Abstract
Several viral vector-based gene therapy drugs have now received marketing approval. A much larger number of additional viral vectors are in various stages of clinical trials for the treatment of genetic and acquired diseases, with many more in pre-clinical testing. Efficiency of gene transfer and ability to provide long-term therapy make these vector systems very attractive. In fact, viral vector gene therapy has been able to treat or even cure diseases for which there had been no or only suboptimal treatments. However, innate and adaptive immune responses to these vectors and their transgene products constitute substantial hurdles to clinical development and wider use in patients. This review provides an overview of the type of immune responses that have been documented in animal models and in humans who received gene transfer with one of three widely tested vector systems, namely adenoviral, lentiviral, or adeno-associated viral vectors. Particular emphasis is given to mechanisms leading to immune responses, efforts to reduce vector immunogenicity, and potential solutions to the problems. At the same time, we point out gaps in our knowledge that should to be filled and problems that need to be addressed going forward.
Keywords: adenovirus, adeno-associated virus, lentivirus, innate immunity, adaptive immunity
Graphical Abstract
Viral vectors are successfully used in human gene therapy. However, immune responses complicate their use, ranging from early innate responses and immunotoxicity to subsequent adaptive immune responses to the vector or transgene product. This article reviews immune response mechanisms against adenoviral, adeno-associated viral, and lentiviral vectors.
Main Text
Gene therapy can treat a variety of both inherited and acquired diseases, and viral vectors have emerged as a preferred platform for gene delivery. Once the viral genome is replaced with a therapeutic gene cassette, stripping the virus of the replicative and pathogenic traits, such vectors are well suited as gene transfer vehicles. An ideal gene therapy vector should reliably and efficiently carry and deliver a therapeutic gene to target cells and direct long-term therapeutic expression. Viruses naturally satisfy these criteria, except that they are prone to host immune responses, as the mammalian immune system has evolved to recognize infectious agents. Past and ongoing clinical trials have utilized several different viral vectors, including adenovirus (Ad), adeno-associated virus (AAV), lentivirus (LV), murine γ-retrovirus, and herpes simplex virus (HSV). Marketing approval has been granted to two AAV-based therapies to treat a form of congenital blindness (Luxturna) and spinal muscular atrophy (Zolgensma), a γ-retrovirus-based therapy for the primary immune deficiency adenosine deaminase severe combined immunodeficiency (ADA-SCID) (Strimvelis), an LV-based therapy for CD19-directed genetically modified chimeric antigen receptor (CAR)-T cell immunotherapies for acute lymphoblastic leukemia and non-Hodgkin lymphoma (Kymriah and Yescarta), and HSV-based oncolytic virotherapy for melanoma (Imlygic).1
The suitability of a viral vector for a given application depends on multiple factors, including target cells or tissues, tropism, use for ex vivo versus in vivo gene transfer, packaging capacity, potential for genome integration (and insertional mutagenesis), and also the propensity for immunotoxicities. While LV vectors are now preferred for ex vivo gene correction (in particular for gene transfer to hematopoietic stem cells [HSCs]), AAV has emerged as the preferred vector for in vivo gene transfer due to its favorable safety profile compared to other vectors, ability to transduce a variety of tissues, and availability of a large number of viral capsids with different tropism.
Although the use of vectors derived from viruses takes advantage of their refined evolutionary fitness to transduce human cells, these advantages have co-evolved with an equally sophisticated human immune system aimed at protecting host tissues by eliminating foreign invaders perceived as dangerous. To the immune system, certain components of viral vectors are indistinguishable from their parent viruses (such as nucleic acids carried in a protein coat). Such vectors are therefore subject to similar innate and adaptive immune responses as wild-type viruses. Innate immune receptors, or pattern recognition receptors (PRRs), detect viruses by recognizing conserved molecular motifs such as unique nucleic acid conformations that trigger antiviral immunity (Figure 1). The virally derived capsid or envelope proteins constitute foreign proteins that can become the target of adaptive immune responses (Figure 2). Furthermore, a therapeutic trasn transgene product that constitutes a neo-antigen may be similarly targeted by both humoral and cellular immune responses (Figure 2). Immune-mediated rejection in viral gene therapy represents one of the most significant hurdles to human gene therapy. A comprehensive understanding of the processes underlying these deleterious responses directed against both the viral vector and transgene product is critical for developing treatment modalities that mitigate immune-mediated rejection. A body of research has interrogated these mechanisms, which are reviewed herein. We will focus on three widely utilized and studied vector systems: Ad, AAV, and LV vectors (Figure 2; Table 1).
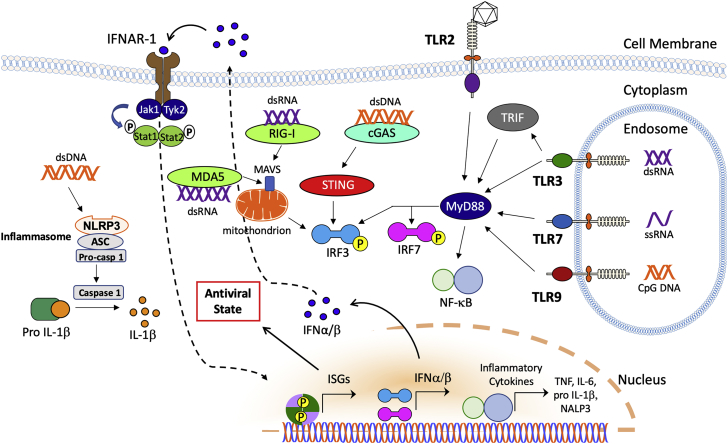
Figure 1.
Innate Immune Sensing and Signaling Pathways that Contribute to Immune Responses to Different Viral Vectors
Note that the figure illustrates some of the most common pathways but is not meant to be exhaustive. Abbreviations are as follows: dsDNA, double-stranded DNA; NLPR3, NACHT, LRR, and PYD domains-containing protein 3; ASC, adaptor protein apoptosis-associated speck-like protein containing CARD; Pro-casp 1, pro-caspase 1; IFNAR-1, interferon α/β receptor 1; Jak1, Janus kinase 1; Tyk2, tyrosine kinase 2; Stat, signal transducer and activator of transcription; P, phosphoryl group; MDA5, melanoma differentiation-associated protein 5; dsRNA, double-stranded RNA; RIG-I, retinoic acid-inducible gene-I; MAVS, mitochondrial antiviral signaling protein; STING, stimulator of interferon genes; IRF, interferon response factor; cGAS, cyclic guanosine monophosphate-AMP synthase; TLR, Toll-like receptor; ISG, interferon-stimulated genes; IFN, interferon; MyD88, myeloid differentiation primary response protein 88; NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; and ssRNA, single-stranded RNA.
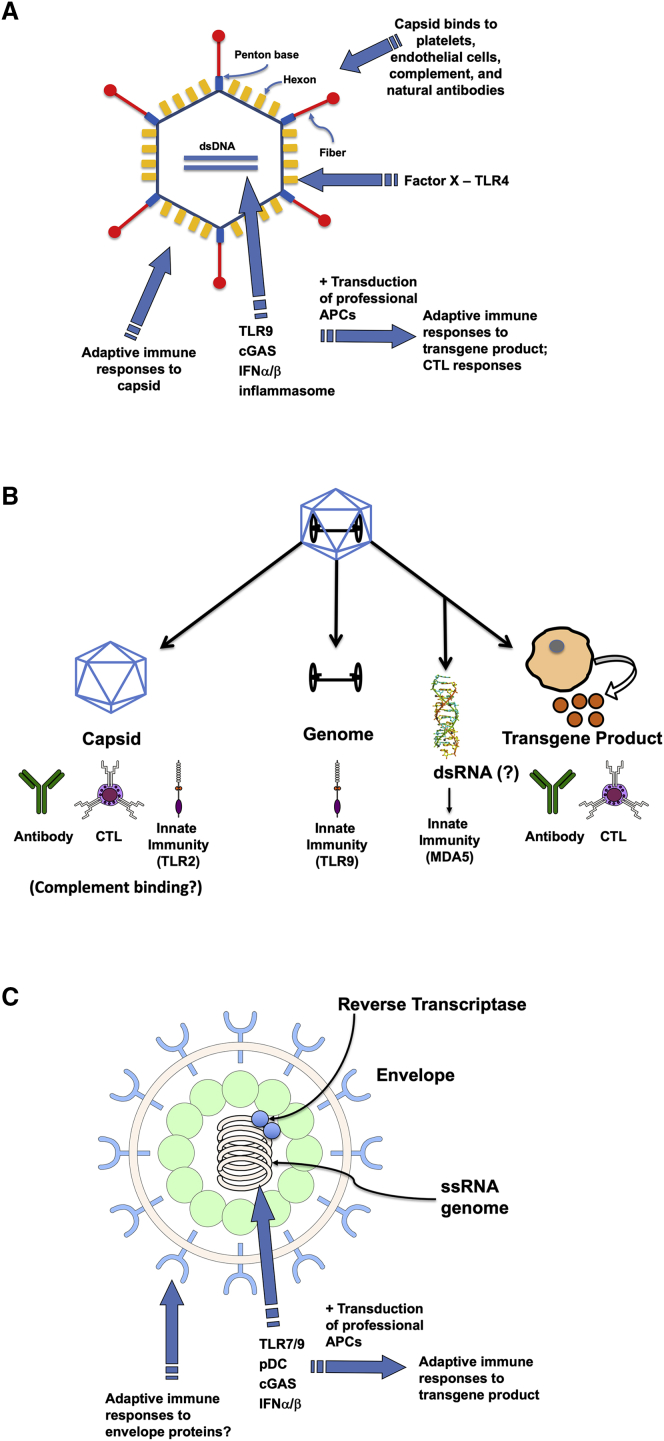
Figure 2.
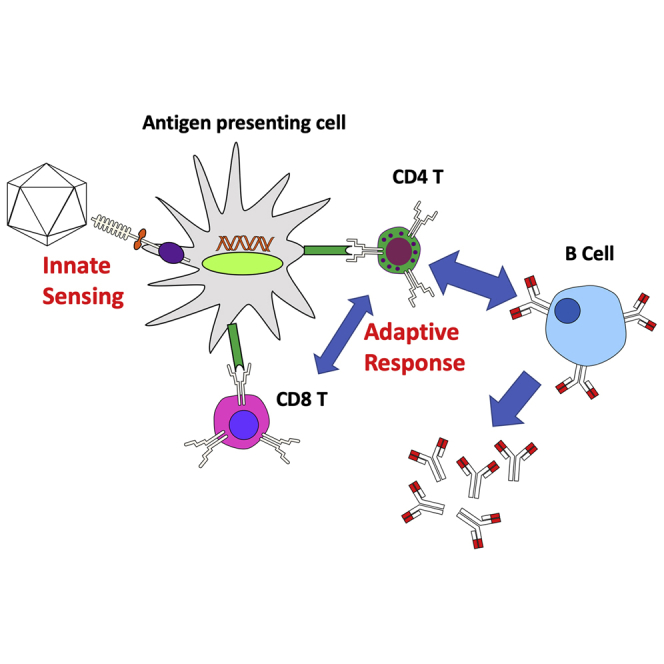
Examples of Molecular Structures and Antigens in Viral Vectors that Are Recognized by Innate Immune Sensors or Targeted by Antigen-Specific Adaptive (B and T Cell) Immune Responses
(A) Adenoviral vectors. (B) AAV vectors. (C) Lentiviral vectors. Abbreviations are as follows: dsDNA, double-stranded DNA; TLR, Toll-like receptor; cGAS, cyclic guanosine monophosphate-AMP synthase; IFN, interferon; APC, antigen-presenting cell; CTL, cytotoxic T lymphocyte; dsRNA, double-stranded RNA; MDA5, melanoma differentiation-associated protein 5; pDC, plasmacytoid dendritic cell; and ssRNA, single-stranded RNA.
