Short abstract
Background
Bone cancer pain is common in patients with advanced cancers as tumor metastasizes to bone. The inefficient clinical treatment severely reduces quality of life of bone cancer pain patients. During the pain status, activated spinal astrocytes and microglia release various inflammatory cytokines, resulting in spinal inflammation and the development of neuron sensitization. Scorpion is the dry body of Buthus martensii Karsch and is often used for various pain management in clinical practice. However, its function on bone cancer pain is unclear.
Methods
We investigated the effects of intragastric administration of scorpion on bone cancer pain induced by left tibial cavity injection of Walker 256 cells. Nociceptive behavior was measured using the von Frey filaments test and the spontaneous ambulatory pain score. The bone destruction was assessed by tibial radiographs. Expression of spinal cord astrocyte marker glial fibrillary acidic protein and microglial marker Iba1 was monitored by Western blot assay and immunofluorescence. Tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, and IL-1β was detected by real-time polymerase chain reaction. The proliferation of Walker 256 cells was evaluated by CCK8 assay.
Results
Intragastric administration of scorpion reduced bone cancer pain behavior and relieved bone destruction, accompanied by decreased expression of spinal glial fibrillary acidic protein and Iba1 protein level and TNF-α, IL-6, and IL-1β mRNA level. Besides, scorpion inhibited proliferation of Walker 256 cells in a dose- and time-dependent manner.
Conclusion
Our results demonstrate that scorpion produces an analgesic effect in a rat model of bone cancer pain via inhibiting bone destruction and activation of spinal cord astrocytes and microglia.
Keywords: bone cancer pain, scorpion, analgesic effect, bone destruction, glia activation
Background
A significant proportion of cancer patients with bone metastases usually suffer from severe pain and have a low quality of life.1–3 Bone complications caused by metastasis are found in 70% of patients with advanced prostate or breast cancer,4,5 and metastasis is considered as the dominant contributor to malignancy-induced bone pain. Moreover, this chronic pain condition can have an unpredictable onset and increase in severity with progression of malignancy. Despite the availability of bisphosphonates, nonsteroidal anti-inflammatory drugs and opioids, many patients with bone cancer pain (BCP) report limited pain relief and adverse side effects, such as neuropsychiatric symptoms and gastric bleeding.6–8 No new pharmacotherapy has emerged, and there is an urgent need for new BCP treatments.
In China, the scorpion Buthus martensii Karsch (BmK) has been an essential material in Chinese traditional medicine for thousands of years. Whole scorpions, scorpion tails, and their extracts have been found to be effective in treating certain neural diseases such as apoplexy, epilepsy, facial paralysis, and hemiplegia, in addition to their use for soothing the nerves and relieving pain caused by meningitis, cerebral palsy, and rheumatism.9,10 Recent study demonstrated that the analgesic effect of scorpion was probably accomplished through opioid receptors on lateral septal nucleus in central nervous system.11 Others found that peripheral and spinal mitogen-activated protein kinases and the voltage-gated calcium channels in dorsal root ganglia neurons may be potential mechanisms of the antinociception provided by scorpion.12–14 However, research on the effects or mechanisms of scorpion in BCP is limited.
Previous studies have exposed the critical importance of glial cells to a variety of biological functions, including pain perception and modulation.15,16 Astrocytes and microglia in the spinal cord participate in the initiation and maintenance of persistent pain induced by tissue inflammation and nerve injury.17–19 During pathological pain status, activated astrocytes and microglia release various inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor-alpha (TNF-α).20,21 The production of proinflammatory mediators modulates pain sensitivity, which leads to the development of peripheral and central sensitization, and induction of chronic pain conditions.22 However, the effects of scorpion on the spinal cord astrocytes and microglia in BCP have not been investigated.
In this study, we demonstrated the analgesic effect of scorpion in a malignant bone pain mouse model and examined the underlying mechanism. Our findings suggested that scorpion may be a choice for BCP treatment.
Materials and methods
Cell culture
Walker 256 mammary gland carcinoma cell line was a gift from Prof. Changsheng Dong (Shanghai Research Institute of Traditional Chinese Medicine, China). Walker 256 cells were cultured in RPMI 1640 medium (GIBCO; MD, USA) containing 10% fetal bovine serum (heat-inactivated) (Hyclone; UT, USA), 1% L-glutamine and 2% penicillin/streptomycin (GIBCO; MD, USA). Cells were detached from the flask by 0.25% trypsin for subsequent preparation of injection. Briefly, the cells were collected by centrifugation and the pellet was resuspended in phosphate-buffered saline (PBS).
