Abstract
The last few decades have been marked by the identification of numerous genes implicated in genetic disorders, helping in the elucidation of the underlying pathophysiology of these conditions. This has allowed new therapeutic approaches to emerge such as cellular therapy, gene therapy, or pharmacological therapy for various conditions. Skeletal dysplasias are good models to illustrate these scientific advances. Indeed, several therapeutic strategies are currently being investigated in osteogenesis imperfecta; there are ongoing clinical trials based on pharmacological approaches, targeting signaling pathways in achondroplasia and fibrodysplasia ossificans progressiva or the endoplasmic reticulum stress in metaphyseal dysplasia type Schmid or pseudoachondroplasia. Moreover, the treatment of hypophosphatasia or Morquio A disease illustrates the efficacy of enzyme drug replacement. To provide a highly specialized multidisciplinary approach, these treatments are managed by reference centers. The emergence of treatments in skeletal dysplasia provides new perspectives on the prognosis of these severe conditions and may change prenatal counseling in these diseases over the coming years.
Keywords: achondroplasia, clinical trials, endoplasmic reticulum stress, fibrodysplasia ossificans progressiva, osteogenesis imperfecta
Introduction
Skeletal dysplasias (SD), also known as osteochondrodysplasia, are a group of rare, heterogeneous disorders characterized by significant skeletal involvement1 with cartilage and bone growth abnormalities resulting in abnormal bone length, shape or density. Although individually rare, with 461 entities recognized so-far,1 SD have an overall prevalence of at least 1 per 5000 births.2 Clinical manifestations are heterogeneous, involving growth, bone shape, or bone density, reflecting the complexity of etiopathogenetic mechanism. There are very few treatments for skeletal dysplasia and therapy is largely supportive at present. The last decades were marked by the identification of a large number of genes responsible for skeletal dysplasia leading to a better understanding of cellular and biological pathways involved in skeletogenesis. Functional studies in cellular and animal models have also allowed the development of novel perspectives in the treatment of SD. Currently, several clinical trials are ongoing and some new drugs are available for patients. Here, we give an overview of some of the most recent achievements, focusing mainly on pharmacological approaches in osteogenesis imperfecta, achondroplasia, fibrodysplasia ossificans progressiva (FOP), pseudoachondroplasia, metaphyseal dysplasia type Schmid, and enzyme therapies in hypophosphatasia (HPP) and Morquio A (Table 1).
Table 1.
Treatments currently available, in phase II/III, in phase I or expected in skeletal dysplasias.
| Available | Phase II/III | Phase I/expected | |
|---|---|---|---|
| Osteogenesis imperfecta | Bisphosphonates | BPS804_ NCT03216486 Denosumab NCT03638128 Mesenchymal stem cells NCT03706482 |
Fresolimumab NCT03064074 |
| Achondroplasia | Vosoritide NCT03583697 NCT03197766 TransCon CNP NCT04085523 |
||
| Fibrodysplasia ossificans progressiva | Palovarotene NCT02279095
REGN2477 NCT03188666 |
||
| Multiple Osteochondromas | Palovarotene NCT03442985 | ||
| Pseudoachondroplasia | Resveratrol NCT03866200 |
||
| Metaphyseal dysplasia type Schmid | Carbamazepine EudraCT number 2018-002633-38 |
||
| Hypophosphatasia | Asfotase alfa | ||
| Morquio A syndrome | Elosulfase alfa | ||
| X-linked hypophosphatemia | Burosumab | ||
| Cherubism | Tankyrase | ||
| Diastrophic dysplasia | N-acetylcysteine |
NCT numbers denote ClinicalTrial.gov identifiers.
Physiopathology of skeletal dysplasia
The skeleton is a complex organ formed through two mechanisms, with endochondral ossification and intramembranous ossification responsible for the formation of long bones and flat bones, respectively. During intramembranous ossification, mesenchymal cells differentiate directly into osteoblasts. Endochondral ossification begins with mesenchymal cell condensation and differentiation into chondrocytes that secrete a matrix rich in type II collagen to form the cartilage template. Chondrocytes near the center undergo hypertrophy that secrete type X collagen. These hypertrophic chondrocytes control the mineralization of their surrounding matrix. Subsequent hypoxia stimulates the secretion of vascular endothelial growth factor that attracts blood vessels. These vessels bring osteoclasts and osteoblasts that digest the cartilage matrix and deposit the bone matrix forming the primary ossification center. At both extremities cartilage continues to proliferate, providing longitudinal growth. After birth, secondary ossification centers in the epiphysis appear following a similar process.3,4
While cartilage extracellular matrix (ECM) is composed mainly of type II collagen5 and proteoglycans produced by chondrocytes, bone mineralized ECM is essentially composed of type I collagen produced by osteoblasts.6 On the bone surface, osteoblasts become buried in mineralized bone and enter apoptosis or transform themselves into osteocytes that produce different proteins of bone matrix, such as dentin matrix protein 1 and sclerostin.7
Bone homeostasis results from two coupled processes, bone resorption and bone formation performed by osteoclasts and osteoblasts, respectively. In addition, osteocytes are key actors in the remodeling process by regulating both action and recruitment in osteoclasts and osteoblasts. An imbalance between the two processes results in significant bone loss or gain.4
Bone formation and bone remodeling are subtly regulated by several signaling pathways such as Indian Hedgehog, parathyroid hormone–related peptide, fibroblast growth factor (FGF), C-type natriuretic peptide (CNP), transforming growth factor beta (TGF-β), bone morphogenetic protein (BMP), Notch, and Wnt signaling pathways.5–8
Defects in bone formation, homeostasis, mineralization, or in one of these signaling pathways are responsible for numerous SD with a wide spectrum of features such as short stature, bone fragility or ectopic ossifications. A better understanding of formation processes and bone homeostasis helps to provide specific targets for therapeutic approaches.
Osteogenesis imperfecta: not a unique strategy
Osteogenesis imperfecta (OI) is the most common bone fragility disorder, affecting 1 in 10,000–20,000 births9 with a broad spectrum of clinical severity from antenatal lethal form to moderate adult disorder.10 Clinical and radiological features are low-impact bone fractures, osteopenia, bowing long bones, vertebral compressions, or growth deficiency.11 Individuals with OI also commonly exhibit dentinogenesis imperfecta. This generalized connective-tissue disorder can also include joint laxity, muscle weakness, fatigue, blue sclerae, hearing loss, decreased pulmonary function, and cardiac valvular regurgitation.11 The original classification by Sillence described four subtypes: (I) typically mild nondeforming OI with blue sclerae; (II) perinatally lethal OI; (III) progressively deforming disease; (IV) common variable disease with normal sclera.12
About 85–90% of cases are related to dominant mutations in the COL1A1 or COL1A2 genes13 coding for the α1(I) and α2(I) chains of type I collagen, the most abundant protein of bone mineralized ECM, also present in ligaments, tendons, dentin, sclera, and skin. Since 2006, advances in genetic analysis allowed the identification of new genes related to OI. These genes, mainly recessive, are implicated in collagen folding or post-translational modifications or in osteoblast differentiation and function.14,15
Patients with OI are managed by calcium and vitamin D supplements, physiotherapy and surgery. Oral and venous bisphosphonates, with their antiresorptive properties, are the main pharmacological interventions in OI.16 New therapeutic strategies are currently being investigated, such as pharmacological strategies with further antiresorptive drugs or stimulating ossification agents and mesenchymal cell transplantation (Figure 1).17,18
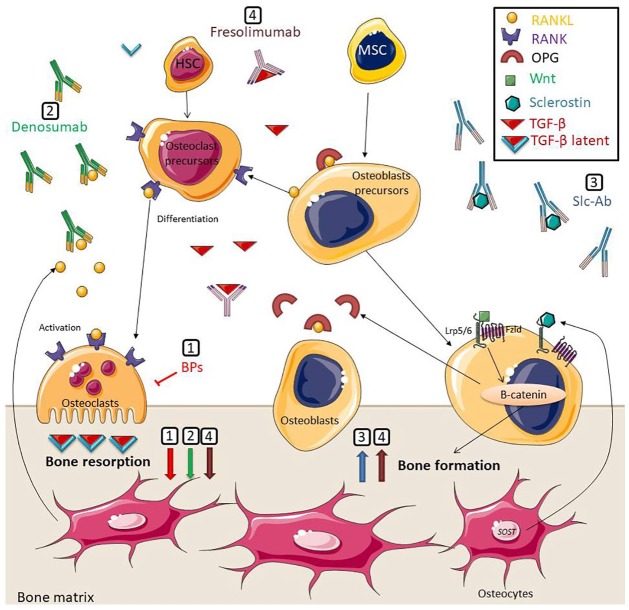
Figure 1.
