Abstract
BACKGROUND
Resection may be appropriate for select patients with recurrent glioblastoma. The incidence of histopathological findings related to prior treatment and their prognostic implications are incompletely characterized.
OBJECTIVE
To quantify the incidence and survival outcomes associated with treatment effect at resection of recurrent glioblastoma (GBM).
METHODS
Patients who underwent resection for recurrent GBM were retrospectively reviewed, and pathology, treatment history, and survival data were collected. Treatment effect was defined as any component of treatment-related changes on pathology.
RESULTS
In total, 110 patients underwent 146 reoperations. Median age at first reoperation was 57.2 yr and overall survival from reoperation was 10.8 mo. Treatment effect of any kind was noted in 81 of 146 reoperations (55%). Increased treatment effect was observed closer to radiotherapy; by quartile of time from radiotherapy, the rates of treatment effect were 77.8%, 55.6%, 40.7%, and 44.4% (P = .028). Treatment effect was associated with earlier reoperation (8.9 vs 13.8 mo after radiotherapy, P = .003), and the presence of treatment effect did not impact survival from primary surgery (25.4 vs 24.3 mo, P = .084). Patients treated with bevacizumab prior to reoperation were less likely to have treatment effect (20% vs 65%, P < .001).
CONCLUSION
Histopathological treatment-related changes are evident in a majority of patients undergoing resection for recurrent glioblastoma. There was no association of treatment effect with overall survival from primary surgery.
Keywords: Bevacizumab, Glioblastoma, Radiation necrosis, Recurrent, Treatment effect
ABBREVIATIONS
- EMR
electronic medical record
- GBM
glioblastoma
- GTR
gross total resection
- IDH1
isocitrate dehydrogenase 1
- MGMT
O(6)-methylguanine-DNA methyltransferase
- OR
odds ratio
- RN
radiation necrosis
- VEGF
vascular endothelial growth factor
Glioblastoma (GBM) is an aggressive neoplasm associated with poor survival and near-inevitable recurrence following initial resection.1 Adjuvant temozolomide and external beam radiotherapy are the standard of care for newly diagnosed GBM.2 However, adjuvant therapy may produce tissue injury (ie radiation injury and necrosis) and associated radiographic changes,3 which can be challenging to distinguish from recurrence. Development of imaging techniques to reliably distinguish these entities is an ongoing effort4,5; tissue diagnosis remains the gold standard for distinguishing radiation-related changes from recurrence.6
Repeat resection is an option for patients with suspected recurrence, good functional status, and localized enhancement amenable to surgical treatment.7,8 Upon histopathological review, treatment-related changes may be observed, such as coagulative necrosis, fibrinous vascular necrosis, and hyalinized vessels.6 However, the prognostic implications of these changes are incompletely characterized, as is their association with adjuvant therapies.
The objective of this study was to evaluate the incidence of treatment effect and survival outcomes in patients who underwent resection for suspected recurrent GBM. The presence of treatment effect was also analyzed for correlation with preoperative therapies, including radiotherapy and bevaci-zumab treatment.
METHODS
Study Design and Inclusion Criteria
A retrospective cohort study was conducted of consecutive patients who underwent resection of recurrent GBM between 2008 and 2015 at a single brain tumor center. Patients were identified via review of operative records. Study size was determined by the number who met inclusion criteria. Inclusion criteria were repeat craniotomy for tumor resection for recurrent primary GBM following standard 6-wk external beam radiation therapy combined with temozolomide per Stupp protocol.2 Diagnosis of primary GBM was confirmed via review of pathology records; patients without primary pathology records were excluded. This study was approved by the Institutional Review Board and patient consent waiver granted. The manuscript was prepared according to strengthening the reporting of observational studies in epidemiology (STROBE) criteria.32
Data Sources, Variables, and Bias
Data were collated from the electronic medical record (EMR). Variables included age, gender, adjuvant treatments, lesion size, extent of resection, pathology findings, and mortality. Extent of resection was recorded as gross total resection (GTR) versus a combined category of “near total” and “subtotal” resection. All adjuvant therapies and timing of adjuvant therapy were recorded. Chemotherapy was classified as either Stupp protocol, clinical trial enrollment, or off-trial use of non-Stupp agents. Mortality data were collected from the Social Security Death Index and the EMR.
Histopathological findings were based on the pathology report. All samples were analyzed by a consistent group of dedicated neuropathologists with no significant changes in protocol during the study period. Patients with any treatment-related effects described in their pathology report were designated “treatment effect,” including hyalinized vessels, fibrinoid necrosis, and radiation necrosis (RN). When available, the quantity of treatment effect and tumor were recorded. O(6)-methylguanine-DNA methyltransferase (MGMT) methylation status and isocitrate dehydrogenase 1 (IDH1) mutation status were recorded when available.
