Abstract
With the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approvals for Zolgensma, Luxturna, and Glybera, recombinant adeno-associated viruses (rAAVs) are considered efficient tools for gene transfer. However, studies in animals and humans demonstrate that intramuscular (IM) AAV delivery can trigger immune responses to AAV capsids and/or transgenes. IM delivery of rAAV1 in humans has also been described to induce tolerance to rAAV characterized by the presence of capsid-specific regulatory T cells (Tregs) in periphery. To understand mechanisms responsible for tolerance and parameters involved, we tested 3 muscle-directed administration routes in rhesus monkeys: IM delivery, venous limb perfusion, and the intra-arterial push and dwell method. These 3 methods were well tolerated and led to transgene expression. Interestingly, gene transfer in muscle led to Tregs and exhausted T cell infiltrates in situ at both day 21 and day 60 post-injection. In human samples, an in-depth analysis of the functionality of these cells demonstrates that capsid-specific exhausted T cells are detected after at least 5 years post-vector delivery and that the exhaustion can be reversed by blocking the checkpoint pathway. Overall, our study shows that persisting transgene expression after gene transfer in muscle is mediated by Tregs and exhausted T cells.
Keywords: AAV, gene transfer, tolerance, exhaustion, regulatory T cells, exhausted T cells, immunity, gene therapy, adeno-associated virus, Tregs
Graphical Abstract
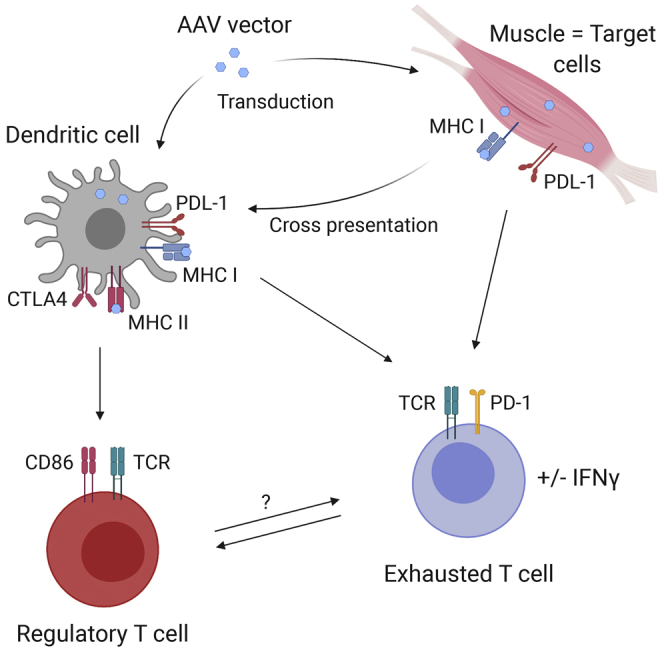
Whereas AAV vector administration in muscle is usually immunogenic, muscle-derived delivery of an AAV1 vector in monkeys and alpha1-antitrypsin-deficient (AATD) patients can lead to persisting transgene expression. Both regulatory and exhausted T cells are involved in maintaining transgene expression.
Introduction
The adeno-associated virus (AAV) is a dependovirus belonging to the parvovirus family. Its 4.7-kb genome carries 2 Rep and Cap genes flanked by 2 inverted terminal repeats (ITRs) and is packaged into an icosahedral protein capsid composed of 3 proteins: VP1, VP2, and VP3 at a 1:1:10 ratio. Despite its low encapsidation capacity, its genetic simplicity and low immunogenicity have made it a promising tool for gene transfer in the past decades. With the recent FDA and EMA approvals for Zolgensma, Luxturna, and Glybera, recombinant AAVs (rAAVs) are now, more than ever, considered efficient and successful viral vectors for the treatment of inherited disorders. Its variety of serotypes offers a large spectrum of abilities to transduce tissues, including the liver,1,2 muscle,3, 4, 5, 6 central nervous system (CNS),7, 8, 9 and retina,10,11 as demonstrated in preclinical studies targeting metabolic,12, 13, 14, 15, 16 neuromuscular,17, 18, 19, 20 neurodegenerative,21,22 and retinal23, 24, 25 disorders. Interestingly, certain serotypes of AAV capsids elicit more robust immune responses than others, with AAV8 being less immunoreactive and AAVrh32.33 being more immunoreactive in mouse models.26,27 Furthermore, when protocols were translated to patients, limits imposed by the host immune system have emerged. Indeed, the study of T cell-mediated immune responses to AAV capsids in patients have shown that, after gene transfer, memory T cells can be reactivated and can lead to transduced cell clearance.28 The first evidence was an elevation of transaminases related to a cellular immune response characterized by interferon (IFN)γ-secreted T cells in response to AAV capsid peptide libraries.28, 29, 30 Studies on pre-existing immunity to AAV have shown that exposure to wild-type AAV2 occurs early in life,31 suggesting that gene transfer past 3–18 years of age using AAV vectors can potentially result in the reactivation of these memory T cell pools. Currently, one way to efficiently manage the cytotoxic immune response and restore gene expression post-vector delivery is transient corticosteroid-based immunosuppression.29,32
Elevation of serum enzymes is not always evidence of a deleterious immune response resulting in loss of transgene expression. In a clinical trial targeting the muscle in the absence of any immunosuppression, Flotte et al.33 showed persistent alpha1-antitrypsin (AAT) protein expression in serum, despite (1) an elevation of serum creatine kinase and (2) the detection of IFNγ-secreting cells to AAV1 capsid in peripheral blood mononuclear cells (PBMCs). This was explained by the presence of muscle-infiltrated regulatory T cells (Tregs) 1 year post-vector delivery in addition to AAV capsid persistence in situ.34 Tregs were also detected in lipoprotein-lipase-deficient (LPLD) patients35 after intramuscular (IM) gene transfer, suggesting that cellular immune response to rAAV does not lead exclusively to cytotoxicity and gene transfer failure but can also result in immune tolerance. Altogether, these data highlight that (1) several factors can manage the nature of the T cell response, including the AAV serotype, the dose, the administration route, the transgene, and/or the promoter;36 and (2) the nature of the tolerance mechanism and its kinetic are still not clearly established.
Here, we studied the mechanisms responsible for persisting transgene expression after gene transfer in muscle in rhesus monkeys by comparing three modes of delivery: IM; a high-volume venous limb perfusion (VLP), also called hydrodynamic intravenous delivery;37,38 and an intra-arterial push and dwell (IAPD) method. To better characterize the mechanisms involved, we focused on Tregs and exhausted T cells. We performed an in situ immune cell analysis shortly after vector dosing (days 21 and 60) instead of analyzing peripheral immune responses. We showed that both Tregs and exhausted T cells might be responsible for the absence of a cytotoxic immune response after IM injection. Because we already had evidenced that IM injection may induce an AAV-specific Treg response in humans at least 1 year post-vector delivery, we focused on the functionality of exhausted T cells in AAT-deficient (AATD) patients. In the present study, we assessed the functionality of these cells by blocking the PD1 pathway and showed an increase of IFNγ secretion when the checkpoint pathway is blocked, demonstrating a capsid-specific T cell exhaustion.
Our results suggest that the absence of a deleterious T cell response to the AAV capsid is mediated by Tregs and exhausted T cells and occurs early after gene transfer to muscle and may offer a new way to interrogate in situ immune responses to the AAV capsid. Importantly, these responses occurred in both humans and non-human primates without either steroid therapy or any other form of immune modulation.
