Abstract
Transfer of genes by adeno-associated virus (AAV) vectors is benefiting patients with particular genetic defects. Challenges remain by rejection of AAV-transduced cells, which may be caused by CD8+ T lymphocytes directed to AAV capsid antigens. Reducing the number of CpG motifs from the genome of AAV vectors reduces expansion of naive T cells directed against an epitope within the capsid. In contrast, AAV capsid-specific memory CD8+ T cells respond more vigorously to AAV vectors lacking CpG motifs than to those with CpG motifs presumably reflecting dampening of T cell expansion by cytokines from the innate immune system. Depending on the purification method, AAV vector preparations can contain substantial amounts of empty AAV particles that failed to package the genome. Others have used empty particles as decoys to AAV-neutralizing antibodies. We tested if empty AAV vectors given alone or mixed with genome-containing AAV vectors induce proliferation of naive or memory CD8+ T cells directed to an antigen within an AAV capsid. Naive CD8+ T cells failed to respond to empty AAV vectors, which in contrast induced expansion of AAV-specific memory CD8+ T cells.
Keywords: AAV gene transfer, mouse model, T cell response, primary T cell response, memory T cell response, CpG motifs
Stimulation of naive, but not memory CD8+ T cells, to capsids of AAV vectors depends on CpG motifs within the vectors’ genomes. Naive T cells respond to CpGhi but not CpGlow or empty vectors. Memory T cells respond vigorously to CpGlow or empty vectors, but not to CpGhigh vectors.
Introduction
Permanent replacement therapy of gene defects by AAV vectors has made impressive progress in recent years. An adeno-associated virus (AAV)-mediated treatment for retinal dystrophy due to mutation of the RPE65 gene,1, 2, 3 called Luxturna, has gained approval by the Federal Drug Administration and is the first licensed in vivo gene therapy in the US to correct an inherited disease. Other successes are being reported for treatment of hemophilia B, an X-linked disease for which AAV vectors expressing human factor 9 (hFIX) have achieved in some patients functional correction of their bleeding disorder.4,5 A more recent trial reported AAV-FVIII-mediated correction of hemophilia A.6
Challenges remain for AAV-mediated gene transfer to liver. Some of the individuals enrolled into early clinical trials for treatment of hemophilia B showed within a few weeks after gene transfer evidence of hepatocyte destruction accompanied by loss of transgene product expression.7,8 This was linked to increases in circulating AAV capsid-specific CD8+ T cells that presumably attacked and destroyed the AAV-transduced cells.8 Most humans are infected with AAVs together with a helper virus during childhood and therefore have immunological B and T cell memory to AAV capsid antigens.9, 10, 11 T cell epitopes between different AAV serotypes are largely cross-reactive,12 so that T cells induced by one serotype respond to others. It was initially postulated that recall of AAV-specific memory CD8+ T cells causes the decline of AAV-transduced cells in humans, although a role for de novo stimulated naive T cells to novel epitopes within the AAV gene transfer vehicles has not been ruled out. De facto, the very slow onset of T cell responses to AAV capsid antigen in some patients may argue for primary rather than secondary T cell responses. Induction of AAV capsid-specific T cell responses can be circumvented by dose reductions upon optimizing transduction rates and/or the biological activity of the transgene product.13,14 Alternatively, the destructive effects of AAV capsid-specific CD8+ T cells can be blunted by immunosuppressive drugs such as prednisone.4,15
Stimulation of CD8+ T cells by AAV vectors has been linked to innate responses induced by Toll-like receptor (TLR)9-stimulating CpG sequences within the vector genome.16, 17, 18 In mice, depletion of all CpG sequences but for those essential for the integrity of the inverted terminal repeat (ITR) sequences was shown to prevent rejection of a highly immunogenic chimeric rhesus macaque AAV serotype called AAVrh32.33.19,20 CpG-reduced AAV8 vectors have been used in clinical trials for correction of hemophilia B; notwithstanding, recipients of high doses of the CpG-reduced double-stranded AAV8 vector still showed increases of AAV capsid-specific CD8+ T cell responses following gene transfer and required immunosuppression to prevent loss of FIX expression.4 Studies in mice assessed the effects of CpG sequences on primary AAV capsid and transgene product-specific CD8+ T cells,16, 17, 18 which may have different requirements for their successful activation than already primed memory CD8+ T cells.
Another challenge faced by systemic AAV vector-mediated gene correction is that many humans have pre-existing neutralizing antibodies to AAV, which even when present at low concentrations significantly reduce AAV vector transduction of liver cells.21 Such antibodies are induced due to natural infections in childhood. Antibodies to AAV2 and AAV1 are most common and can be detected in 40%–60% of adults with higher prevalence rates in Africa than Europe or the US. Seroprevalence rates of neutralizing antibodies to AAV5, 7, 8, or 9 are slightly lower but still well above 30% in most countries.9,10 Furthermore, AAV vector-mediated gene transfer induces AAV-neutralizing antibodies,22 which precludes a second administration of the vector for additional treatment.23 As some neutralizing antibodies cross-react between different AAV serotypes,24 options for changing serotypes for sequential gene transfer are limited. Mixing an excess amount of empty AAV capsids, i.e., AAV particles that during production failed to incorporate a genome, also called “empties,” with genome-containing vectors was shown to blunt the impact of AAV-neutralizing antibodies on transduction rates of intravenously injected AAV vectors.25
As mentioned above, AAV vector-mediated gene transfer is hampered by rejection of AAV-transduced cells by human CD8+ T cells directed against epitopes present within AAV capsids.4,8,26 Applying genome-containing AAV vectors together with a large dose of empties could potentially increase induction of AAV capsid-specific CD8+ T cells and thus negate the benefits of modifications that allow for lower vector doses.
Here, we tested the effects of CpG motifs within the genome of AAV8 or AAV2 vectors on primary and memory CD8+ T cell responses to an epitope within the vector’s capsid. As wild-type AAV8 capsid fails to be recognized by mouse CD8+ T cells, we inserted, as described previously,27 10 copies of SIINFEKL, the immunodominant H-2b-binding epitope of ovalbumin, into VP2 of AAV8 (AAV8SIINFEKL). Vectors expressed a CpG-depleted form of β- galactosidase (β-Gal) (LacZ) under the control of the human elongation factor-1 alpha (EF1) promoter, which lacks CpG sequences, or the CpG-rich chicken β-actin (CB) promoter. Our results show that CpG motifs are essential to drive proliferation of naive capsid-specific CD8+ T cells but blunt and delay expansion of memory CD8+ T cells. We assessed if adding empty AAV particles to genome-containing AAV vectors increases the proliferative response of naive or memory capsid-specific CD8+ T cells. Our results show that empty AAV capsids do not drive proliferation of naive CD8+ T cells even if given together with genome-containing vectors. In contrast, they drive proliferation of AAV capsid-specific memory CD8+ T cells.
Results
Vector Characteristics
Vectors carry a CpG-depleted sequence of β-Gal under the control of the EF1 (AAV8SIINFEKL-EF1-LacZ) or CB (AAV8SIINFEKL-CB-LacZ) promoter. The CpG content of these vectors is as follows: The AAV8-CB-LacZ vector has 113 CpGs (16 in each of the 2 ITRs, 11 in the enhancer, 45 in the CB promoter, 7 in the intron, and 18 in the poly(A) tail). The AAV8-EF1-LacZ vector has 46 CpGs (16 in each of the 2 ITRs, 1 in the enhancer, and 13 in the poly(A) tail). Vectors were tested for incorporation of SIINFEKL into the vector capsid by gel electrophoresis followed by silver stains, which, as described previously,27 showed the expected shift of VP2 due to the added SIINFEKL sequences. Vectors were tested in vitro for expression of β-Gal in transduced HEK293 cells, where equal doses of vectors achieved equal numbers of transgene product expressing cells (data not shown). In contrast, upon intravenous (i.v.) injection into male C57BL/6 mice, using an equal dose of 1011 vector genomes (vg) per animal, there was a striking difference in numbers of hepatocytes expressing β-Gal (Figure S1). Most cells of livers of mice that received the AAV8SIINFEKL-EF1-LacZ vector were β-Gal positive when tested 2 weeks later, while upon transfer of the AAV8SIINFEKL-CB-LacZ vector, less than 5% of cells showed β-Gal expression.