Table 1.
Overview of Viral Vectors and Their Immune Responses
| Viral Vector | Ad | AAV | LV |
|---|---|---|---|
| Virion and genome | capsid; 36-kb dsDNA genome | capsid, ≤5-kb ssDNA genome (or ∼2.5-kb scDNA genome) | enveloped virus containing capsid and ∼10-kb ssRNA genome |
| Innate immunity | potent innate response, including activation of vascular endothelial cells and platelets, inflammatory cytokine production, and macrophage cell death | comparatively weak and transient innate response; TLR9 signaling promotes CD8+ T cell responses; complement activation and other immunotoxicities seen in some patients receiving high-dose systemic gene transfer | strong IFN-α/β response limits transduction and drives adaptive responses |
| Immunity in human population | pre-existing immunity to human serotypes | pre-existing immunity varies between serotypes and geographic location | low pre-existing immunity |
| In vivo transduction of antigen-presenting cells | efficient | inefficient | efficient |
| Use as a vaccine carrier | vaccine and cancer gene therapy applications | limited | targeting of dendritic cells for vaccine development |
| Adaptive immune responses to vector | NAB formation; CD8+ T cell responses to viral gene products (except for high-capacity vectors) | NAB formation; CD8+ T cell responses to capsid | NAB formation; possibly T cell responses to envelope protein |
| Adaptive immune responses to transgene product | efficient inducer of CD8+ T cell responses; antibody formation possible | least efficient inducer of CD8+ T cells compared to Ad and LV; risk of CD8+ T cell and antibody responses highly variable depending on vector design and dose, route of administration, and host factors | efficient inducer of B and T cell responses unless transgene expression is tightly controlled by miRNA and promoter and expression is professional APCs is eliminated |
dsDNA, double-stranded DNA; ssDNA, single-stranded DNA; scDNA, self-complementary DNA; TLR9, Toll-like receptor 9; IFN, interferon; NAB, neutralizing antibody; miRNA, microRNA; APC, antigen-presenting cell.
Overview of Immune Responses to Viral Vectors
The immune system is comprised of a complex interwoven network of multiple different cell types that collaborate to protect host tissues from further infection and mobilize a cadre of effectors tailored to specifically eliminate the invading pathogen. The cellular network can be generally broken up into innate and adaptive arms. Innate immune responses occur early, are not antigen specific, and do not result in immunological memory. Adaptive immune responses are conditioned by the inflammatory environment created by innate immune sensing, rely on activation and clonal expansion of antigen-specific (effector) B and T cell differentiation, and generate immunological memory. Viral vectors share many commonalities with natural viruses but are distinctly different in that they are non-replicative, delivered in a single high-titer bolus, and introduced at an unnatural site. So, although canonical immunological concepts can be applied, the unwanted immune response to viral vectors also has inherently unique aspects. Vector particles containing viral proteins that are identical or similar to antigens that humans are exposed to as a result of natural infection may be neutralized by antibodies upon injection into in some humans because of pre-existing immunity. Recognition of viral structures (e.g., capsids or nucleic acids) by innate immune sensors may cause tissue infiltration by innate immune cells, may trigger the production of interferon (IFN)-α/β (type 1 IFN, hereafter abbreviated as T1 IFN), thereby inducing an antiviral state in the tissue and reducing transduction, and provides an activation signal for adaptive immune responses. Activation of, and subsequent antigen presentation by, dendritic cells (DCs) is a critical step in linking innate to adaptive immunity, leading to activation/differentiation and expansion of T cells. While major histocompatibility complex (MHC) class I (MHC I)-restricted CD8+ T cells (cytotoxic T lymphocytes [CTLs]) are capable of lysing virally infected cells, MHC class II (MHC II)-restricted CD4+ T cells provide help for optimal CD8+ T cell activation and for B cell activation, leading to antibody formation. T helper (Th) cells are also critical for generation of memory responses.
Early Lessons from Ad-Based Vectors
Ad is one of the first viruses investigated as a potential gene therapy vector and was also the subject of early failures for in vivo gene transfer that highlight how pivotal host inflammatory responses are on the durability of therapeutic gene expression and the overall safety of this type of intervention.2 Early enthusiasm for Ad vectors was largely based on their high transduction efficiency and packaging capacity. However, robust transgene expression was met with an equally high inflammatory response that resulted in transient expression and a potential for severe immunotoxicity, resulting in the death of a patient. Because of their ability to effectively activate CD8+ T cells, subsequent efforts shifted toward their use as vaccine carriers and in cancer gene therapy.
Ad vectors contain an ∼36-kb double-stranded DNA genome packaged into a viral protein capsid. Various viral genes are removed to render the virus replication defective. It is also possible to remove as viral coding sequences and produce “gutted” or “helper-dependent” adenoviral vectors. Some serotypes such as AdHu5 efficiently transduce a variety of cell types in vivo (with particularly high tropism for hepatocytes), while serotypes infecting hematopoietic cells have also been described. Upon transduction, the vector genome remains episomal. Ad vectors activate a large spectrum of innate immune pathways and have therefore been ideal tools to study innate immunity to viruses.
Early Innate Responses to Systemically Delivered Ad
Hepatic gene transfer is achieved by intravenous injection of adenoviral vector. However, innate responses may occur within minutes to hours, leading to blood pressure changes, thrombocytopenia, inflammation, and fever.3 Dysregulation of coagulation can spread to multiple organs and lead to DIC (disseminated intravascular coagulation). Activation of vascular endothelial cells by Ad vectors results in release of ultra-large-molecular-weight multimers of von Willebrand factor (vWF), a blood protein that is critical for platelet adhesion. Ad vectors also activate platelets and induce exposure of the adhesion molecule P-selectin and formation of platelet-leukocyte aggregates, ultimately causing thrombocytopenia and thus a risk for bleeding.4 Important cellular interactions occurring early after the systemic Ad vector involve vascular and hepatic endothelial cells, platelets, Kupffer cells, hepatocytes, and splenic macrophages (MFs) and DCs.5
Once the virus is blood-borne, the hexon component of the adenoviral capsid binds to coagulation factor X (FX), as shown by Shayakhmetov and colleagues.6,7 Viral particles decorated with FX activate Toll-like receptor (TLR)4 on the surface of splenic MFs and thereby trigger a nuclear factor κB (NF-κB)-dependent activation of interleukin (IL)-1β, attracting polymorphonuclear leukocytes to the marginal zone of the spleen. These mechanisms, reflecting a co-evolution of the immune and coagulation systems to defend against pathogens, contribute to rapid clearance of the virus from the spleen. Upon delivery into a blood vessel, interactions with molecules and cells of the blood and immune organs that survey the systemic circulation critically influence the response to Ad vectors. Besides binding to coagulation proteins with γ-carboxyglutamic acid (GLA) domains, adenoviral particles bind complement component C3 and natural immunoglobulin (Ig)M antibodies, resulting for instance in neutrophil activation.8, 9, 10, 11, 12 Antibody-virus complexes can trigger inflammatory cytokine and chemokine responses in macrophages through the intracellular antibody receptor TRIM21.13, 14, 15, 16 Interestingly, binding of FX appears to compete with adenoviral interactions with complement and antibodies, shielding it from these components while subsequently promoting TLR4 signaling in the spleen.17 Ad vectors also interact with shed cellular receptors, the effect of which on immune responses requires more extensive studies.18,19
Innate Sensing in Antigen-Presenting Cells
Gene transfer with Ad results in innate inflammation at the site of gene transfer and at high doses in macrophage death. Much of the innate signaling in response to Ad was learned from studies of macrophages. While primary receptors for Ad typically bind the fiber knob of the capsid, RGD loops in the penton bind to secondary receptors such as integrins. For example, splenic macrophages of the macrophage receptor with collagenous structure (MARCO) subset trap Ad.20 Binding to integrin β3 results in release of IL-1α, which in turn causes signaling through the IL-1 receptor, production of chemokines, and recruitment of other innate immune cells in order to kill virally infected macrophages.21 Resident macrophages in the liver (Kupffer cells) undergo necrotic cell death at high vector doses through a mechanism that is not fully understood but that depends on IFN regulatory factor 3 (IRF3).22 Ad vectors also activate the NALP3 inflammasome, a process that requires intracellular DNA sensing (independent of TLR9 and thus likely representing cytosolic sensing), results in IL-1β expression, and also leads to necrotic cell death.23 Cytosolic sensing of adenoviral DNA through cyclic guanosine monophosphate-AMP synthase (cGAS; see “Immune Responses to LV Vectors” below for more detail on this pathway) results in production of T1 IFN, which promotes an antiviral state that can lead to transgene silencing in hepatic gene transfer.24,25 Nonetheless, adenoviral DNA is also sensed through the endosomal receptor TLR9, resulting, for example, in IL-6 production during hepatic gene transfer and T1 IFN production in plasmacytoid DCs (pDCs). Roles for other TLRs have also been demonstrated. Finally, there are nuclear sensing mechanisms of adenoviral DNA that can increase or decrease immunity.26,27
Adaptive Responses to Adenoviral Vectors and Transgene Products
Pre-existing immunity to human Ad-derived vectors has led to the development of alternative serotype vectors such as chimpanzee Ads.28 As expected from viral vectors, adenoviral vectors elicit neutralizing antibody (NAB) responses that prevent re-administration. Moreover, adenoviral vectors activate both conventional DCs and pDCs and transduce DCs in vivo. Gene expression in DCs is considered a major contributor to the generation of adaptive immune responses to the transgene product and to viral gene products.29,30 Adenoviral vectors are particularly potent in inducing CD8+ T cell responses, facilitated by potent induction of Th1 immunity.31 Elimination of all viral genes in high-capacity vectors and the use of tissue-specific promoters or promoters that are weak in professional antigen-presenting cells (APCs) are strategies that have been employed to reduce T cell responses. Blockage of co-stimulatory pathways that are required for B and T cell activation has also been successful in pre-clinical studies.32,33 However, the potent innate response to adenoviral vectors substantially complicates translation of such approaches, and use of adenoviral vectors for in vivo gene transfer in the treatment of genetic disease has been largely abandoned.