Results
Experimental Design, Transgene Expression, and Cellular Immune Response
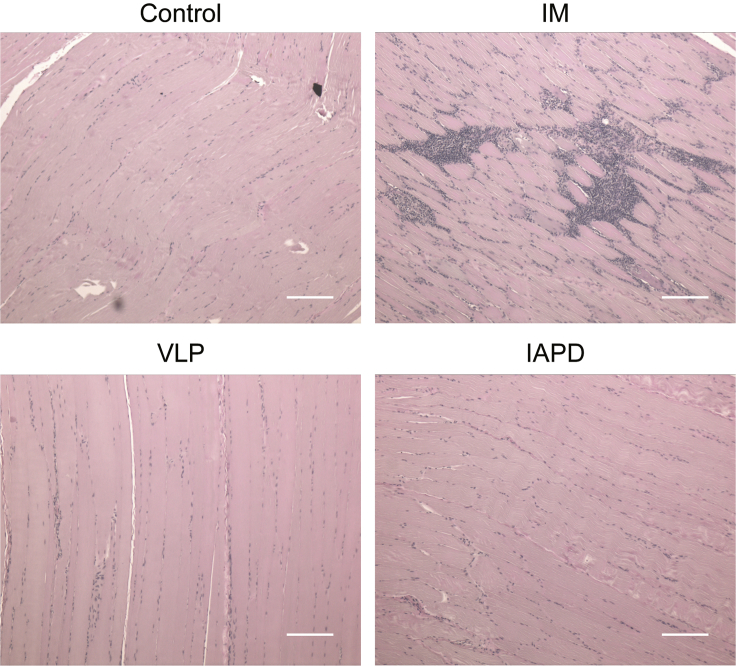
Rhesus macaques were injected with a single-stranded (ss)AAV1-CB-AAT vector at a dose of 6e12 viral genomes per kilogram (vg/kg), corresponding to the highest dose injected in AATD patients (ClinicalTrials.gov: NCT01054339).33 Three different methods of delivery were tested: IM (n = 3), VLP (n = 3), and IAPD (n = 2) (Table 1).39 As previously described by our group,39 the procedures are well tolerated, and the vector biodistribution analysis showed a broader distribution when the vector is delivered by VLP compared to IM injection. To track protein expression and differentiate endogenous AAT from recombinant AAT, a c-Myc tag was added to the recombinant rhesus AAT (rhAAT) sequence. The rhAAT-cMyc fusion protein expression was measured at the mRNA level by qRT-PCR and at the protein level in serum by a semiquantitative immunoblot over time.39 Our results show that the protein expression was detected in all groups, with a higher protein expression in the VLP group: up to an average of 90 μg/mL at 60 days post-injection. The level of protein expression is respectively VLP > IM > IAPD (Table 1).39 As expected, all the animals seroconverted after rAAV administration, as illustrated by detection of anti-AAV1 neutralizing antibodies (NAbs) and immunoglobulin G (IgG) (Table 2). In contrast to results obtained in the phase II AATD clinical trial, these animals showed sporadic and transient elevation in serum creatine kinase or liver transaminases (alanine aminotransferase [ALT]/aspartate aminotransferase [AST]). IFNγ secretion was measured by ELISpot on PBMCs prior to dosing and at necropsy. Only one animal (RA0332, VLP group) showed a positive cellular immune response to AAV1 capsid protein at day 60 post-injection (Table 2),39 despite evidence of cell infiltrates in situ. Analysis of cell infiltrates in the injected limbs reveals that IM vector injection leads to a higher cell infiltration than the VLP and IAPD administration routes (Figure 1).
Table 1.
Experimental Groups
| Vector | Route | Dose | Animal No. | Serum rhAAT-cMyc Concentration at Endpoint (μg/mL) |
|---|---|---|---|---|
| ssAAV1-CB-rhAAT-cMyc | IM | 6e12 vg/kg | RA1598 | 12 |
| RA1683 | 50 | |||
| RA0764 | 51 | |||
| ssAAV1-CB-rhAAT-cMyc | VLP | 6e12 vg/kg | RA1567 | 92 |
| RA0770 | 116 | |||
| RA0332 | 61 | |||
| ssAAV1-CB-rhAAT-cMyc | IAPD | 6e12 vg/kg | RA1562 | 20 |
| RA1709 | 17 |
rhAAT, rhesus alpha1-antitrypsin; ss, single-stranded; CB, chicken β-actin promoter; IM, intramuscular; VLP, venous limb perfusion; IAPD, intra-arterial push and dwell; vg/kg, viral genomes per kilogram.
Table 2.
Humoral and Cellular Immune Responses to AAV Capsid
| Route and Animal No. | Anti-AAV1 IgG Concentration (μg/mL) |
Anti-AAV1 NAb Titer |
IFNγ Secretion to rAAV1 Capsid | ||||
|---|---|---|---|---|---|---|---|
| Pre-dose | Day 21 | Day 60 | Pre-dose | Day 21 | Day 60 | ||
| IM | |||||||
| RA1598 | ND | 18.2 | 76.4 | <1:10 | 1:640 | 1:1,280 | − |
| RA1683 | 0.6 | NA | 1,080.2 | 1:10 | 1:1,280 | 1:20,480 | − |
| RA0764 | ND | 30.0 | 530.1 | <1:10 | 1:1,280 | 1:10,240 | − |
| VLP | |||||||
| RA1567 | ND | 91.2 | 139.7 | <1:10 | 1:2,560 | 1:5,120 | − |
| RA0770 | ND | 55.4 | 170.4 | <1:10 | 1:1,280 | 1:5,120 | − |
| RA0332 | ND | 51.3 | 159.7 | 1:10 | 1:1,280 | 1:5,120 | + |
| IAPD | |||||||
| RA1562 | ND | 19.8 | 120.4 | <1:10 | 1:160 | 1:1,280 | − |
| RA1709 | 0.3 | 32.0 | 241.3 | <1:10 | 1:320 | 1:1,280 | − |
IM, intramuscular; VLP, venous limb perfusion; IAPD, intra-arterial push and dwell; NAb, neutralizing antibodies; ND, not detected, NA, not available; −, no response; +, positive response.
Figure 1.
Detection of Cellular Infiltrates in the Injected Muscle or Perfused Limb after rAAV1 Dosing
Nonhuman primates were injected IM (n = 3), VLP (n = 3), or IAPD (n = 2) with a ssAAV1 vector (6e12 vg/kg). Muscles were harvested 60 days after dosing. Infiltrated cell populations were analyzed by hematoxylin and eosin (H&E) staining in a control monkey, quadriceps receiving an IM injection (RA1683), VLP perfused quadriceps (RA0770), and IAPD perfused quadriceps (RA1709). Each illustration is representative of each group. Scale bars, 200 μm.
Activated Tregs Are Detected in Muscle after IM Injection in Nonhuman Primates
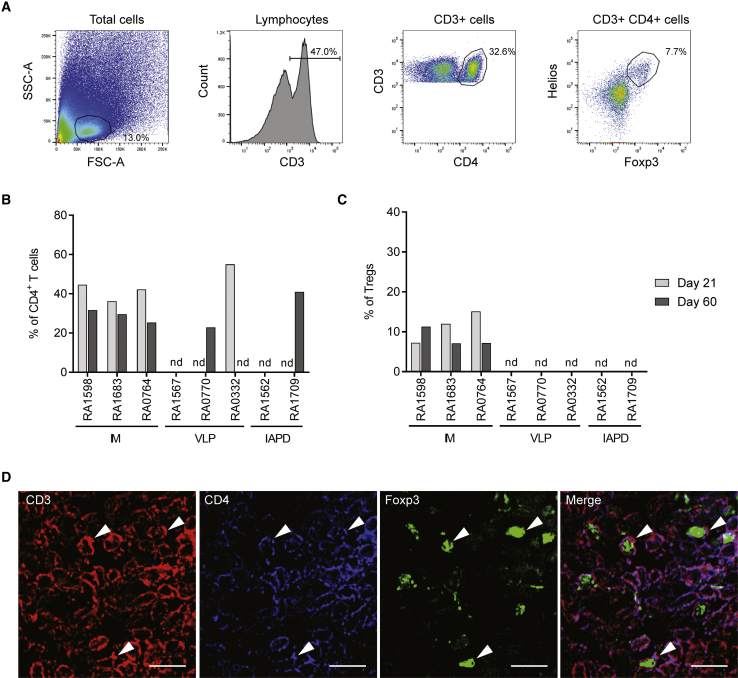
To date, two studies have shown that AAV1 IM injection can lead to Treg infiltration in injected muscles in AATD and LPLD patients.34,35 To verify that Tregs can be detected in injected or perfused muscles post-AAV1 administration in non-human primates, muscle biopsies were performed at day 21 post-vector administration, and muscles were harvested at necropsy (day 60). After tissue dissociation, samples were analyzed for the presence of regulatory T cells in situ. This analysis was performed by flow cytometry. Regulatory T cells are defined as CD3+CD4+Helios+Foxp3+ cells (Figures 2A and S1A for isotype controls), and activated Tregs are defined as Tregs expressing CD25. For each nonhuman primate, the injected or perfused muscle and a contralateral muscle were analyzed at days 21 and 60 after dosing (Figures 2B and 2C; Table S1). Our results show that all animals that were injected IM have infiltrated Tregs at day 21 (7%–15% of CD4+ T cells), and these cells are still detected 60 days post-dosing (7%–8% of CD4+ T cells) (Figures 2B and 2C; Table S1). Some of the animals injected via VLP or the IAPD method had infiltrated CD4+ T cells (Figure 2B), but none of these cells were Tregs (Figure 2C). These results were then confirmed by immunohistochemistry. Since no Tregs were detected by flow cytometry after VLP and IAPD deliveries, we focused on muscle tissue from the IM group (Figures 2D and S2A for negative control). Our data confirm that Tregs are present in the muscle and suggest that these cells play a role in maintaining transgene expression in situ after IM vector administration in nonhuman primates.