Schematic representation of therapeutic approaches for osteogenesis imperfecta. (1) Bisphosphonates inhibit osteoclastic function. (2) Denosumab links RANKL, preventing the interaction with its receptor, RANK, on osteoclasts and osteoclasts precursors leading to inhibition of osteoclast formation and function. (3) Scl-Ab prevents binding of sclerostin to LRP5/6 and Frizzled coreceptors, thus inhibition of the Wnt/β-catenin signaling pathway. (4) Fresolimumab links TGF-β leading to beneficiary effects in bone remodeling. BPs, denosumab, and fresolimumab decrease bone resorption. Anti-sclerostin and fresolimumab increase bone formation.
BPs, bisphosphonates; Fzld, Frizzled; HSC, hematopoietic stem cells; MSC, mesenchymal stroma cells; OPG, osteoprotegerin; RANK, receptor activator of nuclear factor κB; RANKL, receptor activator of nuclear factor κB ligand; Slc-Ab, sclerostin Antibodies; TGF-β, transforming growth factor-beta.
Pharmacological approaches
Antiresorptive drugs
Bisphosphonates
Bisphosphonates inhibit osteoclastic function, leading to a significant decrease in bone remodeling.19 Current evidence demonstrates that this treatment increases bone mineral density (BMD) in patients with OI, even though the long-term fracture reduction and improvement in quality of life still remains uncertain.20 According to the latest guidelines on the use of bisphosphonate therapy in children and adolescents, intravenous bisphosphonates should be considered for use in children with severe OI (e.g. type III), children with vertebral compression fractures or children who have had two or more long-bone fractures per year. Oral bisphosphonates should only be considered for those with mild to moderate OI in the absence of vertebral compression fractures.16,21 However, the most efficient agent, dose and frequency is still undefined.16 Although pamidronate is the most frequent drug used in children younger than 2 years of age, with a dose between 9 and 12 mg/kg/year, zoledronate is used in older children with moderate to severe OI and commenced at 0.1 mg/kg/year in two divided doses.16
Denosumab
Some patients with OI-IV without a mutation in COL1A1/2 genes show a poor response to bisphosphonates, suggesting another etiopathogenic mechanism in OI. Thus, recessive mutations in the SERPINF1 gene encoding for pigment epithelium-derived factor, were identified in few patients with OI-VI22 with a poor response to bisphosphonates.23 These mutations lead to an overactivation of osteoclasts via the receptor activator of nuclear factor κB ligand (RANK/RANKL) pathway, essential for the osteoclast differentiation and function.22 Denosumab, a human monoclonal antibody against RANKL, is an antiresorptive agent approved for the treatment of postmenopausal osteoporosis.24 This antibody links RANKL, preventing the interaction with its receptor, RANK, to osteoclasts and osteoclast precursors, leading to the inhibition of osteoclast formation and function, decreasing bone resorption, and increasing bone density.24
First, subcutaneous injections of denosumab (1 mg/kg body weight every 12 weeks) allowed the suppression of bone resorption and consequently an increase of BMD in four patients with a severe phenotype of OI-VI related to SERPINF1 mutations after 2 years of treatment.25,26 Then, several clinical trials showed an improvement of areal BMD27,28 in children and adults with OI-I related to COL1A1/2 mutations. One of the adverse effects of denosumab is hypocalcemia, caused by the inhibition of bone resorption. Supplementation with vitamin D and calcium is recommended during this treatment.
While bisphosphonates can suppress bone resorption for several years, denosumab has a short duration of action. Thus, hypercalcemia 7–9 weeks after denosumab injection was observed.29 This side effect could be due to the short life of denosumab, and a reduction of the injection-interval could be sufficient to prevent it. Moreover, an excess of bone resorption with a rapid decrease in bone density was also observed as soon as denosumab was discontinued. Finally, some children developed hypercalciuria and nephrocalcinosis.
To date, the pharmacodynamics and the pharmacokinetics of anti-RANKL remain unknown. Clinical trials are still necessary to determine an efficient and well-tolerated protocol for denosumab administration in patients with OI.
Stimulating ossification agents
Anti-sclerostin
The Wnt/β-catenin pathway plays a major role in the regulation of bone formation and regeneration. Wnt ligands are glycoproteins expressed by osteocytes, which bind its receptor LRP5/6 and Frizzled to the osteoblasts and initiate a downstream intracellular signaling cascade, leading to activation of β-catenin and, thus, upregulation of its target gene expression that are implicated in osteoblast differentiation, proliferation, and activity.30 Sclerostin, encoded by the SOST gene and expressed by osteocytes and articular chondrocytes, is a monomeric glycoprotein that binds the LRP5/6 and Frizzled coreceptors leading to the inhibition of the Wnt/β-catenin signaling pathway and resulting in reduced osteoblastic bone formation.31
Anti-sclerostin (Scl-Ab) is a monoclonal antibody against sclerostin. Preclinical studies demonstrated that administration of Scl-Ab induced an enhancement of Wnt/β-catenin signaling, thereby causing an increase in bone formation, BMD, and bone strength32,33 and an acceleration of bone repair.34 Likewise, Scl-Ab reduces long-bone fractures in mouse models of OI.31
Scl-Ab including romosozumab, blosozumab, and BPS804, an Scl-Ab antibody, has already proved to have beneficial effects in patients with osteoporosis, with effects such as an increase in bone density with a reduction in fracture risk.35,36
A randomized phase IIa trial demonstrated that multiple, dose-escalating, intravenous infusions of BPS804 significantly increased bone formation biomarkers, decreased bone resorption biomarkers, and improved lumbar spinal bone density in adults with moderate OI.37 In the next few years, further studies are necessary to investigate the effects of Scl-Ab treatment in patients with OI.
Transforming growth factor β inhibition
The ECM is a reservoir of various growth factors and cytokines such as TGF-β, a key factor of cell proliferation, lineage determination, and cell differentiation. During osteoclast-mediated bone resorption, TGF-β, released from the bone matrix, plays a key role in bone remodeling by coupling bone resorption with formation.33 High levels of active TGF-β have been shown in mouse models of OI (Crtap−/− and Col1a2tm1.1Mcbr mouse models for recessive OI type VII and dominant OI related to COL1A2 gene, respectively)38 and a TGF-β neutralizing antibody improved bone mass in these models.39 This data suggests that increased signaling of TGF-β could be a common mechanism contributing to the OI phenotype and, thus, a new target for treatment in patients with OI. Nevertheless, these results seem to depend on the mouse model and therefore the effect of TGF-β inhibition may vary with the underlying genetic cause of the disorder.40 A phase I randomized study is currently testing the safety profile of fresolimumab, an antibody targeting TGF-β in moderate to advanced OI disease (ClinicalTrials.gov identifier: NCT03064074).
