
Disclosures
Drs Mohammadi, Chiang, Ahluwalia, Kim, Barnett, Fecci, and Leuthardt are consultants for Monteris medical company. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
COMMENTS
Since treatment options for deep inaccessible glioblastomas remain limited, Laser Interstitial Thermal Therapy (LITT) has recently gained popularity as a potential surgical cytoreductive strategy. Previously, we have published our institutional results reviewing outcomes utilizing LITT as a safe primary treatment for deep inaccessible nGBM with mean PFS of 14.3 months.1
The authors of this study report the largest multi-center series of LITT as a primary treatment for nGBM with a matched cohort of biopsy-only patients. The study reports favorable results in patients with maximal tumor ablation compared to biopsy-alone cohorts. Although no difference was found in OS between the biopsy alone and LITT treatment groups, the authors suggest that favorable tumor coverage (maximal ablation) was associated with improved disease-specific outcomes. However, the heterogeneity of patient-selection, inconsistent ablation volumes, lack of molecular sub-classification (IDH1, MGMT status), and short-term follow-up limit the general applicability of this study. The authors’ findings that maximal ablation portends an improved prognosis suggest that patient-selection remains critical, ie, LITT should be reserved for lesions that can be ablated favorably. Nevertheless, we commend the authors for conducting a large multi-center study that expands the applicability of LITT for nGBM. Neurosurgeons should continue to exercise a patient-specific approach when utilizing LITT as a primary treatment for deep inaccessible gliomas. We are confident that LITT will serve as a safe effective primary treatment for deep inaccessible nGBM in properly-selected patients.
Ashish H. Shah
Christopher A. Sarkiss
Ricardo J. Komotar
Miami, Florida
Reference
- 1. Shah AH, Burks JD, Buttrick SS, Debs L, Ivan ME, Komotar RJ. Laser Interstitial Thermal Therapy as a Primary Treatment for Deep Inaccessible Gliomas. Neurosurgery. 2019;84(3):768-777. [DOI] [PubMed] [Google Scholar]
In this manuscript, the authors describe their multi-institutional experience using laser interstitial thermal ablation for newly diagnosed deep-seated glioblastomas compared against a contemporaneous matched cohort of patients at another tertiary care institution. The authors should be commended for performing a comparative study, which is sorely needed to effectively evaluate the role of laser ablation in the treatment of this dreaded disease. Interestingly, overall, the biopsy-only cohort of patients survived longer compared to the laser ablation cohort. However, in the subset of patients who had complete or near complete ablations, they tended to do better than the biopsy only patients. This does suggest that a cytoreductive benefit can be achieved with laser ablation, even for deep seated tumors typically thought to be unresectable. Based on this interesting data, prospective comparative multi-institutional studies should proceed.
Ian Lee
Detroit, Michigan
REFERENCES
- 1. Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ et al.. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Andersen AP. Postoperative irradiation of glioblastomas: Results in a randomized series. Acta Radiol Oncol Radiat Phys Biol. 1978;17(6):475–484. [DOI] [PubMed] [Google Scholar]
- 4. Walker MD, Alexander E Jr., Hunt WE et al.. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49(3):333–343. [DOI] [PubMed] [Google Scholar]
- 5. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. [DOI] [PubMed] [Google Scholar]
- 6. Stupp R, Hegi ME, Mason WP et al.. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 7. Lacroix M, Abi-Said D, Fourney DR et al.. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95 (2):190–198. [DOI] [PubMed] [Google Scholar]
- 8. Stummer W, Reulen HJ, Meinel T et al.. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564–576; discussion 564–576. [DOI] [PubMed] [Google Scholar]
- 9. McGirt MJ, Chaichana KL, Gathinji M et al.. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. [DOI] [PubMed] [Google Scholar]
- 10. Sanai N, Polley M-Y, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. [DOI] [PubMed] [Google Scholar]
