Short abstract
Objectives
To explore the effects of trigeminal neuralgia on neurodegeneration in rats and the underlining mechanism.
Methods
Sixty adult male Sprague Dawley rats were divided randomly into Chronic Constriction Injury of the Rat’s Infraorbital Nerve (ION-CCI) group and sham group (n = 30). Right suborbital nerve was ligated in ION-CCI group to establish a trigeminal neuralgia model. In sham group, suborbital nerve was exposed without ligation. Pain thresholds were measured before surgery and 1, 7, 15, and 30 days after surgery (n = 10). Morris water maze tests (n = 10) were conducted at 1, 15, and 30 days after surgery to evaluate the changes in learning and memory ability of rats. At 5, 19, and 34 days after surgery, serum S100β protein concentration and hippocampal Aβ1-42 protein expression were detected by enzyme-linked immunosorbent assay; total tau protein expression was detected by Western blotting; changes of neurons in hippocampus were observed by Nissl staining; and the expression of ser404p-tau, cluster of differentiation (CD)95, CD95L, and cleaved caspase-3 proteins was detected by immunofluorescence and Western blotting.
Results
Hyperalgesia occurred in ION-CCI group, and mechanical pain threshold decreased significantly (P < 0.05). On the 15th and 30th days after surgery, ION-CCI group showed lower learning and memory ability than sham group (P < 0.05). Serum S100β protein concentration, hippocampal A β1-42, and ser404p-tau protein expression increased in the ION-CCI group 19 and 34 days after surgery (P < 0.05), hippocampal CD95 expression increased in the ION-CCI group after surgery (P < 0.05), hippocampal CD95L expression increased at 19 and 34 days after surgery (P < 0.05), and cleaved caspase-3 expression increased at 5 and 19 days after surgery (P < 0.05). Nissl bodies in ION-CCI group decreased significantly at 15 days after surgery. The expression of cleaved caspase-3 protein in ION-CCI group was positively correlated with the expression of CD95 and CD95L (P < 0.05).
Conclusions
Trigeminal neuralgia may lead to neuronal inflammation and neuronal apoptosis associated with upregulation of CD95/CD95L expression, thus causing neurodegeneration.
Keywords: Trigeminal neuralgia, CD95/CD95L, neuroinflammation, cell apoptosis, neurodegeneration, hippocampus, cognitive dysfunction, Nissl staining
Introduction
Recent studies have found that patients with chronic pain are often accompanied by cognitive dysfunction, which seriously affects the quality of life of patients.1–3 Existing studies suggested that changes in hippocampal synaptic structure, changes in the expression of neurotransmitters (cytokines, brain-derived neurotrophic factors), and overuse of opioids might be the mechanisms of cognitive dysfunction in patients,3,4 but the exact mechanism remains to be further studied.
Trigeminal neuralgia is a typical clinical chronic neuropathic pain. Meskal et al.5 suggested that patients with trigeminal neuralgia are at risk of cognitive defects. In addition, studies have reported that trigeminal neuralgia can damage spatial learning and memory ability of rats.6,7 The hippocampus is involved in the regulation of learning, memory, pain, and emotional response, and damage to the hippocampus can lead to impaired learning and memory;8–10 after nerve injury, the transmission of nociceptive stimulation to the central nervous system can lead to increased expression of cytokines in the hippocampus, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, etc.11–14 These factors act on neurons, microglia, or astrocytes; activate inflammatory responses; participate in the regulation of pain; and induce and maintain neuropathic pain.15–17 In addition, neuropathic pain also increased apoptosis of hippocampal neurons in rats.18,19 These evidences suggest that hippocampus is closely related to cognitive impairment in neuropathic pain animal models.
Among the related diseases with cognitive impairment as the main symptom, neurodegenerative disease is the most typical and common, presenting as progressive cognitive dysfunction. Neuroinflammation and neuronal apoptosis are important mechanisms of neurodegenerative disease, among which Alzheimer’s disease (AD) is more common. Amyloid β-protein (Aβ) deposits form senile plaques (SPs) and neurofibrillary tangles (NFTs), which are formed by hyperphosphorylation of tau protein. Cluster of differentiation (CD)95 expression was upregulated in patients with AD;20,21 it is suggested that CD95 is closely related to neurodegeneration, but whether CD95 is related to trigeminal neuralgia is not clear at present.