Mesenchymal stem cells
Although osteoclasts are derived from hematopoietic stem cells, osteoblasts and chondrocytes are derived from mesenchymal stroma cells (MSCs). MSCs are able to engraft into target tissues and differentiate into different types of cells such as chondrocytes or osteoblasts.41,42 Moreover, MSCs secrete a wide range of factors such as cytokines, chemokines, and growth factors, modifying the microenvironment and, thus, stimulating cell proliferation and preventing apoptosis.43 MSCs also release extracellular vesicles containing lipids, microRNAs, and peptides, stimulating chondrocyte proliferation in the growth plate and resulting in improved bone growth in a mouse model of OI.44
The first studies that demonstrated MSC derived from bone marrow transplantation (BMT) were able to migrate to bone in children with OI and give rise to osteoblasts with an improvement in bone structure and function.45 These results were improved by an infusion of bone marrow-derived mesenchymal cells from the patients’ original donors that engraft in bone, marrow stroma, and skin without the requirement for preparative chemotherapy in five children with OI after BMT. MSCs secrete a soluble mediator that indirectly stimulates growth.46 Experiments in mouse models corroborated these observations.
Severe cases of OI are detectable during pregnancy. Preclinical studies of OI on mouse models showed that MSC transplanted in utero or in early neonatal life resulted in a significant reduction in bone fractures and increased bone strength.47,48 To date, two patients with type III and type IV OI with a prenatal transplantation of allogenic human first-trimester liver-derived MSCs at 31 weeks of gestational age have been reported. Observed over several years, their clinical condition was better than expected with their mutation. Owing to a decrease of the lengthwise growth, the increase of fracture frequency, or both, they received several booster doses from the same donor at 8 years old and 19 months old, resulting in a significant clinical improvement.49,50 Indeed, although these healthy cells can survive in damaged tissue, they tend to decrease with time.
MSC transplantation is a promising treatment; nevertheless, clinical experience with MSC for OI is limited and further studies are currently ongoing. One of them, the BOOSTB4 (Boost Brittle Bones Before Birth, ClinicalTrials.gov identifier: NCT03706482) study (a European multicenter phase I/II study), is evaluating the efficacy of prenatal and postnatal or postnatal only transplantation of fetal-derived MSCs in patients with OI type III or IV.
Targeting a signaling pathway
Endochondral ossification involves actions and crosstalk of numerous signaling pathways in the cartilage, including FGFs, sonic hedgehog, BMPs, and Wnts. Biological studies in achondroplasia and FOP demonstrated an overactivation of one of these pathways, leading to the emergence of therapeutic strategies targeting these specific pathways.
Achondroplasia
Achondroplasia is the most common skeletal dysplasia, occurring in 1/15,000–25,000 births, characterized by a severe disproportionate short stature, typical facial features with frontal bossing, and midfacial hypoplasia.51 The severe complications are craniocervical junction compression, upper-airway obstruction, or thoracolumbar kyphosis, leading to serious life-threatening health issues.52,53
The diagnosis can easily be established in early infancy based on specific physical and radiographic features. Thanks to the improvement of prenatal ultrasounds, diagnosis tends to be easier and most frequent during the third trimester of the pregnancy.
Achondroplasia is related to a recurrent gain-of-function heterozygous mutation in the gene encoding the FGF receptor type 3 (FGFR3).54,55 FGFR3 is a key negative regulator of endochondral ossification by inhibiting both the proliferation and terminal differentiation of chondrocytes in growth plate. FGF ligands induce dimerization of the receptor leading to transphosphorylation of key tyrosine residues within the receptors’ kinase domain. Activation of FGFR3 induces several downstream pathways, including the signal transducer and activator of transcription and mitogen-activated protein kinase (MAPK).56 Recurrent mutation G380R is responsible for a constitutive activation of the FGFR3 receptor leading to an increased signal transduction, thus a negative regulation of bone growth chondrocytes through shortening of the proliferative phase and accelerating terminal differentiation.57
Management of patients with achondroplasia is still challenging. Limb-lengthening surgery is controversial because of the potential for severe complications.58 No clear long-term benefit of recombinant human growth hormone (rhGH) treatment has been established in achondroplasia. Some studies have suggested that rhGH may improve growth patterns, but the effect on adult height is still unknown.59
During the last few decades, several therapeutic strategies emerged. These molecules interfere with different steps of the FGFR3 signaling pathway, including its synthesis, activation, tyrosine-kinase activity, degradation, and downstream signals.
The most promising therapy up to now is a recombinant CNP analogue called BMN-111 (Vosoritide).60,61 CNP and its receptor natriuretic peptide receptor 2 (NPR2) are expressed in proliferating and prehypertrophic chondrocytes and play a crucial role in stimulating chondrocyte proliferation via inhibition of MAPK signaling pathway at the level of RAF-1. Loss-of-function mutations in the NPR2 gene are responsible for acromesomelic dysplasia characterized by severe short stature with both axial and appendicular involvement.62 Mice lacking CNP show severe dwarfism with impaired endochondral ossification and their skeletal phenotype are histologically similar to those seen in patients with achondroplasia.63 Furthermore, CNP-overexpressing transgenic mouse studies exhibit skeletal overgrowth. These results suggest that CNP administration could rescue the skeletal phenotype in achondroplasia. In this way, preclinical studies have shown promising findings in mouse models of achondroplasia, with a correction of the dwarfism phenotype.60 A multinational study of 35 children (5–14 years of age) receiving daily subcutaneous vosoritide at a dose of 15 µg/kg demonstrated a sustained increase in the annualized growth velocity of approximately 1.5–2.0 cm/year over 42 months of treatment. Side-effects appeared to be generally mild.64 A long-term extension study and a phase II, randomized, double-blind, placebo-controlled clinical trial (ClinicalTrials.gov identifier: NCT03583697) of vosoritide in infants and younger children (age range, 0 to <60 months) are ongoing. Moreover, a phase III, randomized, double-blind, placebo-controlled trial (ClinicalTrials.gov identifier: NCT03197766) is currently evaluating the efficacy and safety of vosoritide in up to 110 children (age range, 5 to <18 years) with achondroplasia. Lastly, a new phase II trial has been developed in patients with achondroplasia, testing a weekly injection of TransCon CNP, a long-acting prodrug of CNP providing continuous exposure to CNP.
Others strategies seem to rescue the skeletal phenotype in Fgfr3 mouse models (Figure 2), such as a soluble form of human FGFR365 acting as a decoy receptor, parathyroid hormone,66 statin,67 meclozine,68 and tyrosine kinase inhibitor NVP-BGJ398.69 These approaches could be potential therapies for children with achondroplasia and related disorders.
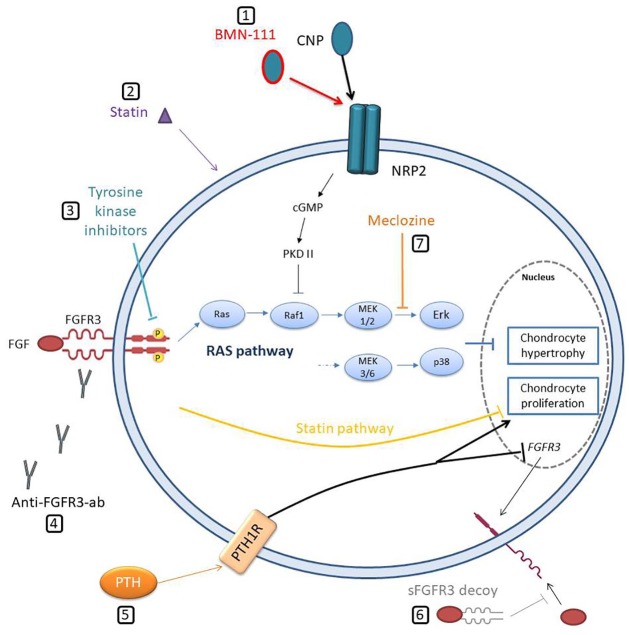
Figure 2.