In Vivo Responses of SIINFEKL-Specific Primary and Memory CD8+ T Cells to the Capsid of CpGhigh and CpGlow AAV8SIINFEKL Vectors
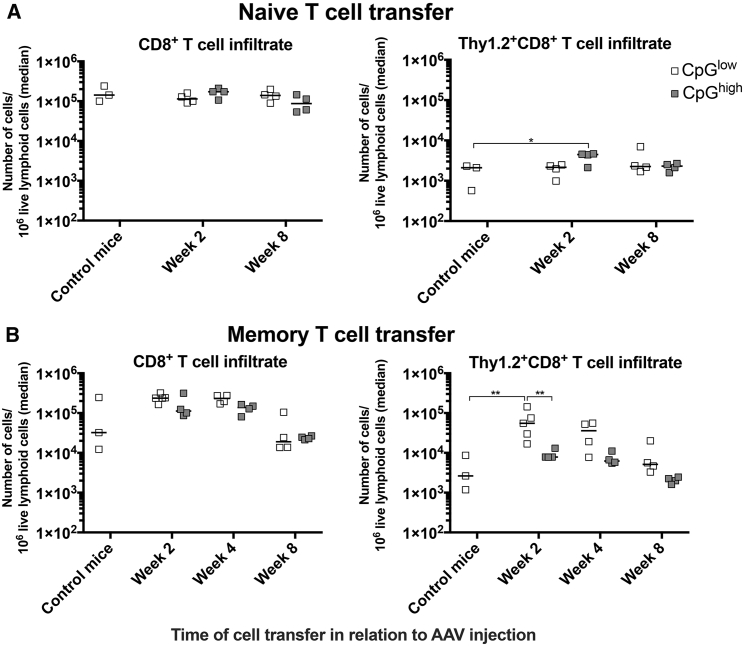
We tested if CpG motifs influence responses of AAV capsid-, i.e., SIINFEKL-specific, primary or memory CD8+ T cells. Groups of male Thy1.1+ C57BL/6 recipient mice were injected i.v. with 1011 vg of AAV8SIINFEKL-EF1-LacZ (CpGlow) or AAV8SIINFEKL-CB-LacZ (CpGhigh) vectors. Control mice were not injected with AAV8 vectors. 2 to 8 weeks after injection of recipient mice, donor memory splenocytes from Thy1.2+ C57BL/6 mice that had been injected at least 2 months earlier with a chimpanzee origin adenovirus vector expressing ovalbumin, which contains the SIINFEKL epitope, as a fusion protein together with the nucleoprotein of influenza A virus and GFP (AdC7-NP-Ova-GFP), or from naive OT-1 mice, which carry a transgenic T cell receptor to SIINFEKL, were isolated and stained with carboxyfluorescein N-hydroxysuccinimidyl ester (CFSE). They were then transferred i.v. into recipient mice. Ten days later, lymphocytes were isolated from livers and spleens of the recipient mice and analyzed for CD8+ T cells. We determined the contribution of all CD8+ T cells as well as of donor-derived Thy1.2+ CD8+ T cells to the hepatic lymphocytic infiltrate. The overall gating strategy for this and all other T cell experiments is shown in Figure S2. In mice injected with naive OT-1 donor cells, presence of the AAV8SIINFEKL-EF1-LacZ vectors failed to increase hepatic infiltration by CD8+ T cells. In contrast, there was a small but statistically significant increase of donor-cell-origin CD8+ T cells in livers of AAV8SIINFEKL-CB-LacZ-injected mice (Figure 1A). Mice injected with SIINFEKL-specific memory T cells 2 or 4 weeks after injection of either of the two AAV vectors showed a trend toward increased hepatic infiltration with CD8+ T cells, which failed to reach significance. There was a significant increase of donor-derived memory CD8+ T cells if cells were transferred at 2 weeks after injection of the AAV8SIINFEKL-EF1-LacZ vector. In mice that received donor cells at 4 weeks after AAV8SIINFEKL-EF1-LacZ injection, there was still a trend toward increased memory donor CD8+ T cell infiltration, which subsided by week 8 (Figure 1B).
Figure 1.
Hepatic T Cell Infiltrates in Donor Mice
Mice were injected with AAV8-LacZ vectors with high (CB promoter, gray symbols) or low (EF1 promoter, white symbols) CpG content. At the indicated time thereafter, they were injected i.v. with CFSE-labeled splenocytes from naive OT-1 mice (A) or with CFSE-labeled splenocytes from mice that had been injected at least 2 months earlier with AdC7-NP-Ova-GFP (B). Three naive mice were injected with splenocytes as well and served as controls. Lymphocytes were isolated from individual mice 10 days after lymphocyte transfer, and upon staining numbers of the indicated cell subsets were determined and normalized to 106 live lymphoid cells. Graphs on the left show relative numbers of CD8+ T cells in liver. Right graphs show numbers of donor-derived Thy1.2+CD8+ cells in livers. Data are shown for individual mice, with lines indicting medians. Significant differences in this and all subsequent graphs are indicated by lines with stars above. Number of stars show level of significance, *p ≤ 0.05 to > 0.01, **p ≤ 0.01 to > 0.001, ***p ≤ 0.01 to > 0.001, ****p ≤ 0.0001.
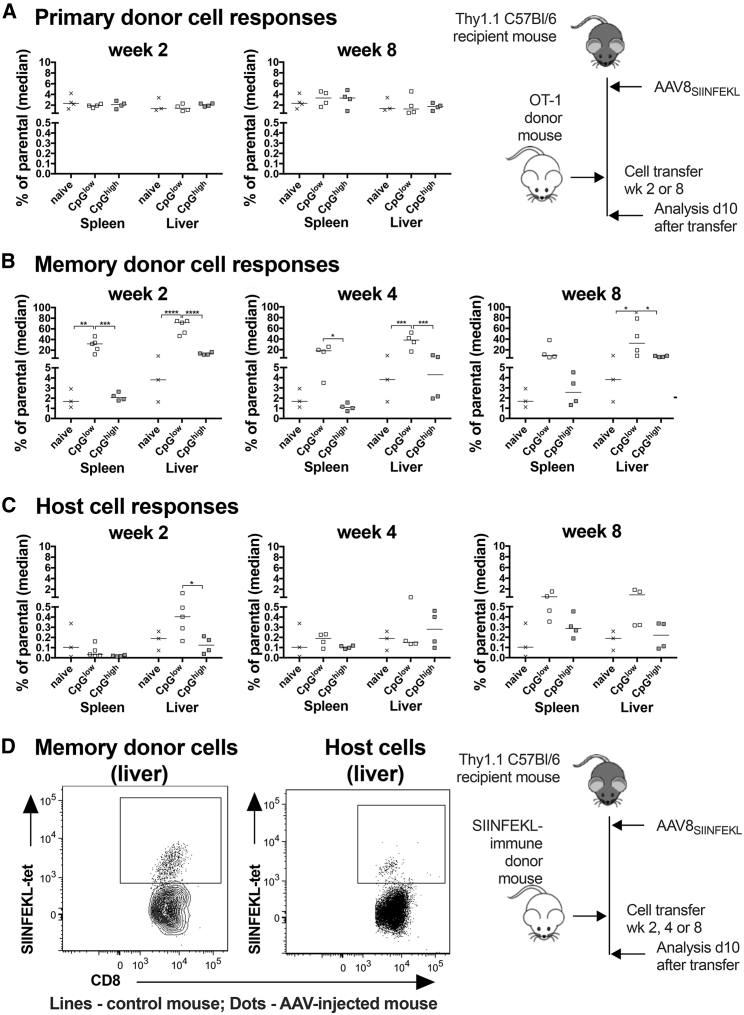
To test if presence of AAV vectors affected frequencies of SIINFEKL-specific naive or memory CD8+ T cells of donor origin, we analyzed lymphocytes from spleens and livers of control mice and mice that had been injected with AAV8SIINFEKL-EF1-LacZ or AAV8SIINFEKL-CB-LacZ vectors 2–8 weeks prior to cell transfer. Frequencies of SIINFEKL-specific CD8+ T cells from OT-1 mice that had been naive prior to transfer were not increased in presence of either of the AAV8SIINFEKL vectors within the host mice (Figure 2A). SIINFEKL-specific memory donor CD8+ T cells, on the other hand, were markedly more frequent in spleens and livers of host mice injected with the AAV8SIINFEKL-EF1-LacZ vector compared to those carrying no vector or the AAV8SIINFEKL-CB-LacZ vector. This difference was especially pronounced if donor cells were transferred early at 2 weeks after AAV8SIINFEKL-EF1-LacZ vector injection (Figures 2B and 2D).