Experimental animals
Female Sprague-Dawley (SD) rats were from Institute of Genome engineered Animal Models for Human Diseases (Dalian, China). All animals were housed with ad libitum access to water and food in a 12/12-h light–dark cycle regime and environmental temperature was controlled at 22 ± 1°C. Animals were housed for seven days to acclimatize before the experimental procedures. All animals were housed and handled in accordance with the Animal Care and Use Committee at Dalian Medical University.
Surgical procedure for establishing a rat model of BCP
A rat model of BCP was established following previous report.23 For cell preparation, ascitic cancer cells 0.5 ml (2 × 107 cells/ml) were injected into the abdominal cavity of 60–80 g female SD juvenile rats. After six to seven days, ascitic fluid was extracted from above rats. Then, cells were collected by centrifugation of 2 ml ascitic fluid for 3 min at 1200 r/min. The pellet was washed with 10 ml PBS and recentrifuged for 3 min at 1200 r/min. Before the final pellet was resuspended in an appropriate volume to achieve final concentrations for injection, the pellet was suspended in 10 ml PBS and cells were counted using a hemocytometer (2 × 107 cells/ml). The cell suspension was kept on ice until injected into 180–220 g female SD adult rats. For the sham group, PBS was prepared in the same volume for injection. For surgery, female SD adult rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneal injection). Bilateral superficial incisions were made in the skin overlying the patella after disinfected with 70% v/v ethanol. Then, more incisions were cut along the patellar ligament in order to expose the tibia head with minimal damage. A 23-gauge needle was inserted at the site of intercondylar eminence and pierced 7 mm below the knee joint into the medullary cavity of tibia. The needle was then removed and replaced with a 29-gauge needle (long thin blunt needle) attached to a 10 μl microinjection syringe. Then, carcinoma cells (2 × 105) in 10 μl PBS or PBS (sham group) 10 μl were slowly injected into the left tibia cavity. The syringe was left in place for an additional 2 min to prevent the carcinoma cells from leaking out along the injection track. The injection site was closed using bone wax while the syringe was removed. The wound was closed and dusted with penicillin powder after the injection site was closed using gelatin sponge.
Drug administration
Intragastric administration of scorpion was used for the treatment of BCP rats. Briefly, rats (n = 40) were randomly divided into four groups: sham group, BCP group, BCP+scorpion (prophylactic) group and BCP+scorpion (reversal), each group contained 10 rats. The scorpion was decocted twice with distilled water for 1 h. The decoction was collected, filtered, merged, and concentrated to 1 g/mL (equivalent to crude herb materials) and stored at −20°C. Scorpion-contained decoction was given to the rats at 108 mg/200 g/d from 4 to 21 days (prophylactic) or 14 to 21 days (reversal) after modeling, and rats in sham group and BCP group were given same volume of saline every day.
Mechanical allodynia test
Rats were placed on a 5 × 5 mm wire mesh grid floor and allowed to habituate for 30 min. Test was blind with respect to group. The von Frey filaments (ranging from 0.4 g to 15 g) (North Coast Medical, CA, USA) were used to apply mechanical stimuli to the hind paw. Each von Frey hair was held about 1–2 s, with a 10-min interval between each application. A trial began with the application of the 2 g von Frey hair. The positive response was defined as a withdrawal of hind paw upon the stimulus. Whenever a positive response to a stimulus occurred, the next lower von Frey hair was applied, and whenever a negative response occurred, the next higher hair was applied. The testing consisted of five more stimuli after the first change in response occurred, and the pattern of response was converted to a 50% von Frey threshold using the method described by Dixon.24 The test was taken at 1 day before modeling and 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, and 21 days after modeling.
Behavioral assays for ambulatory pain
Rats were placed in a large plastic observation box with smooth floor. According to the extent of limb use during spontaneous ambulation, scores were given as follows: (0) normal use, (1) slight limp, (2) extent between (1) and (3), (3) severe limp, and (4) complete lack of limb use. Testing was blind with respect to group. The test was taken at 1 day before modeling and 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 21 days after modeling.
Radiology
Rats were anesthetized with sodium pentobarbital and exposed to an X-ray source (RayNova Pet DR). Roentgenography of the tibia was performed on postoperative day 21. Radiographs were taken from hind limbs of the rats and then analyzed by NIH ImageJ (ImageJ, 1.47 v).
