ABSTRACT
The management of Chiari I malformation (CMI) is controversial because treatment methods vary and treatment decisions rest on incomplete understanding of its complex symptom patterns, etiologies, and natural history. Validity of studies that attempt to compare treatment of CMI has been limited because of variable terminology and methods used to describe study subjects. The goal of this project was to standardize terminology and methods by developing a comprehensive set of Common Data Elements (CDEs), data definitions, case report forms (CRFs), and outcome measure recommendations for use in CMI clinical research, as part of the CDE project at the National Institute of Neurological Disorders and Stroke (NINDS) of the US National Institutes of Health. A working group, comprising over 30 experts, developed and identified CDEs, template CRFs, data dictionaries, and guidelines to aid investigators starting and conducting CMI clinical research studies. The recommendations were compiled, internally reviewed, and posted online for external public comment. In October 2016, version 1.0 of the CMI CDE recommendations became available on the NINDS CDE website. The recommendations span these domains: Core Demographics/Epidemiology; Presentation/Symptoms; Co-Morbidities/Genetics; Imaging; Treatment; and Outcome. Widespread use of CDEs could facilitate CMI clinical research trial design, data sharing, retrospective analyses, and consistent data sharing between CMI investigators around the world. Updating of CDEs will be necessary to keep them relevant and applicable to evolving research goals for understanding CMI and its treatment.
Keywords: Chiari Malformation, Common Data Elements
ABBREVIATIONS
- CDE
Common Data Elements
- CMI
Chiari malformation
- CRF
case report form
- CSF
Chiari & Syringomyelia Foundation
- MRI
magnetic resonance imaging
- NIH
National Institutes of Health
- NINDS
National Institute of Neurological Disorders and Stroke
- WG
working group
Chiari Malformation (CMI) Type I has been generally recognized as a disorder of the cervical-medullary junction that includes crowding and compression of neural structures.1-3,4 Because of the complexity of the region, Chiari presentation may involve headache or other pain, cranial nerve dysfunction or extremity deficits due to cerebellar, brainstem or spinal cord involvement,5,6 leading to variable differential diagnosis with great potential for misdiagnosis. While the consequences of failure to recognize and surgically treat this disorder can be severe, the morbidity and cost of overtreatment can be as consequential.
To facilitate research required to answer critical questions about patient selection and Chiari management, the Chiari & Syringomyelia Foundation (CSF) proposed an international, multicenter clinical research project using a common database. The group recognized that this effort would require a dataset that could be utilized by many researchers around the world with data that are consistently recorded, shared, and interpreted. A first step in this process would require the development of a common language for data collection.
In 2005, the National Institute of Neurological Disorders and Stroke (NINDS) initiated the Common Data Elements (CDEs) project to assist NINDS-funded investigators in collecting clinical trial research data in a standard and consistent fashion. The NINDS CDE project is not itself a database—rather, a collection of metadata and data standards. The CDE recommendations identify common definitions, validated outcome measures, and standardized case report forms (CRFs). The goals of the NINDS CDE Project are to disseminate standards, create easily accessible study tools, encourage focused and simplified data collection, and improve data quality.7 To date, the NINDS CDE project has collected metadata with data standards for 23 neurological diseases. Since no CDEs had previously existed for CMI, the CMI CDE Working Group (WG) was assembled and followed National Institutes of Health (NIH) guidelines to establish a common language for Chiari research.
The expected outcome of the project was the creation of CDEs, CRFs, and recommended standardized outcomes measures and guidelines that would be publicly posted on the NINDS CDE website to facilitate integration across the global Chiari research community. The process was designed to involve a broad group of researchers and stakeholders. It was unique in its openness to comments from both the patient populations and general public. This article describes the process by which the CMI CDEs were developed.
METHODS
The CMI CDE WG was formed by inviting United States and international representatives with expertise and experience in CMI. When selecting members of the WG, an effort was made to achieve diversity of subspecialty, location, practice type, and focus. Though this effort originates primarily from 1 CMI advocacy group, the CSF, whose mission is most strongly aligned with this project, an effort was made to reach out to other groups. This was facilitated by significant overlap in CMI-interested physicians on the medical boards of these groups. In fact, 6 authors listed in this paper are on boards for multiple advocacy groups.