Schematic representation of therapeutic approaches for achondroplasia. (1) Stabilized CNP (BMN-111) links NPR2 and thus inhibits RAF activation through protein kinase II activated by a guanylyl cyclase. (2) Statin induces degradation of FGFR3. (3) Tyrosine kinase inhibitors block receptor transphosphorylation of key tyrosine residues within the receptors’ kinase domain. (4) Anti-FGFR3 antibodies (Anti-FGFR3-ab) block FGF binding to the receptor and, thus, its dimerization. (5) PTH increases chondrocyte proliferation and inhibits FGFR3 expression. (6) Soluble FGFR3 (sFGFR3) decoy binds and sequesters FGF ligands. (7) Meclozine attenuates ERK phosphorylation and, thus, decreases RAS pathway hyperactivation.
cGMP, cyclic guanosine monophosphate; CNP, C-type natriuretic peptide; FGFR3, fibroblast growth factor receptor type 3; FGF, fibroblast growth factor; NPR2, natriuretic peptide receptor 2; PKD I, protein kinase I; PTH, parathormone.
Fibrodysplasia ossificans progressiva
FOP is a very rare genetic condition characterized by malformations of the great toes, the only apparent sign in neonatal period, and progressive heterotopic endochondral ossification. The first decade is characterized by the onset of episodic, painful inflammatory soft tissue swellings triggered by minor trauma. Although some of them regress, most transform soft connective tissues, including aponeuroses, fascia, ligaments, tendons and skeletal muscles, into mature heterotopic bone.70 During the second decade, patients develop progressive limitations and deformations with a frequent loss of independence in the third decade. Life expectancy is reduced because of respiratory insufficiency or thrombosis.70
The BMP signaling pathway plays a key role in the regulation of cartilage and bone development and growth. FOP is caused by a dominant mutation in the ACVR1 gene encoding activin receptor type IA, a type I BMP receptor. The mutant receptor loses some essential inhibitory interactions with its ligands and co-receptors, thereby conferring an aberrant phosphorylation of the downstream Smad 1/5/8 signaling pathway, triggering ectopic chondrogenesis, osteogenesis, and joint fusions.71
BMP signaling is antagonized by the nuclear retinoic acid receptor γ (RARγ)72 that inhibits Smad phosphorylation and promotes the degradation of phosphorylated Smad1/5/8.73 Palovarotene, a RARγ agonist, binds RARγ, thus inhibiting heterotopic ossification, and maintains limb mobility and growth in a mouse model.74 Phase II clinical trials suggest that Palovarotene decreased heterotopic ossification, time to flare-up resolution and patient-reported pain.75 A phase III multicenter open-label study is currently evaluating the efficacy and safety of a chronic/flare-up dosing regimen of palovarotene.
Other therapeutic strategies, such as anti-activin antibody (REGN2477) and rapamycin for patients with FOP, are currently being investigated (Figure 3).
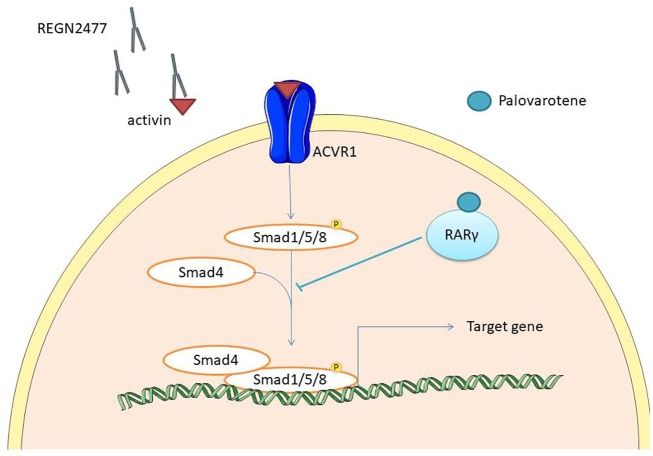
Figure 3.
Schematic representation of therapeutic approaches in fibrodysplasia ossificans progressiva. Palovarotene binds RARγ that inhibits Smad phosphorylation and promotes the degradation of phosphorylated Smad1/5/8. Anti-activin antibody (REGN2477) binds and sequesters activin, inhibiting ACVR1 activation.
ACVR1, activin receptor type IA.
Multiple osteochondromas
Multiple osteochondromas (MO) is an autosomal dominant disorder characterized by the development of multiple cartilaginous/bony tumors (osteochondromas or exostoses) within perichondrium in bones. This disorder is related to heterozygous loss-of-function mutations in the EXT1 or EXT2 genes,76 encoding a glycosyltransferase essential for heparan sulfate (HS) biosynthesis. This HS deficiency results in enhanced BMP signaling,77 suggesting that palovarotene could be a promising remedy for targeting MO. A preclinical study reported that palovarotene reduced osteochondroma formation in Fsp1-EXt1CKO mice,77 thus the treatment seems to be a potential therapeutic approach for children with MO (ClinicalTrials.gov identifier: NCT03442985).
Endoplasmic reticulum stress in matrix related skeletal dysplasia
The endoplasmic reticulum (ER) is an essential organelle for synthesis, folding, and trafficking of proteins entering the secretory pathway. Accumulation of misfolded proteins in ER, known as ER stress, leads to cell death.78 Type I collagen synthesis requires post-translational modifications in ER,9 thus, some forms of OI are related to ER stress,79 in particular those caused by mutations in the CREB3L180 or TMEM38B81 genes encoding an ER-stress transducer and an ER membrane monovalent cation channel, respectively. Moreover, pseudoachondroplasia and metaphyseal chondrodysplasia type Schmid (MCDS) are two forms of disproportionate short stature in which ER stress is the core disease mechanism.82 Owing to this, pharmacological drugs targeting ER stress are currently under investigation.
Pseudoachondroplasia
Pseudoachondroplasia is a form of disproportionate short-limb short stature. Length is generally normal at birth and the growth rate falls below the standard growth curve by approximately age 2 years,83 with a mean adult height of 116 cm and 120 cm for women and men, respectively.84 The diagnosis is generally evoked at the onset of walking when children present with significant waddling gait and joint pain. Natural history is marked by joint laxity, early onset osteoarthritis and dysplasia of the spine, epiphysis, and metaphysis.84,85 This autosomal dominant disease is related to heterozygous mutations in the COMP gene, encoding a noncollagenous ECM glycoprotein.83 Its accumulation induces ER stress, leading to inflammation and chondrocyte death.86 A preclinical study in a MT-COMP mice model demonstrated that antioxidant or anti-inflammatory agents, such as aspirin and resveratrol, interrupted the lethal chondrocyte pathological process induced by mutant COMP retention, thereby partially restoring chondrocyte proliferation, resulting in a significantly increased femur length. A phase II randomized clinical trial of resveratrol in adults (age range between 18 and 70 years) with pseudoachondroplasia is planned to start in 2019 (ClinicalTrials.gov identifier: NCT03866200).
Metaphyseal dysplasia type Schmid
MCDS is a very rare form of disproportionate short stature with bone deformities. Patients present after 2 years of age with short stature and short limbs, waddling gait, varus or valgus of the knee, and coxa vara. The major concern is chronic pain caused by bone deformities. Adult height varies between 135 and 160 cm. MCDS is caused by heterozygous mutations in the COL10A1 gene.87,88 Abnormal collagen type X produced by chondrocytes89 is misfolded and retained within the ER, leading to an increase of ER stress in hypertrophic chondrocytes, which is the primary cause of the MCDS phenotype.90 Carbamazepine (CBZ) is an autophagy-stimulating drug, already used in epilepsy, bipolar disorder, and neuropathic pain. Preclinical studies have shown that CBZ stimulated proteolysis of misfolded collagen X by autophagy or proteasomal degradation lead to a decrease of its accumulation and thus reduced ER stress in vitro and in vivo. Mouse model studies demonstrated increased bone growth and reduced skeletal dysplasia.91 An open label phase I/IIa trial repurposing Carbamazepine in children with MCDS is ongoing (EudraCT number 2018-002633-38).