Figure 2.
Regulatory T Cells Infiltrate the Muscle after rAAV1 IM Injection
Nonhuman primates were injected IM, VLP, or IAPD with an ssAAV1 vector (6e12 vg/kg). Muscles were harvested 21 and 60 days after dosing and were dissociated as described in the Materials and Methods section. Regulatory T cell population was analyzed by flow cytometry. (A) Gating strategy. RA1683, right quadriceps (injected muscle), 60 days after dosing. T cells are gated as CD3+ cells in the lymphocyte subset, CD4 T cells are gated as CD3+ CD4+ cells in the CD3+ subset, and Tregs are gated as Helios+Foxp3+ cells in the CD3+ CD4+ subset. (B and C) Percentage of CD4 T cells (B) and Tregs (C) in injected muscle or perfused limb (right quadriceps) at day 21 and day 60 after dosing. nd, not detected. (D) Detection of Tregs by immunohistochemistry in injected muscle after IM delivery (RA0764, day 60 post-injection). Scale bars, 10 μm. SS, side scatter; FSC, forward scatter.
Evidence of Exhausted T Cells in Injected Limb after Gene Transfer in Muscle in Nonhuman Primates
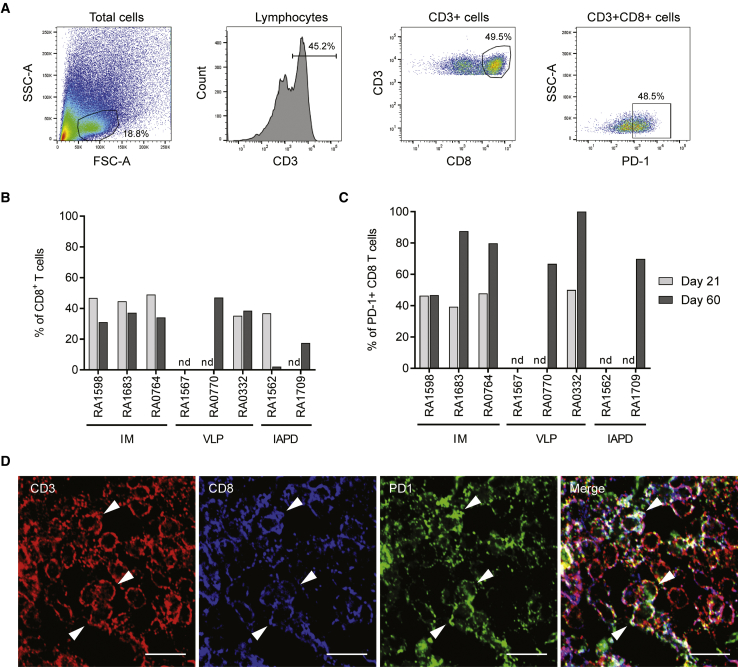
Regulatory T cells are not the only mediator of immune regulation. As we described in an AATD patient 1 year and 5 years after vector delivery,34,40 exhausted T cells can also be involved in AAV capsid tolerance. As performed for the Tregs, we analyzed by flow cytometry the muscle tissues for the presence of exhausted T cells at day 21 and day 60 after vector administration (Figure 3). Exhausted T cells are defined as CD3+CD8+PD1+ cells (Figures 3A and S1B for isotype controls) and activated exhausted T cells are defined as CD3+CD8+PD1+CD69+ cells. For each nonhuman primate, the injected or perfused muscle and a contralateral muscle were analyzed at both time points (Figures 3B and 3C; Table S2). Our results show that all animals that were injected IM have infiltrated exhausted T cells at day 21 (39%–48% of CD8+ T cells), and these cells are still detected (47%–87% of CD8+ T cells) (Figure 3C; Table S1) and express the CD69 activation marker (Table S3) 60 days post-dosing. Some of the animals injected by VLP or IAPD method have infiltrated CD8+ T cells expressing the PD1 marker as well (Figures 3B and 3C). These results were then confirmed by immunohistochemistry, as illustrated in Figure 3D (Figure S2B for negative control). Altogether, our data suggest that T cell exhaustion might also be involved in maintaining transgene expression after muscle-directed gene transfer.
Figure 3.
Exhausted T Cells Infiltrate the Muscle after rAAV1 Administration
Nonhuman primates were injected IM, VLP, or IAPD with an ssAAV1 vector (6e12 vg/kg). Muscles were harvested 21 and 60 days after dosing and were dissociated as described in Materials and Methods. The exhausted T cell population was analyzed by flow cytometry. (A) Gating strategy. RA1683, right quadriceps (injected muscle), 60 days after dosing. T cells are gated as CD3+ cells in the lymphocyte subset, CD8 T cells are gated as CD3+ CD8+ cells in the CD3+ subset, and exhausted T cells are gated as PD-1+ cells in the CD3+ CD8+ subset. (B and C) Percentages of CD8 T cells (B) and exhausted T cells (C) in injected muscle or perfused limb at day 21 and day 60 after dosing. nd, not detected. (D) Detection of exhausted T cells by immunohistochemistry in injected muscle after IM delivery (RA0764, day 60 post-injection). Scale bars: 10 μm.
Persistence of AAT Expression after Gene Transfer Is Driven by T Cell Exhaustion in AATD Patients
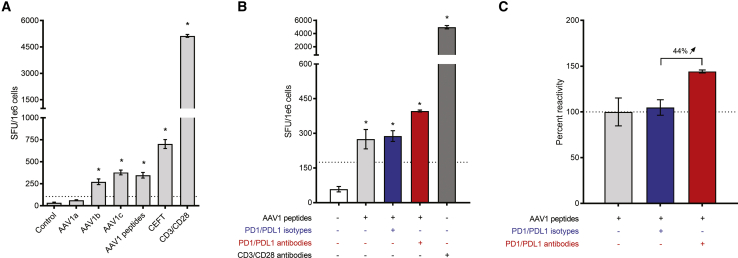
Our AATD clinical trial has shown that, despite an IFNγ immune response to AAV capsid, the transgene expression is maintained for years after gene transfer, suggesting an unresponsiveness toward the AAV capsid. Indeed, in situ analysis has shown that Tregs and exhausted T cells are present up to 5 years post-dosing in injected muscle.34,40 Because several studies have shown the reversal of the exhaustion by in vitro blockade of these inhibitory pathways, we have developed an assay to verify that functional exhausted T cells are involved in persisting AAT expression in the patient still expressing the transgene 5 years post-vector administration. First, we confirmed by ELISpot that the PBMCs were still secreting IFNγ in response to AAV capsid peptides but also in response to AAV1 immunodominant peptides described by Hui et al.41 (Figure 4A; Table S4). Then, PBMCs were restimulated in vitro for 48 h with the AAV1 immunodominant peptides with or without PD1/PDL1 blocking antibodies or isotype controls. An IFNγ-ELISpot assay was performed and showed an increase in IFNγ secretion when blocking the PD1/PDL1 pathway (Figure 4B). The cell relative reactivity was assessed and showed that the IFNγ secretion increased by 44% when the checkpoint pathway is blocked, compared to the reference condition (stimulation with AAV1 peptides only; Figure 4C). Altogether, these data suggest that the lack of a cytotoxic T cell response to rAAV1 capsid is, indeed, mediated by exhausted T cells.