To ensure a diverse knowledge base, the committee was expanded to include members of industry, various clinical specialists, researchers, and patient advocates. The participants attended the 2014 CSF Meeting in San Francisco, California. International participation was available through in-person or remote attendance. The invited group consisted of 73 persons, including 45 practicing physicians, 35 of whom were neurosurgeons with expertise in CMI. Fifty-nine of the group were from the United States and 14 were from Europe, Asia, and Australia (Table 1). At the initial meeting, there were 38 in-person participants, and 9 via web conferencing or phone.
Table 1.
Specialty/Geography of Chiari Malformation Type 1 Common Data Element Working Group Members
| Profession | n |
| Neurosurgeon | 35 |
| Neuroradiologist | 4 |
| Geneticist | 2 |
| Radiologist | 5 |
| Industry Representative | 5 |
| Patient Advocate | 10 |
| Othera | 9 |
| Location | n |
| United States | 59 |
| Europe | |
| Croatia | 1 |
| France | 2 |
| Germany | 1 |
| Italy | 1 |
| Spain | 2 |
| UK | 4 |
| Asia | |
| China | 1 |
| Japan | 1 |
| Australia | 1 |
aOther: neurology, ophthalmology, internal medicine, pain management, bioengineering, nursing, NIH representation, veterinary
General Process
The process of the CMI CDE WG was facilitated through twice-annual face-to-face meetings over 2.5 yr. In addition to these face-to-face meetings, the steering committee and subgroups organized weekly teleconferences. The steering committee met weekly over the project period to monitor progress.
WG Subgroup Formation
The CMI CDE Project WG was initially divided into 6 subgroups (Core Demographics/Epidemiology; Presentation/Symptoms; Co-morbidities/Genetics; Imaging; Treatment; and Outcomes). Each subgroup reviewed the existing literature, came to consensus agreement, and proposed CDE recommendations that were then shared with the complete WG for discussion. Subgroup composition was curated to assure that all viewpoints were heard, including both sides of controversial topics.
Common Data Element Production Process
Individual CDE assignment and subgroup creation: Subgroup chairs solicited individually written CDEs for subgroup discussion by teleconference and online cloud-based file sharing.
- Intergroup editing:
- Subgroup chair presentation: Each subgroup chair presented their work at a weekly steering committee teleconference.
- The biannual face-to face CDE Project Meeting: During face-to-face general WG meetings, the work of the 6 subgroups was presented in greater detail. At the end of the process, the subgroup chairs presented the commented documents for WG revision and review.
- Presentation to the general CSF membership:
- The WG presented a progress report to the general CSF meeting, biannually.
- A draft of the CDE documents was distributed to all CSF members.
Final editing and formatting by NINDS: Completed CDEs were sent to the NINDS CDE Team for formatting and then forwarded to NIH/NINDS for preliminary review. Once all documents were officially completed, they were posted for final public review on the NINDS CDE website for 1 mo. of public critique. Public review comments were used to make final edits, and the CMI CDEs were made available on the NINDS CDE website in October 2016.
RESULTS
Over a period of approximately 2 yr, a CDE set for CMI was developed and made public via the NINDS CDE website. The total work included 794 distinct CDEs and 62 CRFs. An overall summary of the CMI CDEs and an example CRF pertaining to the Presentation subgroup are available as appendices to this publication (Appendices A and B, Supplemental Digital Content 1 and 2).
Following NINDS CDE guidelines, potential CDEs were classified as (1) Core, n = 54; (2—Highly Rec.) Supplemental–Highly Recommended, n = 75; (2) Supplemental, n = 554; or (3) Exploratory, n = 111. Core CDEs may be considered essential information for any clinical research on CMI. Supplemental–Highly Recommended CDEs are considered important for most CMI research but may not be included in all CMI research if the focus of the research makes the CDE in question less relevant. Supplemental CDEs may or may not be relevant for a given research question. Exploratory CDEs are those that are emerging or that warrant further study in CMI.
As an example, the CDE written for cerebellar tonsil position was heavily scrutinized. It was discovered in developing this CDE that there was considerable variability in methodology for measuring this value. After deliberation, the WG drew up a consensus CDE for cerebellar tonsil position that defined the foramen magnum using a midline, or near-midline image, illustrated in Figure. Additional morphometric indicators and magnetic resonance imaging (MRI) measurements determined to have utility were debated and parsed into more and less important categories, based on feedback from the WG.
Figure.