Pathology specimens were not available, and all clinical data were collected via review of the EMR. This study is thus subject to observer bias: only data recorded in the chart could be used. All patients meeting inclusion criteria were included.
Statistical Methods
Fisher exact tests and Wilcoxon rank sum tests were used for categorical and continuous variable comparison, respectively. Chi-squared tests were used for the comparison of multiple categorical variables. Kaplan-Meier analyses with log-rank tests were used for univariate survival analysis. Multivariate Cox proportional hazard analysis was used for multivariate survival analysis. The threshold for statistical significance was set at P < .05. All statistical analyses were performed using JMP statistical software version 12 (SAS Institute Inc, Cary, North Carolina) and SPSS Statistics version 24.0 (IBM, Armonk, New York).
RESULTS
A total of 110 patients met inclusion criteria (Table 1). Most patients (n = 74, 67%) underwent a single reoperation for recurrent disease; 36 (33%) underwent 2 reoperations. Median age at primary resection was 54.9 yr. Extent of resection at primary surgery was available for 108 patients, of whom 42 achieved GTR (39%). All patients completed radiotherapy with temozolomide, with a majority completing a standard course of adjuvant temozolomide. A total of 60 patients underwent interval treatment with additional agents, most frequently bevacizumab (Avastin, Genentech, South San Francisco, California; n = 25). Median follow-up from primary resection was 25.4 mo (4.6-133).
TABLE 1.
Cohort Characteristics
| n | 110 |
| Gender | |
| Male (%) | 66 (60%) |
| Female (%) | 44 (40%) |
| Number of patients undergoing resection of recurrent disease | |
| One reoperation (%) | 110 (100%) |
| Second reoperation (%) | 36 (33%) |
| Total number of resections of recurrent disease | 146 |
| Median age at first reoperation, yr (range) | 57.2 (21-78) |
| Median time from primary resection to first reoperation, mo (range) | 12.9 (1.4-114) |
| Median survival after first reoperation, mo (95% CI) | 10.8 (8-13) |
CI, confidence interval.
Reoperations for Recurrent Disease
First reoperation for suspected recurrence occurred at median age 57.2 yr (Table 2). Reoperation followed primary resection by median 12.9 mo and radiotherapy median 10.5 mo. Median preoperative karnofsky performance status (KPS) was 90 (range 60-100). Median maximum dimension was 3.4 cm. GTR was achieved in 35 patients (32%). Treatment effect of any kind was present in 60 operations (54%). The amount of treatment effect was not reported in 41 of 60 patients. In 54 patients, both treatment effect and viable tumor were observed; in 6 patients, only treatment effect was reported. Sarcoma elements were observed in 8 patients (7%). Following resection, 90 patients (82%) received adjuvant chemotherapy (Table 3). Of 60 patients with any treatment effect, 85% received adjuvant chemotherapy after their reoperation. Of 6 patients with pure treatment effect: 2 were observed with serial imaging, 2 were started on off-trial chemotherapeutic agents due to concern for early postoperative progression on imaging, 1 was re-started on temozolomide per patient preference, and 1 underwent treatment with bevacizumab for RN. One patient enrolled in a clinical trial prior to reoperation and continued on protocol, and 1 patient underwent reoperation prior to the conclusion of adjuvant temozolomide and resumed treatment with temozolomide postoperatively.
TABLE 2.
Reoperation Details
| Overall | |
| n | 110 |
| Median size at primary resection, cm (range) | 4.2 (1.5-8) |
| GTR at primary resection (%) | 42 (38%) |
| Median age at primary resection, yr (range) | 54.9 (20-75) |
| Median OS from primary resection, mo (95% CI) | 16.2 (21-31) |
| First reoperation for recurrent disease | |
| n | 110 |
| Treatment effect present (%) | 60 (54%) |
| Sarcoma elements present (%) | 8 (7%) |
| Median tumor size, cm (range) | 3.4 (0.7-7.8) |
| GTR (%) | 35 (32%) |
| Median age, yr (range) | 57.2 (21-78) |
| Median time after primary resection, mo (range) | 12.9 (1.4-114) |
| Median time after XRT, mo (range) | 10.5 (0.2-111) |
| Median OS after reoperation, mo (95% CI) | 10.8 (8-13) |
| Second reoperation for recurrent disease | |
| n | 36 |
| Treatment effect present (%) | 21 (58%) |
| Sarcoma changes present (%) | 6 (17%) |
| Median tumor size, cm (range) | 3.7 (1-8.1) |
| GTR (%) | 10 (28%) |
| Median age, yr (range) | 57.6 (27-72) |
| Median time after first reoperation, mo (range) | 7.4 (0.4-30) |
| Median time from last XRT, mo (range) | 14.9 (3-109) |
| Median OS after reoperation, mo (95% CI) | 9.5 (5-13) |
GTR, gross total resection; OS, overall survival; XRT, radiation therapy.