Bone and spinal cord histology
On day 21 following inoculation of Walker 256 cells, rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneal injection). After transcardial perfused with 0.9% saline followed with 4% paraformaldehyde. Spinal cord (L4-6) were isolated from animals and fixed in 4% paraformaldehyde at 4°C for 24 h and then transferred to 30% sucrose buffer until the tissues drop to the bottom of the container. Then the spinal cords were embedded in paraffin and sections were cut into 8 μm (Leica RM 2165). The other unfixed spinal cords were stored at −80°C. The tibial bones were preserved in 4% paraformaldehyde.
Western blot
The spinal cord was homogenized in ice-cold radioimmunoprecipitation assay (RIPA) buffer containing a cocktail of protease inhibitors (Sigma). After centrifugation at 12,000 g for 15 min, supernatant was used for Western blot analysis. Protein concentrations were determined by BCA method. Equal amounts of protein samples were separated in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred onto a polyvinylidene difluoride membrane and then incubated with the appropriate primary antibodies at 4°C overnight. The following antibodies were used in this study (ProteinTech): rabbit anti-GFAP (1:1000, 16825–1-AP), rabbit anti-Iba1 (1:1000, 10904–1-AP), and mouse anti-GAPDH (1:5000, 60004–1-Ig). Horseradish peroxidase-conjugated secondary antibodies (1:5000, Thermo Fisher Scientific, 31460/31430) were used to visualize the primary antibodies. Imaging System (Bio-Rad, CA, USA) was applied to detect immunoreactive bands.
Immunofluorescence
Frozen spinal sections (8 μm) were fixed with cold acetone for 10 min and rinse with PBS for three times. The sections were then blocked with 10% normal donkey serum for 1 h at room temperature. Subsequently, the sections were incubated with primary antibody, rabbit anti-GFAP (1:100, 16825–1-AP) or rabbit anti-Iba1 (1:50, 10904–1-AP), overnight at 4°C. After incubation and being washed with PBS, sections were incubated with goat anti-mouse IgG H&L (FITC) (1:200, Abcam, ab6785) at room temperature for 1 h and then examined with a fluorescence microscope.
Real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) and used to generate cDNA by EasyScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech) with an oligo-dT primer. Real-time polymerase chain reaction (PCR) was performed using SYBR Select Master Mix (Life Technology) as recommended by the manufacturer. GAPDH was used as the internal control. All primers are designed by Thermo Fisher (Beijing) and listed below:
TNF-α-F: 5′-CAAGAGCCCTTGCCCTAAGG-3′
TNF-α-R: 5′-CGGACTCCGTGATGTCTAAGTACTT-3′
IL-1β-F: 5′-TCAGGAAGGCAGTGTCACTCA-3′
IL-1β-R: 5′-CATCATCCCACGAGTCACAGA-3′
IL-6-F: 5′-CTGATTGTATGAACAGCGATGATG-3′
IL-6-R: 5′-GGTAGAAACGGAACTCCAGAAGAC-3′
rGAPDH-F: 5′-GCATCTTCTTGTGCAGTGCC-3′
rGAPDH-R: 5′-TACGGCCAAATCCGTTCACA-3′
CCK8 assay
Walker 256 cells (1 × 103) were plated onto 96-well flat bottom plates in a final volume of 100 μl/well. After attached, cells were exposed to scorpion at the concentration of 0 mg/ml, 0.5 mg/ml, 2 mg/ml, and 8 mg/ml for 24 h and 48 h. At the end of the above stated exposure periods, CCK-8 was placed in each of the wells containing the control samples as well as those containing scorpion exposed samples. The samples were kept in an incubator for 60 min, after which the absorbance was measured with MultiskanGo Spectrophotometer (USA) at 450 nm optical density. Suppression of cell proliferation was determined by the following mathematical relation: [(Mean absorbance of control – Mean absorbance of exposed)/Mean absorbance of control) × 100].
Statistical analysis
Each in vivo and in vitro experiment was performed in triplicate and repeated at least three times. Data analysis was achieved with SPSS software (version 19.0). Differences between variables were assessed by one-way analysis of variance, where appropriate. Data are shown as mean ± standard error of the mean (SEM). p < 0.05 is considered statistically significant.