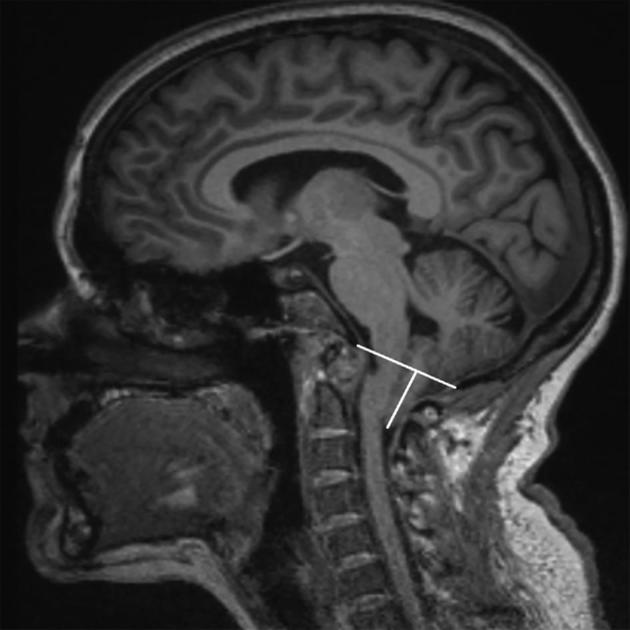
Common Data Element: measurement of cerebellar tonsil position. CDE instructions are as follows: Define foramen magnum using midline image. Draw line from basion to opisthion. Define basion as the confluence of cortical bone (most inferior point) making up the anterior foramen magnum. Define opisthion as the confluence of cortical bone (most inferior) of the posterior aspect of the foramen magnum (ventral most point). Measure tonsillar position perpendicular to that line. Tonsillar measurement may be taken parasagittally to assure the largest possible number is recorded. Record negative number for tonsil position above line, based onT1 sagittal or coronal MRI. Recognizing the tonsils is not normally midline structure; we define tonsillar position by a midline or near-midline image because they are displaced.
Outcomes measures to determine quality of life were also a high priority for the CMI population. Table 2 summarizes the important major outcome measures included in this iteration of the CMI CDEs and their classifications. Summaries of all subgroup work and research recommendations are available on the NINDS CDE website, as well.
Table 2.
Common Data Elements—Outcomes Measures with Classifications
| Domain | Outcomes Measure | CDE Classificationa |
|---|---|---|
| Functional Outcomes | Nurick (Ambulatory Function) Grade8 | 2—Highly Rec. |
| ASIA Motor Scale9 | 2—Highly Rec. | |
| Functional Status Karnofsky Score10 | 2 | |
| Functional Status Lansky Score11 | 2 | |
| ASIA Sensory Scale9 | 2 | |
| Functional Independence Measure12 | 2 | |
| Functional Independence Measure for Children (WeeFIM)12 | 2 | |
| Modified Japanese Orthopaedic Association Score13 | 2 | |
| McCormick Scale8,14 | 3 | |
| Chicago Chiari Outcome Scale15,16 | 3 | |
| Emotional and Cognitive Status | Zung Depression Score13 | 3 |
| Other Non-Motor | Assessment of Shunt Functionb | 2, 3 |
| Pain | McGill Pain Questionnaire17 | 2 |
| Brief Pain Inventory (Short Form) | 2 | |
| Numeric Rating Scale—Neck Pain13 | 2 | |
| Quality of Life | Headache Disability Index13 | 2—Highly Rec. |
| Meaningful Improvement18 | 2—Highly Rec. | |
| Chiari Symptom Profile5 | 2 | |
| EuroQoL-5 Dimension Questionnaire—Youth (EQ-5DY)13 | 2 | |
| Health Transition Index13 | 2 | |
| Neck Disability Index13 | 2 | |
| PROMIS | 2 | |
| Neuro-QOL | 2 | |
| Short Form 36-Item Health Survey (SF-36) | 2 | |
| Short Form Health Survey-12 | 2 | |
| The North American Spine Society Satisfaction Questionnaire13 | 2 | |
| PROMIS—Pediatric | 3 | |
| Neuro-QOL—Pediatric Functional Health | 3 |
aCDEs classified as 2—Highly Rec. are not required, but highly recommended in CMI research; CDEs classified as 2 are not required, but relatively important to CMI research, depending on the framework of the study; and CDEs classified as 3 are novel concepts in CMI research that can be considered experimental or exploratory based on the current state of the literature.
bIndividual data points included in the Assessment of Shunt Function tool may be classified as either 2 or 3, depending on the data point.