TABLE 3.
Postoperative Adjuvant Therapy at First Recurrence
| Treatment effect | No treatment effect | P-value | |
|---|---|---|---|
| Postoperative adjuvant chemotherapy | 51 (85%) | 39 (78%) | .343 |
| Continuation of Stupp adjuvant TMZ | 3 (5%) | 0 (0%) | .109 |
| Off trial agent | 27 (45%) | 24 (48%) | .753 |
| Bevacizumab | 10 (16.7%) | 10 (20%) | .652 |
| Clinical trial | 22 (36.7%) | 15 (30%) | .461 |
| Postoperative adjuvant XRT | 2 (3.3%) | 5 (10%) | .154 |
TMZ, temozolomide; XRT, radiation therapy.
Of 110 patients, 36 (33%) underwent a second reoperation at median age 57.6 yr. The second reoperation occurred median 7.4 mo after the first reoperation and median 14.9 mo after radiotherapy. Median maximum dimension of the enhancing mass was 3.7 cm. GTR was achieved in 10 of 36 patients (28%). Histologic treatment effect was noted to be present in 21 patients (58%), of whom 20 had concurrent viable tumor; 1 patient of 21 had pure treatment effect. Of 21 patients with treatment effect at second reoperation, 15 (71.4%) had treatment effect at first reoperation (P = .3). Sarcoma changes were observed in 6 patients (16.7%).
Incidence of Treatment Effect
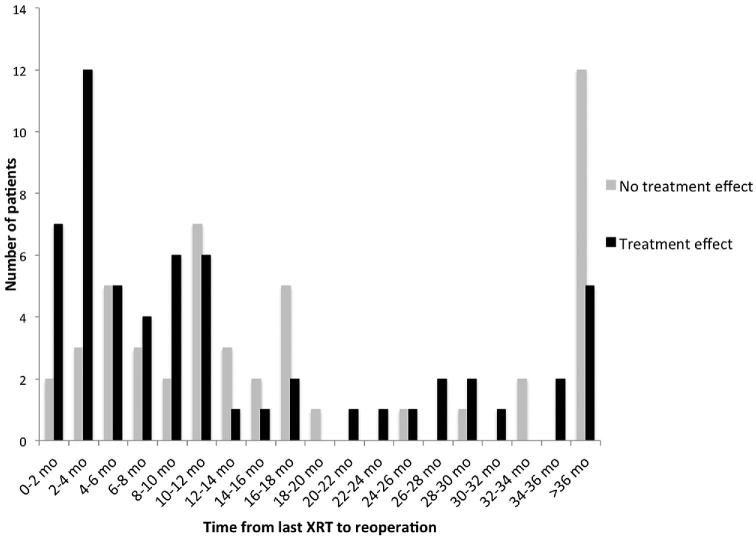
Combining first and second reoperations, treatment effect was observed in 81 cases following 146 reoperations (55%). There was a significant relationship between time from last radiotherapy to reoperation and incidence of treatment effect (Table 4, Figure 1). Treatment effect was observed in 77.8% of patients who underwent reoperation within 4.5 mo of completing radiotherapy (first quartile of time from radiotherapy to first reoperation), as compared to 55.6% treatment effect in patients who underwent reoperation 4.5 to 10.5 mo after radiotherapy, 40.7% treatment effect in patients who underwent reoperation 10.5 to 25.8 m after radiotherapy, and 44.4% treatment effect in patients who underwent reoperation over 25.8 mo after last radiotherapy (P = .028).
TABLE 4.
Incidence of Treatment Effect by Time After Radiation Therapy Quartile
| Time after XRT | Treatment effect | No treatment effect | P-value |
|---|---|---|---|
| < 4.5 mo | 21 (77.8%) | 6 (22.2%) | .028 |
| 4.5-10.5 mo | 15 (55.6%) | 12 (44.4%) | |
| 10.5-25.8 mo | 11 (40.7%) | 16 (59.3%) | |
| >25.8 mo | 12 (44.4%) | 15 (55.6%) |
XRT, radiation therapy.