Figure 2.
SIINFEKL-Specific CD8+ T Cell Responses within Hosts
AAV vector-injected host mice were injected with donor cells from naive OT-1 mice (A) or AdC7-NP-Ova-GFP-immune (B) donors at the indicated times after gene transfer. A naive host mouse served as a control for each time point. Data from naive mice were pooled, and the same data are shown for each time point. (A and B) Frequencies of SIINFEKL-tetramer+ CD8+ donor cells in spleens and livers of host mice over all CD44+CD8+ cells. (C) Frequencies of host-origin SIINFEKL-specific CD8+ T cells. Lines with stars above indicate significant differences as detailed in legend to Figure 1. (D) Frequencies of SIINFEKL-specific donor or host CD8+ T cells comparing non-AAV-injected mice (contour blot) to mice that had been injected 8 weeks prior to cell transfer with a CpGlow AAV vector (dot blot). The cartoon to the right of (A) shows the experimental design for the naive cell transfer. The cartoon to the right of (D) shows the experimental design for the naive cell transfer.
To assess if transfer of SIINFEKL-specific memory CD8+ T cells promoted induction of host CD8+ T cell responses, we analyzed frequencies of SIINFEKL-tetramer+Thy1.2− CD8+ T cells. A significant host origin SIINFEKL-specific CD8+ T cell response was only observed in spleens and livers of mice that had received the AAV8SIINFEKL-EF1-LacZ vector 2 or 8 weeks before cell transfer (Figures 2C and 2D), suggesting that activation of the host CD8+ T cells had been driven by the strong donor cell response.
Functions of SIINFEKL-Specific CD8+ T Cells after Their Transfer into AAV8SIINFEKL-Injected Mice
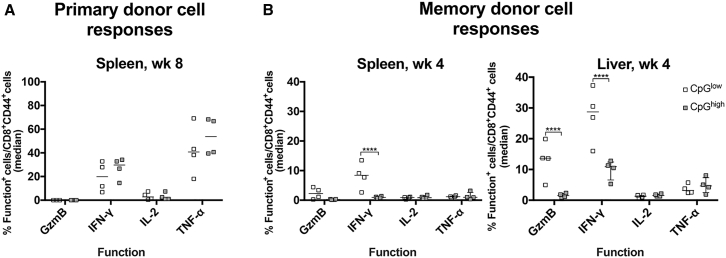
Staining for cell-surface markers such as the Thy1 antigen or the T cell receptor tested for by the SIINFEKL-specific tetramer informs on the origin and antigen-specificity of the CD8+ T cells but allows no insight into the cells’ functions. We therefore tested OT-1 CD8+ T cells transferred into mice injected with the AAV8SIINFEKL vectors 8 weeks after and memory donor-derived CD8+ T cells transferred into mice 4 weeks after AAV injection in vitro by intracellular cytokine staining (ICS) for production of granzyme B (GzmB), interferon (IFN)-γ, interleukin (IL)-2, and/or tumor necrosis factor (TNF)-α in response to the SIINFEKL peptide. Transfer of splenocytes from naive OT-1 mice or AdC7-NP-Ova-GFP-immunized mice into naive hosts failed to trigger a cytokine response (data not shown). Naive OT-1 cells transferred into mice injected with the AAV8SIINFEKL-CB-LacZ or the AAV8SIINFEKL-EF1-LacZ vector and isolated from spleens 10 days later produced mainly TNF-α followed by IFN-γ; responses were comparable in mice injected with either of the two vectors (Figure 3A). In mice that received memory CD8+ T cells, IFN-γ responses were significantly higher in spleens and liver of mice that had been injected with the AAV8SIINFEKL-EF1-LacZ vector, while in the same comparison GzmB+ CD8+ T cell frequencies were significantly higher only in livers (Figure 3B).
Figure 3.
Functions of Donor-Derived SIINFEKL-Specific CD8+ T Cells
CD8+ T cells from donor splenocytes isolated from host mice that had been transferred with naive OT-1 cells 8 weeks after injection with AAV vector (A) or from donor lymphocytes from spleens and livers of mice that had been injected with AdC7-NP-Ova-GFP-immune donor cells 4 weeks after AAV gene transfer (B) were tested for release of cytokines in response to the SIINFEKL peptide. Background data obtained upon culture with an irrelevant peptide were subtracted.
Proliferation of Donor-Derived SIINFEKL-Specific CD8+ T Cells to AAV Capsid
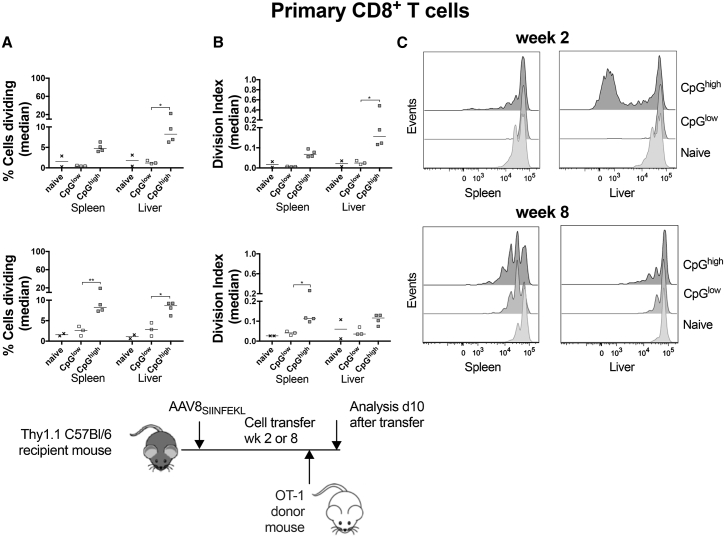
Proliferation of donor cells was tested for by determining loss of CFSE in donor-origin SIINFEKL-specific CD8+ T cells at 10 days after splenocyte transfer into congenic host mice (Figure 4). Donor-derived naive OT-1 CD8+ T cells showed some minor homeostatic proliferation upon transfer into naive mice that had not been injected with an AAV8SIINFEKL vector. OT-1 CD8+ T cells transferred 2 weeks after injection of AAV8SIINFEKL vectors with or without CpG motifs failed to show any significant proliferation in spleen. In livers, these cells showed enhanced proliferation upon transfer into mice carrying the AAV8SIINFEKL-CB-LacZ vector, as evidenced by increases in the percent of proliferating cells as well as in the division index, which takes numbers of cell cycles into account; they failed to proliferate in response to the AAV8SIINFEKL-EF1-LacZ vector (Figure 4A). In mice transferred 8 weeks after AAV8SIINFEKL vector injection, OT-1 CD8+ T cells showed significant proliferation in spleen of mice that received the AAV8SIINFEKL-CB-LacZ; they failed to respond to the AAV8SIINFEKL-EF1-LacZ vector. In liver, this difference was only significant for the percent of cells that divided (Figure 4B). Overall, these data show that stimulation of naive CD8+ T cells requires the presence of CpG motifs within the AAV8 vector’s genome.
Figure 4.
Proliferation of Naive OT-1 Cells in AAVSIINFEKL-Injected Host Mice
Proliferation was assessed by measuring loss of CFSE expression within Thy1.2+CD44+CD8+SIINFEKL-tetramer+ cells using host mice that received the naive OT-1 cell transfer 2 or 8 weeks after AAV injection. Mice that had not been injected with an AAV vector but received the cell transfer served as controls (naive). Thy1.2+CD44+CD8+SIINFEKL-tetramer+ cells were gated manually on generations 0–6. Percentages of cells that proliferated and the division index were calculated as described in http://v9docs.flowjo.com/html/proliferation.html. (A) Percent of cells that proliferated. (B) Division index. (C) Representative histograms. The cartoon at the bottom shows the experimental design.