Figure 4.
PD1/PDL1 Blocking Leads to Exhaustion Reversal in Patient PBMCs
PBMCs were isolated 5 years post-injection. (A) PBMCs were stimulated in vitro with overlapping peptides (15-mers overlapping by 10 aa) spanning the VP1 capsid protein sequence or AAV1 immunodominant peptides. (B) PBMCs were stimulated in vitro with AAV1 immunodominant peptides for 48 h in the presence or absence of PD1/PDL1 antibodies or isotype controls. For each experiment in (A) and (B), the negative control consisted of unstimulated cells (medium), whereas CD3/CD28 stimulation was used as a positive control for cytokine secretion. Responses were considered positive when the number of spot-forming units (SFUs) per 1e6 cells was >50 and at least 3-fold higher than in the control condition (dotted line); asterisks denote positive response. (C) IFNγ secretion fold change expressed as percent reactivity relative to the AAV1 peptide stimulation condition. Each condition was performed in triplicate. Error bars represent SEM.
Discussion
Despite the evidence of efficacy of AAV vectors for the treatment of inherited diseases, some limitations imposed by the host immune system, such as cellular immune responses to AAV vector and/or transgene, persist. Depending on the administration route, the dose, and/or the AAV serotype, gene transfer can result in a deleterious immune response30,42, 43, 44 or tolerance.34,35 Because immune tolerance initiation after AAV-based gene transfer in muscle remains unknown, we studied the mediators involved in persisting transgene expression after rAAV1 injection in muscle in rhesus monkeys by comparing three modes of delivery (IM, VLP, and IAPD) and in an AATD patient who was injected IM with an AAV1 vector . We showed that both Tregs and exhausted T cells are detected early after muscle-directed gene transfer and are involved in modulating immune response to AAV capsid in nonhuman primates and humans.
In this study, we confirmed in nonhuman primates that the injection of an AAV1 encoding for the AAT protein in muscle leads to a sustained transgene expression for up to 60 days with no evidence of an IFNγ-positive response to the capsid. This is expected, since a CD8 T cell-mediated cellular immune response to the capsid had not been anticipated in preclinical models16,38,45 and had been described for the first time in hemophilia B patients treated with a rAAV2.30 Although a capsid-specific immune response is not detected in the periphery (PBMCs), we analyzed and characterized the inflammation in situ. Our data confirmed that the IM delivery results in higher inflammation than VLP and IAPD deliveries.38,46 So far, IM delivery has been described as immunogenic with the induction of antibodies to the transgene and loss of transgene expression. In this study, gene transfer in muscle leads to sustained transgene expression explained by the presence of Tregs and exhausted T cell infiltrates in situ. In our model, Tregs were detected only after IM injection, whereas exhausted T cells were detected in animals that were injected IM but also via VLP (2/3) and IAPD (1/2) deliveries but only by flow cytometry and at a lower frequency. These findings in nonhuman primates led us to analyze the same cell population in humans after gene transfer in muscle. In AATD patients, we have shown that, after 5 years post-vector delivery, Tregs and exhausted T cells are still detected in muscle.40 Here, we focused on exhausted T cell properties. These cells are usually found in chronic diseases and are described to express PD1 receptor and to lose their ability to secrete cytotoxic cytokines such as IFNγ, interleukin-2 (IL-2), and tumor necrosis factor alpha (TNF-α).47 In the present study, we show that AAV vectors can actually induce T cell exhaustion in AATD patients that can be reversed by immune checkpoint inhibitors. Altogether, our data suggest that both Tregs and exhausted T cells are involved in modulating the immune response to the AAV capsid. More importantly, our results indicate that these mechanisms seem to occur early after gene transfer, as soon as day 21 post-dosing, and last up to 5 years post-vector delivery.
Surprisingly, in our model, the absence of a detrimental immune response is observed when the vector is injected in the muscle. In gene transfer protocols, the liver has more often been described as a tolerogenic organ, as illustrated in animal model.48, 49, 50 However, a systemic injection has shown limits due to a capsid-specific cellular immune response observed in high-dose patient groups injected with either rAAV2,28 rAAV8,29 or rAAV932 and was explained by the presentation of AAV-capsid-derived antigen on major histocompatibility complex class I (MHC I) expressed on hepatocytes.51 The fact that it was not predicted in animal models can be due to the pre-existing immunity to AAV capsid in humans.30,52,53 Even if monkeys are natural hosts of AAV, their pre-existing capsid-specific T cells differ from those of humans, with an effector phenotype compared to a memory phenotype in humans, and also have more restricted function: human T cells produce mainly IFNγ and IL-2 cytokines, while nonhuman primate T cells produce mainly IL-2.54
In contrast to liver, where capsid clearance presumably occurs up to 16 weeks after gene transfer depending on vector serotype,55 in muscle, the AAV vector seems to be trapped in muscle for years after IM injection.34 This results in a potential persistent exposure of T cells to capsid antigens that might be presented by antigen-presenting cells mimicking a chronic disease and explaining the induction of Tregs and exhausted T cells. Indeed, both cell types have shown the ability to mediate tolerance in the case of chronic diseases due to long duration of antigen exposure.56, 57, 58, 59 The initiation of a regulatory response can also be explained by the requirement of a certain concentration of epitopes to induce an active tolerance in opposition to ignorance.60,61 In the case of a rAAV1 IM delivery, the vector stays concentrated at the site of injection, as illustrated by the vector biodistribution data: the same dose is administered via IM, VLP, or IAPD delivery, but the vector biodistribution is different, with a less concentrated and more diffuse distribution in the injected limb after VLP or IAPD delivery.37, 38, 39,62, 63, 64 Tregs can also mediate T cell exhaustion,65 but this does not seem to be the case here. Indeed, both cell types are detected early post-injection; more importantly, exhausted T cells are detected after VLP and IAPD delivery, whereas Tregs are not.
In this study, we were able to correlate results obtained in monkeys to those obtained with patients, but our data confirm that monkeys do not completely mimic the immune response to the AAV capsid observed in humans. These observations highlight the complexity of the human immune system that cannot be completely understood with animal models, including those of nonhuman primates, which differ in their immune repertoire and reactivity to AAV capsids. Nevertheless, our study shows that, if analysis is performed in situ, we may observe similarities between our two models.
In summary, we showed that AAV vector delivery in muscle mediates T cell exhaustion and/or Treg response without preventing the formation of anti-AAV IgG or NAbs, which are critical for AAV readministration.66, 67, 68 One major point to take into consideration is that these results were obtained when gene transfer is made in healthy muscles in opposition to some previous preclinical and clinical trials where the IM delivery was used to treat neuromuscular disorders, such as Duchenne muscular dystrophy (DMD)42 or limb-girdle muscular dystrophy type 2D (LGM2D).69 In these models, the immunogenicity of the IM route might be enhanced because of the inflammatory environment caused by the disease itself.70,71 A good alternative to the IM delivery would be the VLP route, which still leads to T cell exhaustion and provides higher transgene expression. Other alternatives can also be explored, such as modulating the dose or using less prevalent72, 73, 74 or less immunogenic27,75 AAV serotypes or even capsid-modified rAAV.76 Indeed, the transduction efficiency of dendritic cells (DCs) depends on AAV serotype and/or genome conformation (ss versus self-complementary)27,77, 78, 79 and might explain why some serotypes are more immunogenic or, conversely, more tolerogenic than others. In the case of capsid-engineered vectors, we can imagine that the capsid circumvents natural pre-existing immunity. Actually, in recent preclinical and clinical trials using engineered capsids, the immune response does not occur as frequently as in previous trials.80
In conclusion, our data show that rAAV gene transfer may result in the absence of a deleterious T cell response to the capsid, and at least two cell populations are involved: Tregs and exhausted T cells. These findings, in addition to the characterization of immune responses in seronegative and seropositive patients,53 will help in developing safer gene transfer protocols and the establishment of new immune modulatory strategies.