Immune Responses to AAV Vectors
AAV is a small non-enveloped parvovirus with a single-stranded genome of about 5 kb that is naturally non-pathogenic and replication defective. AAV vectors contain no viral coding sequences, elicit only weak inflammatory or T1 IFN responses when compared to other viruses, and their V genomes persist mostly in episomal form. These features contribute to a favorable safety profile, although immunotoxicities may still occur following systemic delivery of very high doses.34,35 AAV vectors are being widely tested in human gene therapy trials for in vivo gene transfer to a large number of different tissues and cells types, including the CNS, liver, skeletal and cardiac muscle, eye, and lung. In addition to two US Food and Drug Administration (FDA)-approved products for treatment of Leber’s congenital amaurosis (LCA) and spinal muscular atrophy (SMA), other products have reached phase III trials as in liver-directed gene therapy for hemophilia A and B.1,36
Pre-existing Immunity and NAB Formation
Initial marketing approval was for an ocular gene transfer with limited vector doses using a subretinal route of injection. Likely facilitated by the immune privilege of the eye, vector administration to one eye could be later on followed by contralateral gene transfer to the second eye, and repeat administration to the same eye is also possible.37,38 Other routes of in vivo vector administration are more typically associated with formation of NABs that prevent re-administration of vector. Furthermore, humans develop NABs to various serotypes during childhood.35 Seroprevalence varies geographically, and some humans have NABs against multiple serotypes, likely reflecting cross-reactivity. Some of the highest prevalences of NABs are against AAV2, the serotype that is similar to AAVs present in the human population, while the more diverse AAV5 has some of the lowest prevalences. Overall, seroprevalences appear to vary from 5% to 60%, depending on capsid.39 Prior to enrollment in a clinical trial, human subjects are typically screened for pre-existing NAB titers against the vector capsid, and only those with titers below a set threshold are enrolled. There is ongoing debate as to what level of pre-existing NABs may prevent gene transfer, depending on serotype, dose, and route of administration.40, 41, 42, 43 Methods such as addition of “decoy” capsids (empty viral capsids that can bind the antibody) to the vector product or plasmapheresis have been explored to overcome or eliminate pre-existing NABs.44,45 Interestingly, pre-existing binding antibodies (that are not neutralizing) do not block gene transfer but may alter biodistribution of the vector.46 Theoretically, repeat administration of the AAV vector is possible by switching the capsid sequence. However, such a strategy is complicated by the tendency of humans to produce cross-reactive antibodies and by the need to develop at least two products. An alternative approach is to apply immunosuppression. One protocol uses antibody-mediated B cell depletion combined with rapamycin, while rapamycin-containing nanoparticles have been tested in animal models.47,48
CD8+ T Cell Responses to Capsid
In the first liver-directed gene therapy trial with AAV vectors (performed with an AAV2 vector in patients with hemophilia B), a loss of factor IX transgene expression and transient mild elevations of liver enzymes in circulation were correlated with a CD8+ T cell response against the viral capsid.49,50 This came as a surprise since none of the animal models, including non-human primates, had shown such a response and because AAV vectors are engineered to not express capsid antigen. However, it was later shown that hepatocytes transduced with AAV present capsid antigen on their surface by MHC I and can be targeted by capsid-specific CD8+ T cells.51 Multiple epitopes have been identified in humans, some of which are conserved among several serotypes.52 It is furthermore known that AAV vector particles are prone to proteasomal degradation following endosomal escape, which would then result in MHC I presentation of capsid-derived peptides. Subsequent clinical trials have utilized liver enzyme levels as a biomarker for the T cell response and employed immunosuppression with steroid drugs (prednisolone) to counter the response, which appears to be vector dose-dependent.53, 54, 55 This immune suppression strategy was successful in several patients. However, T cell responses were not seen in all trials, raising questions about the role of factors such as serotype, vector design, or manufacturing method. It is also possible that hepatotoxicity seen in some trials, such as for factor VIII (FVIII) expression, may not always be related to T cell responses but perhaps relate to overexpression of certain transgenes.56
It has been proposed that the lack of CD8+ T cell responses in animal models is because they are not natural hosts for AAVs, while memory T cells stemming from natural infection were activated in humans (and that these may be present even in the absence of NABs). While non-human primates harbor AAVs, their CD8+ T cells to capsid have been found to be distinct in differentiation status and in their functions from those in humans. However, more recent data suggest that CD8+ T cell responses against capsid in patients following vector administration reflect primary immune responses, while empty capsids (not containing vector DNA) or vectors with genomes that have been largely depleted of immune stimulatory CpG motifs primarily stimulate memory CD8+ T cells.57 Studies in mice (and to some extent in human cells) have shown that IFN-α/β production and priming of CD8+ T cells in AAV gene transfer is dependent on TLR9 (an endosomal DNA receptor that is particularly stimulated by unmethylated CpG sequences as found in viral or bacterial DNA; see below for further details). Therefore, CpG depletion of expression cassettes is one current approach to try and “deimmunize” AAV vectors. Data from clinical trials suggest that CpG enrichment negatively affected the outcome of liver-directed gene therapy for hemophilia.56,58 Recently presented data suggest that long-term FIX expression despite CpG-enriched vector sequences may be associated at least in some patients with polymorphism in the IL6R gene.59 Another strategy is to remove tyrosine residues from the capsid that may be phosphorylated, which in turn serves as a signal for ubiquitination and proteasomal degradation. As a result, such modified capsids may be less MHC I presented.60 Also, note that CD8+ T cell responses to AAV capsid can be observed in animals such as mice or non-human primates. However, the time course of their appearance in peripheral blood is very different from humans. Responses in mice occur with 1–2 weeks, while 1 to several months are typical in humans. Furthermore, the response in the animals does not result in destruction of transduced cells unless the T cells are further ex vivo expanded and then adoptively transferred.60 These differences between the animal and human responses remain unresolved.
CD8+ T cell responses to AAV capsid have also been observed in muscle-directed gene transfer. For example, a prolonged inflammatory response was seen in muscle of patients who received AAV1 vector for treatment of α1-antitrypsin deficiency.61 Interestingly, transgene expression was somewhat reduced but not eliminated and seemed to recover over time. Moreover, CD4+CD25+FoxP3+ regulatory T cells (Tregs) infiltrated the tissue and likely contributed to the resolution of this immune response, while CD8+ T cells acquired a phenotype resembling exhausted T cells.62 Similar observations were made in muscle gene transfer for lipoprotein lipase deficiency.63
Innate Immunity and Links to Adaptive Responses
Innate immune responses in tissues transduced with AAV gene therapy are relatively mild compared to other viruses, resulting in a rapid (within 1–2 h) but often limited and highly transient (<12 h) immune infiltration of macrophages, natural killer (NK) cells, and neutrophils and expression of NF-κB-dependent pro-inflammatory cytokines and T1 IFN. In the mouse liver, these responses are dependent on the endosomal innate DNA receptor TLR9 and partially dependent on Kupffer cells.64 In culture of human liver cells, TLR2-dependent cytokine expression was observed in Kupffer cells.65 Therefore, the viral genome and the capsid may contribute to innate immune recognition of AAV. A recent report provides evidence that transduction of cells with AAV vectors through a yet-to-be-defined mechanism results in formation of double-stranded RNA (dsRNA), which can be sensed by cytoplasmic dsRNA sensors such as MDA5, resulting in IFN-β expression.66
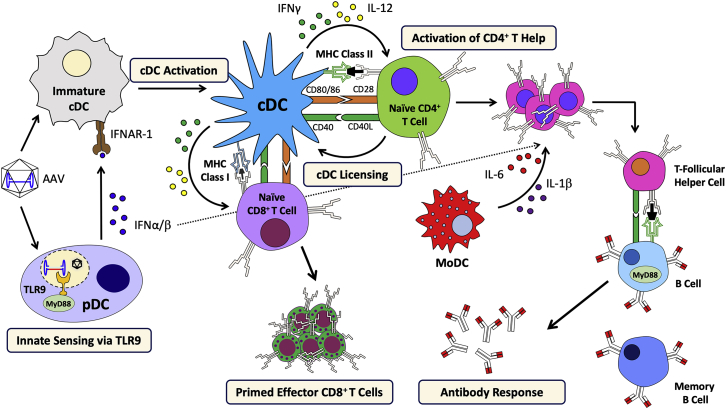
AAV vectors activate T1 IFN expression in human and murine pDCs but not in CD11chi conventional DCs (cDCs).67 T1 IFN production is the result of TLR9-myeloid differentiation primary response protein 88 (MyD88) signaling in pDCs and promotes activation of CD8+ T cells. Hence, CD8+ T cell responses against transgene product or capsid are markedly reduced in mice deficient in TLR9, MyD88, or receptor of T1 IFN.67, 68, 69 Studies of cross-priming of AAV capsid-specific CD8+ T cells showed that pDCs and cDCs cooperate to achieve CD8+ T cell activation (Figure 3). Sensing of the AAV genome by TLR9 occurs in pDCs, while cDCs carry out the task of antigen presentation by MHC I.69 This process requires T1 IFN, which binds to its receptor on cDCs, suggesting a direct effect of cytokine production by pDCs on cDC activation.70 However, NK cells, which may indirectly mediate the effect of pDCs on cDCs, are not required. In addition to T1 IFN, CD40-CD40L co-stimulation, which is carried out by CD4+ Th cells, is required for cross-priming of CD8+ T cells against AAV capsid.
Figure 3.
Model for the Immune Response Mechanism Against AAV Vectors
Cooperation between pDCs (that sense the AAV genome via TLR9 and produce T1 IFN), cDCs (that present antigen), and CD4+ T helper cells (that provide co-stimulation via the CD40-CD40L pathway) leads to activation of CD8+ T cells. Antibody formation also depends on CD4+ T helper cells and is augmented by activation of moDCs, resulting in IL-1β and IL-6 pro-inflammatory cytokine production, and is modulated by T1 IFN. Abbreviations are as follows: IFNAR-1, interferon α/β receptor 1; cDC, conventional dendritic cell; pDC, plasmacytoid dendritic cell; IFN, interferon; TLR, Toll-like receptor; MyD88, myeloid differentiation primary response protein 88; MHC, major histocompatibility complex; moDC, monocyte-derived dendritic cell; and CD, cluster of differentiation.