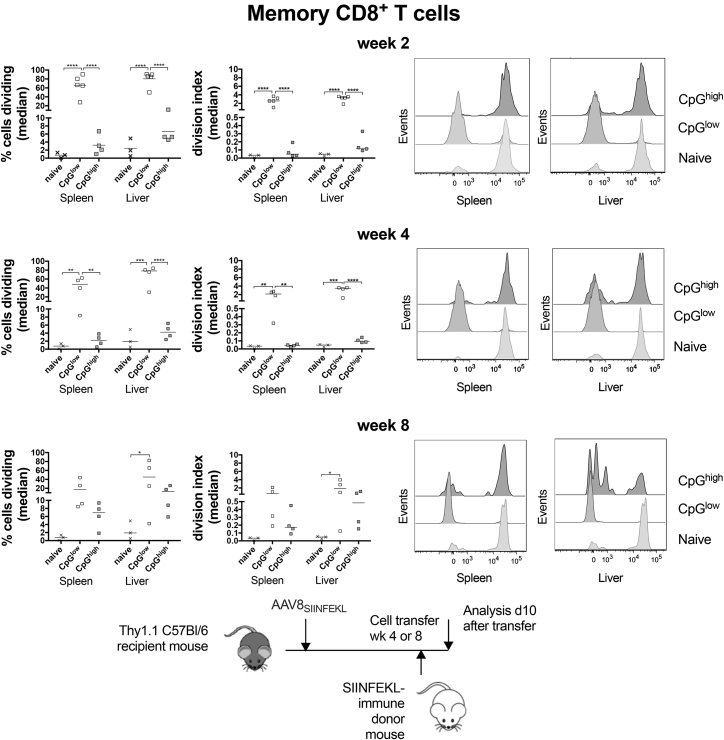
Opposite results were obtained for SIINFEKL-specific memory CD8+ T cells; they showed robust proliferation in spleens and liver upon their transfer into mice that had received the AAV8SIINFEKL-EF1-LacZ vector 2 (Figure 5A) or 4 weeks (Figure 5B) earlier. Upon later cell transfer at week 8 after vector injection, SIINFEKL-specific CD8+ T cells of one of the mice in the AAV8SIINFEKL-EF1-LacZ vector group showed loss of proliferation, while in the other three mice, these CD8+ T cells proliferated vigorously, as evidenced by complete loss of CFSE (Figure 5C). Cells transferred into mice injected with the AAV8SIINFEKL-CB-LacZ vector showed marginal proliferation if transferred 2 or 4 weeks after vector injection. Proliferation became more pronounced upon later transfer at week 8. These data show that CpG motifs are not needed to drive expansion of capsid-specific memory CD8+ T cells but instead appear to delay their proliferative response.
Figure 5.
Proliferation of Memory CD8+ T Cells in AAVSIINFEKL-Injected Host Mice
Loss of CFSE expression within Thy1.2+CD44+CD8+SIINFEKL-tetramer+ cells using host mice that received splenocytes from AdC7-NP-Ova-GFP-immune donors. The figure design mirrors that of Figure 4. The cartoon at the bottom shows the experimental design.
To test if antigen able to drive proliferation of memory CD8+ T cells to the AAV8SIINFEKL-CB-LacZ vector came up very early and then disappeared by week 2, we transferred cells into mice 2 days after vector injection. Proliferation was marginal, with an increase of division index from a median of 0.03 (range, 0–0.05) in naive mice to a median of 0.14 (range, 0.05–0.3) in AAV8SIINFEKL-CB-LacZ-injected mice, indicating that lack of a response to the CpG motif-containing vector was not linked to accelerated degradation of the vector (data not shown).
The Effect of Empty AAV Particles on Primary CD8+ T Cell Responses to AAV Capsid
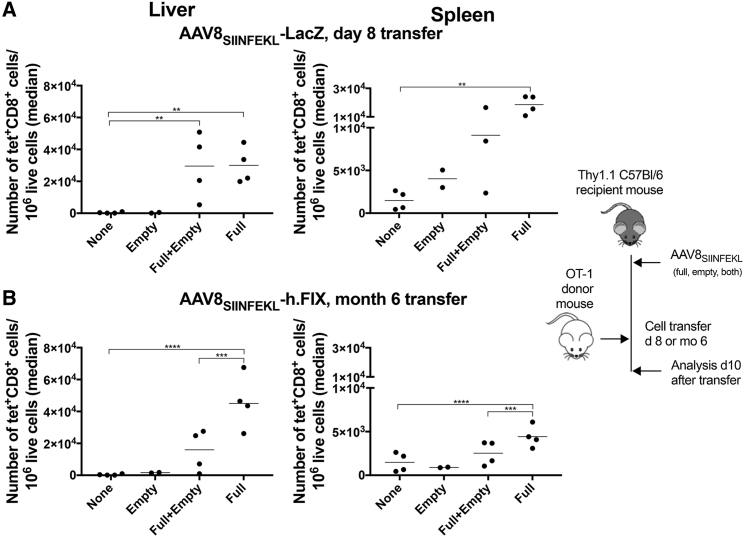
We reported previously that AAV particles without genome fail to elicit strong proliferative CD8+ T cell responses to AAV capsid antigens in mice.27 To assess if empty AAV8 particles increase primary AAV capsid-specific CD8+ T cell responses to AAV genome-containing vectors, we mixed AAV8SIINFEKL-CB-LacZ vectors given at 1 × 1011 vg/mouse with the equivalent of 5 × 1011 vg of empty AAV8SIINFEKL particles. Other mice received either only the empty particles or the genome-containing vectors at the same doses or were left untreated. All vectors were given i.v. Two weeks after AAV injection mice, were transferred i.v. with 5 × 106 CFSE-labeled OT-1 splenocytes. In a follow-up experiment to assess if empty AAV particles would delay the kinetics of T cell proliferation, mice were injected with 1 × 1011 vg/mouse of an AAV8SIINFEKL-hAAT-hFIX vector given alone or mixed with 5 × 1011 vg of empty AAV8SIINFEKL particles. Other mice received only the empty particles or nothing. Mice were injected with 5 × 106 CFSE-labeled OT-1 splenocytes 6 months after vector injection. In both experiments, mice were euthanized 10 days after OT-1 cell transfer, and lymphocytes from spleens and livers were analyzed.
In both experiments (Figures 6A and 6B), the genome-containing vectors increased recovery of SIINFEKL-specific CD8+ T cells from livers and spleens. Empty AAV8SIINFEKL particles given either alone or with genome-containing vector did not affect numbers of SIINFEKL-specific CD8+ T cells in spleens or liver if cells were transferred 2 weeks after AAV injection (Figure 6A) but decreased their numbers in the late transfer experiment (Figure 6B).
Figure 6.
Effect of “AAV8SIINFEKL Empties” on Hepatic T Cell Infiltrates upon Transfer of Naive SIINFEKL-Specific CD8+ T Cells
(A) Thy1.1+ C57BL/6 mice were injected with nothing (none), empty AAV8SIINFEKL particles alone (empty), or mixed with AAV8 SIINFEKL-CB-LacZ (A) or AAV8SIINFEKL-hAAT-h.FIX vectors (B). 8 days (A) or 6 months (B) after AAV injection, they were transferred with OT-1 splenocytes. The graphs show number of donor-derived SIINFEKL-specific CD8+ T cells identified by staining with an antibody to the congenic Thy1 marker and a specific tetramer and normalized to 106 live lymphoid cells in livers and spleens of individual host mice at 10 days after cell transfer, with medians shown by lines. The lines with stars above show significant differences between the connected groups. The cartoon to the right shows the experimental design.
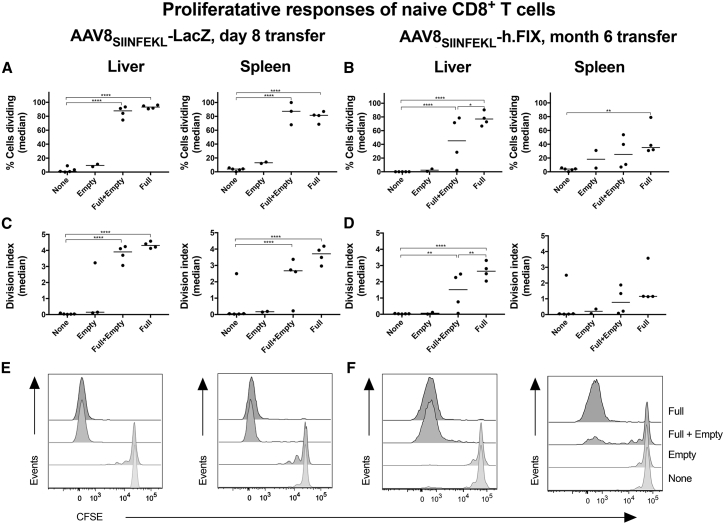
Accordingly, when donor-derived OT-1 CD8+ T cells were assessed for proliferation within the host mice by measuring loss of CFSE, early transfer into mice that had received the genome-containing vectors caused robust proliferative responses, as shown by increases in percentages of cycling cells (Figure 7A) and the division index (Figure 7C). Empty AAV8SIINFEKL particles failed to elicit proliferative responses of donor-derived CD8+ T cells beyond the homeostatic responses also observed upon their transfer into mice that had not been injected with an AAV vector. Empty AAV8SIINFEKL particles also failed to increase proliferation to the genome-containing AAV8vvSIINFEKL vectors.