Materials and Methods
Vector Production
Nonhuman Primate Study
Vector design and manufacturing has been previously described by Gruntman et al.39 Briefly, the CB-rhAAT-cMyc expression cassette has been packaged into an ssAAV1 capsid using the herpes simplex virus 1 (rHSV1) system, concentrated by tangential flow, exchanged to Ringer’s buffer, and filtered through a 0.2-μm filter. The vector was manufactured by Applied Genetic Technologies(AGTC).
AATD Phase II Clinical Trial
The rAAV1-CB-hAAT vector used in the AATD clinical trial has been previously described.33,81 Briefly, the rAAV1-CB-hAAT vector has been made using the rHSV1 system and purified by chromatography followed by AVB Sepharose affinity chromatography.
Study Design
Nonhuman Primate Study
Rhesus monkeys with a NAb titer to AAV1 at or below 1:10 were selected. The NAb titer was determined by the University of Massachusetts Gene Therapy Vector Core using an in vitro assay. At study day 0, three groups of animals were injected with 6e12 vg/kg of an ssAAV1-CB-rhAAT-cMyc by IM (n = 3), VLP (n = 3), or IAPD (n = 2) delivery. At study day 21, blood was drawn, and muscle biopsies were collected. At study day 60, animals were euthanized; blood and tissues were collected for evaluation. All the animal work was done under the approval and supervision of the Lovelace Respiratory Research Institute Animal Care and Use Committee.
The injection procedures are detailed by Gruntman et al.39 Briefly, the IM injection procedure consisted of four 0.5-mL injections in each quadriceps and gastrocnemius muscles in the right hindlimb.
The VLP animals received a volume of 50 mL/kg of the rAAV1 vector diluted in Lactated Ringer’s solution (LRS). An intravenous catheter was placed into the saphenous vein of the right pelvic limb. Then, a tourniquet was placed around the level of the proximal thigh. The vector was infused over 5–10 min. After infusion, the tourniquet remained tight for 15 min and then was released.
Animals in the IAPD group received a volume of 12.5 mL/kg rAAV1 vector diluted in LRS. An incision was made in the lower anterior thigh of the right pelvic limb, and the superficial femoral artery and vein were dissected and isolated with silk suture. Arterial and venous access was obtained with sheath catheters. The arterial and venous balloon catheters were then placed through their respective sheathes. After a pre-flush with LRS (5 mL/kg), the ssAAV1-CB-rhAAT-cMyc vector was infused in a volume of 12.5 mL/kg through the arterial catheter sheath port. After infusion, the vector solution was allowed to dwell for 15 min. Then, a post-flush of 5 mL/kg LRS was injected into the arterial catheter. After the infusion had been completed, the balloons were deflated, and the catheters were removed.
AATD Phase II Clinical Trial
The study design was previously reported by Flotte et al.33 This study is registered with ClinicalTrials.gov (ClinicalTrials.gov: NCT01054339) and was conducted under an IND (investigational new drug) with the approval of the University of Massachusetts Medical School and Cincinnati Children’s Hospital institutional review boards and institutional biosafety committees and in accordance with the tenets of the Declaration of Helsinki. This study was conducted as an open-label, non-randomized, multicenter, sequential, 3-arm, phase II clinical trial to determine the safety and efficacy of IM administration of a rAAV1-CB-hAAT vector. Nine subjects (three per cohort) received IM doses of rAAV1-CB-hAAT (6e11, 1.9e12, or 6e12 vg/kg body weight). For the present study, blood was collected on subject 308 (high-dose cohort) at 5 years post-dosing.
NAb Assay
Sera sampled prior to dosing, day 21, and at necropsy were heat inactivated for 30 min at 56°C. AAV1-CMV-LacZ (109 gc/well) was incubated with 2-fold serial dilutions of samples for 1 h at 37°C, 5% CO2. The mixture was added to Huh7 cells (1e5 cells) previously infected with an adenovirus (100 particles per well) and incubated for 18–22 h at 37°C, 5% CO2. Cells were washed and developed with the Galacto-Star Kit (Applied Biosystems). Luminescence was measured with a luminometer. The NAb titers are expressed as the highest dilution that inhibited β-galactosidase expression by at least 50% compared to a negative mouse serum control.
Anti-AAV1 IgG ELISA
Anti-AAV1 IgG antibodies were detected by enzyme-linked immunosorbent assay (ELISA). Plates were coated with 5e9 vg of rAAV1 vector in coating buffer (carbonate-bicarbonate, 0.5 M [pH = 9.6]) at 4°C overnight. The plates were blocked with PBS-Tween 0.1% for 1 h at 37°C. Monkey sera were serially diluted and incubated for 2 h at 37°C. Plates were then incubated with an anti-human IgG horseradish peroxidase (HRP) antibody (A80-104P, Bethyl Laboratories) for 1 h at room temperature (RT). Substrate (tetramethylbenzidine [TMB]; BD Biosciences) was added, and plates were incubated for 7 min at RT. The reaction was stopped using 2 N H2SO4. Plates were read in a spectrometer (VersaMax, Molecular Devices) at 450 nm.
H&E Staining
Skeletal muscle paraffin sections (8 μm) were collected on slides. H&E staining was performed as per standard histological protocols using formolin-fixed and paraffin-embedded muscle sections. The slides were observed using a Leica DM5500B microscope.
Muscle Dissociation
Muscle biopsies and muscles were harvested 21 and 60 days after AAV vector injection. Tissues were cut into approximately 5-mm3 pieces and incubated in RPMI media supplemented with DNase I (62.5 U/mL; Zymo Research) and collagenase (0.25 mg/mL; Roche) for 2 h at 37°C. Cell suspension was homogenized and filtered using a 70-μm cell strainer before staining for flow cytometry analysis.
Flow Cytometry
Regulatory T cells and exhausted CD8+ T cell populations were analyzed using CD3/CD4/Helios/FoxP3/CD25 and CD3/CD8/PD1/CD69 markers, respectively. Extracellular staining consisted of cell incubation on ice for 30 min. Cells were then washed twice with 1× PBS and re-suspended in 1× PBS supplemented with 0.5% fetal bovine serum (FBS) and 2 mM EDTA. FoxP3 staining and Helios intracellular staining were performed according to the manufacturer’s recommendations (anti-human FoxP3 Flow Kit, BioLegend). Cells were acquired using a BD FACS LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo software (v.10.1; Tree Star).
Immunohistochemistry
Skeletal muscle cryosections (5 μm) were collected on slides, air dried, and fixed with 3% paraformaldehyde (PFA) (Thermo Scientific) for 3 min. Saturation was performed in 10% goat serum (S-1000, Vector Laboratories) and 5% BSA (BP1600-100, Thermo Scientific) for 45 min at RT. Then, sections were incubated in blocking buffer antibodies overnight at 4°C. Polyclonal rabbit anti-human CD3 (1:100) (A0452, Dako), monoclonal mouse anti-human CD4 human (1:100) (317402, BioLegend), monoclonal rat anti-human CD8 (1:100) (MCA351GT, Bio-Rad), monoclonal mouse anti-human FoxP3 (1:100) (14-4777-82, Invitrogen), and monoclonal mouse anti-human PD1 (1:100) (329902, BioLegend) antibodies were used to detect Tregs (CD3+CD4+FoxP3+) and exhausted T cells (CD3+CD8+PD1+). After washing with PBS, sections were incubated with goat anti-rabbit Alexa Fluor 555-conjugated antibody (1:500) (A32732, Invitrogen) combined with goat anti-mouse (A21242, Invitrogen) or goat anti-rat (1:500) (A21247, Invitrogen) Alexa Fluor 647-conjugated antibodies or with goat anti-mouse Alexa Fluor 488-conjugated antibody (1:500) (A21121, Invitrogen) for 1 h at RT. Nuclei were counterstained with DAPI for 5 min at RT (0.001 mg/mL) (10236276001, Sigma Aldrich). Finally, slides were mounted with Clear-Mount (17985-12, Electron Microscopy Sciences). Images were captured using a Leica DM5500B microscope and Leica Application Suite AF v.3.1.0 build 8587 imaging software.