Enzyme replacement therapy
Inherited metabolic disorders are caused by enzyme deficiency, leading to substrate accumulation, and produce deficiency resulting in various multisystemic conditions. In recent years, enzyme replacement therapies have emerged, consisting of enzyme substitution. HPP and Morquio disease are two metabolic disorders characterized by severe skeletal phenotypes.
Hypophosphatasia
HPP is a rare metabolic disorder ranging from a prenatal lethal form with no skeletal mineralization to a mild adult form with late onset presenting with premature exfoliation of teeth without any other symptoms. Vitamin-B6-dependent seizures occur in children with the severe form. This disease is caused by loss-of-function mutations in the alkaline phosphatase bio-mineralization-associated gene encoding the tissue nonspecific alkaline phosphatase (TNSALP), a central regulator of mineralization. Although recessive mutations are associated with severe or benign forms, dominant mutations are responsible for the benign form only. The diagnosis is suggested with clinical and radiologic features and confirmed by biochemical (low alkaline phosphatase activity in serum) and genetic analysis.92
Deficient TNSALP activity results in extracellular accumulation of its substrates, inorganic pyrophosphate (PPi) and pyridoxal 5’-phosphate (PLP). PPi prohibits hydroxyapatite crystal formation, and thus disrupts endochondral and intramembranous bone formation.93 PLP is the principal circulating form of vitamin B6. Its dephosphorylation by TNSALP in pyridoxal is essential to its intracellular transport,94 explaining B6-dependant seizures observed in the severe form of HPP.
Asfotase alfa (Strensiq, Alexion Pharmaceuticals, Boston, MA, USA) is a human, recombinant, TNSALP replacement therapy approved and now available for pediatric-onset HPP. A single intravenous infusion followed by subcutaneous injections three times per week showed a rapid and substantial improvement of bone mineralization and respiratory and motor functions, with an increased survival rate in patients with perinatal or infantile hypophosphatasia over 7 years. Asfotase alfa was generally well tolerated, with minor adverse events of injection site reactions, lipodystrophy, hypercalcemia, and hypocalcemia.95
Likewise, Asfotase alfa treatment in adult and adolescent HPP patients improved skeletal radiographic findings, enhanced fracture healing and reduced muscle pain96,97 with a recovery of bone mineralization.98,99
Owing to the requirement for recurrent injections of Asfotase alfa, alternative treatment using cell and gene therapy is currently being investigated. The first studies have shown that genetic correction in two childhood HPP patient-derived induced pluripotent stem cells recovers enzyme activity and calcification in vitro.100
Morquio A syndrome
Morquio A syndrome, also called mucopolysaccharidosis type IVA (MPS IVA), is an autosomal recessive lysosomal storage disease caused by mutations in the gene encoding the enzyme N-acetylgalactosamine-6-sulfate sulfatase (GALNS), leading to glycosaminoglycans accumulation in the lysosomes. This accumulation results in short stature and progressive skeletal dysplasia with bone deformity. The visual, auditory, cardiovascular, and respiratory systems can be involved. The central nervous system, however, is preserved in MPS IVA. Diagnosis is confirmed by biochemical analysis and genetic testing.101,102
Elosulfase alfa (recombinant human GALNS, BMN 110) enzyme replacement therapy is approved for Morquio A syndrome and recommended in all patients as soon as possible after a confirmed diagnosis.103 This treatment seems to slow down the natural progression of the disease.104 Patients receiving weekly intravenous infusions showed sustained increases in improvements to mobility, self-care, and caregiver assistance over 2 years.
Hematopoietic stem cell transplantation (HSCT) is recommended in MPS IH (Hurler’s disease) because it is the only treatment that has been shown to attenuate the developmental decline.105 Four patients with MPS IV received a successful HSCT with an increase of enzyme activity. Given the mean age at HSCT of 10.5 years, it is unclear whether HSCT may be efficient on bone growth for younger children.105 To date, HSCT is not recommended for patients with MPS IVA.103
X-linked hypophosphatemia
X-linked hypophosphatemia is the most common form of hereditary rickets caused by loss of function mutations in the X-linked phosphate-regulating endopeptidase homolog (PHEX) gene, which results in excess circulating FGF-23,106 a key regulator of phosphate homeostasis. This excess FGF-23 is responsible for a decrease in the tubular reabsorption of phosphate and a suppression of the production of 1,25-dihydroxyvitamin D leading to chronic hypophosphatemia. Patients present with a disproportionately short stature, low mineral density and rickets or osteomalacia, but the severity of phenotype is highly variable. So far, pharmacologic treatment includes multiple daily doses of oral phosphate supplementation and 1-alpha hydroxyvitamin D. Long-term risks, however, including nephrocalcinosis, hypercalciuria, and hyperparathyroidism in adults are still concerning. rhGH seems to improve growth velocity in these patients without clear evidence of the benefit on final height.107
Recently, burosumab, a fully human monoclonal antibody against FGF-23, has been approved for the treatment of X-linked hypophosphatemia in the US, EU, and Canada. Several clinical trials have shown that this treatment normalizes phosphate homeostasis leading to an improvement of osteomalacia. A phase II randomized open-label trial in 52 children with X-linked hypophosphatemia aged from 5 to 12 years and treated by subcutaneous burosumab (either every 2 weeks or every 4 weeks) showed an improvement of renal tubular phosphate reabsorption, serum phosphorus levels, linear growth, physical function, pain, and rickets.108 A phase III open-label single-arm trial investigating the efficacy of subcutaneous burosumab every 4 weeks in 14 adults showed a significant improvement of osteomalacia, and thus an improvement of fracture healing and an amelioration of skeletal complications.109 No notable adverse events have been observed with this treatment.
Other potentials therapies investigated in animal models
Other therapies are currently being investigated in animal models. Tankyrase is a potential drug in cherubism, a disorder characterized by bilateral symmetric fibro-osseous lesions in the maxilla and mandible during childhood. This genetic disorder is related to mutations in the SH3BP2 gene encoding a tankyrase substrate.110,111
An off-label therapeutic role of N-acetylcysteine (N-Ac) is currently being tested in diastrophic dysplasia (DTD), a severe nonlethal chondrodysplasia due to recessive mutations in the DTST gene, a cell membrane sulfate–chloride antiporter, which allows the recruitment of sulfate from the extracellular space into the cytoplasm. Preclinical studies showed promising results of N-Ac administration that acts as an intracellular sulfate source for macromolecular sulfatation in mouse models of DTD.112,113
Conclusion
The development of genomic technology leads to a dramatic improvement of the pathophysiological understanding of rare genetic conditions, including skeletal disorders. This permits the definition of new therapeutic targets such as a cell type, a cell deficiency or a signaling pathway and, thus, aids the development of new therapeutic strategies with real benefit for patients. Moreover, skeletal dysplasia represents a good model for clinical trials repurposing drugs already in clinical use. These advances provide real hope for significant positive life changes for patients and their families. Because these very rare conditions have a limited number of patients, the optimal therapy, timing, and dosage is still to be documented for most of these treatments. In addition, natural history studies are essential before all clinical trials to evaluate the benefits of each treatment. The next few years represent an exciting time in genetic disease healthcare, with the potential for a real transformation of prognosis and management through these new treatments.
Acknowledgments
The authors thank Michael Smith for his thorough re-reading of this article.
Footnotes
Authors’ note: Pauline Marzin and Valérie Cormier-Daire have both contributed to the writing of the manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare no conflict of interest.
ORCID iD: Valérie Cormier-Daire  https://orcid.org/0000-0002-2839-9856
https://orcid.org/0000-0002-2839-9856
Contributor Information
Pauline Marzin, Clinical Genetics, INSERM UMR 1163, Paris Descartes-Sorbonne Paris Cité University, IMAGINE Institute, Necker Enfants Malades Hospital, Paris, France.