Figure 7.
Effect of “AAV8SIINFEKL Empties” on Proliferation of Naive SIINFEKL-Specific CD8+ T Cells
Donor-derived SINFEKL-specific CD8+ T cells from the same groups as in Figure 6 were analyzed for loss of CFSE. (A) Percentages of dividing donor cells transferred 8 days after AAV injection. (B) Percentages of dividing donor cells transferred 6 months after AAV injection. (C) Division index of donor cells transferred 8 days after AAV injection. (D) Division index of donor cells transferred 6 months after AAV injection. (E) Histograms of CFSE loss in donor cells transferred 8 days after AAV injection. (F) Histograms of CFSE loss in donor cells transferred 6 months after AAV injection.
We repeated the experiment using a later time for transfer and an AAV8 vector with a clinically relevant transgene, i.e., hFIX controlled by a hepatocyte-specific promoter. Upon delayed transfer of CFSE-labeled OT-1 cells donor-derived CD8+ T cells, proliferation of donor-derived CD8+ T cells remained high within livers of mice injected 6 months early with an AAV8SIINFEKL-hAAT-hFIX vector (Figures 7B and 7D). Proliferation decreased in spleens. The livers of mice that received the mixtures of genome-containing vectors and empty particles showed a reduction of proliferation of OT-1 CD8+ T cells compared to mice that had only been injected with the genome-containing vector. Again, SIINFEKL-specific CD8+ T cells failed to show loss of CFSE upon their transfer into mice injected 6 months earlier with empty vectors. Overall, the results show that empty AAV particles fail to trigger proliferation of AAV-capsid-specific naive CD8+ T cells.
The Effect of Empty AAV Particles on Secondary CD8+ T Cell Responses to AAV Capsid
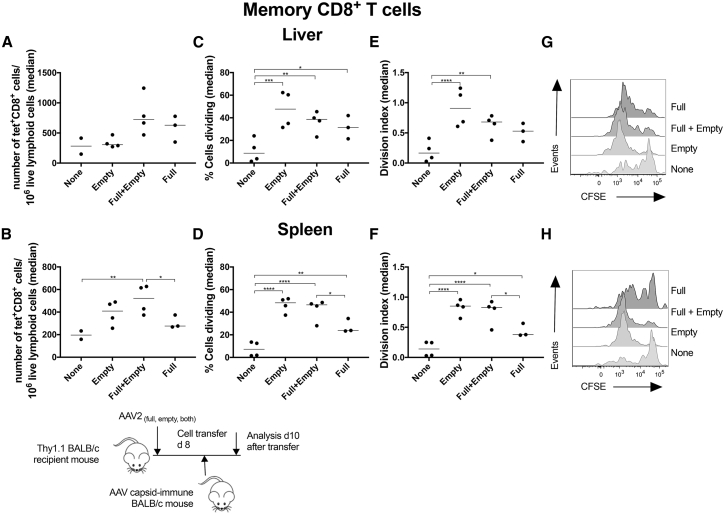
In this experiment, rather than relying on proliferation of CD8+ T cells to an artificial AAV capsid antigen, i.e., SIINFEKL, we immunized Thy1.2+ BALB/c mice with a simian adenovirus vector of serotype 7 (AdC7) expressing the capsid antigen of AAV8, which shares cross-reactive CD8+ T cell epitopes with AAV2 (AdC7-AAVcap). As the capsid antigen of AAV8 is not appropriately processed for recognition by capsid-specific CD8+ T cells,27 we used 1 × 1011 vg of a CpG-reduced AAV2-EF1-LacZ vector to inject Thy1.1+ BALB/c host mice. As in the above-described experiment, other host mice were injected with 1 × 1011 vg of the AAV2-EF1-LacZ vector with the equivalent of 5 × 1011 vg of empty AAV2 particles. Control mice received no vector or empties only at the equivalent of 5 × 1011 vg/mouse. CFSE-labeled donor splenocytes (8 × 107/mouse) were transferred 14 days after AAV2 vector injection and then analyzed from spleens and livers 10 days later. There was a trend toward increased recovery of donor-derived AAV capsid-specific CD8+ T cells from livers of mice injected with the AAV2-EF1-LacZ vector with or without empties (Figure 8A), while in spleens, significantly higher numbers of such cells were detected in mice that were injected with the AAV2 EF1-LacZ vector together with empties (Figure 8B). In livers and spleens of mice, which received either the genome-containing vector, the empties, or both, percentages of cells that proliferated significantly increased compared to those in control mice. Furthermore, in spleens, significantly higher percentages of cells cycled in mice that received mixtures of genome-containing vectors and empty particles compared to mice that only received the genome-containing vectors. These results were mirrored by the division indices, which increased most significantly over those in controls in mice that had received empty particles with or without genome-containing particles. The results show that empty AAV particles drive proliferation of capsid-specific memory CD8+ T cells. Ideally, we would have liked to confirm our results for primary AAV capsid-specific T cells using the endogenous epitope, but this was technically not feasible due to a paucity such T cells in a naive host.
Figure 8.
Effect of “AAV Empties” on Hepatic Infiltration by and Proliferation of Memory AAV Capsid-Specific CD8+ T Cells
BALB/c host mice were injected with an AAV2-EF1-LacZ. They were injected i.v. 14 days later with CFSE-labeled splenocytes from congenic donor mice that had been immunized several months earlier with an AdAAV vector. Cells were isolated from liver and spleens 10 days later and analyzed. (A) Recovery of SIINFEKL-specific donor CD8+ T cells from liver. (B) Recovery of SIINFEKL-specific donor CD8+ T cells from spleen. (C) Percentages of SIINFEKL-specific donor CD8+ T cells that divided in liver. (D) Percentages of SIINFEKL-specific donor CD8+ T cells that divided in spleen. (E) Division index of SIINFEKL-specific donor CD8+ T cells in liver. (F) Division index of SIINFEKL-specific donor CD8+ T cells in spleen. (G) Histograms for CFSE expression in SIINFEKL-specific CD8+ T cells from liver. (H) Histograms for CFSE expression in SIINFEKL-specific CD8+ T cells from spleen.
Discussion
Induction of immune responses to AAV upon gene transfer can be prevented by immunosuppression, which has been used successfully in a number of clinical trials for different diseases.4, 5, 6,15 Some trials have used immunosuppression starting at or shortly before the time of gene transfer;6,28, 29, 30 others carefully monitored their patients and initiated treatment only upon clinical evidence of damage of transduced cells, such as increases in liver enzymes.4 The former protocol exposes all participants to immunosuppression, including those who would not reject the vector; the latter is cumbersome, as it requires testing liver enzymes frequently and carries the risk that treatment might be initiated too late. Most patients thus far who required prednisone have been treated for 8–20 weeks with prednisone.4,31 The duration of prednisone treatment needed to prevent rejection of AAV vectors depends on the time frame of vector degradation, which produces peptides of the AAVs’ capsid for recognition by CD8+ T cells and which is likely to vary depending on the AAV serotype, its genome, composition, the target tissue, and characteristics of a given patient.