DISCUSSION
The WG, consisting of a broad group of clinicians and scientists with expertise and experience in CMI, has developed and published an initial version of CMI CDEs, with the goal of facilitating more standard methods in CMI clinical research. This version is understood as being subject to change in subsequent revisions, based on advancements in research. While CDEs from other neurological disease domains were assessed, variables and considerations unique to CMI necessitated new development.
Presentation and Comorbidities
The presentation of CMI is complex, due to various factors that influence the anatomy of the cervical-medullary junction. One of the goals for this project is to provide tools necessary to more effectively describe the presentation and natural history of individuals with cerebellar tonsils positioned below the foramen magnum from any cause.
The phenotype of low cerebellar tonsil position may arise from a variety of primary and comorbid conditions of the brain, skull, spine, and connective tissue, through a variety of pathophysiological mechanisms.5,6 In response to this, the CDEs include a focus on defining other primary and comorbid conditions. Recognized potential comorbidities, such as hereditary connective tissue disorders, tethered cord, and pseudotumor cerebri, occur in a relatively small percentage of the overall CMI population.6 The symptoms and findings associated with the co-morbidities were broken down and detailed separately.
The CDEs covering clinical presentation were based on the classical symptoms described in patients with the established diagnosis of CMI as documented in the medical literature. These include headache, symptoms of imbalance, and visual symptoms. When syringomyelia is associated with CMI, symptoms of altered sensation in limbs or trunk, as well as weakness of one or more limbs, may be among the presenting complaints.5,6 The CDEs aim to define these symptoms more specifically. For example, quantification of symptoms such as headache has thus far been generally suboptimal in Chiari research. Thus, the CDEs for headache specify onset, frequency, duration, location and other factors.
CDEs detailing the physical examination cover the standard neurological examination of cranial nerves, motor and sensory systems, with an emphasis on those modalities likely to show abnormalities in CMI patients, with or without syringomyelia. Absolute quantification of findings such as limb strength and limb tone is not possible, but reasonable quantification has been previously established for limb strength19 and limb tone.20 These measures are widely accepted and endorsed for CMI studies.
Imaging
Because the diagnosis of CMI relies heavily on findings from neuroimaging, it was crucially important to define these parameters in standard fashion. A review of existing CDEs outside of CMI revealed no previously defined neuroimaging elements that could be used for CMI purposes. The literature did provide measures and angles derived primarily from images of cervical-medullary bone structures. However, in many cases, there was no well-established definition of a given imaging parameter. In these cases, the WG arrived at a definition by group consensus. For example, much consideration was given to the exact anatomical evaluation of the position of the cerebellar tonsils—historically, a key parameter. Due to a surprising variety of the landmarks and image types utilized, and known variability in measurement amongst readers, a single method was reached by consensus and described. Further, the mandatory, ongoing review process by the international CDE Oversight Committee seeks to correct bias going forward.
Surgical Treatment
Surgical care for CMI centers on the decompression of the posterior fossa. The common factors in CMI decompression have been suboccipital craniectomy and cervical laminectomy.21 One of the important controversies in CMI is whether expansion of the underlying dura (duraplasty) should also routinely be performed.22 Therefore, the Treatment subgroup defined CDEs to allow description of the various suboccipital decompression techniques in sufficient detail that studies to explore differences in surgical treatment could be performed. Surgical treatment of commonly associated disorders, such as ventriculoperitoneal shunt placement, release of tethered spinal cord, and spinal fusion, were included.