Valérie Cormier-Daire, Clinical Genetics, INSERM UMR 1163, Paris Descartes-Sorbonne Paris Cité University, IMAGINE Institute, Necker Enfants Malades Hospital, 149 rue de sevres, Paris, 75015, France.
References
- 1. Mortier GR, Cohn DH, Cormier-Daire V, et al. Nosology and classification of genetic skeletal disorders: 2019 revision. Am J Med Genet A 2019; 179: 2393–2419. [DOI] [PubMed] [Google Scholar]
- 2. Orioli IM, Castilla EE, Barbosa-Neto JG. The birth prevalence rates for the skeletal dysplasias. J Med Genet 1986; 23: 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kronenberg HM. Developmental regulation of the growth plate. Nature 2003; 423: 332–336. [DOI] [PubMed] [Google Scholar]
- 4. Rodan GA. Bone homeostasis. Proc Natl Acad Sci 1998; 95: 13361–13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayrapetyan A, Jansen JA, van den Beucken JJ. Signaling pathways involved in osteogenesis and their application for bone regenerative medicine. Tissue Eng Part B Rev 2015; 21: 75–87. [DOI] [PubMed] [Google Scholar]
- 6. Teixeira CC, Agoston H, Beier F. Nitric oxide, C-type natriuretic peptide and cGMP as regulators of endochondral ossification. Dev Biol 2008; 319: 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elango J, Rahman SU, Henrotin Y, et al. Parathyroid hormone-related protein (PTHrP) accelerates soluble RANKL signals for downregulation of osteogenesis of bone mesenchymal stem cells. J Clin Med 2019; 8: pii: E836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morsczeck C, Reck A, Beck HC. The hedgehog-signaling pathway is repressed during the osteogenic differentiation of dental follicle cells. Mol Cell Biochem 2017; 428: 79–86. [DOI] [PubMed] [Google Scholar]
- 9. Lim J, Grafe I, Alexander S, et al. Genetic causes and mechanisms of osteogenesis imperfecta. Bone 2017; 102: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andersen PE, Hauge M. Osteogenesis imperfecta: a genetic, radiological, and epidemiological study. Clin Genet 1989; 36: 250–255. [DOI] [PubMed] [Google Scholar]
- 11. Forlino A, Marini JC. Osteogenesis imperfecta. Lancet Lond Engl 2016; 387: 1657–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sillence DO, Rimoin DL. Classification of osteogenesis imperfect. Lancet Lond Engl 1978; 1(8072): 1041–2. [DOI] [PubMed] [Google Scholar]
- 13. Byers PH, Wallis GA, Willing MC. Osteogenesis imperfecta: translation of mutation to phenotype. J Med Genet 1991; 28: 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bardai G, Moffatt P, Glorieux FH, et al. DNA sequence analysis in 598 individuals with a clinical diagnosis of osteogenesis imperfecta: diagnostic yield and mutation spectrum. Osteoporos Int 2016; 27: 3607–3613. [DOI] [PubMed] [Google Scholar]
- 15. Besio R, Chow CW, Tonelli F, et al. Bone biology: insights from osteogenesis imperfecta and related rare fragility syndromes. FEBS J. Epub ahead of print 5 July 2019. DOI: 10.1111/febs.14963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simm PJ, Biggin A, Zacharin MR, et al. Consensus guidelines on the use of bisphosphonate therapy in children and adolescents. J Paediatr Child Health 2018; 54: 223–233. [DOI] [PubMed] [Google Scholar]
- 17. Tauer JT, Robinson ME, Rauch F. Osteogenesis imperfecta: new perspectives from clinical and translational research. JBMR Plus 2019; 3: e10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marini JC, Forlino A, Bächinger HP, et al. Osteogenesis imperfecta. Nat Rev Dis Primer 2017; 3: 1–19. [DOI] [PubMed] [Google Scholar]
- 19. Fisher JE, Rogers MJ, Halasy JM, et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci U S A 1999; 96: 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dwan KM, Phillipi CA, Steiner RD, et al. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev 2016; 10: CD005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi CG, Zhang Y, Yuan W. Efficacy of bisphosphonates on bone mineral density and fracture rate in patients with osteogenesis imperfecta: a systematic review and meta-analysis. Am J Ther 2016; 23: e894–e904. [DOI] [PubMed] [Google Scholar]
- 22. Becker J, Semler O, Gilissen C, et al. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am J Hum Genet 2011; 88: 362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trejo P, Palomo T, Montpetit K, et al. Long-term follow-up in osteogenesis imperfecta type VI. Osteoporos Int J 2017; 28: 2975–2983. [DOI] [PubMed] [Google Scholar]
- 24. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361: 756–765. [DOI] [PubMed] [Google Scholar]
- 25. Semler O, Netzer C, Hoyer-Kuhn H, et al. First use of the RANKL antibody denosumab in osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact 2012; 12: 183–188. [PubMed] [Google Scholar]
- 26. Hoyer-Kuhn H, Netzer C, Koerber F, et al. Two years’ experience with denosumab for children with osteogenesis imperfecta type VI. Orphanet J Rare Dis 2014; 9: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoyer-Kuhn H, Stark C, Franklin J, et al. Correlation of bone mineral density on quality of life in patients with osteogenesis imperfecta during treatment with denosumab. Pediatr Endocrinol Rev 2017; 15(Suppl. 1): 123–129. [DOI] [PubMed] [Google Scholar]
- 28. Kobayashi T, Nakamura Y, Suzuki T, et al. Efficacy and safety of denosumab therapy for osteogenesis imperfecta patients with osteoporosis-case series. J Clin Med 2018; 7: pii: E479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trejo P, Rauch F, Ward L. Hypercalcemia and hypercalciuria during denosumab treatment in children with osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact 2018; 18: 76–80. [PMC free article] [PubMed] [Google Scholar]
- 30. MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009; 17: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cardinal M, Tys J, Roels T, et al. Sclerostin antibody reduces long bone fractures in the oim/oim model of osteogenesis imperfecta. Bone 2019; 124: 137–147. [DOI] [PubMed] [Google Scholar]
- 32. Ominsky MS, Vlasseros F, Jolette J, et al. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 2010; 25: 948–959. [DOI] [PubMed] [Google Scholar]
- 33. Sinder BP, Lloyd WR, Salemi JD, et al. Effect of anti-sclerostin therapy and osteogenesis imperfecta on tissue-level properties in growing and adult mice while controlling for tissue age. Bone 2016; 84: 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ominsky MS, Niu QT, Li C, et al. Tissue-level mechanisms responsible for the increase in bone formation and bone volume by sclerostin antibody. J Bone Miner Res 2014; 29: 1424–1430. [DOI] [PubMed] [Google Scholar]
- 35. Lewiecki EM. Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther Adv Musculoskelet Dis 2014; 6: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McClung MR. Sclerostin antibodies in osteoporosis: latest evidence and therapeutic potential. Ther Adv Musculoskelet Dis 2017; 9: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Glorieux FH, Devogelaer JP, Durigova M, et al. BPS804 anti-sclerostin antibody in adults with moderate osteogenesis imperfecta: results of a randomized phase 2a trial. J Bone Miner Res 2017; 32: 1496–1504. [DOI] [PubMed] [Google Scholar]
- 38. Grafe I, Yang T, Alexander S, et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat Med 2014; 20: 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bi X, Grafe I, Ding H, et al. Correlations between bone mechanical properties and bone composition parameters in mouse models of dominant and recessive osteogenesis imperfecta and the response to anti-TGF-β treatment. J Bone Miner Res 2017; 32: 347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tauer JT, Abdullah S, Rauch F. Effect of Anti-TGF-β treatment in a mouse model of severe osteogenesis imperfecta. J Bone Miner Res 2019; 34: 207–214. [DOI] [PubMed] [Google Scholar]