IFNγ ELISpot Assay
PBMCs were isolated 5 years post-injection, and the IFNγ ELISpot assay was performed according to the manufacturer’s recommendations (Human IFN-γ ELISpotBASIC, MABTech). Briefly, PBMCs were stimulated in vitro for 48 h with overlapping peptides spanning the AAV1 capsid VP1 sequences and divided into 3 pools (2 μg/mL, 15-mers overlapping by 10 aa, Mimotopes, Mulgrave, VIC, Australia) or stimulated with the AAV1 immunodominant peptides (5μg/ml, Mimotopes, Mulgrave, VIC, Australia; Table S4). A negative control consisted of unstimulated cells (medium only), whereas CEFT (JPT Peptide Technologies, Berlin, Germany) and CD3/CD28 stimulation were used as a positive control for cytokine secretion. Spot number was determined using an iSpot ELISpot Reader ELR068IFL (AID) and analyzed with AID ELISpot Reader software v.6.0. Responses were considered positive when the number of spot-forming units (SFUs) per 1e6 cells were >50 and at least 3-fold higher than in the control condition.
PD1/PDL1 Blocking Assay
The PD1/PDL1 assay was conducted as described in the previous IFNγ ELISpot Assay section, except that PBMCs were stimulated in vitro for 48 h with the AAV1 immunodominant peptides (5 μg/mL, Mimotopes, Mulgrave, VIC, Australia; Table S4) with either no antibodies or 1 μL/100 μL cells of PD1/PDL1 antibodies (BioLegend) or their respective isotypes (BioLegend).
Author Contributions
G.G., A.M.G., T.R.F., and C.M. designed the study. G.G. and C.M. designed experiments and wrote and edited the manuscript. G.G. conducted and analyzed all the experiments. A.M.G. provided nonhuman primate tissues. M.B. performed the anti-AAV ELISA and neutralizing antibody assay. M.Z. performed H&E staining and immunohistochemistry. T.R.F. and C.M. supervised the study.
Conflicts of Interest
T.R.F. is a paid consultant for Beam Therapeutics and was a scientific founder of AGTC. C.M. is a cofounder of Apic Bio and holds equity in the company. C.M. is an inventor on patents with potential royalties licensed to Apic Bio. G.G., A.M.G., M.B., and M.Z. declare no competing financial interests.
Acknowledgments
This work was performed at the University of Massachusetts Medical School, Worcester, MA, USA. We would like to acknowledge the University of Massachusetts Flow Cytometry Core and Morphology Core for their assistance; all the staff at the Lovelace Respiratory Research Institute (LRRI), where the nonhuman primate work was done; and, more particularly, Denise Marks for her assistance and support. We thank Drs. G. Gao, Q. Su, and J. Xie from the University of Massachusetts Viral Vector Core for providing the AAV1-LacZ vector. We thank Margaret Humphries for coordinating patient blood draws and our study subject, 308, for her interest and participation in this study. The graphical abstract was created with Biorender.com. This study was supported by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK] R01 DK098252), National Heart, Lung, and Blood Institute (NHLBI, P01 HL131471 and69 Gene Therapy Resource Program), the Alpha-1 Foundation, and AGTC.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2020.01.004.
Supplemental Information
References
- 1.Inagaki K., Fuess S., Storm T.A., Gibson G.A., Mctiernan C.F., Kay M.A., Nakai H. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L., Bell P., Somanathan S., Wang Q., He Z., Yu H., McMenamin D., Goode T., Calcedo R., Wilson J.M. Comparative study of liver gene transfer with AAV vectors based on natural and engineered AAV capsids. Mol. Ther. 2015;23:1877–1887. doi: 10.1038/mt.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao W., Chirmule N., Berta S.C., McCullough B., Gao G., Wilson J.M. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao H., Liu Y., Rabinowitz J., Li C., Samulski R.J., Walsh C.E. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- 5.Gregorevic P., Blankinship M.J., Allen J.M., Crawford R.W., Meuse L., Miller D.G., Russell D.W., Chamberlain J.S. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z., Zhu T., Qiao C., Zhou L., Wang B., Zhang J., Chen C., Li J., Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 7.Klein R.L., Dayton R.D., Tatom J.B., Diaczynsky C.G., Salvatore M.F. Tau expression levels from various adeno-associated virus vector serotypes produce graded neurodegenerative disease states. Eur. J. Neurosci. 2008;27:1615–1625. doi: 10.1111/j.1460-9568.2008.06161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dayton R.D., Wang D.B., Klein R.L. The advent of AAV9 expands applications for brain and spinal cord gene delivery. Expert Opin. Biol. Ther. 2012;12:757–766. doi: 10.1517/14712598.2012.681463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang B., Li S., Wang H., Guo Y., Gessler D.J., Cao C., Su Q., Kramer J., Zhong L., Ahmed S.S. Global CNS transduction of adult mice by intravenously delivered rAAVrh.8 and rAAVrh.10 and nonhuman primates by rAAVrh.10. Mol. Ther. 2014;22:1299–1309. doi: 10.1038/mt.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebherz C., Maguire A., Tang W., Bennett J., Wilson J.M. Novel AAV serotypes for improved ocular gene transfer. J. Gene Med. 2008;10:375–382. doi: 10.1002/jgm.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber M., Rabinowitz J., Provost N., Conrath H., Folliot S., Briot D., Chérel Y., Chenuaud P., Samulski J., Moullier P., Rolling F. Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term transduction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol. Ther. 2003;7:774–781. doi: 10.1016/s1525-0016(03)00098-4. [DOI] [PubMed] [Google Scholar]
- 12.Snyder R.O., Miao C., Meuse L., Tubb J., Donahue B.A., Lin H.F., Stafford D.W., Patel S., Thompson A.R., Nichols T. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat. Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 13.Niemeyer G.P., Herzog R.W., Mount J., Arruda V.R., Tillson D.M., Hathcock J., van Ginkel F.W., High K.A., Lothrop C.D., Jr. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross C.J.D., Twisk J., Meulenberg J.M., Liu G., van den Oever K., Moraal E., Hermens W.T., Rip J., Kastelein J.J., Kuivenhoven J.A., Hayden M.R. Long-term correction of murine lipoprotein lipase deficiency with AAV1-mediated gene transfer of the naturally occurring LPL(S447X) beneficial mutation. Hum. Gene Ther. 2004;15:906–919. doi: 10.1089/hum.2004.15.906. [DOI] [PubMed] [Google Scholar]
- 15.Song S., Morgan M., Ellis T., Poirier A., Chesnut K., Wang J., Brantly M., Muzyczka N., Byrne B.J., Atkinson M., Flotte T.R. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc. Natl. Acad. Sci. USA. 1998;95:14384–14388. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathwani A.C., Rosales C., McIntosh J., Rastegarlari G., Nathwani D., Raj D., Nawathe S., Waddington S.N., Bronson R., Jackson S. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol. Ther. 2011;19:876–885. doi: 10.1038/mt.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yue Y., Ghosh A., Long C., Bostick B., Smith B.F., Kornegay J.N., Duan D. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol. Ther. 2008;16:1944–1952. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Guiner C., Servais L., Montus M., Larcher T., Fraysse B., Moullec S., Allais M., François V., Dutilleul M., Malerba A. Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy. Nat. Commun. 2017;8:16105. doi: 10.1038/ncomms16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mack D.L., Poulard K., Goddard M.A., Latournerie V., Snyder J.M., Grange R.W., Elverman M.R., Denard J., Veron P., Buscara L. Systemic AAV8-mediated gene therapy drives whole-body correction of myotubular myopathy in dogs. Mol. Ther. 2017;25:839–854. doi: 10.1016/j.ymthe.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodino-Klapac L.R., Lee J.S., Mulligan R.C., Clark K.R., Mendell J.R. Lack of toxicity of alpha-sarcoglycan overexpression supports clinical gene transfer trial in LGMD2D. Neurology. 2008;71:240–247. doi: 10.1212/01.wnl.0000306309.85301.e2. [DOI] [PubMed] [Google Scholar]