FIGURE 1.
Histogram of histopathological findings by time of first reoperation relative to completion of radiotherapy. The incidence of treatment effect was significantly higher closer to completion of radiotherapy.
Factors Influencing the Incidence of Treatment Effect
Patients with any treatment effect at first reoperation underwent reoperation median 8.9 mo after radiotherapy, as compared to 13.8 mo in patients without treatment effect (P = .003, Table 5). Patients with treatment effect at second reoperation underwent reoperation median 10.7 mo after radiotherapy, as compared to median 26.5 mo in patients without treatment effect (P = .018). The presence of any treatment effect was associated with time from primary resection to first reoperation, but not with time from first reoperation to second reoperation (P = .003 and P = .6, respectively). The presence of treatment effect was not associated with significant difference in age at first or second reoperation (P = .3 and P = .470, respectively), or lesion size at first or second reoperation (P = .5 and P = .4, respectively).
TABLE 5.
Comparison of Patients With and Without Treatment Effect
| Treatment effect | No treatment effect | P-value | |
|---|---|---|---|
| Median time from last XRT, mo (range) | |||
| Time from XRT to reoperation 1 | 8.9 (0.6-112) | 13.8 (0.2-79) | .003 |
| Time from XRT to reoperation 2 | 10.7 (3.4-43) | 26.5 (4.9-109) | .018 |
| Median time from prior resection, mo (range) | |||
| Time from primary resection to reoperation 1 | 11.3 (1.4-81) | 16.1 (3.6-114) | .003 |
| Time from reoperation 1 to 2 | 9.6 (0.4-30) | 5.9 (0.7-29) | .596 |
| Median age, yr (range) | |||
| Age at first reoperation | 57.2 (27-78) | 58.1 (21-72) | .259 |
| Age at second reoperation | 59.1 (27-72) | 56.6 (36-70) | .47 |
| Median size, cm (range) | |||
| Tumor size at first resection | 3.5 (0.7-6.3) | 3.2 (0.8-7.8) | .484 |
| Tumor size at second resection | 3.3 (1.0-6.1) | 4.0 (1.4-8.1) | .363 |
| Viable tumor cells present (%) | |||
| No tumor cells present | 6 (100%) | 0 (0%) | .031 |
| Tumor cells tumor cells present | 54 (52%) | 50 (48%) | |
| MGMT methylation status (%) | |||
| Negative | 13 (59%) | 9 (41%) | .752 |
| Positive | 9 (56%) | 7 (44%) | |
| Interval bevacizumab (%) | |||
| No interval bevacizumab | 55 (65%) | 30 (35%) | <.001 |
| Interval bevacizumab | 5 (20%) | 20 (80%) | |
| Sarcoma present (%) | |||
| No sarcoma present | 58 (57%) | 44 (43%) | .138 |
| Sarcoma present | 2 (25%) | 6 (75%) | |
| Survival, mo (95% CI) | |||
| OS after first reoperation | 13.1 (8.8-17.3) | 8.1 (6.5-9.5) | .015 |
| OS after second reoperation | 11.8 (7.6-16.0) | 5.2 (4.4-6.0) | .026 |
CI, confidence interval; GTR, gross total resection; OS, overall survival; XRT, radiation therapy; MGMT, O(6)-methylguanine-DNA methyltransferase.
MGMT methylation status was available for 38 patients (34.5%) and was not significantly associated with treatment effect (P = .8) or survival after reoperation (P = .7). IDH1 status was available for 70 patients (64%), of whom 7 (10% of 70, 6.4% overall) were IDH1 mutant. IDH1 mutation was not significantly correlated with treatment effect (P = .5) or survival after reoperation (P = .274). Interval treatment with bevacizumab was associated with decreased incidence of treatment effect at reoperation: treatment effect was observed in 20% of the 25 patients who received bevacizumab, as compared to 65% of patients who did not (P < .001).
Survival Analysis
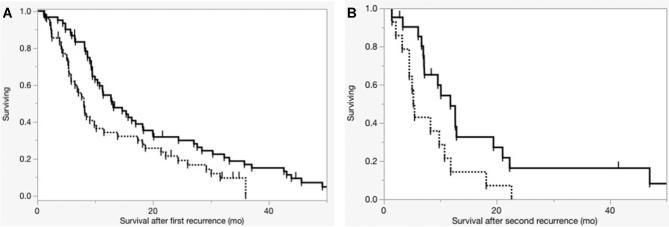
Median overall survival was 26.2 mo after primary resection, 10.8 mo after first reoperation, and 9.5 mo after second reoperation. Overall survival from primary resection was 23.3% at 5 yr and 3.9% at 10 yr. GTR at primary resection was associated with increased time to reoperation (9.1 vs 17.9 mo, P = .019) and increased overall survival (28.5 vs 24.2 mo, P = .047). Median overall survival from primary resection was 25.4 mo in patients with treatment effect and 24.3 in patients without (P = .084). Median survival after first reoperation was 13.1 mo in patients with treatment effect and 8.1 mo in patients without (P = .015, Figure 2A). Median survival after second reoperation was 11.8 mo for patients with treatment effect and 5.2 mo for patients without (P = .026, Figure 2B).