- 41. Granero-Moltó F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009; 27: 1887–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sagar R, Götherström C, David AL, et al. Fetal stem cell transplantation and gene therapy. Best Pract Res Clin Obstet Gynaecol. Epub ahead of print 5 March 2019. DOI: 10.1016/j.bpobgyn.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 43. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006; 98: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 44. Otsuru S, Desbourdes L, Guess AJ, et al. Extracellular vesicles released from mesenchymal stromal cells stimulate bone growth in osteogenesis imperfecta. Cytotherapy 2018; 20: 62–73. [DOI] [PubMed] [Google Scholar]
- 45. Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999; 5: 309–313. [DOI] [PubMed] [Google Scholar]
- 46. Otsuru S, Gordon PL, Shimono K, et al. Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood 2012; 120: 1933–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vanleene M, Saldanha Z, Cloyd KL, et al. Transplantation of human fetal blood stem cells in the osteogenesis imperfecta mouse leads to improvement in multiscale tissue properties. Blood 2011; 117: 1053–1060. [DOI] [PubMed] [Google Scholar]
- 48. Ranzoni AM, Corcelli M, Hau KL, et al. Counteracting bone fragility with human amniotic mesenchymal stem cells. Sci Rep 2016; 6: 39656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Le Blanc K, Götherström C, Ringdén O, et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation 2005; 79: 1607–1614. [DOI] [PubMed] [Google Scholar]
- 50. Götherström C, Westgren M, Shaw SWS, et al. Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: a two-center experience. Stem Cells Transl Med 2014; 3: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rankin G, Mackay EC. Achondroplasia. Br Med J 1906; 1: 1518–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hunter AG, Bankier A, Rogers JG, et al. Medical complications of achondroplasia: a multicentre patient review. J Med Genet 1998; 35: 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pauli RM. Achondroplasia: a comprehensive clinical review. Orphanet J Rare Dis 2019; 14: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rousseau F, Bonaventure J, Legeai-Mallet L, et al. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature 1994; 371: 252–254. [DOI] [PubMed] [Google Scholar]
- 55. Shiang R, Thompson LM, Zhu YZ, et al. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell 1994; 78: 335–342. [DOI] [PubMed] [Google Scholar]
- 56. Narayana J, Horton WA. FGFR3 biology and skeletal disease. Connect Tissue Res 2015; 56: 427–433. [DOI] [PubMed] [Google Scholar]
- 57. Segev O. Restrained chondrocyte proliferation and maturation with abnormal growth plate vascularization and ossification in human FGFR-3G380R transgenic mice. Hum Mol Genet 2000; 9: 249–258. [DOI] [PubMed] [Google Scholar]
- 58. Ko KR, Shim JS, Chung CH, et al. Surgical results of limb lengthening at the femur, tibia, and humerus in patients with achondroplasia. Clin Orthop Surg 2019; 11: 226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miccoli M, Bertelloni S, Massart F. Height outcome of recombinant human growth hormone treatment in achondroplasia children: a meta-analysis. Horm Res Paediatr 2016; 86: 27–34. [DOI] [PubMed] [Google Scholar]
- 60. Wendt DJ, Dvorak-Ewell M, Bullens S, et al. Neutral endopeptidase-resistant C-type natriuretic peptide variant represents a new therapeutic approach for treatment of fibroblast growth factor receptor 3-related dwarfism. J Pharmacol Exp Ther 2015; 353: 132–149. [DOI] [PubMed] [Google Scholar]
- 61. Lorget F, Kaci N, Peng J, et al. Evaluation of the therapeutic potential of a CNP analog in a Fgfr3 mouse model recapitulating achondroplasia. Am J Hum Genet 2012; 91: 1108–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bartels CF, Bükülmez H, Padayatti P, et al. Mutations in the transmembrane natriuretic peptide receptor NPR-B impair skeletal growth and cause acromesomelic dysplasia, type Maroteaux. Am J Hum Genet 2004; 75: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chusho H, Tamura N, Ogawa Y, et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A 2001; 98: 4016–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Savarirayan R, Irving M, Bacino CA, et al. C-Type natriuretic peptide analogue therapy in children with achondroplasia. N Engl J Med 2019; 381: 25–35. [DOI] [PubMed] [Google Scholar]
- 65. Garcia S, Dirat B, Tognacci T, et al. Postnatal soluble FGFR3 therapy rescues achondroplasia symptoms and restores bone growth in mice. Sci Transl Med 2013; 5: 203ra124. [DOI] [PubMed] [Google Scholar]
- 66. Xie Y, Su N, Jin M, et al. Intermittent PTH (1-34) injection rescues the retarded skeletal development and postnatal lethality of mice mimicking human achondroplasia and thanatophoric dysplasia. Hum Mol Genet 2012; 21: 3941–3955. [DOI] [PubMed] [Google Scholar]
- 67. Yamashita A, Morioka M, Kishi H, et al. Statin treatment rescues FGFR3 skeletal dysplasia phenotypes. Nature 2014; 513: 507–511. [DOI] [PubMed] [Google Scholar]
- 68. Matsushita M, Hasegawa S, Kitoh H, et al. Meclozine promotes longitudinal skeletal growth in transgenic mice with achondroplasia carrying a gain-of-function mutation in the FGFR3 gene. Endocrinology 2015; 156: 548–554. [DOI] [PubMed] [Google Scholar]
- 69. Komla-Ebri D, Dambroise E, Kramer I, et al. Tyrosine kinase inhibitor NVP-BGJ398 functionally improves FGFR3-related dwarfism in mouse model. J Clin Invest 2016; 126: 1871–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pignolo RJ, Bedford-Gay C, Liljesthröm M, et al. The natural history of flare-ups in fibrodysplasia ossificans progressiva (FOP): a comprehensive global assessment. J Bone Miner Res 2016; 31:650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hildebrand L, Stange K, Deichsel A, et al. The fibrodysplasia ossificans progressiva (FOP) mutation p.R206H in ACVR1 confers an altered ligand response. Cell Signal 2017; 29: 23–30. [DOI] [PubMed] [Google Scholar]
- 72. Sheng N, Xie Z, Wang C, et al. Retinoic acid regulates bone morphogenic protein signal duration by promoting the degradation of phosphorylated Smad1. Proc Natl Acad Sci U S A 201; 107: 18886–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pacifici M. Retinoid roles and action in skeletal development and growth provide the rationale for an ongoing heterotopic ossification prevention trial. Bone 2018; 109: 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chakkalakal SA, Uchibe K, Convente MR, et al. Palovarotene inhibits heterotopic ossification and maintains limb mobility and growth in mice with the human ACVR1(R206H) fibrodysplasia ossificans progressiva (FOP) Mutation. J Bone Miner Res 2016; 31: 1666–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. PR Newswire. Clementia announces top-line results from phase 2 trial of palovarotene for treatment of patients with fibrodysplasia ossificans progressiva, https://www.prnewswire.com/news-releases/clementia-announces-top-line-results-from-phase-2-trial-of-palovarotene-for-treatment-of-patients-with-fibrodysplasia-ossificans-progressiva-300345138.html (2016).