It is currently unknown if memory CD8+ T cells induced by natural infections and recalled by AAV-mediated gene transfer, de novo stimulated naive T cells induced to epitopes of the vector that the individual had not encountered previously, or both cause rejection of AAV-transduced cells. The kinetics of rejection, which in most gene transfer recipients starts several weeks after AAV injection, argues for a primary T cell response that would come up more slowly than a recall response. Successful use of steroids to blunt the AAV capsid-specific T cell response would also point toward primary T cells, which are more sensitive to immunosuppression than memory T cells. Induction of naive T cells requires mature dendritic cells that not only present the T cell receptor’s antigen associated with major histocompatibility complex (MHC) molecules but also provide a second signal in form of a co-stimulator such as CD80 or CD86.32 Maturation of dendritic cells in turn requires stimulation of innate responses that through the release of cytokines and chemokines initiate inflammatory reactions.32 The genome of AAV vectors carries unmethylated CpG-motifs. Previous studies have shown that AAV transduces plasmacytoid dendritic cells and through CpG-mediated activation of the TLR9-MyD88 pathway stimulates production of type I IFN (IFN-1), which in turn is crucial for activation of primary CD8+ T cell responses.18 Nevertheless, considering their short lifespan, it is unlikely that dendritic cells activated upon direct AAV transduction or presenting AAV capsid upon cross-priming33 are driving the prolonged proliferative response of naive OT-1 T cells. Here, we extended these studies to assess the effects of CpG motifs within the AAV vector’s genome on proliferation of naive or memory CD8+ T cells directed to an epitope displayed by the capsid of AAV8 or AAV2 vectors. As AAV8 fails to induce capsid-specific CD8+ T cells in mice,27 we used AAV8 vectors that carry 10 copies of SIINFEKL, the immunodominant epitope of ovalbumin, within V2. This model offers the advantage that OT-1 mice, which carry a transgenic CD8+ T cell receptor for SIINFEKL associated with H-2Kb, are available, which in turn allows for a reliable analysis of naive CD8+ T cell responses to an epitope within the AAV capsid.
Our initial studies that aimed to characterize transgene product expression by AAV8SIINFEKL-EF1LacZ or AAV8SIINFEKL-CB-LacZ vectors showed no difference upon in vitro transduction of HEK293 cells. Using equal low doses of the vectors showed expression of the transgene product in approximately equal percentages of cells (data not shown). Results were markedly different upon transfer of the vectors into mice; the AAV8SIINFEKL-EF1LacZ vector achieved β-Gal expression in nearly all hepatocytes, while upon injection of the AAV8SIINFEKL-CBLacZ vector, only a fraction of cells stained positive when tested 14 days later, although numbers of β-Gal+ hepatocytes increased over time (data not shown). The only difference between these two vectors is in the promoter driving LacZ expression. As previous studies showed that both the EF1 and CB promoter are active in liver34 and as both promoters performed equally well in vitro, the result suggests that differences in the CpG content of the promoters and the resultant innate immune responses may have affected transgene expression levels. Innate immune responses to AAV vectors have been described to come up rapidly and then decline.35 Nevertheless, it should be pointed out that AAV vectors uncoat very slowly,36,37 so that low levels of DNA containing CpG motifs are released over a lengthy period of time maintaining activation of innate sensors and thereby a localized inflammatory response. Innate responses may dampen transgene product expression by various mechanisms, such as by retarding intracellular trafficking and uncoating of the vectors or by reducing the activity of the promoter.
Our other results confirm those of previous studies,18,20 which showed that CpG motifs are essential to drive proliferation of naive CD8+ T cells to AAV capsids. Curiously, although cycling of naive SIINFEKL-specific CD8+ T cells in response to their cognate antigen expressed by the AAV capsid was only observed in response to CpG-rich vectors, naive SIINFEKL-specific CD8+ T cells gained the ability to produce cytokines in response to both CpGhigh and CpGlow vectors, indicating segregation of these two pathways, as has been reported previously.38 Proliferation of naive T cells to AAV vectors that contain CpG motifs within their CB promotor was modest and did not result in detectable increases in the overall hepatic infiltrates or increased frequencies of donor-derived SIINFEKL-specific CD8+ T cells within spleens or livers. Results differed for memory CD8+ T cells, which vigorously responded to SIINFEKL displayed within the capsid of an AAV8 vector that was largely depleted of CpG sequences but for those essential for the integrity of the ITRs. Frequencies of donor-derived SIINFEKL-specific CD8+ T cells, which in naive mice comprised <5% of the entire CD8+ T cell population in livers, increased to over 70% in host mice that carried the CpG-reduced AAV8SIINFEKL vector. This was caused by their vigorous proliferation that was still detectable if cells were transferred 8 weeks after gene transfer. The strong donor memory CD8+ T cell response within livers of mice carrying a CpG-reduced AAV8SIINFEKL vector also drove activation of the hosts’ naive SIINFEKL-specific CD8+ T cells. Vigorous proliferation of memory CD8+ T cells to the CpG-reduced vectors was to be expected as T cell recall responses are relatively independent of innate responses and require less antigen compared to naive T cells.
Unexpectedly, the proliferative response of memory CD8+ T cells was attenuated in mice injected with a CpG motif containing AAV8SIINFEKL vector, especially if cells were transferred within 2–4 weeks after vector injection; upon later transfer, proliferation of memory CD8+ T cells started to increase. This shift in proliferation is suggestive of inhibition of T cell proliferation by innate immune responses to the CpG-motif-containing AAV8SIINFEL vector. Cytokines induced by CpG motifs include IFN-1, which increases MHC class I expression and the activity of lytic enzymes that process antigen; both would be expected to increase stimulation of T cells.39 Previous studies showed that IFN-1 augments T cell proliferation, but depending on its timing in relation to stimulation by the T cells’ cognate antigen it can also suppress T cells.40 IFN-1-mediated suppression is more pronounced for memory CD8+ T cells, which express higher levels of the IFN-1 receptor than naive T cells. Exposure of T cells to IFN-1 prior to antigenic stimulation reduces the proliferative response of naive T cells and causes apoptosis of memory CD8+ T cells. T cells that encounter IFN-1 later during their activation phase can immediately differentiate into effector cells at the expense of further proliferation. Alternative mechanisms, such as induction of regulatory cells or release of cytokines that selectively inhibit proliferation of memory CD8+ T cells, could also explain the reduced proliferation of SIINFEKL-specific memory CD8+ T cells transferred early after injection of the AAV8SIINFEKL-CB-LacZ vector.
Vector preparations can contain substantial amounts of empty AAV particles. Empty AAV particles can also be added to genome-containing vectors to circumvent AAV-neutralizing antibodies.25 This in turn might have immunological consequences, as it increases the load of antigen able to trigger an AAV capsid-specific CD8+ T cell response. As shown here, empty AAV particles neither stimulated naive T cells directed to an epitope within AAV capsid nor increased activation triggered by genome-containing vectors. The former is evidenced by absence of specific CD8+ T cell proliferation in mice that were only injected with empty AAV particles. Empty AAV particles appear to shorten stimulation of naive AAV capsid-specific CD8+ T cells to genome-containing AAV vectors. These results are in agreement with our finding that CpG-reduced AAV8 vector failed to induce proliferation of capsid-specific CD8+ T cells. Also, both sets of our results with capsid-specific memory CD8+ T cells using CpG-reduced or empty AAV vectors concur and show conclusively that capsid-specific memory CD8+ T cells can be activated without a trigger of TLR9 activation. A few caveats should be stressed. From an experimental standpoint, our assay system allows for the detection of CD8+ T cell proliferation, which in turn reflects their activation due to AAV capsid-derived peptides displayed on MHC class I molecules. The consistently higher levels of proliferation in liver suggest enrichment of such peptides on hepatocytes, which are readily targeted by AAV8 and AAV2 vectors. Nevertheless, unlike humans, mice fail to reject AAV8 vector-transduced hepatocytes,41 indicating that the observed T cell proliferation fails to lead to a fully functional effector CD8+ T cell response.11 From a clinical standpoint, it should be noted that, in a trial of an AAV vector expressing a highly specific activity variant of blood coagulation factor IX, supplemented with a 3- to 5-fold excess of empty capsid, of 10 adult men with hemophilia infused with AAV, 8/10 did not manifest any clinical evidence of an immune response to AAV capsid (elevation in aminotransferases or decline in FIX levels), and of the 2/10 who did, the immune response was readily controlled with oral glucocorticoids, resulting in rescue of FIX expression.31 Thus, the relationship of the findings in the current study to clinical results is not yet clear but could potentially suggest that rejection of human AAV-transduced hepatocytes is mediated by capsid-specific de novo activated rather than recalled memory CD8+ T cells.