Outcome
The determination of outcome in CMI treatment should account for the high variability in presenting symptoms and severity. Importantly, CMI is a condition currently defined by MRI measures, including tonsillar position, obliteration of the retrocerebellar CSF space,23-25 and the presence or absence of hydrocephalus and/or syringomyelia. Therefore, outcome studies for CMI should include MRI measures as well as the previously described clinical features. To improve comparison of outcome between research studies, the Outcomes subgroup supported the use of standard tools for measuring pain, headache, quality of life, and neurological function. Complications of surgical therapy are also included. A single Chiari-specific surgical outcome scale was included as exploratory.15,16
The relationship between the historically accepted anatomical definition of CMI, often cited as tonsillar position 5 mm below the foramen magnum,26 Chiari symptoms, and surgical response is uncertain. The purpose of creating CMI CDEs was to improve research methods and not to clinically define CMI or influence surgical treatment selection. This is an important distinction, since the number of anatomically defined patients identified radiologically is growing and a label of CMI is passed on directly to the general physician and patient, regardless of symptoms. In the past decade, a significant increase in MRI27-29 has resulted in an increase in the radiological diagnosis of symptomatic and asymptomatic30 Chiari with a 1%, 2%, and 3% incidence in males, females, and children, respectively.31,32 Many clinicians suspect the number of symptomatic surgical candidates to be significantly smaller, with annual incidence estimates as low as 0.06%.33 Studying these discrepancies will require the standardized methods recommended.
Future Directions
CDEs were originally developed as a means of standardizing terminology and reporting. Therefore, one obvious next step is to test inter-rater reliability of the imaging measurements included on the imaging CRF. Such a study is underway. In addition, in 2017, a new CMI CDE steering committee was formed from CSF and other CMI advocacy group membership, external experts and 2–3 original WG members. The next steps in this process involve an annual review by this oversight committee. This committee will monitor comments and proposed changes to develop a second iteration of CDEs.
Expected areas of expansion include presentation, comorbidities, more specific or improved outcome tools and clear radiological elements. In this first edition, imaging elements were largely constrained to long-utilized tonsillar position and the relationship of bone structures. The imaging CDEs are being studied for reproducibility in a blind-reader validation study. In addition, the potential importance of fluid movement in CSF spaces and of CNS dynamic deformation over the cardiac cycle make the development of soft tissue and CSF-space quantification an immediate priority for addition to the CMI CDEs, classified as Exploratory. Dynamic and physiological measures involving pressure, flow and resistance will also be considered.34-36
Finally, a clearly defined separation between the anatomical demonstration of CMI on the one hand, and the possible association with other anatomic findings, comorbidities, attributable symptoms, and appropriate consideration of a surgical decompression on the other hand is of great importance. The goal is to provide a more consistent grouping and study of CMI patients by anatomy, etiology, symptoms or co-morbidities and to standardized CDE definitions for them.
CONCLUSION
The care of CMI patients is presently impeded by the complexity of symptom pattern, historic reliance on anatomical radiological findings regardless of symptoms, uncertain etiology, symptom pathophysiology and natural history, association of other congenital and acquired conditions, and controversy over the best surgical treatment. The growing number of MRI-identified CMI patients provides an opportunity to help more patients, but also holds great potential for overtreatment. The common language provided by the first version of CMI CDEs is a prerequisite for large-scale research coordination that seeks to appropriately address key questions in CMI. The CMI CDEs can facilitate trial design, data sharing and retrospective analyses as well as foster cooperation between researchers and institutions which will have a significant impact on the diagnosis and treatment of patients.
Disclosures
Logistics support for this project was provided in part through NIH Contract HHSN271201200034C. The CMI CDE project was made possible from financial contributions of the Chiari & Syringomyelia Foundation. The views expressed here are those of the authors and do not represent those of the National Institutes of Health (NIH), the National Institute of Neurological Disorders and Stroke (NINDS) or the US Government. Dr Rocque receives other support from NIH Grant 1KL2TR001419. Dr Martin receives other support from NIH NIGMS Grants P20 GM103408, 4U54GM104944-04, NIH NIMH Grant 1R44MH112210-01A1 and NASA Grant NNX16AT06G. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Acknowledgments
This work was made possible thanks to the great investment of time and effort of WG members and members of the NINDS CDE team, participating from 2014 to 2017.
Notes
Presented at the 2017 American Academy of Neurology Annual Meeting as poster, April 22-28, 2017, Boston, Massachusetts, and at the 2017 Congress of Neurological Surgeons Annual Meeting as e-poster, October 7-11, 2017, Boston, Massachusetts.
Supplemental Digital Content 1. Appendix A. Overview of Chiari malformation common data elements and recommendations.
Supplemental Digital Content 2. Appendix B. Example case report formstrain-related headache.
COMMENTS
This manuscript represents a significant investment of time and effort by the authors to establish a framework for future Chiari I Malformation research. Widespread use of the Common Data Elements in future Chiari Malformation Type I research projects will allow better cross study data analysis.