FIGURE 2.
Overall survival after resection for recurrent disease. Dotted lines denote patients without treatment effect present on histopathology and solid lines denote patients with treatment effect. Panel A illustrates survival after the first reoperation for recurrent disease. Presence of treatment effect was associated with a statistically significant increase in survival (P = .014). Panel B illustrates survival after the second reoperation for recurrent disease. Presence of treatment effect was associated with a statistically significant increase in survival (P = .026).
On multivariate analysis of survival after first reoperation (Table 6), prior treatment with bevacizumab (P = .001, odds ratio [OR] 2.391) and presence of sarcoma (P = .002, OR = 3.532) were associated with reduced survival. Increased time from radiotherapy (P = .008, OR 0.987) and treatment effect (P = .040, OR 0.609) were associated with increased survival. Extent of resection at reoperation and age at reoperation were not significantly associated with survival after reoperation (P = .6 and P = .7, respectively). On analysis of survival after primary resection, time to re-resection was associated with increased survival and interval bevacizumab was associated with decreased survival (P < .001 and P = .026, respectively). The presence of treatment effect at reoperation did not impact survival from primary resection (P = .134).
TABLE 6.
Cox Proportional Hazards Multivariate Analysis of Overall Survival
| P-value | HR | 95% CI | |
|---|---|---|---|
| Survival after primary resection | |||
| Time to reoperation (mo) | <.001 | 0.931 | 0.887-0.931 |
| Interval bevacizumab | .025 | 3.187 | 1.083-3.187 |
| Sarcoma present at reoperation | .055 | 4.816 | 0.984-4.816 |
| Treatment effect present at reoperation | .172 | 1.162 | 0.431-1.162 |
| GTR (primary resection) | .861 | 1.486 | 0.623-1.486 |
| Age at recurrence | .92 | 1.022 | 0.981-1.022 |
| Survival after first reoperation | |||
| Interval bevacizumab | .001 | 2.391 | 1.418-4.034 |
| Sarcoma present at reoperation | .002 | 3.532 | 1.592-7.838 |
| Time from prior XRT (mo) | .008 | 0.987 | 0.977-0.997 |
| Treatment effect present | .04 | 0.609 | 0.38-0.979 |
| GTR (recurrence) | .561 | 1.137 | 0.738-1.752 |
| Age at recurrence | .674 | 0.996 | 0.977-1.015 |
CI, confidence interval; GTR, gross total resection; HR, hazard ratio; XRT, radiation therapy.
DISCUSSION
Distinguishing between recurrence and treatment-related processes remains a challenge in management of patients with previously treated GBM. While the majority of studies have primarily concerned cases of pure or majority (greater than 80%) treatment effect and predictive radiographic findings and outcomes,9-14 the incidence and implications of having any treatment-related histologic findings at the time of reoperation are incompletely characterized.
Our data indicate that some quantity of treatment-related changes are present in a majority of patients at time of resection of recurrent disease.
Challenges Associated with Assessing Treatment Effect
The combination of radiotherapy and temozolomide has been the standard of care for newly diagnosed GBM since the publication of the Strupp protocol in 2005, and has been shown to improve overall survival.2 However, radiation and chemotherapy are injurious to brain parenchyma, especially when used in conjunction, and may result in treatment effects (predominantly ascribed to radiotherapy and designated radiation injury/necrosis).3,6,12,15 Following adjuvant therapy, tumor recurrence and treatment effects may present with similar magnetic resonance imaging changes, presenting a challenge for diagnosis and management.