CD95 is type I transmembrane protein, known as “death receptors,” and CD95L is type II transmembrane protein; it is the ligand of CD95, both belong to the TNF superfamily member. It was found that CD95/CD95L played an important role in the development, regeneration, and apoptosis of the nervous system;22,23 under physiological conditions, CD95/CD95L is only slightly expressed in the brain to maintain immunosuppression, while under pathological stimulation such as craniocerebral injury or stroke, CD95L may be highly expressed in neurons and glial cells. CD95/CD95L was found to promote the secretion of cytokines,24,25 while cytokines such as IL-6, IL-8, and IL-1β have been proved to be key factors in inducing neuroinflammatory responses, so CD95/CD95L can induce neuroinflammatory responses by promoting the secretion of inflammatory cytokines. Meanwhile, CD95/CD95L can activate the cascade reaction of caspase family and lead to apoptosis. Combined with the above studies, neuropathic pain can lead to neuroinflammatory response and neuronal apoptosis, and neuroinflammatory response and neuronal apoptosis play an important role in neurodegeneration. Therefore, we hypothesized that CD95/CD95L pathway may be involved in the pathogenesis of cognitive impairment caused by trigeminal neuralgia.
Based on the above results, this study intends to explore whether trigeminal neuralgia affects the neurodegeneration of rats through the CD95/CD95L pathway in the model rats of trigeminal neuralgia.
Methods
Animals
Sixty-seven healthy and clean adult male Sprague Dawley rats weighing 200 to 250 g were used in this experiment, provided by animal experiment center of Southwest Medical University. All animal procedures were approved by the Ethical Committee of Southwest Medical University, Luzhou, China. Additionally, all animal procedures in the experiment in strict accordance with the regulations of the People’s Republic of China on the management of experimental animals and the methods for the quality management of experimental animals.
Three days before the experiment, the animals were trained to adapt the environment. The rats were knocked on the cage wall with a plastic rod, and the rats’ tails were lifted. After the rats were calm, the whisker pads were stimulated with a self-made brush for three times on each side. The bilateral stimulation was carried out alternately until the rats changed from initial exploration, fear curling, nose whipping, rapid escape, aggression, and other behaviors to silence. After three days of the above training, those who showed calmness and did not lose their whiskers were selected.
Rats were randomly divided into surgery group (Chronic Constriction Injury of the Rat’s Infraorbital Nerve (ION-CCI) group) and sham group, with 30 rats in each group and sham group as the control group. Chronic Constriction Injury of the Infraorbital Nerve (ION-CCI) was performed in the ION-CCI group to establish a trigeminal neuralgia model. In the Sham group, only the infraorbital nerve was exposed without ligating.
Surgical procedures
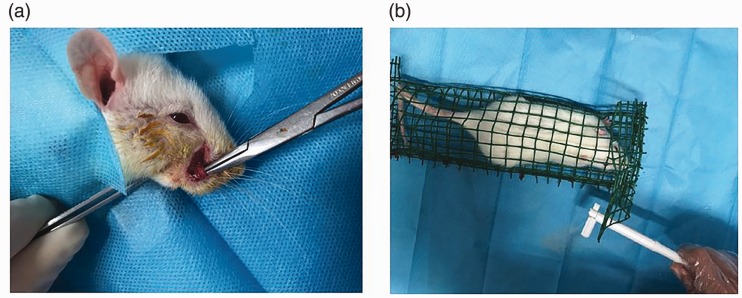
Anesthesia was given by intraperitoneal injection of sodium pentobarbital (40 mg/kg), a 1-cm incision was made along the upper margin of the right vibrissa pad of the rat to separate and expose the infraorbital nerve (Figure 1(a)), and then two chromium-enteric wires (4-0) were used to ligate the nerve at the proximal and distal ends, with an interval of about 2 mm. The standard of ligation was that the diameter of the infraorbital nerve became slightly thinner, but the conduction was not completely blocked; the blood circulation of the nerve outer membrane was unobstructed to prevent nerve ischemia and necrosis, and the incision was closed after surgery. The suborbital nerve was exposed in the sham group by the same method without ligation.
Figure 1.
(a) Surgical procedures: After anesthesia by intraperitoneal injection of sodium pentobarbital (40 mg/kg), a 1-cm incision was made along the upper margin of the right vibrissa pad of the rat to separate and expose the infraorbital nerve; (b) Mechanical pain threshold measurement: The rat was placed using a reticulated rat cage and waited until the rats were silent; the test subjects used the von Frey fiber filament to stimulate the rat’s surgical side whiskers.
Behavioral testing
Mechanical pain threshold measurement was performed to evaluate whether the establishment of trigeminal neuralgia model is successful.
Rats were placed in a mesh cage and waited until the rats were silent; the test subjects used the von Frey fiber filament to stimulate the rat’s surgical side whiskers (Figure 1(b)). Starting from the minimum stimulus intensity of 0.008 g, and gradually increasing, until the rats showed withdrawal and avoidance, aggression, and asymmetric face scratching behavior, it was considered as a positive reaction. Each stimulus intensity was tested 3 times/side, and when the positive number of the 3 stimuli was 2, the corresponding fibrofilament strength was the mechanical pain threshold of the rat. If the stimulation intensity was 26 g and the rats still did not have the above positive reaction, the test was stopped, and 26 g was taken as the threshold value of this test. The test time included 5 time points: before surgery and 1, 7, 15, and 30 days after surgery. At each time point, two groups of rats were randomly selected for pain threshold measurement (n = 10).