- 21.Borel F., Gernoux G., Sun H., Stock R., Blackwood M., Brown R.H., Jr., Mueller C. Safe and effective superoxide dismutase 1 silencing using artificial microRNA in macaques. Sci. Transl. Med. 2018;10:eaau6414. doi: 10.1126/scitranslmed.aau6414. [DOI] [PubMed] [Google Scholar]
- 22.Meyer K., Ferraiuolo L., Schmelzer L., Braun L., McGovern V., Likhite S., Michels O., Govoni A., Fitzgerald J., Morales P. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose-response study in mice and nonhuman primates. Mol. Ther. 2015;23:477–487. doi: 10.1038/mt.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennicelli J., Wright J.F., Komaromy A., Jacobs J.B., Hauck B., Zelenaia O., Mingozzi F., Hui D., Chung D., Rex T.S. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol. Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petit L., Lhériteau E., Weber M., Le Meur G., Deschamps J.Y., Provost N., Mendes-Madeira A., Libeau L., Guihal C., Colle M.A. Restoration of vision in the pde6β-deficient dog, a large animal model of rod-cone dystrophy. Mol. Ther. 2012;20:2019–2030. doi: 10.1038/mt.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Meur G., Stieger K., Smith A.J., Weber M., Deschamps J.Y., Nivard D., Mendes-Madeira A., Provost N., Péréon Y., Cherel Y. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 2007;14:292–303. doi: 10.1038/sj.gt.3302861. [DOI] [PubMed] [Google Scholar]
- 26.Mays L.E., Vandenberghe L.H., Xiao R., Bell P., Nam H.J., Agbandje-McKenna M., Wilson J.M. Adeno-associated virus capsid structure drives CD4-dependent CD8+ T cell response to vector encoded proteins. J. Immunol. 2009;182:6051–6060. doi: 10.4049/jimmunol.0803965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mays L.E., Wang L., Lin J., Bell P., Crawford A., Wherry E.J., Wilson J.M. AAV8 induces tolerance in murine muscle as a result of poor APC transduction, T cell exhaustion, and minimal MHCI upregulation on target cells. Mol. Ther. 2014;22:28–41. doi: 10.1038/mt.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 29.Nathwani A.C., Tuddenham E.G.D., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mingozzi F., Maus M.V., Hui D.J., Sabatino D.E., Murphy S.L., Rasko J.E.J., Ragni M.V., Manno C.S., Sommer J., Jiang H. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 31.Calcedo R., Morizono H., Wang L., McCarter R., He J., Jones D., Batshaw M.L., Wilson J.M. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 33.Flotte T.R., Trapnell B.C., Humphries M., Carey B., Calcedo R., Rouhani F., Campbell-Thompson M., Yachnis A.T., Sandhaus R.A., McElvaney N.G. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results. Hum. Gene Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller C., Chulay J.D., Trapnell B.C., Humphries M., Carey B., Sandhaus R.A., McElvaney N.G., Messina L., Tang Q., Rouhani F.N. Human Treg responses allow sustained recombinant adeno-associated virus-mediated transgene expression. J. Clin. Invest. 2013;123:5310–5318. doi: 10.1172/JCI70314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira V., Twisk J., Kwikkers K., Aronica E., Brisson D., Methot J., Petry H., Gaudet D. Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPL(S447X)) in a phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum. Gene Ther. 2014;25:180–188. doi: 10.1089/hum.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gernoux G., Wilson J.M., Mueller C. Regulatory and exhausted T cell responses to AAV capsid. Hum. Gene Ther. 2017;28:338–349. doi: 10.1089/hum.2017.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toromanoff A., Chérel Y., Guilbaud M., Penaud-Budloo M., Snyder R.O., Haskins M.E., Deschamps J.Y., Guigand L., Podevin G., Arruda V.R. Safety and efficacy of regional intravenous (RI) versus intramuscular (IM) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle. Mol. Ther. 2008;16:1291–1299. doi: 10.1038/mt.2008.87. [DOI] [PubMed] [Google Scholar]
- 38.Haurigot V., Mingozzi F., Buchlis G., Hui D.J., Chen Y., Basner-Tschakarjan E., Arruda V.R., Radu A., Franck H.G., Wright J.F. Safety of AAV factor IX peripheral transvenular gene delivery to muscle in hemophilia B dogs. Mol. Ther. 2010;18:1318–1329. doi: 10.1038/mt.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gruntman A.M., Gernoux G., Tang Q., Ye G.J., Knop D.R., Wang G., Benson J., Coleman K.E., Keeler A.M., Mueller C. Bridging from intramuscular to limb perfusion delivery of rAAV: optimization in a non-human primate study. Mol. Ther. Methods Clin. Dev. 2019;13:233–242. doi: 10.1016/j.omtm.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller C., Gernoux G., Gruntman A.M., Borel F., Reeves E.P., Calcedo R., Rouhani F.N., Yachnis A., Humphries M., Campbell-Thompson M. 5 Year expression and neutrophil defect repair after gene therapy in alpha-1 antitrypsin deficiency. Mol. Ther. 2017;25:1387–1394. doi: 10.1016/j.ymthe.2017.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hui D.J., Edmonson S.C., Podsakoff G.M., Pien G.C., Ivanciu L., Camire R.M., Ertl H., Mingozzi F., High K.A., Basner-Tschakarjan E. AAV capsid CD8+ T-cell epitopes are highly conserved across AAV serotypes. Mol. Ther. Methods Clin. Dev. 2015;2:15029. doi: 10.1038/mtm.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendell J.R., Campbell K., Rodino-Klapac L., Sahenk Z., Shilling C., Lewis S., Bowles D., Gray S., Li C., Galloway G. Dystrophin immunity in Duchenne’s muscular dystrophy. N. Engl. J. Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross C.J., Twisk J., Bakker A.C., Miao F., Verbart D., Rip J., Godbey T., Dijkhuizen P., Hermens W.T., Kastelein J.J. Correction of feline lipoprotein lipase deficiency with adeno-associated virus serotype 1-mediated gene transfer of the lipoprotein lipase S447X beneficial mutation. Hum. Gene Ther. 2006;17:487–499. doi: 10.1089/hum.2006.17.487. [DOI] [PubMed] [Google Scholar]
- 44.Herzog R.W., Mount J.D., Arruda V.R., High K.A., Lothrop C.D., Jr. Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol. Ther. 2001;4:192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- 45.Rodino-Klapac L.R., Montgomery C.L., Bremer W.G., Shontz K.M., Malik V., Davis N., Sprinkle S., Campbell K.J., Sahenk Z., Clark K.R. Persistent expression of FLAG-tagged micro dystrophin in nonhuman primates following intramuscular and vascular delivery. Mol. Ther. 2010;18:109–117. doi: 10.1038/mt.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toromanoff A., Adjali O., Larcher T., Hill M., Guigand L., Chenuaud P., Deschamps J.Y., Gauthier O., Blancho G., Vanhove B. Lack of immunotoxicity after regional intravenous (RI) delivery of rAAV to nonhuman primate skeletal muscle. Mol. Ther. 2010;18:151–160. doi: 10.1038/mt.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wherry E.J. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 48.Cao O., Dobrzynski E., Wang L., Nayak S., Mingle B., Terhorst C., Herzog R.W. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooper M., Nayak S., Hoffman B.E., Terhorst C., Cao O., Herzog R.W. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum. Gene Ther. 2009;20:767–776. doi: 10.1089/hum.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mingozzi F., Liu Y.L., Dobrzynski E., Kaufhold A., Liu J.H., Wang Y., Arruda V.R., High K.A., Herzog R.W. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J. Clin. Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pien G.C., Basner-Tschakarjan E., Hui D.J., Mentlik A.N., Finn J.D., Hasbrouck N.C., Zhou S., Murphy S.L., Maus M.V., Mingozzi F. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J. Clin. Invest. 2009;119:1688–1695. doi: 10.1172/JCI36891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veron P., Leborgne C., Monteilhet V., Boutin S., Martin S., Moullier P., Masurier C. Humoral and cellular capsid-specific immune responses to adeno-associated virus type 1 in randomized healthy donors. J. Immunol. 2012;188:6418–6424. doi: 10.4049/jimmunol.1200620. [DOI] [PubMed] [Google Scholar]