Radiation injury/necrosis has been reported to occur in 3 to 24% of patients following radiotherapy,12,13,16 with rates increasing as much as 3-fold when radiotherapy is combined with chemotherapy.15,16 However, no standard exists with regard to the quantity of radiation-related changes on histopathological examination necessary for a lesion to be designated RN.12,14
Multiple series have reported higher rates of “pure” RN than mixed tumor and necrosis13,17; however, other authors have reported that the most common histopathological finding following standard chemoradiation treatment is a mix of both residual or recurrent tumor and radiation injury.6,14 In our experience, patients with treatment effect and without viable tumor represented only 5.4% of the overall cohort, lower than the 13.5% to 35.7% previously reported in some postradiation glioma series,9,13,17 and comparable to the 3.9% reported in a mixed population of grade III/IV glioma patients status post combined temozolomide and radiotherapy by Rusthoven et al,14 and the 6% observed by Forsyth et al18 via biopsy of a population of low- and high-grade glioma patients following radiotherapy.9
Prognostic Implications of Treatment Effect
Limited data exist regarding the prognostic implications of treatment-related changes on histopathology. In the largest series limited to GBM, Grossman et al12 found that RN (defined as a radiation injury comprising over 80% of resected tissue) was not associated with significant survival benefit, with a mean survival of 15 vs 17.5 mo in 159 patients undergoing resection for recurrence. Similarly, in a cohort of 51 patients with WHO grade III/IV glioma in which 14 patients were found to have treatment effect (more than 80% radiation injury), patients with RN had increased survival of 21.8 vs 7 mo; this difference was not statistically significant (P > .1).14
However, other series describing mixed glioma grades and smaller GBM cohorts have found an association between treatment effect and survival. Forsyth et al18 found that mixed RN (defined as any quantity of RN with greater than 5% recurrent tumor) was significantly associated with survival in 51 patients with recurrent primarily low-grade gliomas (survival 1.86 vs 0.83 yr, P = .008), and pure RN was also associated with increased survival. In a series of 23 GBM patients, Fitzek et al10 reported that pure RN was associated with a mean overall survival of 29 mo, as compared to 16 mo in patients without RN (P = .001). Similarly, Peca et al11 reported significantly increased survival in association with pure RN in a cohort of 11 patients following Strupp protocol for GBM. In this series, the presence of any treatment-related changes at reoperation for suspected recurrence was associated with increased survival after reoperation (20.8 vs 13.0 mo, P = .015). However, treatment effect was not associated with a significant difference in survival from primary surgery (24.3 vs 25.4 mo, P = .084). Treatment effect was strongly correlated with proximity to prior treatment; therefore, it is likely the observed relationship of treatment effect and survival after reoperation was primarily reflective of lead-time bias, where patients with treatment effect underwent repeat resection earlier and survived longer postreoperation, but had similar survival from primary resection overall.
Impact of Bevacizumab on Treatment Effect
Following promising phase II trials, adjuvant GBM therapy with bevacizumab, an antiangiogenic antibody that neutralizes vascular endothelial growth factor (VEGF)-A, was initially met with excitement.19,20 However, phase III trials have failed to demonstrate significant improvements in overall survival in newly diagnosed GBM patients treated with bevacizumab.21,22 VEGF has been hypothesized to play a role in RN, as regional hypoxia in irradiated tissues increases VEGF, in turn increasing vascular permeability, edema, and necrosis.15 Studies have demonstrated that bevacizumab treatment for RN reduces contrast-enhancing volumes and fluid-attenuated inversion-recovery abnormalities,23-25 reduces glucocorticoid requirements, and produces symptomatic improvement.18,22-24 In a prospective, randomized, placebo-controlled trial, Levin et al25 found that use of bevacizumab for RN in a mix of primary central nervous system and head and neck tumors reduced contrast-enhancing volumes and improved symptoms, providing class I evidence for the use of bevacizumab for the treatment of RN.
Histopathological changes associated with bevacizumab administration for glioblastoma have been previously characterized as reduced mean vessel density without other overt changes or immunohistochemistry findings.26 We found that prior treatment with bevacizumab was associated with a reduction in the incidence of treatment effect on histopathology. While causation cannot be established due to the retrospective nature of this series, our findings suggest that bevacizumab may reduce radiation-related changes on histopathology in a manner consistent with the imaging changes previously described to follow bevacizumab administration for RN.