- 76. Philippe C, Porter DE, Emerton ME, et al. Mutation screening of the EXT1 and EXT2 genes in patients with hereditary multiple exostoses. Am J Hum Genet 1997; 61: 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Inubushi T, Lemire I, Irie F, et al. Palovarotene inhibits osteochondroma formation in a mouse model of multiple hereditary exostoses. J Bone Miner Res 2018; 33: 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016; 529: 326–335. [DOI] [PubMed] [Google Scholar]
- 79. Scheiber AL, Guess AJ, Kaito T, et al. Endoplasmic reticulum stress is induced in growth plate hypertrophic chondrocytes in G610C mouse model of osteogenesis imperfecta. Biochem Biophys Res Commun 2019; 509: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Symoens S, Malfait F, D’hondt S, et al. Deficiency for the ER-stress transducer OASIS causes severe recessive osteogenesis imperfecta in humans. Orphanet J Rare Dis 2013; 8: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cabral WA, Ishikawa M, Garten M, et al. Absence of the ER cation channel TMEM38B/TRIC-B disrupts intracellular calcium homeostasis and dysregulates collagen synthesis in recessive osteogenesis imperfecta. PLoS Genet 2016; 12: e1006156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Briggs MD, Bell PA, Wright MJ, et al. New therapeutic targets in rare genetic skeletal diseases. Expert Opin Orphan Drugs 2015; 3: 1137–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Briggs MD, Wright MJ. Pseudoachondroplasia. In: Adam MP, Ardinger HH, Pagon RA, et al. (eds) GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle, 1993, http://www.ncbi.nlm.nih.gov/books/NBK1487/ (accessed 28 July 2019). [PubMed] [Google Scholar]
- 84. McKeand J, Rotta J, Hecht JT. Natural history study of pseudoachondroplasia. Am J Med Genet 1996; 63: 406–410. [DOI] [PubMed] [Google Scholar]
- 85. Wynne-Davies R, Hall CM, Young ID. Pseudoachondroplasia: clinical diagnosis at different ages and comparison of autosomal dominant and recessive types. A review of 32 patients (26 kindreds). J Med Genet 1986; 23: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Suleman F, Gualeni B, Gregson HJ, et al. A novel form of chondrocyte stress is triggered by a COMP mutation causing pseudoachondroplasia. Hum Mutat 2012; 33: 218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Warman ML, Abbott M, Apte SS, et al. A type X collagen mutation causes Schmid metaphyseal chondrodysplasia. Nat Genet 1993; 5: 79–82. [DOI] [PubMed] [Google Scholar]
- 88. Wallis GA, Rash B, Sweetman WA, et al. Amino acid substitutions of conserved residues in the carboxyl-terminal domain of the alpha 1(X) chain of type X collagen occur in two unrelated families with metaphyseal chondrodysplasia type Schmid. Am J Hum Genet 1994; 54: 169–178. [PMC free article] [PubMed] [Google Scholar]
- 89. Schmid TM, Linsenmayer TF. Developmental acquisition of type X collagen in the embryonic chick tibiotarsus. Dev Biol 1985; 107: 373–381. [DOI] [PubMed] [Google Scholar]
- 90. Rajpar MH, McDermott B, Kung L, et al. Targeted induction of endoplasmic reticulum stress induces cartilage pathology. PLoS Genet 2009; 5: e1000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mullan LA, Mularczyk EJ, Kung LH, et al. Increased intracellular proteolysis reduces disease severity in an ER stress-associated dwarfism. J Clin Invest 2017; 127: 3861–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mornet E. Hypophosphatasia. Metabolism 2018; 82: 142–155. [DOI] [PubMed] [Google Scholar]
- 93. Whyte MP. Hypophosphatasia and how alkaline phosphatase promotes mineralization. In: Thakker RV, Whyte MP, Eisman JA, et al. (eds) Genetics of bone biology and skeletal disease. 2nd ed. London: Academic Press, 2018, pp.481–505, http://www.sciencedirect.com/science/article/pii/B9780128041826000289 (accessed 28 July 2019). [Google Scholar]
- 94. Baumgartner-Sigl S, Haberlandt E, Mumm S, et al. Pyridoxine-responsive seizures as the first symptom of infantile hypophosphatasia caused by two novel missense mutations (c.677T>C, p.M226T; c.1112C>T, p.T371I) of the tissue-nonspecific alkaline phosphatase gene. Bone 2007; 40: 1655–1661. [DOI] [PubMed] [Google Scholar]
- 95. Kishnani PS, Rush ET, Arundel P, et al. Monitoring guidance for patients with hypophosphatasia treated with asfotase alfa. Mol Genet Metab 2017; 122: 4–17. [DOI] [PubMed] [Google Scholar]
- 96. Kishnani PS, Rockman-Greenberg C, Rauch F, et al. Five-year efficacy and safety of asfotase alfa therapy for adults and adolescents with hypophosphatasia. Bone 2019; 121: 149–162. [DOI] [PubMed] [Google Scholar]
- 97. Klidaras P, Severt J, Aggers D, et al. Fracture healing in two adult patients with hypophosphatasia after asfotase alfa therapy. JBMR Plus 2018; 2: 304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Freitas TQ, Franco AS, Pereira RMR. Improvement of bone microarchitecture parameters after 12 months of treatment with asfotase alfa in adult patient with hypophosphatasia: case report. Medicine (Baltimore) 2018; 97: e13210.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rolvien T, Schmidt T, Schmidt FN, et al. Recovery of bone mineralization and quality during asfotase alfa treatment in an adult patient with infantile-onset hypophosphatasia. Bone 2019; 127: 67–74. [DOI] [PubMed] [Google Scholar]
- 100. Nakano C, Kitabatake Y, Takeyari S, et al. Genetic correction of induced pluripotent stem cells mediated by transcription activator-like effector nucleases targeting ALPL recovers enzyme activity and calcification in vitro. Mol Genet Metab 2019; 127: 158–165. [DOI] [PubMed] [Google Scholar]
- 101. Hendriksz CJ, Harmatz P, Beck M, et al. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol Genet Metab 2013; 110: 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Peracha H, Sawamoto K, Averill L, et al. Molecular genetics and metabolism, special edition: diagnosis, diagnosis and prognosis of mucopolysaccharidosis IVA. Mol Genet Metab 2018; 125: 18–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Akyol MU, Alden TD, Amartino H, et al. Recommendations for the management of MPS IVA: systematic evidence- and consensus-based guidance. Orphanet J Rare Dis 2019; 14: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hendriksz CJ, Parini R, AlSayed MD, et al. Impact of long-term elosulfase alfa on activities of daily living in patients with Morquio A syndrome in an open-label, multi-center, phase 3 extension study. Mol Genet Metab 2018; 123: 127–134. [DOI] [PubMed] [Google Scholar]
- 105. Sawamoto K, Stapleton M, Alméciga-Díaz CJ, et al. Therapeutic options for mucopolysaccharidoses: current and emerging treatments. Drugs 2019; 79: 1103–1134. [DOI] [PubMed] [Google Scholar]
- 106. Carpenter TO. The expanding family of hypophosphatemic syndromes. J Bone Miner Metab 2012; 30: 1–9. [DOI] [PubMed] [Google Scholar]
- 107. Fuente R, Gil-Peña H, Claramunt-Taberner D, et al. X-linked hypophosphatemia and growth. Rev Endocr Metab Disord 2017; 18: 107–115. [DOI] [PubMed] [Google Scholar]
- 108. Carpenter TO, Whyte MP, Imel EA, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med 2018; 378: 1987–1998. [DOI] [PubMed] [Google Scholar]
- 109. Insogna KL, Rauch F, Kamenický P, et al. Burosumab improved histomorphometric measures of osteomalacia in adults with X-linked hypophosphatemia: a phase 3, single-arm, international trial. J Bone Miner Res. Epub ahead of print 1 October 2019. DOI: 10.1002/jbmr.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ricalde P, Ahson I, Schaefer ST. A paradigm shift in the management of cherubism? A preliminary report using imatinib. J Oral Maxillofac Surg 2019; 77: 1278.e1–1278.e7. [DOI] [PubMed] [Google Scholar]
- 111. Mukai T, Fujita S, Morita Y. Tankyrase (PARP5) inhibition induces bone loss through accumulation of its substrate SH3BP2. Cells 2019; 8 pii: E195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pecora F, Gualeni B, Forlino A, et al. In vivo contribution of amino acid sulfur to cartilage proteoglycan sulfation. Biochem J 2006; 398: 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Monti L, Paganini C, Lecci S, et al. N-acetylcysteine treatment ameliorates the skeletal phenotype of a mouse model of diastrophic dysplasia. Hum Mol Genet 2015; 24: 5570–5580. [DOI] [PubMed] [Google Scholar]