TLR9-MD88 signaling and T1 IFN production can have a modulating effect on antibody formation against capsid or transgene product but is not as strictly required as for CD8+ T cell priming (although MyD88 has an intrinsic role in B cells in Th1-dependent antibody class switching).68,70,71 This is in contrast to CD4+ T help and related co-stimulatory pathways, which are required for antibody formation and could therefore potentially be targeted for prevention of both T cell and antibody responses. A recent study investigating antibody formation against AAV in healthy humans found that production of IL-1β and IL-6 by circulating monocyte-derived DCs (moDCs) upon pulsing with AAV particles or AAV capsid-derived peptides activated B cells (Figure 3).72 Blockage of either cytokine suppressed antibody formation against AAV in vitro and in vivo (in mice). While prior studies found no correlation between the presence of NABs and AAV-reactive IFN-γ-producing CD8+ T cells in humans, this new study found a good correlation between the presence of NABs and with transforming growth factor α (TNF-α)-producing memory CD8+ T cells. Interestingly, NK cells from seronegative individuals seemed to respond to both AAV capsid and AAV capsid peptide pools when stimulated in vitro. Studies in mice demonstrated a unique ability of TLR9 agonists to activate antibody responses to the transgene product in muscle gene transfer, which occurred through induction of moDC responses that enhance activation of T follicular helper T cells.73,74 Therefore, moDC activation is a driver of T cell responses that promote antibody formation. Complement may also be involved in NAB formation. For instance, AAV2 capsid was found to bind to iC3b complement protein (which, however, did not result in complement activation) and to complement regulatory protein factor H, and C3 deficient mice had impaired antibody responses to capsid.75
Risks of Adaptive Responses to the Transgene Product
The risk of an antibody response to the transgene product is influenced by many factors, including the underlying mutation in replacement therapy for genetic disease, the route of vector administration/target tissue, specific vector design, AAV serotype, and vector dose. Additional host factors may include disease-specific aspects such as tissue inflammation. For systemic delivery of proteins, muscle gene transfer bears an elevated risk of B cell activation, which, for example, complicates AAV delivery of antibodies against viruses that cause infectious disease such as human immunodeficiency virus (HIV).76,77 Transient immune suppression protocols, in particular those that preserve Tregs and promote tolerance, may prevent unwanted immune responses in gene transfer to muscle and other organs.78, 79, 80 Risks of CD8+ T cell responses are also affected by multiple factors. While AAV vectors gained popularity because of their inefficient activation of CTLs when compared to Ad vectors, thereby substantially increasing the chance for long-term transgene expression, CD8+ T cell responses against dystrophin and in rare cases against α1-antitrypsin transgene products were nonetheless observed in patients.81,82 Some patients with Duchenne muscular dystrophy may actually have pre-existing T cell immunity because of occasional endogenous dystrophin expression in revertant fibers.81 Because overexpression of utrophin can partially compensate for lack of dystrophin, AAV vectors expressing this “self-gene” that is widely expressed in muscle may avoid these immune complications.83,84 α1-Antitrypsin deficiency is typically caused by a missense mutation. Interestingly, CD8+ T cell responses in rare human leukocyte antigen (HLA) types may be directed not against an epitope that spans the mutation but rather against a polymorphic sequence that may differ from the therapeutic transgene.82
Given the link between TLR9 signaling and CD8+ T cell activation, CpG depletion of the expression cassette has been incorporated into vector design to reduce this risk.85 Interestingly, CD8+ T cell responses against the transgene product are often not fully functional in muscle gene transfer in mice when using conventional vectors with single-stranded DNA genome. They fail to eliminate the transduced muscle, do not respond to boost vaccination, and upregulate immunoinhibitory molecules such as PD-1.86 Self-complementary vectors (which can be generated by elimination of a nicking site in one of the inverted terminal repeats [ITRs]), however, elicit more functional responses, possibly because of enhanced TLR9 signaling and/or different kinetics of transgene expression.64,87,88 These vectors do not require second-stand synthesis and thus can more rapidly express but can only be half the size of a conventional AAV genome in order not to exceed the packaging limit.
Immune Tolerance Induction by Hepatic Gene Transfer
Even in the context of a gene deletion, long-term expression of a secreted transgene product without antibody formation can be achieved by hepatic gene transfer with AAV vectors. These observations reflect the ability of the liver environment to aid in tolerance induction.89 Tolerance is induced through a combination of mechanisms. Programmed cell death is required to delete effector T cells, while induction of FoxP3+ Tregs is requisite for both induction and maintenance of tolerance.90, 91, 92, 93, 94 The ability to induce tolerance and the extent of Treg induction depend on levels of transgene expression. Antigen presentation leading to Treg induction likely takes place in liver-draining lymph nodes and in the environment of the liver itself, which provides immunosuppressive cytokines and specialized cell types capable of presenting antigen such as Kupffer cells, hepatic DCs, and liver sinusoidal endothelial cells (LSECs).95,96 Additional mechanisms include T cell anergy, T cell exhaustion, and suppression of CD8+ T cell responses through IL-10 production.97, 98, 99, 100 The dominant nature of induced tolerance allows for introduction of the therapeutic protein through gene transfer to other tissues or systemic protein delivery such as in enzyme replacement therapy for storage disorders.101,102 Hepatic-induced tolerance can also reverse pre-existing immune responses and may be synergistic with conventional immunosuppression in treatment of autoimmune disease.90,103,104 Finally, hepatic gene transfer with microRNA (miRNA)-regulated LV vectors can similarly induce immune tolerance (see below).105,106
Immunotoxicities
With the development of systemic gene therapies for neuromuscular, neurodegenerative, and storage disorders, very large vector doses, ∼1014 AAV vector genomes/kg, are being employed. The immune mechanisms described above were typically not studied in this range, and immunotoxicties are emerging in more recent pre-clinical and clinical studies.107,108 Some of these may relate to AAV itself, others to impurities in the product, and others to effects of the transgene product. Complement activation has been reported in some patients treated with high-dose AAV9, albeit it is unclear whether this follows the classical antibody-mediated pathway or direct binding of virus to complement components.109 At such high-vector doses, it is conceivable that cell surfaces are being decorated with virus and targeted by antibodies. Broader toxicities, including thrombocytopenia, have also been reported in a patient treated for muscular dystrophy (https://www.genengnews.com). CD8+ T cell responses could also be a cause of wider toxicity for instance in multi-organ or CNS transduction.110 Substantially more studies are needed to address these issues.
Immune Responses to LV Vectors
LV vectors are derived from human immunodeficiency virus (HIV) and are capable of transducing non-dividing as well as dividing cells. They are enveloped viruses that contain a single-stranded RNA genome, which upon infection is reverse transcribed into DNA in the host cell’s cytoplasm. After transport to the nucleus, stable integration into the host cell genome leads to long-term expression of the transgene if the cell persists. LV vectors are popular for ex vivo gene transfer to HSCs in the treatment of genetic disease and to T cells for CAR-T cell therapy against cancer.111 They are also being developed for in vivo gene transfer, e.g., to the liver, and integration-deficient LV vectors have also been designed. In vivo gene transfer to DCs is a more recent strategy in vaccine development. Pseudotyping with vesicular stomatitis virus (VSV)-G is common, because this envelope protein allows LVs to efficiently infect a large variety of target cells. However, VSV pseudotyped LV vectors do not infect B cells which prompted the development of CD20-targeted envelopes that contain a single variable fragment against CD20 fused to a measles virus envelope protein.112 Similarly, envelopes for specific targeting of distinct subsets of B and T cells have been created.113,114
Innate Immune Responses against LV Vectors
While pre-existing immunity to LV in humans is low, efficacy of in vivo hepatic gene transfer with LV vectors is generally limited by several factors, including phagocytosis. To this end, incorporation of human phagocytosis inhibitor CD47 into LV membrane reduces uptake by phagocytic cells and increases distribution to hepatocytes.115 Another severely limiting factor is production of T1 IFN, so that mice deficient in T1 IFN signaling show substantially higher numbers of transduced hepatocytes.116 Similarly, pharmacological suppression of IFN production (e.g., with dexamethasone) increases transduction efficiency.117 Although LV vectors elicit weaker IFN-α responses from pDCs compared to the parent HIV-I virus, it is thought that pDCs play a key role in the T1 IFN response against LVs. TLR7, the PRR that senses single-stranded RNA molecules in endosomes, and TLR9 contribute to induction of T1 IFN.118,119 It has been proposed that VSV-G protein-pseudotyped LV vectors may contain tubulovesicular structures with DNA fragments that promote TLR9 signaling.120 The strength of TLR7 and TLR9 downstream signaling is in part regulated by the mammalian target of rapamycin (mTOR) pathway. Interestingly, work by Brown and colleagues121 identified a miRNA (miR126, which has been known to have a critical function in vascular endothelial cells during angiogenesis) to be uniquely expressed in pDCs within the immune system.122 In pDCs, miR126 targets a negative regulator of mTOR for degradation. An overactive mTOR pathway is important for generation of pDCs and for TLR-mediated innate responses to nucleic acids and LV vectors in pDCs. Furthermore, one can take advantage of this observation in vector design to avoid transgene expression in pDCs by incorporation of target sequences into the transcript (see below). However, blocking TLR7 or TLR9 signaling is insufficient to prevent LV vectors from inducing IFN-α responses. This is likely because of sensing of viral DNA genomes (which result from reverse transcription) through a cytoplasmic mechanism that involves the cGAS-stimulator of IFN genes (STING) pathway.123,124 cGAS is a cytosolic sensor of DNA. Upon binding to DNA, the enzyme catalyzes the generation of cyclic guanosine monophosphate-AMP (cGAMP), which binds to STING. STING predominantly localizes to the endoplasmic reticulum (ER) and is a signaling adaptor for production of T1 IFN. Re-localization to the Golgi apparatus triggers phosphorylation of IRF3 via TBK1, leading to T1 IFN expression.
Adaptive Responses against LV Gene Transfer, miRNA Regulation Strategy, and Tolerance Induction
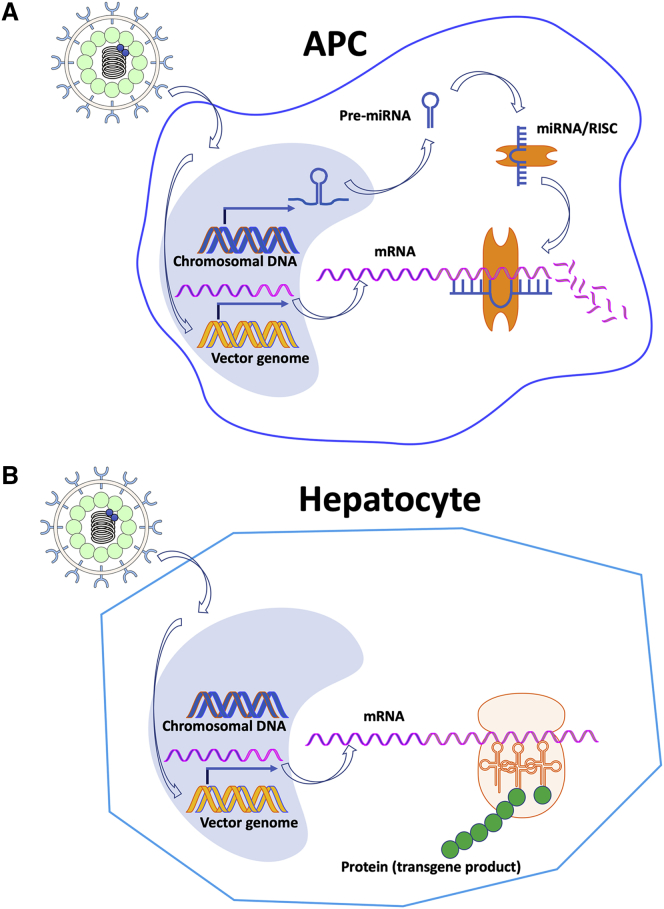
LV vectors efficiently transduce professional APCs such as MFs and DCs, which has been recognized to strongly promote immune responses against the transgene product. In fact, in vivo and ex vivo transduction of DCs, e.g., using integration-deficient LV vectors, is being exploited in vaccine development.125,126 In hepatic gene transfer, use of a hepatocyte-specific promoter has not been sufficient to entirely prevent expression in APCs and activation of immune responses. In an elegant solution to this problem, Naldini and colleagues127 took advantage of miRNA regulation of transgene expression and incorporated multiple copies of a target for a miRNA (miR142) that is highly expressed in hematopoietic cells, including professional APCs (Figure 4). Combined with use of a hepatocyte-specific promoter, CTL and antibody responses against the transgene product were avoided. Moreover, immune tolerance to transgene products such as GFP or factor IX was induced.128,129 CD8+ T cell activation was abortive, while transgene product-specific CD4+CD25+FoxP3+ Tregs were induced that actively suppressed immune responses. Using such an optimized hepatocyte-restricted LV vector expression insulin B chain, onset of type 1 diabetes could be prevented in nonobese diabetic (NOD) mice.130 When combined with monoclonal antibody immunmodulation directed against CD3, reversal of the autoimmune disorder was achieved.