- 53.Kuranda K., Jean-Alphonse P., Leborgne C., Hardet R., Collaud F., Marmier S., Costa Verdera H., Ronzitti G., Veron P., Mingozzi F. Exposure to wild-type AAV drives distinct capsid immunity profiles in humans. J. Clin. Invest. 2018;128:5267–5279. doi: 10.1172/JCI122372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H., Lasaro M.O., Jia B., Lin S.W., Haut L.H., High K.A., Ertl H.C. Capsid-specific T-cell responses to natural infections with adeno-associated viruses in humans differ from those of nonhuman primates. Mol. Ther. 2011;19:2021–2030. doi: 10.1038/mt.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H., Tuyishime S., Wu T.-L., Giles-Davis W., Zhou D., Xiao W., High K.A., Ertl H.C. Adeno-associated virus vectors serotype 2 induce prolonged proliferation of capsid-specific CD8+ T cells in mice. Mol. Ther. 2011;19:536–546. doi: 10.1038/mt.2010.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brooks D.G., McGavern D.B., Oldstone M.B.A. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J. Clin. Invest. 2006;116:1675–1685. doi: 10.1172/JCI26856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Angelosanto J.M., Blackburn S.D., Crawford A., Wherry E.J. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J. Virol. 2012;86:8161–8170. doi: 10.1128/JVI.00889-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoop J.N., van der Molen R.G., Baan C.C., van der Laan L.J., Kuipers E.J., Kusters J.G., Janssen H.L. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771–778. doi: 10.1002/hep.20649. [DOI] [PubMed] [Google Scholar]
- 59.Keynan Y., Card C.M., McLaren P.J., Dawood M.R., Kasper K., Fowke K.R. The role of regulatory T cells in chronic and acute viral infections. Clin. Infect. Dis. 2008;46:1046–1052. doi: 10.1086/529379. [DOI] [PubMed] [Google Scholar]
- 60.Kelly M.E., Zhuo J., Bharadwaj A.S., Chao H. Induction of immune tolerance to FIX following muscular AAV gene transfer is AAV-dose/FIX-level dependent. Mol. Ther. 2009;17:857–863. doi: 10.1038/mt.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar S.R.P., Hoffman B.E., Terhorst C., de Jong Y.P., Herzog R.W. The balance between CD8+ T cell-mediated clearance of AAV-encoded antigen in the liver and tolerance is dependent on the vector dose. Mol. Ther. 2017;25:880–891. doi: 10.1016/j.ymthe.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arruda V.R., Stedman H.H., Nichols T.C., Haskins M.E., Nicholson M., Herzog R.W., Couto L.B., High K.A. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. 2005;105:3458–3464. doi: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Childers M.K., Joubert R., Poulard K., Moal C., Grange R.W., Doering J.A., Lawlor M.W., Rider B.E., Jamet T., Danièle N. Gene therapy prolongs survival and restores function in murine and canine models of myotubular myopathy. Sci. Transl. Med. 2014;6:220ra10. doi: 10.1126/scitranslmed.3007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodino-Klapac L.R., Janssen P.M.L., Montgomery C.L., Coley B.D., Chicoine L.G., Clark K.R., Mendell J.R. A translational approach for limb vascular delivery of the micro-dystrophin gene without high volume or high pressure for treatment of Duchenne muscular dystrophy. J. Transl. Med. 2007;5:45. doi: 10.1186/1479-5876-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Penaloza-MacMaster P., Kamphorst A.O., Wieland A., Araki K., Iyer S.S., West E.E., O’Mara L., Yang S., Konieczny B.T., Sharpe A.H. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J. Exp. Med. 2014;211:1905–1918. doi: 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moskalenko M., Chen L., van Roey M., Donahue B.A., Snyder R.O., McArthur J.G., Patel S.D. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J. Virol. 2000;74:1761–1766. doi: 10.1128/jvi.74.4.1761-1766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scallan C.D., Jiang H., Liu T., Patarroyo-White S., Sommer J.M., Zhou S., Couto L.B., Pierce G.F. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- 68.Wang L., Calcedo R., Bell P., Lin J., Grant R.L., Siegel D.L., Wilson J.M. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum. Gene Ther. 2011;22:1389–1401. doi: 10.1089/hum.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mendell J.R., Rodino-Klapac L.R., Rosales-Quintero X., Kota J., Coley B.D., Galloway G., Craenen J.M., Lewis S., Malik V., Shilling C. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann. Neurol. 2009;66:290–297. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuasa K., Sakamoto M., Miyagoe-Suzuki Y., Tanouchi A., Yamamoto H., Li J., Chamberlain J.S., Xiao X., Takeda S. Adeno-associated virus vector-mediated gene transfer into dystrophin-deficient skeletal muscles evokes enhanced immune response against the transgene product. Gene Ther. 2002;9:1576–1588. doi: 10.1038/sj.gt.3301829. [DOI] [PubMed] [Google Scholar]
- 71.Ferrand M., Galy A., Boisgerault F. A dystrophic muscle broadens the contribution and activation of immune cells reacting to rAAV gene transfer. Gene Ther. 2014;21:828–839. doi: 10.1038/gt.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Calcedo R., Vandenberghe L.H., Gao G., Lin J., Wilson J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leborgne C., Latournerie V., Boutin S., Desgue D., Quéré A., Pignot E., Collaud F., Charles S., Simon Sola M., Masat E. Prevalence and long-term monitoring of humoral immunity against adeno-associated virus in Duchenne muscular dystrophy patients. Cell. Immunol. 2019;342:103780. doi: 10.1016/j.cellimm.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M.F., Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 75.Lin S.-W., Hensley S.E., Tatsis N., Lasaro M.O., Ertl H.C.J. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J. Clin. Invest. 2007;117:3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li C., Diprimio N., Bowles D.E., Hirsch M.L., Monahan P.E., Asokan A., Rabinowitz J., Agbandje-McKenna M., Samulski R.J. Single amino acid modification of adeno-associated virus capsid changes transduction and humoral immune profiles. J. Virol. 2012;86:7752–7759. doi: 10.1128/JVI.00675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aldrich W.A., Ren C., White A.F., Zhou S.Z., Kumar S., Jenkins C.B., Shaw D.R., Strong T.V., Triozzi P.L., Ponnazhagan S. Enhanced transduction of mouse bone marrow-derived dendritic cells by repetitive infection with self-complementary adeno-associated virus 6 combined with immunostimulatory ligands. Gene Ther. 2006;13:29–39. doi: 10.1038/sj.gt.3302601. [DOI] [PubMed] [Google Scholar]
- 78.Veron P., Boutin S., Martin S., Chaperot L., Plumas J., Davoust J., Masurier C. Highly efficient transduction of human plasmacytoid dendritic cells without phenotypic and functional maturation. J. Transl. Med. 2009;7:10. doi: 10.1186/1479-5876-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Veron P., Allo V., Rivière C., Bernard J., Douar A.M., Masurier C. Major subsets of human dendritic cells are efficiently transduced by self-complementary adeno-associated virus vectors 1 and 2. J. Virol. 2007;81:5385–5394. doi: 10.1128/JVI.02516-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brantly M.L., Spencer L.T., Humphries M., Conlon T.J., Spencer C.T., Poirier A., Garlington W., Baker D., Song S., Berns K.I. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum. Gene Ther. 2006;17:1177–1186. doi: 10.1089/hum.2006.17.1177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.