David Wrubel
Atlanta, Georgia
The authors of this manuscript should be congratulated on their diligence in completing a first attempt to define and collect CDEs for the study of Chiari malformation type 1. The process described seems to be one of high integrity and demonstrates a great deal of hard work. Individuals who work in this field will surely benefit from this effort for its use as a framework for discovery. As to whether this particular group of Chiari CDEs will be fruitfully used and will substantially help move the field forward, only time will tell. However, each step we take towards clarifying commonalities in order to reduce ambiguity can only sharpen our abilities to conduct a conversation that grows ever more precise. I, for one, am looking forward to that enhanced precision and to the discovery that it will enable.
David M. Frim
Chicago, Illinois
REFERENCES
- 1. Chiari H. Über Veränderungen des Kleinhirns infolge von Hydrocephalie des Grosshirns. Dtsch med Wochenschr. 1891;17(42):1172–1175. [Google Scholar]
- 2. Chiari H. Über Veränderungen des Kleinhirns des Pons und der Medulla Oblongata infolge von kongentialer Hydrocephalie des Grosshirns. Denkschriften der Kais Akad Wiss math-naturw. 1896;63:71–116. [Google Scholar]
- 3. Chiari H. Concerning alterations in the cerebellum resulting from cerebral hydrocephalus. 1987. Pediatr Neurosurg. 1987;13(1):3–8. [DOI] [PubMed] [Google Scholar]
- 4. National Institute of Neurological Disorders and Stroke: Chiari Malformation Fact Sheet. Available at: http://www.ninds.nih.gov/disorders/chiari/detail_chiari.htm#282873087. Updated July 27, 2017. Accessed February 8, 2018. [Google Scholar]
- 5. Milhorat TH, Chou MW, Trinidad EM et al.. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 1999;44(5):1005–1017. [DOI] [PubMed] [Google Scholar]
- 6. Milhorat TH, Nishikawa M, Kula RW, Dlugacz YD. Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir (Wien). 2010;152(7):1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grinnon ST, Miller K, Marler JR et al.. National Institute of Neurological Disorders and Stroke Common Data Element Project—approach and methods. Clin Trials. 2012;9(3):322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giurado VM, Taricco MA, Nobre MR et al.. Quality of life in adult intradural primary spinal tumors: 36-Item Short Form Health Survey correlation with McCormick and Aminoff-Logue scales. J Neurosurg Spine. 2013;19(6):721–735. [DOI] [PubMed] [Google Scholar]
- 9. Ditunno JF Jr, Young W, Donovan WH, Creasey G. The international standards booklet for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Paraplegia. 1994;32(2):70–80. [DOI] [PubMed] [Google Scholar]
- 10. Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1(4):634–656. [Google Scholar]
- 11. Lansky SB, List MA, Lansky LL, Ritter-Sterr C, Miller DR. The measurement of performance in childhood cancer patients. Cancer. 1987;60(7):1651–1656. [DOI] [PubMed] [Google Scholar]
- 12. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6–18. [PubMed] [Google Scholar]
- 13. Parker SL, Godil SS, Zuckerman SL, Mendenhall SK, Tulipan NB, McGirt MJ. Effect of symptomatic pseudomeningocele on improvement in pain, disability, and quality of life following suboccipital decompression for adult Chiari malformation type I. J Neurosurg. 2013;119(5):1159–1165. [DOI] [PubMed] [Google Scholar]
- 14. Samandouras G. The Neurosurgeon's Handbook. Oxford: Oxford University Press; 2010. [Google Scholar]
- 15. Aliaga L, Hekman KE, Yassari R et al.. A novel scoring system for assessing Chiari malformation type I treatment outcomes. Neurosurgery. 2012;70(3):656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yarbrough CK, Greenberg JK, Smyth MD, Leonard JR, Park TS, Limbrick DD Jr. External validation of the Chicago Chiari Outcome Scale. J Neurosurg Pediatr. 2014;13(6):679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Turk DC, Dworkin RH, Allen RR et al.. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337–345. [DOI] [PubMed] [Google Scholar]
- 18. Godil SS, Parker SL, Zuckerman SL, Mendenhall SK, McGirt MJ. Accurately measuring outcomes after surgery for Adult Chiari I malformation. Neurosurgery. 2013;72(5):820–827. [DOI] [PubMed] [Google Scholar]