Figure 4.
Strategy to Eliminate Transgene Expression from Lentiviral Vectors in Professional Antigen-Presenting Cells
This approach can also be applied to other viral vectors such as AAV and adenovirus. (A) Incorporation of multiple repeats of a target for a miRNA that is highly expressed in hematopoietic cells into the transcript of the transgene results in efficient degradation of the transgene message, thereby preventing transgene expression in an APC. (B) Transduction of a target cell for therapeutic gene expression such as a hepatocyte that does not express the miRNA results in transgene expression. Abbreviations are as follows: APC, antigen-presenting cell; miRNA, micoRNA; mRNA, messenger RNA; and RISC, RNA-induced silencing complex.
Interestingly, Follenzi and colleagues131,132 showed an improved success rate of tolerance induction to factor VIII (FVIII) in hemophilia A mice when transgene expression was restricted to hepatic endothelial cells (the normal site of FVIII biosynthesis), which merely required an endothelial cell-specific promoter but not miRNA target sequences. Additionally, this line of research identified transgene expression in pDCs as a major driver of immune responses, while expression restricted to myeloid cells may not result in immune responses.131,132 Another potential source of T cell responses against LV vectors is producer cell-derived polymorphic MHC I molecules. This, however, can be eliminated by disruption of the β2-microglobulin gene in producer cells, thereby generating MHC-free LV.133
Ex vivo gene transfer has been widely viewed as a way to avoid immune responses. However, adaptive responses against gene-modified cells can occur but may be mitigated by combined myeloablation and immunosuppression regimens.134,135 Moreover, a recent study has shown that enzyme replacement therapy for treatment of lysosomal storage disorders (LSDs) may induce CD8+ T cells that can target LV HSC gene therapy.136,137 Therefore, ex vivo gene therapy for LSDs in patients with prior protein therapy may require adjunct immunotherapy. Others are using ex vivo HSC gene transfer with LV vectors to target transgene expression to megakaryocytes for protein delivery via platelets. This approach is designed to release proteins such as FVIII upon platelet activation while otherwise “hiding” the protein antigen from the immune system.138 Over time, platelet-targeted expression may also induce immune tolerance.139
Conclusions
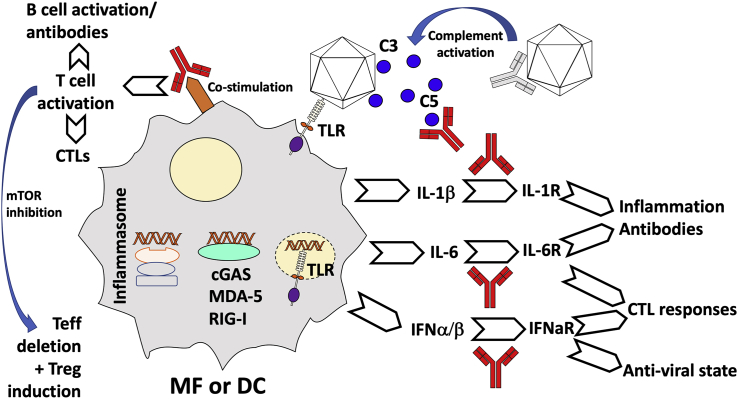
After decades of research, viral-based vectors are now becoming the first clinically approved gene therapies, with AAV and LV vectors increasingly taking the lead. However, immune responses against vectors and transgene products remain a hurdle to uniform efficacy. A better understanding of these immune responses will lead to improved vector designs and more targeted immune modulations. This holds potential to expand gene therapy to a wider set of human conditions. For instance, steroid drugs are currently widely employed in systemic, hepatic, and ocular AAV gene therapy to counter inflammation and antiviral CD8+ T cell responses. Lessons from CAR-T cell therapy, where physicians learned to prevent cytokine release syndrome by inclusion of monoclonal antibodies in the immune modulation regimen to counter IL-6 signaling, may be helpful in management of immunotoxicities in viral vector gene therapy. Targeted blockage of specific cytokines such as IL-1, IL-6, or T1 IFN, monoclonal antibodies that disrupt the complement cascade, co-stimulation blockers, or transient mTOR inhibition may be tools in addition to vector engineering to subdue deleterious innate and adaptive immune responses (Figure 5).
Figure 5.
Potential Targets for Directed Pharmacological Interventions to Prevent Immunotoxicities and Lower the Risk of Innate and Adaptive Immune Responses in Viral Vector Gene Transfer
Immunoglobulins in red indicate blockage of a pathway with a monoclonal antibody. Abbreviations are as follows: cGAS, cyclic guanosine monophosphate-AMP synthase; CTL, cytotoxic T lymphocyte; DC, dendritic cell; IL, interleukin; INF, interferon; MDA5, melanoma differentiation-associated protein 5; MF, macrophage; mTOR, mammalian target of rapamycin; RIG-I, retinoic acid-inducible gene I; Teff, effector T cell; TLR, Toll-like receptor; and Treg, regulatory T cell. Receptor for a specific cytokine is indicated with “R” after the cytokine name.
Author Contributions
J.L.S., Y.P.d.J., C.T., and R.W.H. wrote the manuscript.
Conflicts of Interest
R.W.H. serves on the scientific advisory boards of Applied Genetic Technologies Corporation (AGCT) and Ally Therapeutics. The remaining authors declare no competing interests.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases Grant R01 AI51390 (to R.W.H.), National Institutes of Health/National Heart, Lung, and Blood Institute Grants R01 HL131093 (to R.W.H. and C.T.) and R01 HL097088 (to R.W.H.), and Indiana Collaborative Initiative for Talent Enrichment (INCITE) funds provided by the Lilly Endowment.
References
- 1.High K.A., Roncarolo M.-G. Gene therapy. N. Engl. J. Med. 2019;381:455–464. doi: 10.1056/NEJMra1706910. [DOI] [PubMed] [Google Scholar]
- 2.Crystal R.G. Adenovirus: the first effective in vivo gene delivery vector. Hum. Gene Ther. 2014;25:3–11. doi: 10.1089/hum.2013.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seiler M.P., Cerullo V., Lee B. Immune response to helper dependent adenoviral mediated liver gene therapy: challenges and prospects. Curr. Gene Ther. 2007;7:297–305. doi: 10.2174/156652307782151452. [DOI] [PubMed] [Google Scholar]
- 4.Othman M., Labelle A., Mazzetti I., Elbatarny H.S., Lillicrap D. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood. 2007;109:2832–2839. doi: 10.1182/blood-2006-06-032524. [DOI] [PubMed] [Google Scholar]
- 5.Atasheva S., Yao J., Shayakhmetov D.M. Innate immunity to adenovirus: lessons from mice. FEBS Lett. 2019;593:3461–3483. doi: 10.1002/1873-3468.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atasheva S., Shayakhmetov D.M. Adenovirus sensing by the immune system. Curr. Opin. Virol. 2016;21:109–113. doi: 10.1016/j.coviro.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doronin K., Flatt J.W., Di Paolo N.C., Khare R., Kalyuzhniy O., Acchione M., Sumida J.P., Ohto U., Shimizu T., Akashi-Takamura S. Coagulation factor X activates innate immunity to human species C adenovirus. Science. 2012;338:795–798. doi: 10.1126/science.1226625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tam J.C.H., Bidgood S.R., McEwan W.A., James L.C. Intracellular sensing of complement C3 activates cell autonomous immunity. Science. 2014;345:1256070. doi: 10.1126/science.1256070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker A.L., Waddington S.N., Nicol C.G., Shayakhmetov D.M., Buckley S.M., Denby L., Kemball-Cook G., Ni S., Lieber A., McVey J.H. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- 10.Shayakhmetov D.M., Gaggar A., Ni S., Li Z.-Y., Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen R.J., Byrnes A.P. Interaction of adenovirus with antibodies, complement, and coagulation factors. FEBS Lett. 2019;593:3449–3460. doi: 10.1002/1873-3468.13649. [DOI] [PubMed] [Google Scholar]
- 12.Cotter M.J., Zaiss A.K., Muruve D.A. Neutrophils interact with adenovirus vectors via Fc receptors and complement receptor 1. J. Virol. 2005;79:14622–14631. doi: 10.1128/JVI.79.23.14622-14631.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwan W.A., Tam J.C.H., Watkinson R.E., Bidgood S.R., Mallery D.L., James L.C. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat. Immunol. 2013;14:327–336. doi: 10.1038/ni.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher A.J., James L.C. Coordinated neutralization and immune activation by the cytosolic antibody receptor TRIM21. J. Virol. 2016;90:4856–4859. doi: 10.1128/JVI.00050-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khare R., Hillestad M.L., Xu Z., Byrnes A.P., Barry M.A. Circulating antibodies and macrophages as modulators of adenovirus pharmacology. J. Virol. 2013;87:3678–3686. doi: 10.1128/JVI.01392-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bottermann M., Foss S., van Tienen L.M., Vaysburd M., Cruickshank J., O’Connell K., Clark J., Mayes K., Higginson K., Hirst J.C. TRIM21 mediates antibody inhibition of adenovirus-based gene delivery and vaccination. Proc. Natl. Acad. Sci. USA. 2018;115:10440–10445. doi: 10.1073/pnas.1806314115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Z., Qiu Q., Tian J., Smith J.S., Conenello G.M., Morita T., Byrnes A.P. Coagulation factor X shields adenovirus type 5 from attack by natural antibodies and complement. Nat. Med. 2013;19:452–457. doi: 10.1038/nm.3107. [DOI] [PubMed] [Google Scholar]
- 18.Houri N., Huang K.-C., Nalbantoglu J. The coxsackievirus and adenovirus receptor (CAR) undergoes ectodomain shedding and regulated intramembrane proteolysis (RIP) PLoS ONE. 2013;8:e73296. doi: 10.1371/journal.pone.0073296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H., Ducournau C., Saydaminova K., Richter M., Yumul R., Ho M., Carter D., Zubieta C., Fender P., Lieber A. intracellular signaling and desmoglein 2 shedding triggered by human adenoviruses Ad3, Ad14, and Ad14P1. J. Virol. 2015;89:10841–10859. doi: 10.1128/JVI.01425-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Paolo N.C., Baldwin L.K., Irons E.E., Papayannopoulou T., Tomlinson S., Shayakhmetov D.M. IL-1α and complement cooperate in triggering local neutrophilic inflammation in response to adenovirus and eliminating virus-containing cells. PLoS Pathog. 2014;10:e1004035. doi: 10.1371/journal.ppat.1004035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Paolo N.C., Miao E.A., Iwakura Y., Murali-Krishna K., Aderem A., Flavell R.A., Papayannopoulou T., Shayakhmetov D.M. Virus binding to a plasma membrane receptor triggers interleukin-1α-mediated proinflammatory macrophage response in vivo. Immunity. 2009;31:110–121. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manickan E., Smith J.S., Tian J., Eggerman T.L., Lozier J.N., Muller J., Byrnes A.P. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol. Ther. 2006;13:108–117. doi: 10.1016/j.ymthe.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Muruve D.A., Pétrilli V., Zaiss A.K., White L.R., Clark S.A., Ross P.J., Parks R.J., Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki M., Bertin T.K., Rogers G.L., Cela R.G., Zolotukhin I., Palmer D.J., Ng P., Herzog R.W., Lee B. Differential type I interferon-dependent transgene silencing of helper-dependent adenoviral vs. adeno-associated viral vectors in vivo. Mol. Ther. 2013;21:796–805. doi: 10.1038/mt.2012.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anghelina D., Lam E., Falck-Pedersen E. Diminished innate antiviral response to adenovirus vectors in cGAS/STING-deficient mice minimally impacts adaptive immunity. J. Virol. 2016;90:5915–5927. doi: 10.1128/JVI.00500-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L., Wen M., Cao X. Nuclear hnRNPA2B1 initiates and amplifies the innate immune response to DNA viruses. Science. 2019;365:eaav0758. doi: 10.1126/science.aav0758. [DOI] [PubMed] [Google Scholar]