Bevacizumab treatment prior to recurrence was associated with reduced survival both from time of primary surgery and from time of reoperation. The impact of bevacizumab on overall survival after diagnosis in a reoperated patient cohort may reflect the poor prognosis associated with progression on bevacizumab.19,27,28
Limitations
The primary limitation of this study was that re-analysis of pathology slides by a single neuropathologist was not feasible, which may have contributed variability in histopathological data. Additionally, the lack of granularity in pathology records precluded quantification of the percent of a sample that was treatment effect versus viable tumor. Treatment effect was therefore defined as the presence of any amount of treatment-related changes, including mild or focal treatment effect. While the definition of treatment effect and corresponding rate has varied amongst published series, the proportion of patients with treatment effect was higher in our series than previously published as a result of our low threshold.12,13,15-17 It is also possible that some patients with small amount of treatment effects with a significant quantity of tumor may not have been described as having treatment effect. Despite this limitation, the proportion of patients in our series with treatment effect was highest closer in time to last radiotherapy, suggesting that our methodology was capturing factors associated with treatment effect. Ultimately, prospective, pathology-based studies and establishment of standards in the description and reporting of treatment-related changes and tumor recurrence are warranted. Additionally, this series reports outcomes in patients who underwent surgical resection of recurrent GBM, and is subject to selection bias. Generally, only 13 to 33% of patients with recurrent high-grade glioma are candidates for re-resection.29 The patients in this series therefore represent a selected cohort of recurrent GBM patients with a higher functional status at time of recurrence and relatively focal disease involvement.30-32 Furthermore, while all patients received standard radiotherapy with temozolomide, patients also received adjuvant therapies, including bevacizumab, in a nonrandomized fashion that may introduce additional selection bias.
Generalizability
These results reflect survival outcomes in a subpopulation of recurrent GBM patients who were candidates for repeat resection following treatment with standard of care adjuvant therapy. These results may not be generalizable to all recurrent GBM patients, but should be generalizable to patients with recurrent GBM status post Stupp protocol therapy who are surgical candidates. Assessment of patients for surgery and interpretation of surgical specimens may vary by center and experience: the results from this study are most generalizable to experienced neurosurgeons and neuropathologists at a high-volume center.
CONCLUSION
Treatment-related changes are present on histopathological examination in a majority of patients undergoing repeat resection for recurrent glioblastoma. The presence of treatment-related changes is associated with earlier re-resection, improved survival from repeat resection, and unchanged overall survival from primary resection. Interval treatment with bevacizumab is associated with a decreased incidence of treatment effect on histopathology and reduced overall survival.
Disclosures
Ankush Chandra and Jonathan Rick are supported by the Howard Hughes Medical Institute (HHMI). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
COMMENT
The standard treatment for GBM can cause additional brain tissue injury and associated radiographic and histopathologic changes that have been termed “treatment effects.” Treatment effects can be difficult to distinguish from recurrence and the association between treatment effects and patient prognosis remain unclear. In this study, the authors present a retrospective review of 110 patients undergoing reoperation for recurrent glioblastoma after standard of care treatment to elucidate the implications of histopathologic findings upon re-resection.
Overall, these data revealed that a histopathologic treatment effect associated with earlier reoperation with no impact on overall survival, and underscored the associated guarantee-time bias, which can mislead one to conclude that patients with treatment effect have a longer overall survival. Given the study's limitations - lack of histopathologic quantification of treatment effect versus viable tumor cells, selection bias for those who underwent reoperation, non-randomized administration of bevacizumab - a definitive treatment or prognosis paradigm cannot be formulated. Despite these limitations, this study expands upon previous reports examining the relevance and implications of histopathologic treatment effect in patients harboring a GBM.
Faith Robertson
Boston, Massachusetts
REFERENCES
- 1. Sughrue ME, Sheean T, Bonney PA, Maurer AJ, Teo C. Aggressive repeat surgery for focally recurrent primary glioblastoma: outcomes and theoretical framework. Neurosurg Focus. 2015;38(3):E11. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ et al.. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. [DOI] [PubMed] [Google Scholar]