- 11. Rubin P, Fischbach J, Isaacson S. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: Results of three consecutive radiation therapy oncology group (RTOG) clinical trials. Int j radiat oncol biol phys. 1993;26(2):239–244. [DOI] [PubMed] [Google Scholar]
- 12. Vuorinen V, Hinkka S, Farkkila M, Jaaskelainen J. Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir (Wien). 2003;145(1):5–10. [DOI] [PubMed] [Google Scholar]
- 13. Gulati S, Jakola AS, Nerland US, Weber C, Solheim O. The risk of getting worse: surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg. 2011;76(6):572–579. [DOI] [PubMed] [Google Scholar]
- 14. Carpentier A, McNichols RJ, Stafford RJ et al.. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery. 2008;63(1 Suppl 1):ONS21–28; discussion ONS28-29. [DOI] [PubMed] [Google Scholar]
- 15. Sloan AE, Ahluwalia MS, Valerio-Pascua J et al.. Results of the neuroblate system first-in-humans phase i clinical trial for recurrent glioblastoma. J Neurosurg. 2013;118(6):1202–1219. [DOI] [PubMed] [Google Scholar]
- 16. Mohammadi AM, Schroeder JL. Laser interstitial thermal therapy in treatment of brain tumors–the neuroblate system. Expert Rev Med Devices. 2014;11(2):109–119. [DOI] [PubMed] [Google Scholar]
- 17. Sharma M, Balasubramanian S, Silva D, Barnett GH, Mohammadi AM. Laser interstitial thermal therapy in the management of brain metastasis and radiation necrosis after radiosurgery: An overview. Expert Rev Neurother. 2016;16(2):223–232. [DOI] [PubMed] [Google Scholar]
- 18. Habboub G, Sharma M, Barnett GH, Mohammadi AM. A novel combination of two minimally invasive surgical techniques in the management of refractory radiation necrosis: Technical note. J Clin Neurosci. 2017;35:117–121. [DOI] [PubMed] [Google Scholar]
- 19. Mohammadi AM, Hawasli AH, Rodriguez A et al.. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer Med. 2014;3(4):971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ali MA, Carroll KT, Rennert RC et al.. Stereotactic laser ablation as treatment for brain metastases that recur after stereotactic radiosurgery: a multiinstitutional experience. Neurosurg Focus. 2016;41(4):E11. [DOI] [PubMed] [Google Scholar]
- 21. Sharma M, Habboub G, Behbahani M, Silva D, Barnett GH, Mohammadi AM. Thermal injury to corticospinal tracts and postoperative motor deficits after laser interstitial thermal therapy. Neurosurg Focus. 2016;41(4):E6. [DOI] [PubMed] [Google Scholar]
- 22. Carpentier A, Chauvet D, Reina V et al.. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med. 2012;44(5):361–368. [DOI] [PubMed] [Google Scholar]
- 23. Hawasli AH, Bagade S, Shimony JS, Miller-Thomas M, Leuthardt EC. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions: single-institution series. Neurosurgery. 2013;73(6):1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jethwa PR, Barrese JC, Gowda A, Shetty A, Danish SF. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Neurosurgery. 2012;71(1 Suppl Operative):133–144; 144–135. [DOI] [PubMed] [Google Scholar]
- 25. Leonardi MA, Lumenta CB. Stereotactic guided laser-induced interstitial thermotherapy (SLITT) in gliomas with intraoperative morphologic monitoring in an open MR: clinical expierence. Minim Invasive Neurosurg. 2002;45(4):201–207. [DOI] [PubMed] [Google Scholar]
- 26. Leonardi MA, Lumenta CB, Gumprecht HK, von Einsiedel GH, Wilhelm T. Stereotactic guided laser-induced interstitial thermotherapy (SLITT) in gliomas with intraoperative morphologic monitoring in an open MR-unit. Minim Invasive Neurosurg. 2001;44(1):37–42. [DOI] [PubMed] [Google Scholar]
- 27. Sakai T, Fujishima I, Sugiyama K, Ryu H, Uemura K. Interstitial laserthermia in neurosurgery. J Clin Laser Med Surg. 1992;10(1):37–40. [DOI] [PubMed] [Google Scholar]
- 28. Schwarzmaier HJ, Eickmeyer F, von Tempelhoff W et al.. MR-guided laser irradiation of recurrent glioblastomas. J Magn Reson Imaging. 2005;22(6):799–803. [DOI] [PubMed] [Google Scholar]
- 29. Schwarzmaier HJ, Eickmeyer F, von Tempelhoff W et al.. MR-guided laser-induced interstitial thermotherapy of recurrent glioblastoma multiforme: preliminary results in 16 patients. Eur J Radiol. 2006;59(2):208–215. [DOI] [PubMed] [Google Scholar]