- 27.Avgousti D.C., Herrmann C., Kulej K., Pancholi N.J., Sekulic N., Petrescu J., Molden R.C., Blumenthal D., Paris A.J., Reyes E.D. A core viral protein binds host nucleosomes to sequester immune danger signals. Nature. 2016;535:173–177. doi: 10.1038/nature18317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ertl H.C. Viral vectors as vaccine carriers. Curr. Opin. Virol. 2016;21:1–8. doi: 10.1016/j.coviro.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Jooss K., Yang Y., Fisher K.J., Wilson J.M. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J. Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y., Su Q., Wilson J.M. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J. Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fields P.A., Kowalczyk D.W., Arruda V.R., Armstrong E., McCleland M.L., Hagstrom J.N., Pasi K.J., Ertl H.C., Herzog R.W., High K.A. Role of vector in activation of T cell subsets in immune responses against the secreted transgene product factor IX. Mol. Ther. 2000;1:225–235. doi: 10.1006/mthe.2000.0032. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y., Wilson J.M. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science. 1996;273:1862–1864. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y., Su Q., Grewal I.S., Schilz R., Flavell R.A., Wilson J.M. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J. Virol. 1996;70:6370–6377. doi: 10.1128/jvi.70.9.6370-6377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penaud-Budloo M., François A., Clément N., Ayuso E. Pharmacology of recombinant adeno-associated virus production. Mol. Ther. Methods Clin. Dev. 2018;8:166–180. doi: 10.1016/j.omtm.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colella P., Ronzitti G., Mingozzi F. Emerging issues in AAV-mediated in vivo gene therapy. Mol. Ther. Methods Clin. Dev. 2017;8:87–104. doi: 10.1016/j.omtm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butterfield J.S.S., Hege K.M., Herzog R.W., Kaczmarek R. A molecular revolution in the treatment of hemophilia. Mol. Ther. 2019 doi: 10.1016/j.ymthe.2019.11.006. Published online November 13, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett J., Ashtari M., Wellman J., Marshall K.A., Cyckowski L.L., Chung D.C., McCague S., Pierce E.A., Chen Y., Bennicelli J.L. AAV2 gene therapy readministration in three adults with congenital blindness. Sci. Transl. Med. 2012;4:120ra15. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weed L., Ammar M.J., Zhou S., Wei Z., Serrano L.W., Sun J., Lee V., Maguire A.M., Bennett J., Aleman T.S. Safety of same-eye subretinal sequential readministration of AAV2-hRPE65v2 in non-human primates. Mol. Ther. Methods Clin. Dev. 2019;15:133–148. doi: 10.1016/j.omtm.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Louis Jeune V., Joergensen J.A., Hajjar R.J., Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods. 2013;24:59–67. doi: 10.1089/hgtb.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kruzik A., Fetahagic D., Hartlieb B., Dorn S., Koppensteiner H., Horling F.M., Scheiflinger F., Reipert B.M., de la Rosa M. Prevalence of anti-adeno-associated virus immune responses in international cohorts of healthy donors. Mol. Ther. Methods Clin. Dev. 2019;14:126–133. doi: 10.1016/j.omtm.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majowicz A., Nijmeijer B., Lampen M.H., Spronck L., de Haan M., Petry H., van Deventer S.J., Meyer C., Tangelder M., Ferreira V. Therapeutic hFIX activity achieved after single AAV5-hFIX treatment in hemophilia B patients and NHPs with pre-existing Anti-AAV5 NABs. Mol. Ther. Methods Clin. Dev. 2019;14:27–36. doi: 10.1016/j.omtm.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meadows A.S., Pineda R.J., Goodchild L., Bobo T.A., Fu H. Threshold for pre-existing antibody levels limiting transduction efficiency of systemic rAAV9 gene delivery: relevance for translation. Mol. Ther. Methods Clin. Dev. 2019;13:453–462. doi: 10.1016/j.omtm.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long B.R., Sandza K., Holcomb J., Crockett L., Hayes G.M., Arens J., Fonck C., Tsuruda L.S., Schweighardt B., O’Neill C.A. The impact of pre-existing immunity on the non-clinical pharmacodynamics of AAV5-based gene therapy. Mol. Ther. Methods Clin. Dev. 2019;13:440–452. doi: 10.1016/j.omtm.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mingozzi F., Anguela X.M., Pavani G., Chen Y., Davidson R.J., Hui D.J., Yazicioglu M., Elkouby L., Hinderer C.J., Faella A. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci. Transl. Med. 2013;5:194ra92. doi: 10.1126/scitranslmed.3005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chicoine L.G., Montgomery C.L., Bremer W.G., Shontz K.M., Griffin D.A., Heller K.N., Lewis S., Malik V., Grose W.E., Shilling C.J. Plasmapheresis eliminates the negative impact of AAV antibodies on microdystrophin gene expression following vascular delivery. Mol. Ther. 2014;22:338–347. doi: 10.1038/mt.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitzpatrick Z., Leborgne C., Barbon E., Masat E., Ronzitti G., van Wittenberghe L., Vignaud A., Collaud F., Charles S., Simon Sola M. Influence of pre-existing anti-capsid neutralizing and binding antibodies on AAV vector transduction. Mol. Ther. Methods Clin. Dev. 2018;9:119–129. doi: 10.1016/j.omtm.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corti M., Liberati C., Smith B.K., Lawson L.A., Tuna I.S., Conlon T.J., Coleman K.E., Islam S., Herzog R.W., Fuller D.D. Safety of intradiaphragmatic delivery of adeno-associated virus-mediated alpha-glucosidase (rAAV1-CMV-hGAA) gene therapy in children affected by Pompe disease. Hum. Gene Ther. Clin. Dev. 2017;28:208–218. doi: 10.1089/humc.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meliani A., Boisgerault F., Hardet R., Marmier S., Collaud F., Ronzitti G., Leborgne C., Costa Verdera H., Simon Sola M., Charles S. Antigen-selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re-administration. Nat. Commun. 2018;9:4098. doi: 10.1038/s41467-018-06621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J.E., Ozelo M.C., Hoots K., Blatt P., Konkle B. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 50.Mingozzi F., Maus M.V., Hui D.J., Sabatino D.E., Murphy S.L., Rasko J.E.J., Ragni M.V., Manno C.S., Sommer J., Jiang H. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 51.Pien G.C., Basner-Tschakarjan E., Hui D.J., Mentlik A.N., Finn J.D., Hasbrouck N.C., Zhou S., Murphy S.L., Maus M.V., Mingozzi F. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J. Clin. Invest. 2009;119:1688–1695. doi: 10.1172/JCI36891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hui D.J., Edmonson S.C., Podsakoff G.M., Pien G.C., Ivanciu L., Camire R.M., Ertl H., Mingozzi F., High K.A., Basner-Tschakarjan E. AAV capsid CD8+ T-cell epitopes are highly conserved across AAV serotypes. Mol. Ther. Methods Clin. Dev. 2015;2:15029. doi: 10.1038/mtm.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nathwani A.C., Tuddenham E.G.D., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nathwani A.C., Reiss U.M., Tuddenham E.G.D., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herzog R.W., Pierce G.F. Liver gene therapy: reliable and durable? Mol. Ther. 2019;27:1863–1864. doi: 10.1016/j.ymthe.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiang Z., Kurupati R.K., Li Y., Kuranda K., Zhou X., Mingozzi F., High K.A., Ertl H.C.J. The effect of CpG sequences on capsid-specific CD8+ T cell responses to AAV vector gene transfer. Mol. Ther. 2019 doi: 10.1016/j.ymthe.2019.11.014. Published online November 21, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nathwani A.C. Gene therapy for hemophilia. Hematology (Am. Soc. Hematol. Educ. Program) 2019;2019:1–8. doi: 10.1182/hematology.2019000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bilic I., Monahan P., Berg V., Scheiflinger F., Reipert B.M. Whole Exome Sequencing of Patients Treated with Adeno- associated Virus Serotype 8- Factor IX (AAV8- FIX) Gene Therapy Reveals Potential Determinants of Persistent Transgene Expression. Res Pract Thromb Haemost. 2019;3(Suppl Supl1):95. [Google Scholar]
- 60.Martino A.T., Basner-Tschakarjan E., Markusic D.M., Finn J.D., Hinderer C., Zhou S., Ostrov D.A., Srivastava A., Ertl H.C., Terhorst C. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood. 2013;121:2224–2233. doi: 10.1182/blood-2012-10-460733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flotte T.R., Trapnell B.C., Humphries M., Carey B., Calcedo R., Rouhani F., Campbell-Thompson M., Yachnis A.T., Sandhaus R.A., McElvaney N.G. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results. Hum. Gene Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mueller C., Chulay J.D., Trapnell B.C., Humphries M., Carey B., Sandhaus R.A., McElvaney N.G., Messina L., Tang Q., Rouhani F.N. Human Treg responses allow sustained recombinant adeno-associated virus-mediated transgene expression. J. Clin. Invest. 2013;123:5310–5318. doi: 10.1172/JCI70314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferreira V., Twisk J., Kwikkers K., Aronica E., Brisson D., Methot J., Petry H., Gaudet D. Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPLS447X) in a phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum. Gene Ther. 2014;25:180–188. doi: 10.1089/hum.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martino A.T., Suzuki M., Markusic D.M., Zolotukhin I., Ryals R.C., Moghimi B., Ertl H.C., Muruve D.A., Lee B., Herzog R.W. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117:6459–6468. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hösel M., Broxtermann M., Janicki H., Esser K., Arzberger S., Hartmann P., Gillen S., Kleeff J., Stabenow D., Odenthal M. Toll-like receptor 2-mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors. Hepatology. 2012;55:287–297. doi: 10.1002/hep.24625. [DOI] [PubMed] [Google Scholar]