- 19. Compston A. Aids to the investigation of peripheral nerve injuries. Medical Research Council: Nerve Injuries Research Committee. His Majesty's Stationery Office: 1942; pp. 48 (iii) and 74 figures and 7 diagrams; with aids to the examination of the peripheral nervous system. By Michael O’Brien for the Guarantors of Brain. Saunders Elsevier: 2010; pp. [8]64 and 94 Figures. Brain. 2010;133:2838–2844. [DOI] [PubMed] [Google Scholar]
- 20. Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–207. [DOI] [PubMed] [Google Scholar]
- 21. Rocque BG, Oakes WJ. Surgical treatment of Chiari I malformation. Neurosurg Clin N Am. 2015;26(4):527–531. [DOI] [PubMed] [Google Scholar]
- 22. Lee A, Yarbrough CK, Greenberg JK, Barber J. Comparison of posterior fossa decompression with or without duraplasty in children with Type I Chiari malformation. Childs Nerv Syst. 2014;30(8):1419–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Batzdorf U, McArthur DL, Bentson JR. Surgical treatment of Chiari malformation with and without syringomyelia: experience with 177 adult patients. J Neurosurg. 2013;118(2):232–242. [DOI] [PubMed] [Google Scholar]
- 24. Oldfield EH, Muraszko K, Shawker TH, Patronas NJ. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. J Neurosurg. 1994;80(1):3–15. [DOI] [PubMed] [Google Scholar]
- 25. Sahuquillo J, Rubio E, Poca MA, Rovira A, Rodriguez-Baeza A, Cervera C. Posterior fossa reconstruction. Neurosurgery. 1994;35(5):874–885. [DOI] [PubMed] [Google Scholar]
- 26. Barkovich AJ, Wippold FJ, Sherman JL, Citrin CM. Significance of cerebellar tonsillar position on MR. AJNR Am J Neuroradiol. 1986;7(5):795–799. [PMC free article] [PubMed] [Google Scholar]
- 27. Lang K, Huang H, Lee DW, Federico V, Menzin J. National trends in advanced outpatient diagnostic imaging utilization: an analysis of the medical expenditure panel survey, 2000-2009. BMC Med Imaging. 2013;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith-Bindman R, Miglioretti DL, Larson EB. Rising use of diagnostic medical imaging in a large integrated health system. Health Aff. 2008;27(6):1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith-Bindman R, Miglioretti DL, Johnson E et al.. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large Integrated Health Care Systems, 1996-2010. JAMA. 2012;307(22):2400–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schijman E, Steinbok P. International survey on the management of Chiari I malformation and syringomyelia. Child's Nervous Syst. 2004;20(5):341–348. [DOI] [PubMed] [Google Scholar]
- 31. Smith BW, Strahle J, Bapuraj JR, Muraszko KM, Garton HJ, Maher CO. Distribution of cerebellar tonsil position: implications for understanding Chiari malformation. J Neurosurg. 2013;119(3):812–819. [DOI] [PubMed] [Google Scholar]
- 32. Strahle J, Muraszko KM, Kapurch J, Bapuraj JR, Garton HJ, Maher CO. Chiari malformation Type I and syrinx in children undergoing magnetic resonance imaging. J Neurosurg Pediatr. 2011;8(2):205–213. [DOI] [PubMed] [Google Scholar]
- 33. Speer MC, Enterline DS, Mehltretter L et al.. Review article: Chiari Type I malformation with or without syringomyelia: prevalence and genetics. J Genet Couns. 2003;12(4):297–311. [DOI] [PubMed] [Google Scholar]
- 34. Martin BA, Kalata W, Shaffer N, Fischer P, Luciano M, Loth F. Hydrodynamic and longitudinal impedance analysis of cerebrospinal fluid dynamics at the craniovertebral junction in type I Chiari malformation. PLoS ONE. 2013;8(10):e75335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martin BA, Yiallourou TI, Pahlavian SH, Thyagaraj S. Inter-operator reliability of magnetic resonance image-based computational fluid dynamics prediction of cerebrospinal fluid motion in the cervical spine. Ann Biomed Eng. 2016;44(5):1524–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yiallourou TI, Asboth L, Kroeger J et al.. Comparison of 4D Phase-Contrast MRI Flow Measurements to Computational Fluid Dynamics Simulations of Cerebrospinal Fluid Motion in the Cervical Spine. PLoS ONE. 2012;7(12):e52284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