- 3. Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003;9(4):180-188. [DOI] [PubMed] [Google Scholar]
- 4. Delgado-Lopez PD, Rinones-Mena E, Corrales-Garcia EM. Treatment-related changes in glioblastoma: a review on the controversies in response assessment criteria and the concepts of true progression, pseudoprogression, pseudoresponse and radionecrosis. Clin Transl Oncol. 2017;20(8):939-953. [DOI] [PubMed] [Google Scholar]
- 5. Yang I, Aghi MK. New advances that enable identification of glioblastoma recurrence. Nat Rev Clin Oncol. 2009;6(11):648-657. [DOI] [PubMed] [Google Scholar]
- 6. Perry A, Schmidt RE. Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol. 2006;111(3):197-212. [DOI] [PubMed] [Google Scholar]
- 7. De Bonis P, Anile C, Pompucci A et al.. The influence of surgery on recurrence pattern of glioblastoma. Clin Neurol Neurosurg. 2013;115(1):37-43. [DOI] [PubMed] [Google Scholar]
- 8. Brandes AA, Bartolotti M, Franceschi E. Second surgery for recurrent glioblastoma: advantages and pitfalls. Expert Rev Anticancer Ther. 2013;13(5):583-587. [DOI] [PubMed] [Google Scholar]
- 9. Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82(1):81-83. [DOI] [PubMed] [Google Scholar]
- 10. Fitzek MM, Thornton AF, Rabinov JD et al.. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg. 1999;91(2):251-260. [DOI] [PubMed] [Google Scholar]
- 11. Peca C, Pacelli R, Elefante A et al.. Early clinical and neuroradiological worsening after radiotherapy and concomitant temozolomide in patients with glioblastoma: Tumour progression or radionecrosis? Clin Neurol Neurosurg. 2009;111(4):331-334. [DOI] [PubMed] [Google Scholar]
- 12. Grossman R, Shimony N, Hadelsberg U et al.. Impact of resecting radiation necrosis and pseudoprogression on survival of patients with glioblastoma. World Neurosurg. 2016;89:37-41. [DOI] [PubMed] [Google Scholar]
- 13. Kumar AJ, Leeds NE, Fuller GN et al.. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217(2):377-384. [DOI] [PubMed] [Google Scholar]
- 14. Rusthoven KE, Olsen C, Franklin W et al.. Favorable prognosis in patients with high-grade glioma with radiation necrosis: the University of Colorado reoperation series. Int J Radiat Oncol Biol Phys. 2011;81(1):211-217. [DOI] [PubMed] [Google Scholar]
- 15. Siu A, Wind JJ, Iorgulescu JB, Chan TA, Yamada Y, Sherman JH. Radiation necrosis following treatment of high grade glioma–a review of the literature and current understanding. Acta Neurochir. 2012;154(2):191-201; discussion 201. [DOI] [PubMed] [Google Scholar]
- 16. Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65(2):499-508. [DOI] [PubMed] [Google Scholar]
- 17. Rock JP, Hearshen D, Scarpace L et al.. Correlations between magnetic resonance spectroscopy and image-guided histopathology, with special attention to radiation necrosis. Neurosurgery. 2002;51(4):912-919; discussion 919-920. [DOI] [PubMed] [Google Scholar]
- 18. Forsyth PA, Kelly PJ, Cascino TL et al.. Radiation necrosis or glioma recurrence: is computer-assisted stereotactic biopsy useful? J Neurosurg. 1995;82(3):436-444. [DOI] [PubMed] [Google Scholar]
- 19. Castro BA, Aghi MK. Bevacizumab for glioblastoma: current indications, surgical implications, and future directions. Neurosurg Focus. 2014;37(6):E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muhsin M, Graham J, Kirkpatrick P. Bevacizumab. Nat Rev Drug Discov. 2004;3(12):995-996. [DOI] [PubMed] [Google Scholar]
- 21. Gilbert MR, Dignam JJ, Armstrong TS et al.. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chinot OL, Wick W, Mason W et al.. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709-722. [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67(2):323-326. [DOI] [PubMed] [Google Scholar]
- 24. Wong ET, Huberman M, Lu XQ, Mahadevan A. Bevacizumab reverses cerebral radiation necrosis. J Clin Oncol. 2008;26(34):5649-5650. [DOI] [PubMed] [Google Scholar]
- 25. Levin VA, Bidaut L, Hou P et al.. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blumenthal DT, Kanner AA, Aizenstein O et al.. Surgery for recurrent high-grade glioma after treatment with bevacizumab. World Neurosurg. 2017;110:e727-3737. [DOI] [PubMed] [Google Scholar]
- 27. Clark AJ, Lamborn KR, Butowski NA et al.. Neurosurgical management and prognosis of patients with glioblastoma that progresses during bevacizumab treatment. Neurosurgery. 2012;70(2):361-370. [DOI] [PubMed] [Google Scholar]
- 28. Magnuson W, Ian Robins H, Mohindra P, Howard S. Large volume reirradiation as salvage therapy for glioblastoma after progression on bevacizumab. J Neurooncol. 2014;117(1):133-139. [DOI] [PubMed] [Google Scholar]
- 29. Ryken TC, Kalkanis SN, Buatti JM, Olson JJ, Committee ACJG. The role of cytoreductive surgery in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118(3):479-488. [DOI] [PubMed] [Google Scholar]
- 30. Clarke JL, Ennis MM, Yung WK et al.. Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro Oncol. 2011;13(10):1118-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bloch O, Han SJ, Cha S et al.. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012;117(6):1032-1038. [DOI] [PubMed] [Google Scholar]
- 32. Lacroix M, Abi-Said D, Fourney DR et al.. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190-198. [DOI] [PubMed] [Google Scholar]