- 30. Sloan AE, Ahluwalia MS, Valerio-Pascua J et al.. Results of the neuroblate system first-in-humans phase i clinical trial for recurrent glioblastoma: clinical article. J Neurosurg. 2013;118(6):1202–1219. [DOI] [PubMed] [Google Scholar]
- 31. Reimer P, Bremer C, Horch C, Morgenroth C, Allkemper T, Schuierer G. MR-monitored LITT as a palliative concept in patients with high grade gliomas: preliminary clinical experience. J Magn Reson Imaging. 1998;8(1):240–244. [DOI] [PubMed] [Google Scholar]
- 32. Hawasli AH, Kim AH, Dunn GP, Tran DD, Leuthardt EC. Stereotactic laser ablation of high-grade gliomas. Neurosurg Focus. 2014;37(6):E1. [DOI] [PubMed] [Google Scholar]
- 33. Eisele SC, Wen PY, Lee EQ. Assessment of brain tumor response: RANO and Its Offspring. Curr Treat Options Oncol. 2016;17(7):35. [DOI] [PubMed] [Google Scholar]
- 34. Sharma M, Sattur M, Vogelbaum M. Chapter 58 - response assessment in Neuro-oncology (RANO): An update A2 - Newton, Herbert B. Handbook of Brain Tumor Chemotherapy, Molecular Therapeutics, and Immunotherapy (Second Edition): Academic Press, Cambridge (MA) 2018:747–759. [Google Scholar]
- 35. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. 1999;94(446):496–509. [Google Scholar]
- 36. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–766. [DOI] [PubMed] [Google Scholar]
- 37. Sanai N, Berger MS. Recent surgical management of gliomas. Adv Exp Med Biol. 2012;746:12–25. [DOI] [PubMed] [Google Scholar]
- 38. Ma. Vogelbaum. Does extent of resection of a glioblastoma matter? Clin neurosurg. 2012;59:79–81. [DOI] [PubMed] [Google Scholar]
- 39. Li XZ, Li YB, Cao Y et al.. Prognostic implications of resection extent for patients with glioblastoma multiforme: a meta-analysis. J Neurosurg Sci. 2016;61(6):631–639. [DOI] [PubMed] [Google Scholar]
- 40. Grabowski MM, Recinos PF, Nowacki AS et al.. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115–1123. [DOI] [PubMed] [Google Scholar]
- 41. Oppenlander ME, Wolf AB, Snyder LA et al.. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg. 2014;120(4):846–853. [DOI] [PubMed] [Google Scholar]
- 42. Stummer W, Reulen HJ, Meinel T et al.. Extent of resection and survival in glioblastoma multiforme. Neurosurgery. 2008;62(3):564–576; discussion 564–576. [DOI] [PubMed] [Google Scholar]
- 43. Brown PD, Maurer MJ, Rummans TA et al.. A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: the impact of the extent of resection on quality of life and survival. Neurosurgery. 2005;57(3):495–504; discussion 495–504. [DOI] [PubMed] [Google Scholar]
- 44. Missios S, Schroeder JL, Barnett GH, Mohammadi AM. Prognostic factors of overall survival after laser interstitial thermal therapy in patients with glioblastoma. Photonics Laser Med. 2014;3(2):143–150. [Google Scholar]
- 45. Ascher PW, Justich E, Schrottner O. Interstitial thermotherapy of central brain tumors with the Nd:YAG laser under real-time monitoring by MRI. J Clin Laser Med Surg. 1991;9(1):79–83. [DOI] [PubMed] [Google Scholar]
- 46. Ascher PW, Justich E, Schrottner O. A new surgical but less invasive treatment of central brain tumours Preliminary report. Acta Neurochir Suppl (Wien). 1991;52:78–80. [DOI] [PubMed] [Google Scholar]
- 47. Bettag M, Ulrich F, Schober R et al.. Stereotactic laser therapy in cerebral gliomas. Acta Neurochir Suppl (Wien). 1991;52:81–83. [DOI] [PubMed] [Google Scholar]
- 48. Nakagawa M, Matsumoto K, Higashi H, Furuta T, Ohmoto T. Acute effects of interstitial hyperthermia on normal monkey brain–magnetic resonance imaging appearance and effects on blood-brain barrier. Neurol Med Chir (Tokyo). 1994;34(10):668–675. [DOI] [PubMed] [Google Scholar]
- 49. Sugiyama K, Sakai T, Fujishima I, Ryu H, Uemura K, Yokoyama T. Stereotactic interstitial laser-hyperthermia using Nd-YAG laser. Stereotact Funct Neurosurg. 1990;54-55 (1-8):501–505. [DOI] [PubMed] [Google Scholar]
- 50. Man J, Shoemake JD, Ma T et al.. Hyperthermia sensitizes Glioma stem-like cells to radiation by inhibiting AKT signaling. Cancer Res. 2015;75(8):1760–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leuthardt EC, Duan C, Kim MJ et al.. Hyperthermic laser ablation of recurrent Glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One. 2016;11(2):e0148613. [DOI] [PMC free article] [PubMed] [Google Scholar]