The Morris water maze (MWM) test was performed to assess learning and memory ability.
Morris water maze consists of a black circular pool (160 cm diameter and 50 cm height), a cylindrical platform (12 cm diameter), and a computer camera analysis system. The water pool was covered by blue floodlight curtains and divided into four quadrants. Different markers were placed in each quadrant to provide visual clues for the rats to find the platform. An escape platform was placed in the middle of one of the quadrants, and the platform was 2 cm below the water surface. The temperature in the pool was maintained at 20°C-22°C, providing a stable environment for the rats to avoid external factors interfering with the behavior of the rats.
The rats trained continuously for four days, four times a day, 30 s apart. In each training, the rats face the inner wall of the pool and were put into the pool from the midpoint of the wall of each quadrant. The time for the rats to stand on the escape platform was the result of the time. If the platform was not found within 120 s, it will be recorded as 120 s. The average of four training sessions per day was used as the day’s escape latency. On the fifth day, the platform was removed, and the rats were put into the pool from the opposite quadrant of the original escape platform. The number of times the rats crossed the original platform and the percentage of time they spent in the target quadrant in 120 s were recorded.
At 1, 15, and 30 days after the surgery, the rats after the pain threshold measurement were subjected to the water maze experiment, which were recorded as Test 1, Test 2, and Test 3, respectively.
Sampling and sample preparation
After the completion of Test 1, Test 2, and Test 3 of the Water maze experiment, namely 5 days, 19 days, and 34 days after the operation, rats were sacrificed, and samples were obtained. At three time points, rats were randomly selected from two groups (n = 5) and anesthetized with sodium pentobarbital (40 mg/kg) to expose the heart and inferior vena cava. Blood was drawn from the inferior vena cava for 3-4 ml.The serum was isolated by centrifugation (3000 r/min,10 min) after placing for 1 h. The serum was lightly sucked out and stored in a refrigerator at -80°C for later use. Heart perfusion was performed with 0.1 M phosphate buffer saline (PBS; pH 7.4), followed by pre-chilled 4% paraformaldehyde for tissue fixation. After decapitation, the brain was fixed in 4% paraformaldehyde for 24 hours, and then dehydrated, waxed and embedded. Continuous coronal sectioning was performed with a Paraffin Slicer, the thickness was 3 μm, and it was placed in a 60°C oven overnight. Store at room temperature for future use. The other 5 rats were perfused with PBS only, then the hippocampus was taken immediately after decapitation on ice, the tissue homogenate was centrifuged at 13000 r/min at 4°C for 15 min. The supernatant was removed and the protein concentration was measured by bicinchoninic acid assay for ELISA detection of Aβ1 -42 protein and Western Blotting.
ELISA: the concentration of S100β protein and the expression of Aβ1-42 protein in the hippocampus of rats were detected
Remove the ELISA Kit (Rat S100βELISA Kit, Rat Aβ1-42 ELISA Kit, Qiaodu Biotechnology, Shanghai, China) and balance at room temperature for 20 min. Follow the manufacturer's instructions, briefly, add standard and sample wells in sequence, and then add horseradish peroxidase (HRP) -labeled detection antibody to each well, block the plate and incubate at 37°C for 60 minutes, wash the plate, add substrate and incubate for 15 minutes in the dark, and finally, add stop solution and measure Optical Density (OD) value.
Western blotting: the expression of CD95, CD95L, cleaved caspase-3, total tau, and ser404p-tau in hippocampal tissues was detected
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was used to isolate the equal amounts of protein and transfer it to polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). The membranes were blocked with 5% skim milk at room temperature, but ser404p-tau protein membrane was blocked with 5% bovine serum albumin (BSA). Then, incubate with primary antibody overnight at 4°C (rabbit anti CD95, 1:300, Santa Cruz Biotechnology, Santa Cruz, CA, USA; rabbit anti-CD95L, 1:400, rabbit anti-cleaved caspase-3, 1:1000, rabbit anti-total tau, 1:1000, rabbit anti-ser404p-tau, 1:1000, Abcam, Cambridge, MA, USA). Then, the membranes were incubated with anti-rabbit secondary antibody (1:500, Proteintech, Chicago, IL, USA) at room temperature for 1 h. Finally, the bands were detected using enhanced chemiluminescence (Beyotime, Shanghai, China). Image J (National Institutes of Health, Bethesda, MD, USA) was used for quantitative band intensity; glyceraldehyde 3-phosphate dehydrogenase (GAPDH,1:500, Abcam, Cambridge, MA, USA) was used as the sample control.