Results
Scorpion alleviates BCP behavior
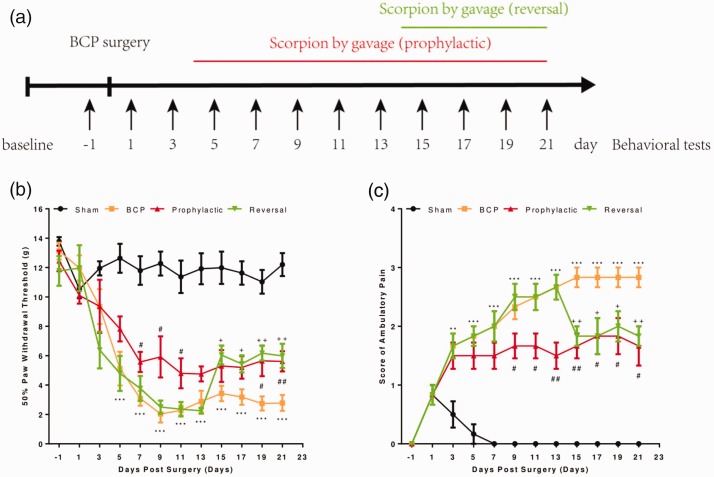
To assess the chronic cancer pain induced by cancer cell injection, pain behavioral change was evaluated by mechanical allodynia. Rats injected with Walker 256 carcinoma cells into the tibia and scorpion was given to rats by gavage according to either prophylactic or reversal paradigms (Figure 1(a)). No signs of pain behavior up to day 5 postinjection, at which point significant development of mechanical allodynia and ambulatory pain started to be seen (Figure 1(b)). Prophylactic treatment was started on day 4 postinjection, since no significant differences in pain behavior between cancer cell-injected or sham rats were detected on day 3. Cancer cell-injected rats showed decreased 50% paw withdraw threshold and increased score of ambulatory pain. Prophylactic treatment with scorpion in cancer cell-injected rats significantly attenuated the development of mechanical allodynia from day 7 to day 21 (Figure 1(b)) and ambulatory pain from day 15 to day 21 (Figure 1(c)). Moreover, to assess the efficacy of scorpion in a way better resembling the clinical setting, scorpion was administered on day 14 in reversal treatment group of carcinoma cell-injected rats. Following the first administration of scorpion, mechanical allodynia was significantly reduced compared with carcinoma cell-injected rats from day 15 to day 21 (Figure 1(b)) and the developed ambulatory pain was significantly reversed from day 15 to day 21 (Figure 1(c)). All behavioral assessments were undertaken by an observer blinded to the treatment given. These findings indicated that scorpion reversed the effect of BCP behavior in both prophylactic and reversal treatment.
Figure 1.
Scorpion alleviates bone cancer pain behavior.Experimental paradigm (a); 50% paw withdrawal threshold (b) to von Frey filaments and score of ambulatory pain (c) in sham and BCP rats with or without scorpion treatment (prophylactic or reversal). All data were expressed as the mean ± SEM (n = 10). BCP versus sham rats (*), prophylactic versus BCP (#), reversal versus BCP (+) at each corresponding time point, #/+p < 0.05, ##/++p < 0.01, ***p < 0.001 (Student’s t test).
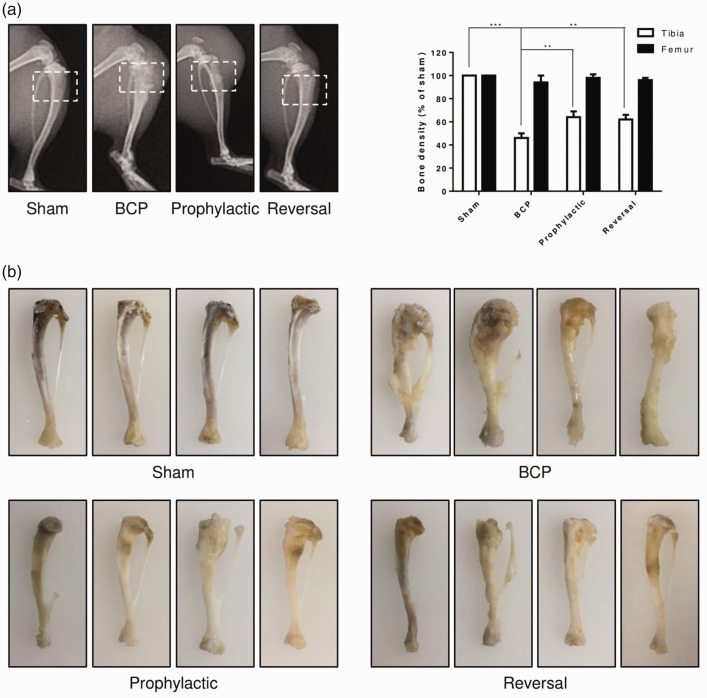
Scorpion attenuates bone destruction
To examine the effect of scorpion on bone destruction, X-ray radiographic images of tibia were taken at the end point of the study to monitor the destruction caused by Walker 256 carcinoma cells. The data showed that no radiological change was found in sham rats (Figure 2(a)). However, the tibia bone showed radiolucent lesion in the proximal epiphysis of cancer cell-injected rats, close to the injection site, and not present in the femur (Figure 2(a)). Prophylactic or reversal treatments of scorpion significantly attenuated the degree of tibia destruction in carcinoma cell-injected rats (Figure 2(a)). Furthermore, representative images of cancer cell-injected tibia displayed similar results. Cancer cell-injected tibia bearing severe tumor burden and bone destruction than those of sham group, and tibia of both prophylactic and reversal treatment of scorpion showed minor bone destruction than cancer cell-injected group (Figure 2(b)). These findings indicated that the analgesic effect of scorpion in BCP might through decreasing the bone destruction caused by cancer cells.