Materials and Methods
Mice
Thy1.1 C57BL/6 or BALB/c and OT-1 breeding pairs were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). These mice were bred at the Wistar Institute. Homozygous mice were used to generate Thy1.1 C57BL/6 or BALB/c pups. OT-1 mice were crossed to C57BL/6 mice. Pups were checked at ∼4 weeks of age for expression of the SIINFEKL-specific T cell receptor by staining followed by analyses by flow cytometry. Male Thy1.2 C57BL/6 and BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were used at 8–16 weeks of age. Mice were housed at the Animal Facility of the Wistar Institute and treated according to institutionally approved protocols.
Vectors
The E1- and E3-deleted Ad chimpanzee serotype 7 vector expressing a fusion protein composed of the nucleoprotein of influenza A virus (NP), ovalbumin and GFP under a cytomegalovirus promoter (AdC7-NP-Ova-GFP), or the AAV2 capsid sequence (AdAAV) were prepared as described before.31 The SIINFEKL-modified AAV8 capsid has been described previously.27 A CpG-depleted LacZ gene was designed and synthesized. It was cloned into a pShuttle vector under the control of the CB or the elongation factor 1 (EF1) promoter using conventional cloning methods. Vectors were produced by quadruplet plasmid transfection with pHelper/pAAV-LacZ/pAAV8SIINFEKL/pAAV8 vectors at ratios of 1:0.5:0.4:0.1 or by triple transfection with pHelper/pAAV-LacZ/pAAV2 vectors at ratios of 1:0.5:0.5.
Purification of Vectors
AAV vectors were produced in HEK293 cells from a batch of 40 T-175 cm2 flasks. Plasmids were transfected with polyethylenimine (PEI) at a 1:3 ratio of DNA:PEI and kept in a 37°C, 5% CO2 incubator for 72 h. After 72 h, cells were harvested by centrifugation at 1,500 rpm for 10 min at 4°C. Medium was decanted, and the cells were washed with PBS by centrifugation. The cell pellet was re-suspended in PBS and sonicated for 5 min with 1 min on and 1 min off at 50% amplitude to release the virus. The cells were further treated with 50 U/mL of Benzonase and 0.5% sodium deoxycholate for 30 min in a 37°C water bath. Cell debris was removed by centrifuging at 4,000 rpm for 30 min at 4°C. Iodixanol step gradients were prepared and added to ultracentrifuge tubes from bottom to top at 60%, 40%, 25%, and 15%. The cleared viral supernatant was loaded on top of 15% iodixanol solution and centrifuged at 67,000 rpm for 2 h at 18°C with maximum acceleration and no brake. Following centrifugation, AAV particles were harvested from 40%–25% interface and dialyzed against PBS in a 100 kDa Millipore Ultra-50 unit. After dialysis, purified AAV was collected, aliquoted, and stored at −80°C.
Titration of AAV Vectors
SYBR green qPCR assays were performed to determine the viral titers with LacZ primers. In brief, 5 μL aliquot of iodixanol-purified vector was treated with DNase I in PCR buffer at 37°C for 30 min. Treatment samples were 10-fold serially diluted in duplicates and the amplified using the primers for LacZ and SYBR green master mix. Standard plasmids with known genome copies were included in each plate. vg copies/mL were calculated based on the plasmid-derived standard curve.
Silver Stain
Samples from purified vectors were loaded on a 10% SDS gel (Bio-Rad) and run using 1× running buffer. After the run, silver staining was performed using a Bio-Rad silver stain plus kit according to manufacturer’s instructions.
β-Gal Expression
HEK293 cells were plated in wells of a 6-well plate. The following day, cells were transduced with various amounts of the AAV8-LacZ vectors. Cells were stained 48 h later for expression of β-Gal as described.32 Alternatively, mice were injected i.v. with the vectors. Individual livers were harvested, frozen, cryo-sectioned (6–14 μm), and placed onto slides. The slides were fixed for 20 min in 0.2% glutaraldehyde and washed twice with phosphate buffer. Thereafter, sections were stained at 37°C overnight with staining solution containing 1 mg/mL of X-gal, 2 mM of MgCl2, 5 mM potassium ferricyanide, 5 mM postassium ferrocyanide, 0.01% Na-deoxycholate, and 0.02% NP-40.
Immunization of Mice
AdC7-NP-Ova-GFP and adenovirus vectors expressing the AAV capsid (AdAAVs) were diluted in sterile PBS to a total volume of 100 μL and intramuscularly injected into the hindlegs of mice. AAV vectors were diluted in 300 μL of sterile PBS and injected i.v. into the tail vein of mice.
Transfer of Donor Cells
Lymphocytes were isolated from spleens of donor mice (Thy1.2 C57BL/6, Thy1.2 BALB/c, or OT-1 mice), and red blood cells were lysed with 1× red blood cell (RBC) lysing buffer (eBioscience, San Diego, CA, USA). Cells were then labeled with 7 μmol/L CFSE using the CellTrace CFSE cell proliferation kit (Invitrogen, Carlsbad, CA, USA) and 5–8 × 107 cells from immunized C67Bl/6 or BALB/c mice or 1 × 106 splenocytes from naive OT-1 mice were transferred into MHC-matched Thy1.1+ recipient mice in 300–500 μL PBS by tail vein injection.
In Vitro T Cell Assay
At 10 days after transfer, splenocytes or liver lymphocytes of recipient mice were harvested, purified, and stained with a live cell stain, antibodies to Thy1.2 (BD clone53-2.1), CD8a (BD53-6.7), CD44 (BioLegend, 1M7), and the SIINFEKL or an AAV capsid-specific tetramer. The stained cells were then analyzed by flow cytometry. Gates were set to identify antigen-specific CD8+ T cells of host (Thy1.2−) or donor (Thy1.2+) origin. Cells were then analyzed for frequencies of different populations including tetramer+ CD8+CD44+ T cells and for decreases in CFSE expression. The division index (DI) and % cells that divided were calculated as before.27 ICS, cells were stimulated for 5 h with SIINFEKL peptide or an irrelevant peptide (both at 1 μg/mL) in presence of brefeldin A. Cells were then stained with a live cell stain for surfaced-expressed CD8, CD44, and Thy1.2 and upon permeabilization of the membranes for intracellular GzmB (BioLegend GB11), IFN-γ (BioLegend, XMG1.2), IL-2 (BioLegend, JES6-5H4), and TNF-α (BioLegend, MP6-XT22). Cells were analyzed by an LSRII. Post-acquisition analyses were conducted with FlowJo.
Statistics
All statistical analyses were conducted using GraphPad Prism 6 (GraphPad). Differences between two populations were calculated by Student’s t test. Multiple comparisons between two groups were performed by multiple t test with type I error correction. Differences among multiple populations were calculated by one- or two-way ANOVA.
Author Contributions
X.Z. prepared the AAV vectors; Z.X., R.K.K., K.K., and Y.L. conducted the immunological experiments; F.M., K.A.H., and H.C.J.E. designed and interpreted experiments and wrote the manuscript.
Conflicts of Interest
K.A.H., F.M., and K.K. are employees of and hold equity in Spark Therapeutics.
Acknowledgments
This work was funded by a Sponsored Research Agreement with Spark Therapeutics, Philadelphia, PA.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2019.11.014.