Immunofluorescence test: expressions of CD95, CD95L, and cleaved caspase-3 proteins was detected
The paraffin sections were dewaxed and gradient ethanol rehydrated, and then washed with distilled water. The sections were then placed in an antigen repair solution (Beyotime, Shanghai, China) and incubated in a water bath for 20 minutes. After the sections were naturally cooled to room temperature, the sections were removed and treated in 0.2% Triton X-100 solution (Amresco, USA) for 15 minutes. The sections were then sealed in 1% BSA for 30 min. They were then incubated with primary antibodies (CD95: 1:50, CD95L: 1:100, cleaved caspase-3: 1:200) Next, add different fluorescent-labeled secondary antibodies (Cy3: 1: 200, Fluorescein 5-isothiocyanate, FITC: 1: 100, CWBIO, Beijing, China) and incubate for 1 hour at room temperature in the dark. 4'-6-Diamidino-2-phenylindole (DAPI,Beyotime, Shanghai, China) was stained for 5 min. The hippocampus was viewed under an optical microscope (Olympus, Tokyo, Japan) (magnification 400).
Nissl staining: observing the changes of neurons in hippocampus
Paraffin slices were dewaxed to water routinely; then, reagent A (Methylene Blue Stain) was dripped for 10 min and then differentiated into reagent B (Nissl differentiation). The slices were observed under a microscope until the Nissl body was clear. Next, reagent C (Ammonium Molybdate Solution) was added to treat the slices for 4 min. The slices were quickly rinsed with distilled water, then dehydrated with anhydrous ethanol, then the slices were made transparent with xylene, and finally blocked slices with resinene.
Statistical analysis
SPSS19.0 statistical software was used. Experimental data were expressed as mean ± standard deviation (χ ± s). Escape latency and swimming speed were analyzed by repetitive measure analysis of variance (ANOVA). The pain threshold, the number of times to cross the original target platform, the target quadrant time ratio, CD95, CD95L, Cleaved-caspase3, S100β, Aβ1-42, and Tau proteins were compared at different time points in the group using one-way ANOVA, Pairwise comparison within the group was performed using the Least Significant Difference (LSD) method, comparison between groups was performed using the Student's t test, and analysis of correlation was performed using the Pearson analysis P < 0.05 was considered statistically significant.
Results
Changes of mechanical pain threshold in rats
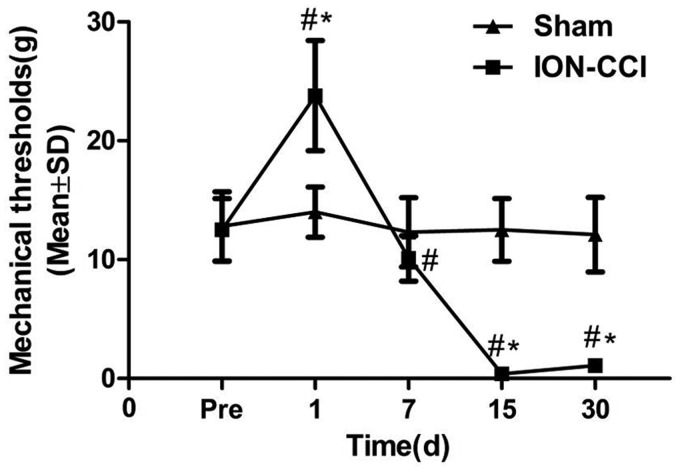
The pain threshold of ION-CCI group rats was higher one day after surgery than that before surgery (P < 0.05), and the subsequent time points were significantly lower than that before surgery (P<0.01). This abnormal sensitivity to pain persisted during the 30-day observation, especially at the 15th day after surgery. The sham group showed no such change (Figure 2).
Figure 2.
Change of the mechanical threshold in two groups. Mechanical threshold. Data are expressed as mean ± SD (#P < 0.05, compared to preoperative; *P < 0.05, compared to sham group).
ION-CCI: Chronic Constriction Injury of the Rat’s Infraorbital Nerve.