Figure 2.
Scorpion attenuates bone destruction. Representative radiographs of the tibia bone (a) in sham and BCP rats with or without scorpion treatment (prophylactic or reversal). All data were expressed as the mean ± SEM (n = 10), **p < 0.01, ***p < 0.001 (Student’s t test). Representative tibia bone images (b) of sham and BCP rats with or without scorpion treatment (prophylactic or reversal).
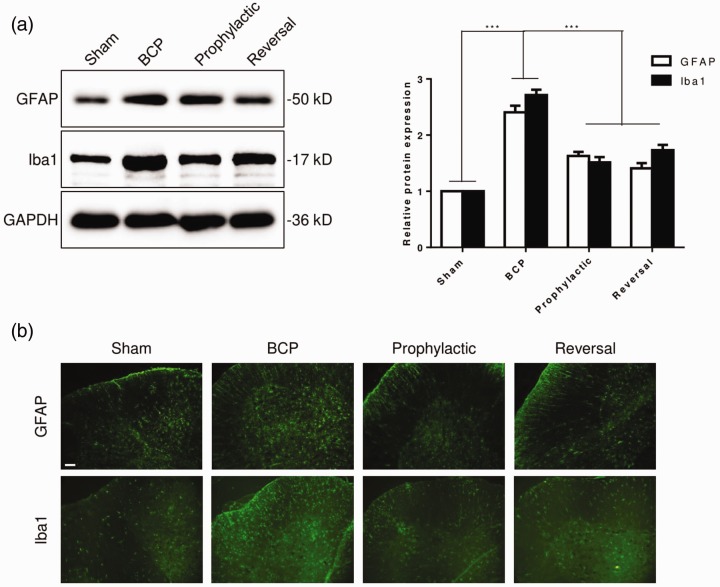
Scorpion inhibits the activation of spinal cord astrocytes and microglia in BCP rats
Previous study showed that spinal cord astrocytes and microglia were involved in the process of pain. Therefore, we examined the activation of spinal cord astrocytes and microglia. The expression of glial fibrillary acidic protein (GFAP), astrocytic biomarker, and ionized calcium binding adapter molecule 1 (Iba1), microglia biomarker, was markedly increased in BCP rats, compared with sham group (Figure 3(a)). This result indicated the activation of spinal cord astrocytes and microglia induced by bone cancer. Interestingly, the level of GFAP and Iba1 was significantly decreased in scorpion treated rats, both prophylactic and reversal treatment (Figure 3(a)). Subsequently, the expression of GFAP and Iba1 was analyzed by immunofluorescence. Consistent with previous finding, the expression of GFAP and Iba1 was upregulated in BCP rats, and scorpion reversed level of GFAP and Iba1 (Figure 3(b)). These data suggested that scorpion inhibited the activation of astrocytes and microglia.
Figure 3.
Scorpion inhibits the activation of spinal cord astrocytes and microglia in BCP rats. Levels of GFAP and Iba1 protein were determined by Western blot (a) and immunofluorescence (b) (n = 3); scale bar, 100 μm. All data were expressed as the mean ± SEM (n = 3), ***p < 0.001 (Student’s t test).