Supplemental Information
References
- 1.Liang F.Q., Dejneka N.S., Cohen D.R., Krasnoperova N.V., Lem J., Maguire A.M., Dudus L., Fisher K.J., Bennett J. AAV-mediated delivery of ciliary neurotrophic factor prolongs photoreceptor survival in the rhodopsin knockout mouse. Mol. Ther. 2001;3:241–248. doi: 10.1006/mthe.2000.0252. [DOI] [PubMed] [Google Scholar]
- 2.Maguire A.M., High K.A., Auricchio A., Wright J.F., Pierce E.A., Testa F., Mingozzi F., Bennicelli J.L., Ying G.S., Rossi S. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett J., Wellman J., Marshall K.A., McCague S., Ashtari M., DiStefano-Pappas J., Elci O.U., Chung D.C., Sun J., Wright J.F. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388:661–672. doi: 10.1016/S0140-6736(16)30371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathwani A.C., Tuddenham E.G.D., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathwani A.C., Reiss U.M., Tuddenham E.G.D., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George L.A., Sullivan S.K., Giermasz A., Ducore J.M., Teitel J.M., Cuker A., Sullivan L.M., Majumdar S., McGuinn C.E., Galvao A.M. Spk-9001: Adeno-associated virus mediated gene transfer for hemophilia B achieves sustained mean Factor IX activity levels of < 30% without immunosuppression. Blood. 2016;128:3. 3. [Google Scholar]
- 7.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 8.Mingozzi F., Maus M.V., Hui D.J., Sabatino D.E., Murphy S.L., Rasko J.E.J., Ragni M.V., Manno C.S., Sommer J., Jiang H. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 9.Calcedo R., Vandenberghe L.H., Gao G., Lin J., Wilson J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M.F., Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 11.Li H., Lasaro M.O., Jia B., Lin S.W., Haut L.H., High K.A., Ertl H.C. Capsid-specific T-cell responses to natural infections with adeno-associated viruses in humans differ from those of nonhuman primates. Mol. Ther. 2011;19:2021–2030. doi: 10.1038/mt.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hui D.J., Edmonson S.C., Podsakoff G.M., Pien G.C., Ivanciu L., Camire R.M., Ertl H., Mingozzi F., High K.A., Basner-Tschakarjan E. AAV capsid CD8+ T-cell epitopes are highly conserved across AAV serotypes. Mol. Ther. Methods Clin. Dev. 2015;2:15029. doi: 10.1038/mtm.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuker A., George L.A. 2016. FIXing hemophilia B: Sustained factor IX expression of 30 percent after gene therapy. The Hematologist: ASH News and Reports.https://www.hematology.org/Thehematologist/Clinical-Trials/5951.aspx Published online August 12, 2016. [Google Scholar]
- 14.Crudele J.M., Finn J.D., Siner J.I., Martin N.B., Niemeyer G.P., Zhou S., Mingozzi F., Lothrop C.D., Jr., Arruda V.R. AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice. Blood. 2015;125:1553–1561. doi: 10.1182/blood-2014-07-588194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flanigan K.M., Campbell K., Viollet L., Wang W., Gomez A.M., Walker C.M., Mendell J.R. Anti-dystrophin T cell responses in Duchenne muscular dystrophy: prevalence and a glucocorticoid treatment effect. Hum. Gene Ther. 2013;24:797–806. doi: 10.1089/hum.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu J., Huang X., Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J. Clin. Invest. 2009;119:2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers G.L., Suzuki M., Zolotukhin I., Markusic D.M., Morel L.M., Lee B., Ertl H.C., Herzog R.W. Unique roles of TLR9- and MyD88-dependent and -independent pathways in adaptive immune responses to AAV-mediated gene transfer. J. Innate Immun. 2015;7:302–314. doi: 10.1159/000369273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers G.L., Martino A.T., Aslanidi G.V., Jayandharan G.R., Srivastava A., Herzog R.W. Innate Immune Responses to AAV Vectors. Front. Microbiol. 2011;2:194. doi: 10.3389/fmicb.2011.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J., Calcedo R., Vandenberghe L.H., Bell P., Somanathan S., Wilson J.M. A new genetic vaccine platform based on an adeno-associated virus isolated from a rhesus macaque. J. Virol. 2009;83:12738–12750. doi: 10.1128/JVI.01441-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faust S.M., Bell P., Cutler B.J., Ashley S.N., Zhu Y., Rabinowitz J.E., Wilson J.M. CpG-depleted adeno-associated virus vectors evade immune detection. J. Clin. Invest. 2013;123:2994–3001. doi: 10.1172/JCI68205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masat E., Pavani G., Mingozzi F. Humoral immunity to AAV vectors in gene therapy: challenges and potential solutions. Discov. Med. 2013;15:379–389. [PubMed] [Google Scholar]
- 22.Calcedo R., Wilson J.M. Humoral Immune Response to AAV. Front. Immunol. 2013;4:341. doi: 10.3389/fimmu.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corti M., Elder M., Falk D., Lawson L., Smith B., Nayak S., Conlon T., Clément N., Erger K., Lavassani E. B-Cell Depletion is Protective Against Anti-AAV Capsid Immune Response: A Human Subject Case Study. Mol. Ther. Methods Clin. Dev. 2014;1:14033. doi: 10.1038/mtm.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harbison C.E., Weichert W.S., Gurda B.L., Chiorini J.A., Agbandje-McKenna M., Parrish C.R. Examining the cross-reactivity and neutralization mechanisms of a panel of mAbs against adeno-associated virus serotypes 1 and 5. J. Gen. Virol. 2012;93:347–355. doi: 10.1099/vir.0.035113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mingozzi F., Anguela X.M., Pavani G., Chen Y., Davidson R.J., Hui D.J., Yazicioglu M., Elkouby L., Hinderer C.J., Faella A. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci. Transl. Med. 2013;5:194ra92. doi: 10.1126/scitranslmed.3005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mingozzi F., Meulenberg J.J., Hui D.J., Basner-Tschakarjan E., Hasbrouck N.C., Edmonson S.A., Hutnick N.A., Betts M.R., Kastelein J.J., Stroes E.S., High K.A. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu T.-L., Li H., Faust S.M., Chi E., Zhou S., Wright F., High K.A., Ertl H.C. CD8+ T cell recognition of epitopes within the capsid of adeno-associated virus 8-based gene transfer vectors depends on vectors’ genome. Mol. Ther. 2014;22:42–51. doi: 10.1038/mt.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendell J.R., Sahenk Z., Malik V., Gomez A.M., Flanigan K.M., Lowes L.P., Alfano L.N., Berry K., Meadows E., Lewis S. A phase 1/2a follistatin gene therapy trial for becker muscular dystrophy. Mol. Ther. 2015;23:192–201. doi: 10.1038/mt.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira V., Twisk J., Kwikkers K., Aronica E., Brisson D., Methot J., Petry H., Gaudet D. Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPL(S447X)) in a phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum. Gene Ther. 2014;25:180–188. doi: 10.1089/hum.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira V., Petry H., Salmon F. Immune Responses to AAV-Vectors, the Glybera Example from Bench to Bedside. Front. Immunol. 2014;5:82. doi: 10.3389/fimmu.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.den Haan J.M.M., Arens R., van Zelm M.C. The activation of the adaptive immune system: cross-talk between antigen-presenting cells, T cells and B cells. Immunol. Lett. 2014;162(2 Pt B):103–112. doi: 10.1016/j.imlet.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Rogers G.L., Shirley J.L., Zolotukhin I., Kumar S.R.P., Sherman A., Perrin G.Q., Hoffman B.E., Srivastava A., Basner-Tschakarjan E., Wallet M.A. Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8+ T cells. Blood. 2017;129:3184–3195. doi: 10.1182/blood-2016-11-751040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen A.T., Dow A.C., Kupiec-Weglinski J., Busuttil R.W., Lipshutz G.S. Evaluation of gene promoters for liver expression by hydrodynamic gene transfer. J. Surg. Res. 2008;148:60–66. doi: 10.1016/j.jss.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martino A.T., Suzuki M., Markusic D.M., Zolotukhin I., Ryals R.C., Moghimi B., Ertl H.C., Muruve D.A., Lee B., Herzog R.W. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117:6459–6468. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas C.E., Storm T.A., Huang Z., Kay M.A. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nonnenmacher M., Weber T. Intracellular transport of recombinant adeno-associated virus vectors. Gene Ther. 2012;19:649–658. doi: 10.1038/gt.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris D.T., Kozumbo W.J., Cerutti P., Cerottini J.C. Molecular mechanisms involved in T cell activation. I. Evidence for independent signal-transducing pathways in lymphokine production vs proliferation in cloned cytotoxic T lymphocytes. J. Immunol. 1987;138:600–605. [PubMed] [Google Scholar]
- 39.Teijaro J.R. Pleiotropic Roles of Type 1 Interferons in Antiviral Immune Responses. Adv. Immunol. 2016;132:135–158. doi: 10.1016/bs.ai.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welsh R.M., Bahl K., Marshall H.D., Urban S.L. Type 1 interferons and antiviral CD8 T-cell responses. PLoS Pathog. 2012;8:e1002352. doi: 10.1371/journal.ppat.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H., Lin S.-W., Giles-Davis W., Li Y., Zhou D., Xiang Z.Q., High K.A., Ertl H.C. A preclinical animal model to assess the effect of pre-existing immunity on AAV-mediated gene transfer. Mol. Ther. 2009;17:1215–1224. doi: 10.1038/mt.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.