Results of rat Morris water maze experiment
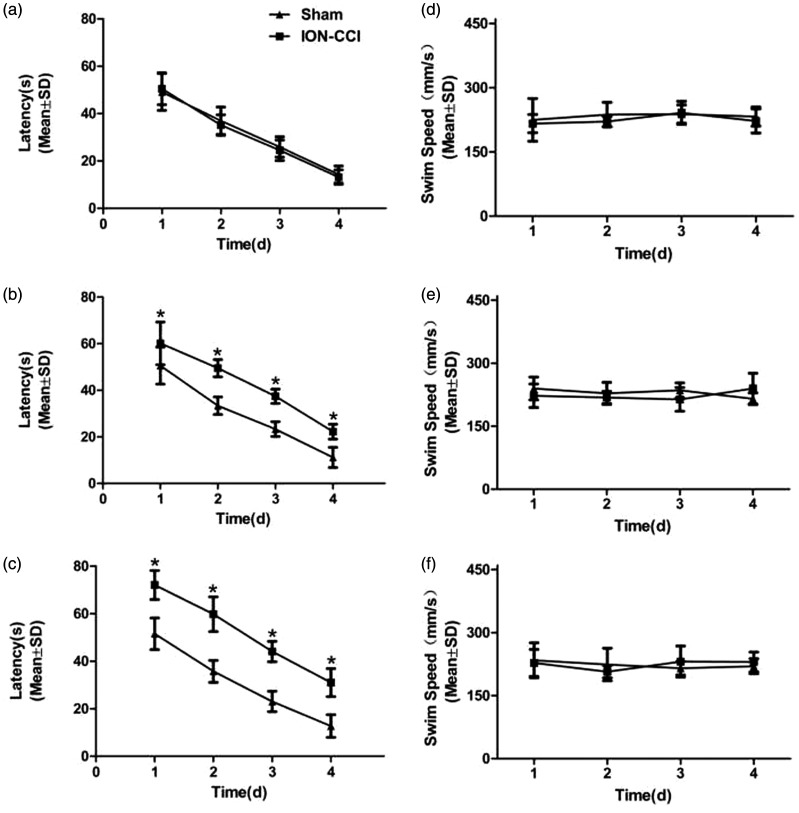
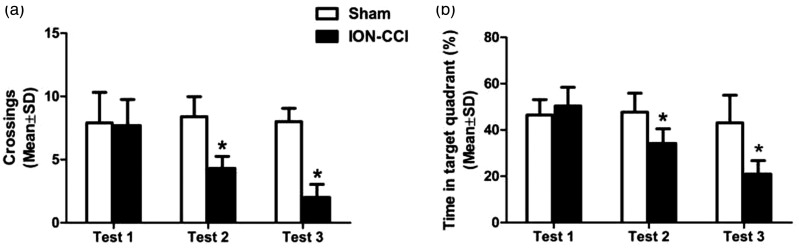
In order to compare the effects of trigeminal neuralgia on cognitive function, we conducted three water maze experiments to observe the behavioral changes of rats, respectively, Test 1, Test 2, and Test 3. Each experiment included four consecutive days of positioning and navigation training and the fifth day of space exploration test. With the prolongation of learning time, the escape latency of rats was gradually shortened. In Test 1, there was no significant difference between the two groups in escape latency (F = 0.153, P = 0.701) (Figure 3(a)) and swimming speed (F = 1.553, P = 0.229) (Figure 3(d)), which means, 1 day after surgery, the learning function of the rats was not significantly impaired. In Test 2, the escape latency of ION-CCI group rats was significantly longer than that of sham group (F = 101.315, P = 0.000) (Figure 3(b)). There was no statistical difference in swimming speed (F = 0.485, P = 0.495) (Figure 3(e)), suggesting that trigeminal neuralgia impaired learning ability 15 days after surgery. In Test 3, the escape latency of ION-CCI group rats was longer than that of sham group (F = 95.984, P = 0.000) (Figure 3(c)), and the swimming speed showed no statistical difference (F = 0.010, P = 0.923) (Figure 3(f)), suggesting that trigeminal neuralgia has sustained damage to learning ability. On the fifth day of training, after the rats were removed from the platform, the space exploration experiment was conducted on the rats. Compared with sham group, the number of rats crossing the platform (Figure 4(a)) and the time percentage in the target quadrant of ION-CCI group were significantly reduced (P < 0.01) (Figure 4(b)), while there was no significant difference between the two groups in Test 1 (Figure 4(a, b)).
Figure 3.
Learning ability in rats on the water maze experiment: (a) escape latency in Test 1 (F = 0.153, P= 0.701); (b) escape latency in Test 2 (F = 101.315, P = 0.000); (c) escape latency in Test 3 (F = 95.984, P = 0.000); (d) swimming speed in Test 1 (F = 1.553, P = 0.229); (e) swimming speed in Test 2 (F = 0.485, P = 0.495); and (f) swimming speed in Test 3 (F = 0.010, P = 0.923). Data are expressed as mean ± SD (*P < 0.05, compared to sham group).
ION-CCI: Chronic Constriction Injury of the Rat’s Infraorbital Nerve.
Figure 4.