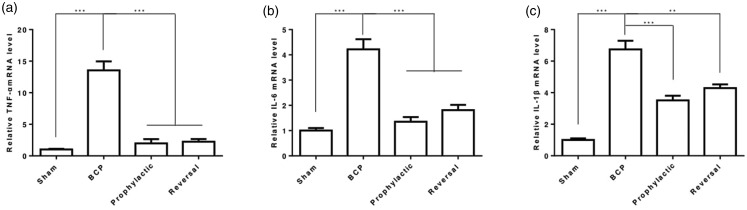
Scorpion suppresses inflammatory cytokines expression
BCP is a unique condition with features of inflammation.25 Thus, we tested the expression of inflammatory cytokines in mRNA level. As expected, TNF-α, IL-6, and IL-1β mRNA level was upregulated in BCP rats, and scorpion treatment significantly reversed TNF-α, IL-6, and IL-1β levels (Figure 4(a)–(c)). These findings indicated that scorpion might inhibit BCP via suppressing inflammatory cytokines expression.
Figure 4.
Scorpion suppresses inflammatory cytokines expression. Levels of TNF-α (a), IL-6 (b) and IL-1β (c) mRNA in spinal cord of bone cancer pain rats were determined by qPCR (n = 3). All data were expressed as the mean ± SEM (n = 3), **p < 0.01, ***p < 0.001 (Student’s t test).
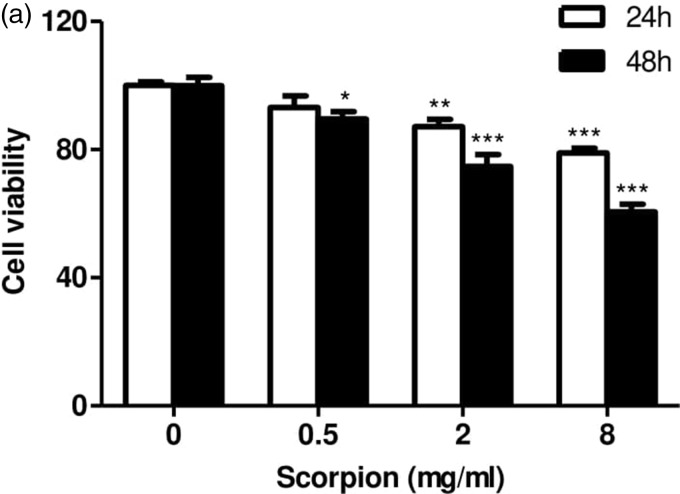
Scorpion represses tumor cell growth
To explore whether scorpion had an effect on tumor cell growth, we tested the cell viability of Walker 256 cell after scorpion treatment for 24 and 48 h. The results indicated that scorpion significantly suppressed Walker 256 cell viability compared with control condition (Figure 5(a)), suggesting that scorpion led to both dose-dependent and time-dependent decrease in Walker 256 cell viability.
Figure 5.
Scorpion represses tumor cell growth. (a) Walker 256 cells were treated with the indicated concentration of scorpion for the indicated times, and cell viability was measured using the CCK8 method (n = 3). All data were expressed as the mean ± SEM (n = 3), *p < 0.05, **p < 0.01, ***p < 0.001 (Student’s t test).
Discussion
The most common types of cancer, including breast, prostate, and lung cancer, tend to metastasize to the bones. As bone remodeling progresses, severe spontaneous pain often occurs, which the occurrence and severity of such pain may be acute and unpredictable.4 And BCP is considered to be one of the most difficult chronic pains to fully control, which seriously affects the quality of life in patients.26 Generally, the treatment of pain from bone metastases involves the use of multiple complementary approaches including radiotherapy, surgery, chemotherapy, bisphosphonates, calcitonin, and analgesics.4,27 However, BCP is a chronic pain that is very difficult to fully control since metastasis is usually not limited to a single site.27 Moreover, the efficacy of commonly used analgesics, such as NSAIDs and opioids is limited, because of their significant adverse side effects in the treatment of cancer pain.27–29 Therefore, we still need to further seek new analgesics and treatment options. The injection of Walker 256 rat breast cancer cells can successfully prepare a rat BCP model similar to human BCP.30,31 The paw withdrawal thresholds in response to mechanical stimulation and the spontaneous ambulatory pain score in rats have significantly decreased or increased from day 3 after the injection of Walker 256 cells into the upper tibia, suggesting that the BCP model was established successfully.
In this study, the effects of nociception on BCP models were investigated by intragastric administration of scorpion. Scorpion not only effectively ameliorated mechanical allodynia and pain behavior but also significantly improved the bone destruction of tibias induced by tumor growth. Moreover, scorpion inhibited proliferation of Walker 256 cells in a dose- and time-dependent manner. Our data suggested that intragastric administration with scorpion could be a potential therapeutic approach for BCP treatment.