- 66.Shao W., Earley L.F., Chai Z., Chen X., Sun J., He T., Deng M., Hirsch M.L., Ting J., Samulski R.J., Li C. Double-stranded RNA innate immune response activation from long-term adeno-associated virus vector transduction. JCI Insight. 2018;3:e120474. doi: 10.1172/jci.insight.120474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu J., Huang X., Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J. Clin. Invest. 2009;119:2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rogers G.L., Suzuki M., Zolotukhin I., Markusic D.M., Morel L.M., Lee B., Ertl H.C., Herzog R.W. Unique roles of TLR9- and MyD88-dependent and -independent pathways in adaptive immune responses to AAV-mediated gene transfer. J. Innate Immun. 2015;7:302–314. doi: 10.1159/000369273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rogers G.L., Shirley J.L., Zolotukhin I., Kumar S.R.P., Sherman A., Perrin G.Q., Hoffman B.E., Srivastava A., Basner-Tschakarjan E., Wallet M.A. Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8+ T cells. Blood. 2017;129:3184–3195. doi: 10.1182/blood-2016-11-751040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shirley J.L., Keeler G.D., Sherman A., Zolotukhin I., Markusic D.M., Hoffman B.E., Morel L.M., Wallet M.A., Terhorst C., Herzog R.W. Type I IFN sensing by cDCs and CD4+ T cell help are both requisite for cross-priming of AAV capsid-specific CD8+ T cells. Mol. Ther. 2019 doi: 10.1016/j.ymthe.2019.11.011. Published online November 15, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sudres M., Ciré S., Vasseur V., Brault L., Da Rocha S., Boisgérault F., Le Bec C., Gross D.A., Blouin V., Ryffel B., Galy A. MyD88 signaling in B cells regulates the production of Th1-dependent antibodies to AAV. Mol. Ther. 2012;20:1571–1581. doi: 10.1038/mt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuranda K., Jean-Alphonse P., Leborgne C., Hardet R., Collaud F., Marmier S., Costa Verdera H., Ronzitti G., Veron P., Mingozzi F. Exposure to wild-type AAV drives distinct capsid immunity profiles in humans. J. Clin. Invest. 2018;128:5267–5279. doi: 10.1172/JCI122372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herzog R.W., Cooper M., Perrin G.Q., Biswas M., Martino A.T., Morel L., Terhorst C., Hoffman B.E. Regulatory T cells and TLR9 activation shape antibody formation to a secreted transgene product in AAV muscle gene transfer. Cell. Immunol. 2019;342:103682. doi: 10.1016/j.cellimm.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Butterfield J.S.S., Biswas M., Shirley J.L., Kumar S.R.P., Sherman A., Terhorst C., Ling C., Herzog R.W. TLR9-activating CpG-B ODN but not TLR7 agonists triggers antibody formation to factor IX in muscle gene transfer. Hum. Gene Ther. Methods. 2019;30:81–92. doi: 10.1089/hgtb.2019.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaiss A.K., Cotter M.J., White L.R., Clark S.A., Wong N.C.W., Holers V.M., Bartlett J.S., Muruve D.A. Complement is an essential component of the immune response to adeno-associated virus vectors. J. Virol. 2008;82:2727–2740. doi: 10.1128/JVI.01990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gardner M.R., Fetzer I., Kattenhorn L.M., Davis-Gardner M.E., Zhou A.S., Alfant B., Weber J.A., Kondur H.R., Martinez-Navio J.M., Fuchs S.P. Anti-drug antibody responses impair prophylaxis mediated by AAV-delivered HIV-1 broadly neutralizing antibodies. Mol. Ther. 2019;27:650–660. doi: 10.1016/j.ymthe.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinez-Navio J.M., Fuchs S.P., Pantry S.N., Lauer W.A., Duggan N.N., Keele B.F., Rakasz E.G., Gao G., Lifson J.D., Desrosiers R.C. Adeno-associated virus delivery of anti-HIV monoclonal antibodies can drive long-term virologic suppression. Immunity. 2019;50:567–575.e5. doi: 10.1016/j.immuni.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nayak S., Sarkar D., Perrin G.Q., Moghimi B., Hoffman B.E., Zhou S., Byrne B.J., Herzog R.W. Prevention and reversal of antibody responses against factor IX in gene therapy for hemophilia B. Front. Microbiol. 2011;2:244. doi: 10.3389/fmicb.2011.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Biswas M., Sarkar D., Kumar S.R.P., Nayak S., Rogers G.L., Markusic D.M., Liao G., Terhorst C., Herzog R.W. Synergy between rapamycin and FLT3 ligand enhances plasmacytoid dendritic cell-dependent induction of CD4+CD25+FoxP3+ Treg. Blood. 2015;125:2937–2947. doi: 10.1182/blood-2014-09-599266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arruda V.R., Favaro P., Finn J.D. Strategies to modulate immune responses: a new frontier for gene therapy. Mol. Ther. 2009;17:1492–1503. doi: 10.1038/mt.2009.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mendell J.R., Campbell K., Rodino-Klapac L., Sahenk Z., Shilling C., Lewis S., Bowles D., Gray S., Li C., Galloway G. Dystrophin immunity in Duchenne’s muscular dystrophy. N. Engl. J. Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calcedo R., Somanathan S., Qin Q., Betts M.R., Rech A.J., Vonderheide R.H., Mueller C., Flotte T.R., Wilson J.M. Class I-restricted T-cell responses to a polymorphic peptide in a gene therapy clinical trial for α-1-antitrypsin deficiency. Proc. Natl. Acad. Sci. USA. 2017;114:1655–1659. doi: 10.1073/pnas.1617726114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song Y., Morales L., Malik A.S., Mead A.F., Greer C.D., Mitchell M.A., Petrov M.T., Su L.T., Choi M.E., Rosenblum S.T. Non-immunogenic utrophin gene therapy for the treatment of muscular dystrophy animal models. Nat. Med. 2019;25:1505–1511. doi: 10.1038/s41591-019-0594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duan D. Micro-utrophin therapy for Duchenne muscular dystrophy. Mol. Ther. 2019;27:1872–1874. doi: 10.1016/j.ymthe.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Faust S.M., Bell P., Cutler B.J., Ashley S.N., Zhu Y., Rabinowitz J.E., Wilson J.M. CpG-depleted adeno-associated virus vectors evade immune detection. J. Clin. Invest. 2013;123:2994–3001. doi: 10.1172/JCI68205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin S.-W., Hensley S.E., Tatsis N., Lasaro M.O., Ertl H.C.J. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J. Clin. Invest. 2007;117:3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu T., Töpfer K., Lin S.-W., Li H., Bian A., Zhou X.Y., High K.A., Ertl H.C. Self-complementary AAVs induce more potent transgene product-specific immune responses compared to a single-stranded genome. Mol. Ther. 2012;20:572–579. doi: 10.1038/mt.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rogers G.L., Martino A.T., Zolotukhin I., Ertl H.C.J., Herzog R.W. Role of the vector genome and underlying factor IX mutation in immune responses to AAV gene therapy for hemophilia B. J. Transl. Med. 2014;12:25. doi: 10.1186/1479-5876-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sack B.K., Herzog R.W., Terhorst C., Markusic D.M. Development of gene transfer for induction of antigen-specific tolerance. Mol. Ther. Methods Clin. Dev. 2014;1:14013. doi: 10.1038/mtm.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Markusic D.M., Hoffman B.E., Perrin G.Q., Nayak S., Wang X., LoDuca P.A., High K.A., Herzog R.W. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol. Med. 2013;5:1698–1709. doi: 10.1002/emmm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao O., Dobrzynski E., Wang L., Nayak S., Mingle B., Terhorst C., Herzog R.W. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Faust S.M., Bell P., Zhu Y., Sanmiguel J., Wilson J.M. The role of apoptosis in immune hyporesponsiveness following AAV8 liver gene transfer. Mol. Ther. 2013;21:2227–2235. doi: 10.1038/mt.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mingozzi F., Liu Y.-L., Dobrzynski E., Kaufhold A., Liu J.H., Wang Y., Arruda V.R., High K.A., Herzog R.W. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J. Clin. Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mingozzi F., Hasbrouck N.C., Basner-Tschakarjan E., Edmonson S.A., Hui D.J., Sabatino D.E., Zhou S., Wright J.F., Jiang H., Pierce G.F. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perrin G.Q., Zolotukhin I., Sherman A., Biswas M., de Jong Y.P., Terhorst C., Davidoff A.M., Herzog R.W. Dynamics of antigen presentation to transgene product-specific CD4+ T cells and of Treg induction upon hepatic AAV gene transfer. Mol. Ther. Methods Clin. Dev. 2016;3:16083. doi: 10.1038/mtm.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Knolle P.A. Staying local-antigen presentation in the liver. Curr. Opin. Immunol. 2016;40:36–42. doi: 10.1016/j.coi.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 97.Dobrzynski E., Mingozzi F., Liu Y.-L., Bendo E., Cao O., Wang L., Herzog R.W. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood. 2004;104:969–977. doi: 10.1182/blood-2004-03-0847. [DOI] [PubMed] [Google Scholar]