Probe trials in rats on the Morris water maze task: (a) the number of times to cross the original target platform in 3 Tests and (b) the target quadrant time ratio in 3 Tests (P < 0.01). Data are expressed as mean ± SD (*P < 0.05, compared to sham group).
ION-CCI: Chronic Constriction Injury of the Rat’s Infraorbital Nerve.
Expression of neurodegenerative-related proteins was increased in trigeminal neuralgia rats
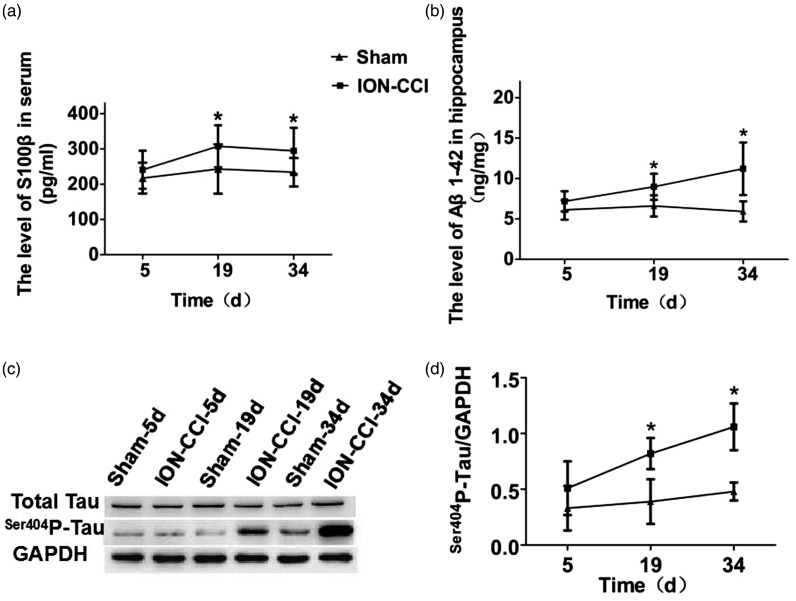
S100β protein is a specific protein of the nervous system, which is closely related to pain and cognitive function. Therefore, we used the ELISA to detect whether S100β protein in serum was upregulated. The results showed that there was no statistically significant difference in the concentration changes at postoperative time points in the sham group (F = 0.602, P = 0.555), while the ION-CCI group was significantly higher at 19 and 34 days after surgery than the sham group (P < 0.05), with the highest concentration at 19 days after surgery (Figure 5(a)). Excessive deposition of Aβ can lead to cognitive dysfunction. Therefore, we detected the expression of Aβ1-42 protein in the hippocampus by ELISA. The results showed that there was no statistical difference in postoperative time points in the sham group (F = 0.379, P = 0.692), and ION-CCI group was significantly higher than that in the sham group at 19 and 34 days after surgery (P < 0.05), with the highest value at 34 days after surgery (Figure 5(b)). Tau protein phosphorylation level in the normal brain tissue is very low; when pathological changes occur, tau protein can be overphosphorylated at multiple sites, which affect cognitive function; therefore, we used the Western blotting to detect the tau protein expression in the hippocampus. The Western Blotting results showed that the changes of Total-Tau protein in the two groups of rats were not statistically different (P> 0.05), while ser404p-tau protein in the hippocampus of the ION-CCI group showed the same continuous increasing trend as Aβ1-42. Compared with the Sham group, it increased significantly at 19 and 34 days after surgery (P <0.05), and was highest at 34 days after surgery (Figure 5(c) and (d)).
Figure 5.
Neurodegenerative-related proteins in trigeminal neuralgia rats: (a) serum S100β protein level; (b) hippocampus Aβ1-42 protein level; (c) hippocampus total tau protein and ser404p-tau protein level; (d) The ratio of ser404p-tau protein to GAPDH.
ION-CCI: Chronic Constriction Injury of the Rat’s Infraorbital Nerve; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
The expression of CD95/CD95L pathway was upregulated in the hippocampus of trigeminal neuralgia rats
Previous clinical studies have shown that CD95 plays an important role in neurodegenerative diseases, such as Alzheimer’s disease. Therefore, we detected whether the CD95/CD95L pathway was associated with cognitive impairment induced by trigeminal neuralgia. We detected the expression of CD95, CD95L, and cleaved caspase-3 in the hippocampus of rats 5, 19, and 34 days after surgery by immunofluorescence and Western blotting.