The mechanism of BCP includes inflammatory pain and neuropathic pain components, but it is not a simple addition of these two types of pain. The BCP includes not only the tumor itself but also the inflammatory reaction, peripheral sensitization and central sensitization.32 Role of astrocytes and microglia during pain pathogenesis has been implicated in several studies.33–37 Inflammatory activation of astrocytes and microglia leaded to proinflammatory cytokines expression and neuronal damage. During BCP, inflammatory signal pathway was activated and a lot of inflammatory mediators were released.17,38 In our study, we found that BCP increased expression of astrocytes and microglia markers (GFAP and Iba1) in spinal cord, and scorpion treatment decreased GFAP and Iba1 expression. Meanwhile, the concentration of TNF-α, IL-6, and IL-1β increased in spinal cord of BCP rats, and scorpion reversed these inflammatory mediators’ levels. Accordingly, scorpion could be a possible candidate for BCP treatment.
Conclusion
In the present study, we demonstrated that intragastric administration of scorpion alleviates BCP through inhibition of bone destruction and glia activation. Our study provided a safety new approach of BCP treatment.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Natural Science Foundation of China (no. 81273923 to QPW) and Basic Research Project of Key Laboratory of Education Department of Liaoning Province (no. LZ2016002 to QPW).
ORCID iD
Qingping Wen https://orcid.org/0000-0002-3311-9230
References
- 1.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002; 2: 584–593. [DOI] [PubMed] [Google Scholar]
- 2.Delaney A, Fleetwood-Walker SM, Colvin LA, Fallon M. Translational medicine: cancer pain mechanisms and management. Br J Anaesth 2008; 101: 87–94. [DOI] [PubMed] [Google Scholar]
- 3.Breivik H, Cherny N, Collett B, de Conno F, Filbet M, Foubert AJ, Cohen R, Dow L. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol 2009; 20: 1420–1433. [DOI] [PubMed] [Google Scholar]
- 4.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain 1997; 69: 1–18. [DOI] [PubMed] [Google Scholar]
- 5.Portenoy RK, Lesage P. Management of cancer pain. Lancet 1999; 353: 1695–1700. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Cancer pain relief and palliative care. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1990; 804: 1–75. [PubMed] [Google Scholar]
- 7.Zech DF, Grond S, Lynch J, Hertel D, Lehmann KA. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain 1995; 63: 65–76. [DOI] [PubMed] [Google Scholar]
- 8.Bruera E, Kim HN. Cancer pain. Jama 2003; 290: 2476–2479. [DOI] [PubMed] [Google Scholar]
- 9.Ding J, Chua PJ, Bay BH, Gopalakrishnakone P. Scorpion venoms as a potential source of novel cancer therapeutic compounds. Exp Biol Med (Maywood) 2014; 239: 387–393. [DOI] [PubMed] [Google Scholar]
- 10.Liu YF, Ma RL, Wang SL, Duan ZY, Zhang JH, Wu LJ, Wu CF. Expression of an antitumor-analgesic peptide from the venom of Chinese scorpion Buthus martensii karsch in Escherichia coli. Protein Expr Purif 2003; 27: 253–258. [DOI] [PubMed] [Google Scholar]
- 11.Huang M, Pan Y Z, Mao H W, Liu M Z, Wang S. Preliminary study of analgesic mechanism of Buthus martensii Karsch (BmK) venom on central nervous system. Zhongguo Ying Yong Sheng Li Xue za Zhi 2001; 17: 85–88. [PubMed] [Google Scholar]
- 12.Mao Q, Ruan J, Cai X, Lu W, Ye J, Yang J, Yang Y, Sun X, Cao J, Cao P. Antinociceptive effects of analgesic-antitumor peptide (AGAP), a neurotoxin from the scorpion Buthus martensii Karsch, on formalin-induced inflammatory pain through a mitogen-activated protein kinases-dependent mechanism in mice. PLoS One 2013; 8: e78239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Li C, Chen J, Du J, Zhang J, Li G, Jin X, Wu C. AGAP, a new recombinant neurotoxic polypeptide, targets the voltage-gated calcium channels in rat small diameter DRG neurons. Biochem Biophys Res Commun 2014; 452: 60–65. [DOI] [PubMed] [Google Scholar]
- 14.Ruan JP, Mao QH, Lu WG, Cai XT, Chen J, Li Q, Fu Q, Yan HJ, Cao JL, Cao P. Inhibition of spinal MAPKs by scorpion venom peptide BmK AGAP produces a sensory-specific analgesic effect. Mol Pain 2018; 14: 1744806918761238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben Achour S, Pascual O. Astrocyte-neuron communication: functional consequences. Neurochem Res 2012; 37: 2464–2473. [DOI] [PubMed] [Google Scholar]