- 98.Kumar S.R.P., Hoffman B.E., Terhorst C., de Jong Y.P., Herzog R.W. The Balance between CD8+ T cell-mediated clearance of AAV-encoded antigen in the liver and tolerance is dependent on the vector dose. Mol. Ther. 2017;25:880–891. doi: 10.1016/j.ymthe.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poupiot J., Costa Verdera H., Hardet R., Colella P., Collaud F., Bartolo L., Davoust J., Sanatine P., Mingozzi F., Richard I., Ronzitti G. Role of regulatory T cell and effector T cell exhaustion in liver-mediated transgene tolerance in muscle. Mol. Ther. Methods Clin. Dev. 2019;15:83–100. doi: 10.1016/j.omtm.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoffman B.E., Martino A.T., Sack B.K., Cao O., Liao G., Terhorst C., Herzog R.W. Nonredundant roles of IL-10 and TGF-β in suppression of immune responses to hepatic AAV-factor IX gene transfer. Mol. Ther. 2011;19:1263–1272. doi: 10.1038/mt.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Keeler G.D., Markusic D.M., Hoffman B.E. Liver induced transgene tolerance with AAV vectors. Cell. Immunol. 2019;342:103728. doi: 10.1016/j.cellimm.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kishnani P.S., Koeberl D.D. Liver depot gene therapy for Pompe disease. Ann. Transl. Med. 2019;7:288. doi: 10.21037/atm.2019.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keeler G.D., Kumar S., Palaschak B., Silverberg E.L., Markusic D.M., Jones N.T., Hoffman B.E. Gene therapy-induced antigen-specific Tregs inhibit neuro-inflammation and reverse disease in a mouse model of multiple sclerosis. Mol. Ther. 2018;26:173–183. doi: 10.1016/j.ymthe.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Finn J.D., Ozelo M.C., Sabatino D.E., Franck H.W.G., Merricks E.P., Crudele J.M., Zhou S., Kazazian H.H., Lillicrap D., Nichols T.C., Arruda V.R. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood. 2010;116:5842–5848. doi: 10.1182/blood-2010-06-288001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mátrai J., Cantore A., Bartholomae C.C., Annoni A., Wang W., Acosta-Sanchez A., Samara-Kuko E., De Waele L., Ma L., Genovese P. Hepatocyte-targeted expression by integrase-defective lentiviral vectors induces antigen-specific tolerance in mice with low genotoxic risk. Hepatology. 2011;53:1696–1707. doi: 10.1002/hep.24230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Annoni A., Cantore A., Della Valle P., Goudy K., Akbarpour M., Russo F., Bartolaccini S., D’Angelo A., Roncarolo M.G., Naldini L. Liver gene therapy by lentiviral vectors reverses anti-factor IX pre-existing immunity in haemophilic mice. EMBO Mol. Med. 2013;5:1684–1697. doi: 10.1002/emmm.201302857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hinderer C., Katz N., Buza E.L., Dyer C., Goode T., Bell P., Richman L.K., Wilson J.M. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum. Gene Ther. 2018;29:285–298. doi: 10.1089/hum.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hordeaux J., Wang Q., Katz N., Buza E.L., Bell P., Wilson J.M. The neurotropic properties of AAV-PHP.B are limited to C57BL/6J mice. Mol. Ther. 2018;26:664–668. doi: 10.1016/j.ymthe.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Duan D. Systemic AAV micro-dystrophin gene therapy for Duchenne muscular dystrophy. Mol. Ther. 2018;26:2337–2356. doi: 10.1016/j.ymthe.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Amado D.A., Rieders J.M., Diatta F., Hernandez-Con P., Singer A., Mak J.T., Zhang J., Lancaster E., Davidson B.L., Chen-Plotkin A.S. AAV-mediated progranulin delivery to a mouse model of progranulin deficiency causes t cell-mediated toxicity. Mol. Ther. 2019;27:465–478. doi: 10.1016/j.ymthe.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guedan S., Calderon H., Posey A.D., Jr., Maus M.V. Engineering and design of chimeric antigen receptors. Mol. Ther. Methods Clin. Dev. 2018;12:145–156. doi: 10.1016/j.omtm.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang X., Herzog R.W., Byrne B.J., Kumar S.R.P., Zhou Q., Buchholz C.J., Biswas M. Immune modulatory cell therapy for hemophilia B based on CD20-targeted lentiviral gene transfer to primary B cells. Mol. Ther. Methods Clin. Dev. 2017;5:76–82. doi: 10.1016/j.omtm.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Frank A.M., Buchholz C.J. Surface-engineered lentiviral vectors for selective gene transfer into subtypes of lymphocytes. Mol. Ther. Methods Clin. Dev. 2018;12:19–31. doi: 10.1016/j.omtm.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jamali A., Kapitza L., Schaser T., Johnston I.C.D., Buchholz C.J., Hartmann J. Highly efficient and selective CAR-gene transfer using CD4- and CD8-targeted lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2019;13:371–379. doi: 10.1016/j.omtm.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Milani M., Annoni A., Moalli F., Liu T., Cesana D., Calabria A., Bartolaccini S., Biffi M., Russo F., Visigalli I. Phagocytosis-shielded lentiviral vectors improve liver gene therapy in nonhuman primates. Sci. Transl. Med. 2019;11:eaav7325. doi: 10.1126/scitranslmed.aav7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brown B.D., Sitia G., Annoni A., Hauben E., Sergi L.S., Zingale A., Roncarolo M.G., Guidotti L.G., Naldini L. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood. 2007;109:2797–2805. doi: 10.1182/blood-2006-10-049312. [DOI] [PubMed] [Google Scholar]
- 117.Agudo J., Ruzo A., Kitur K., Sachidanandam R., Blander J.M., Brown B.D. A TLR and non-TLR mediated innate response to lentiviruses restricts hepatocyte entry and can be ameliorated by pharmacological blockade. Mol. Ther. 2012;20:2257–2267. doi: 10.1038/mt.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Borsotti C., Borroni E., Follenzi A. Lentiviral vector interactions with the host cell. Curr. Opin. Virol. 2016;21:102–108. doi: 10.1016/j.coviro.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 119.Rossetti M., Gregori S., Hauben E., Brown B.D., Sergi L.S., Naldini L., Roncarolo M.G. HIV-1-derived lentiviral vectors directly activate plasmacytoid dendritic cells, which in turn induce the maturation of myeloid dendritic cells. Hum. Gene Ther. 2011;22:177–188. doi: 10.1089/hum.2010.085. [DOI] [PubMed] [Google Scholar]
- 120.Pichlmair A., Diebold S.S., Gschmeissner S., Takeuchi Y., Ikeda Y., Collins M.K., Reis e Sousa C. Tubulovesicular structures within vesicular stomatitis virus G protein-pseudotyped lentiviral vector preparations carry DNA and stimulate antiviral responses via Toll-like receptor 9. J. Virol. 2007;81:539–547. doi: 10.1128/JVI.01818-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Agudo J., Ruzo A., Tung N., Salmon H., Leboeuf M., Hashimoto D., Becker C., Garrett-Sinha L.A., Baccarini A., Merad M., Brown B.D. The miR-126-VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat. Immunol. 2014;15:54–62. doi: 10.1038/ni.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rogers G.L., Herzog R.W. One microRNA controls both angiogenesis and TLR-mediated innate immunity to nucleic acids. Mol. Ther. 2014;22:249–250. doi: 10.1038/mt.2013.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gao D., Wu J., Wu Y.-T., Du F., Aroh C., Yan N., Sun L., Chen Z.J. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lahaye X., Satoh T., Gentili M., Cerboni S., Conrad C., Hurbain I., El Marjou A., Lacabaratz C., Lelièvre J.D., Manel N. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity. 2013;39:1132–1142. doi: 10.1016/j.immuni.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 125.Norton T.D., Zhen A., Tada T., Kim J., Kitchen S., Landau N.R. Lentiviral vector-based dendritic cell vaccine suppresses HIV replication in humanized mice. Mol. Ther. 2019;27:960–973. doi: 10.1016/j.ymthe.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wee E.G., Ondondo B., Berglund P., Archer J., McMichael A.J., Baltimore D., Ter Meulen J.H., Hanke T. HIV-1 conserved mosaics delivered by regimens with integration-deficient DC-targeting lentiviral vector induce robust T cells. Mol. Ther. 2017;25:494–503. doi: 10.1016/j.ymthe.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brown B.D., Venneri M.A., Zingale A., Sergi Sergi L., Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat. Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- 128.Brown B.D., Cantore A., Annoni A., Sergi L.S., Lombardo A., Della Valle P., D’Angelo A., Naldini L. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood. 2007;110:4144–4152. doi: 10.1182/blood-2007-03-078493. [DOI] [PubMed] [Google Scholar]
- 129.Annoni A., Brown B.D., Cantore A., Sergi L.S., Naldini L., Roncarolo M.-G. In vivo delivery of a microRNA-regulated transgene induces antigen-specific regulatory T cells and promotes immunologic tolerance. Blood. 2009;114:5152–5161. doi: 10.1182/blood-2009-04-214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Akbarpour M., Goudy K.S., Cantore A., Russo F., Sanvito F., Naldini L., Annoni A., Roncarolo M.G. Insulin B chain 9–23 gene transfer to hepatocytes protects from type 1 diabetes by inducing Ag-specific FoxP3+ Tregs. Sci. Transl. Med. 2015;7:289ra81. doi: 10.1126/scitranslmed.aaa3032. [DOI] [PubMed] [Google Scholar]
- 131.Merlin S., Cannizzo E.S., Borroni E., Bruscaggin V., Schinco P., Tulalamba W., Chuah M.K., Arruda V.R., VandenDriessche T., Prat M. A novel platform for immune tolerance induction in hemophilia A mice. Mol. Ther. 2017;25:1815–1830. doi: 10.1016/j.ymthe.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Merlin S., Follenzi A. Transcriptional targeting and microRNA regulation of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2019;12:223–232. doi: 10.1016/j.omtm.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Milani M., Annoni A., Bartolaccini S., Biffi M., Russo F., Di Tomaso T., Raimondi A., Lengler J., Holmes M.C., Scheiflinger F. Genome editing for scalable production of alloantigen-free lentiviral vectors for in vivo gene therapy. EMBO Mol. Med. 2017;9:1558–1573. doi: 10.15252/emmm.201708148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Uchida N., Nassehi T., Drysdale C.M., Gamer J., Yapundich M., Bonifacino A.C., Krouse A.E., Linde N., Hsieh M.M., Donahue R.E. Busulfan combined with immunosuppression allows efficient engraftment of gene-modified cells in a rhesus macaque model. Mol. Ther. 2019;27:1586–1596. doi: 10.1016/j.ymthe.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Drysdale C.M., Tisdale J.F., Uchida N. Immunoresponse to gene-modified hematopoietic stem cells. Mol. Ther. Methods Clin. Dev. 2019;16:42–49. doi: 10.1016/j.omtm.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Squeri G., Passerini L., Ferro F., Laudisa C., Tomasoni D., Deodato F., Donati M.A., Gasperini S., Aiuti A., Bernardo M.E. Targeting a pre-existing anti-transgene T cell response for effective gene therapy of MPS-I in the mouse model of the disease. Mol. Ther. 2019;27:1215–1227. doi: 10.1016/j.ymthe.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Markusic D.M. ERT degrades gene therapy for storage disorder. Mol. Ther. 2019;27:1207–1208. doi: 10.1016/j.ymthe.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shi Q. Platelet-targeted gene therapy for hemophilia. Mol. Ther. Methods Clin. Dev. 2018;9:100–108. doi: 10.1016/j.omtm.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen Y., Schroeder J.A., Kuether E.L., Zhang G., Shi Q. Platelet gene therapy by lentiviral gene delivery to hematopoietic stem cells restores hemostasis and induces humoral immune tolerance in FIXnull mice. Mol. Ther. 2014;22:169–177. doi: 10.1038/mt.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]