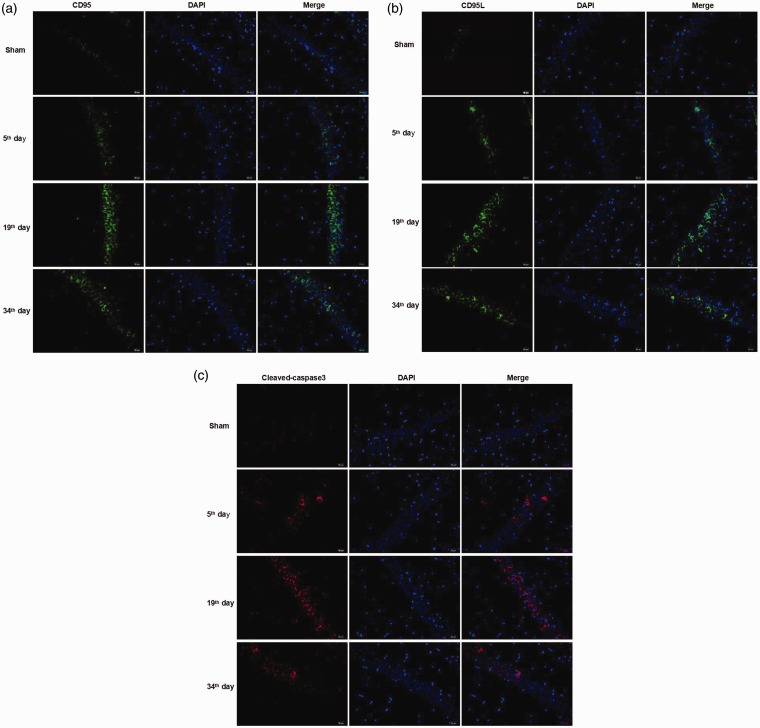
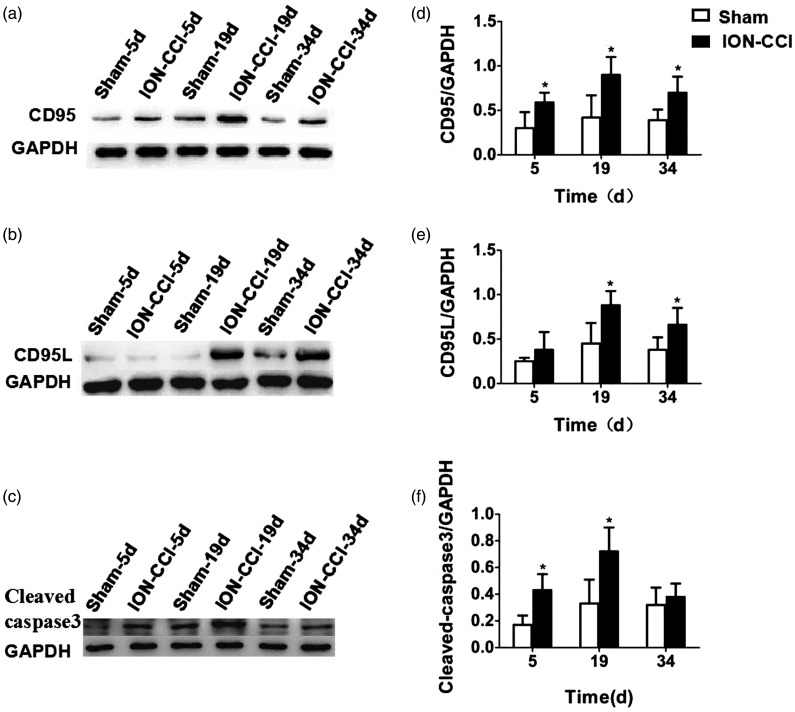
Immunofluorescence results showed that the green fluorescence expression of CD95 and CD95L protein and the red fluorescence expression of cleaved caspase-3 protein were weak in the sham group, while the fluorescence expression of ION-CCI group was obvious (Figure 6(a) to (c)). Quantitative detection of hippocampal CD95, CD95L, and cleaved caspase-3 protein expression by Western blotting showed that no significant differences were found in CD95 (F = 0.496, P = 0.621), CD95L (F = 2.054, P = 0.171), and cleaved caspase-3 (F = 2.463, P = 0.127) expressions at various time points in the sham group. CD95 expression in ION-CCI group was significantly increased at each time point after surgery (P < 0.05), with the highest expression at 19 days after surgery (Figure 7(a) and (d)). CD95L showed a similar trend to that of CD95, which was significantly higher at 19 and 34 days after surgery than in the sham group (P < 0.05), with the highest expression at 19 days after surgery (Figure 7(b) and (e)). Cleaved caspase-3 expression was significantly higher at 5 and 19 days after surgery than in the sham group (P < 0.05), with the highest expression at 19 days after surgery (Figure 7(c) and (f)). We also measured the expression level of CD95, CD95L, cleaved caspase-3, total tau, and ser404p-tau proteins in prefrontal cortex. To our surprise, we did not observe the same change trend in prefrontal cortex as in hippocampus, and they did not show a stable and consistent trend of change (data not shown).
Figure 6.