- 16.Suter MR. Microglial role in the development of chronic pain. Curr Opin Anaesthesiol 2016; 29: 584–589. [DOI] [PubMed] [Google Scholar]
- 17.Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev 2002; 82: 981–1011. [DOI] [PubMed] [Google Scholar]
- 18.Watkins LR, Milligan ED, Maier SF. Spinal cord glia: new players in pain. Pain 2001; 93: 201–205. [DOI] [PubMed] [Google Scholar]
- 19.Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochem Int 2004; 45: 389–395. [DOI] [PubMed] [Google Scholar]
- 20.Hansen RR, Malcangio M. Astrocytes–multitaskers in chronic pain. Eur J Pharmacol 2013; 716: 120–128. [DOI] [PubMed] [Google Scholar]
- 21.Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 2017; 35: 441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen G, Luo X, Qadri MY, Berta T, Ji RR. Sex-dependent glial signaling in pathological pain: distinct roles of spinal microglia and astrocytes. Neurosci Bull 2018; 34: 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao-Ying QL, Zhao J, Dong ZQ, Wang J, Yu J, Yan MF, Zhang YQ, Wu GC, Wang YQ. A rat model of bone cancer pain induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells. Biochem Biophys Res Commun 2006; 345: 1292–1298. [DOI] [PubMed] [Google Scholar]
- 24.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980; 20: 441–462. [DOI] [PubMed] [Google Scholar]
- 25.Colvin L, Fallon M. Challenges in cancer pain management–bone pain. Eur J Cancer 2008; 44: 1083–1090. [DOI] [PubMed] [Google Scholar]
- 26.Aielli F, Ponzetti M, Rucci N. Bone metastasis pain, from the bench to the bedside. IJMS 2019; 20: E280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercadante S, Fulfaro F. Management of painful bone metastases. Curr Opin Oncol 2007; 19: 308–314. [DOI] [PubMed] [Google Scholar]
- 28.Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, Dale O, De Conno F, Fallon M, Hanna M, Haugen DF, Juhl G, King S, Klepstad P, Laugsand EA, Maltoni M, Mercadante S, Nabal M, Pigni A, Radbruch L, Reid C, Sjogren P, Stone PC, Tassinari D, Zeppetella G, European Association for Palliative Care. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012; 13: e58–e68. [DOI] [PubMed] [Google Scholar]
- 29.Lussier D, Huskey AG, Portenoy RK. Adjuvant analgesics in cancer pain management. Oncologist 2004; 9: 571–591. [DOI] [PubMed] [Google Scholar]
- 30.Shen W, Hu XM, Liu YN, Han Y, Chen LP, Wang CC, Song C. CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflammation 2014; 11: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Liu YP, Song WB, Song XJ. EphrinB-EphB receptor signaling contributes to bone cancer pain via Toll-like receptor and proinflammatory cytokines in rat spinal cord. Pain 2013; 154: 2823–2835. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Zhao J, Meng Q. AAV-mediated siRNA against TRPV1 reduces nociception in a rat model of bone cancer pain. Neurol Res 2019; 41: 972–978. [DOI] [PubMed] [Google Scholar]
- 33.Lin SX, Lisi L, D, Russo C, Polak PE, Sharp A, Weinberg G, Kalinin S, Feinstein DL. The anti-inflammatory effects of dimethyl fumarate in astrocytes involve glutathione and haem oxygenase-1. ASN Neuro 2011; 3: AN20100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindia JA, McGowan E, Jochnowitz N, Abbadie C. Induction of CX3CL1 expression in astrocytes and CX3CR1 in microglia in the spinal cord of a rat model of neuropathic pain. J Pain 2005; 6: 434–438. [DOI] [PubMed] [Google Scholar]
- 35.Jiang W, Wang Y, Sun W, Zhang M. Morin suppresses astrocyte activation and regulates cytokine release in bone cancer pain rat models. Phytother Res 2017; 31: 1298–1304. [DOI] [PubMed] [Google Scholar]
- 36.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996; 19: 312–318. [DOI] [PubMed] [Google Scholar]
- 37.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther 2003; 306: 624–630. [DOI] [PubMed] [Google Scholar]
- 38.Lozano-Ondoua AN, Symons-Liguori AM, Vanderah TW. Cancer-induced bone pain: mechanisms and models. Neurosci Lett 2013; 557 PtA: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]