Expression of CD95 protein, CD95L protein, and cleaved caspase-3 protein in hippocampus of rats: (a) CD95 protein (the green fluorescence represents the CD95 protein, and the blue fluorescence represents DAPI, 400×); (b) CD95L protein (the green fluorescence represents the CD95L protein, and the blue fluorescence represents DAPI, 400×); and (c) cleaved caspase-3 protein (the red fluorescence represents the cleaved caspase-3 protein, and the blue fluorescence represents DAPI, 400×).
CD: cluster of differentiation.
Figure 7.
Quantitative detection of hippocampal CD95, CD95L, and cleaved caspase-3 protein at various time points: (a) and (d) CD95 expression; (b) and (e) CD95L expression; (c) and (f) cleaved caspase-3 expression.
CD: cluster of differentiation; ION-CCI: Chronic Constriction Injury of the Rat’s Infraorbital Nerve; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
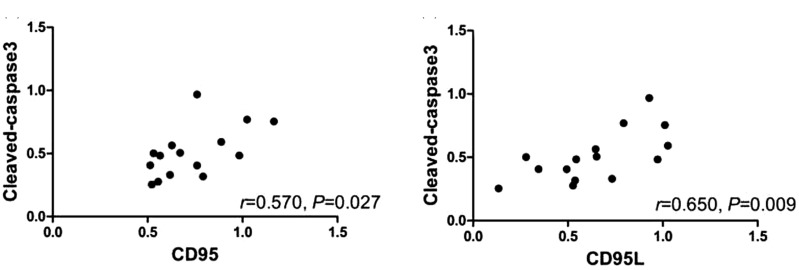
The cleaved caspase-3 protein in hippocampus of trigeminal neuralgia rats was correlated with CD95/CD95L protein
The expression of cleaved caspase-3 protein in the hippocampal tissues of ION-CCI rats was positively correlated with the expression of CD95 protein (r = 0.570, P = 0.027) (Figure 8(a)), and the expression of cleaved caspase-3 protein was positively correlated with the expression of CD95L protein (r = 0.650, P = 0.009) (Figure 8(b)).
Figure 8.
Correlation between cleaved caspase-3 protein and CD95/CD95L protein level in hippocampus in ION-CCI group. (a) The expression of cleaved caspase-3 and CD95 was positively related (r = 0.570, P = 0.027); (b) the expression of cleaved caspase-3 and CD95L was positively related (r = 0.650, P = 0.009); P < 0.05; there is statistical significance.
CD: cluster of differentiation.
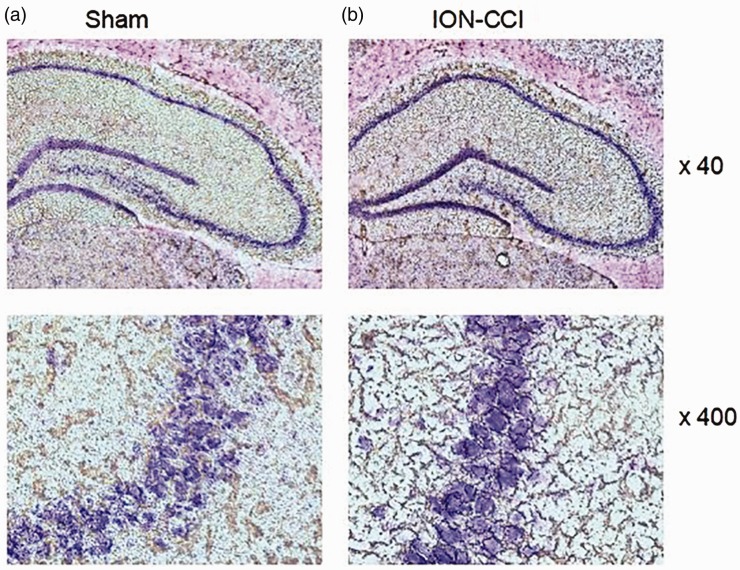
Changes of neurons in hippocampus
In sham group, the structure of neurons in CA3 area of hippocampus was intact, nucleolus was obvious, and a large number of granular and tigroid Nissl bodies could be seen in the cytoplasm (Figure 9(a)). While in ION-CCI group, the neurons in CA3 area of hippocampus were mostly vacuolar, with no obvious nucleolus, and Nissl bodies dissolved or even disappeared (Figure 9(b)).
Figure 9.
Nissl staining: the changes of neurons in hippocampus: (a) morphology of hippocampal neurons in sham group and (b) morphology of hippocampal neurons in ION-CCI group.
ION-CCI: Chronic Constriction Injury of the Rat’s Infraorbital Nerve.
Discussion
Recent studies have found that head and facial pain is closely related to cognitive dysfunction.26–30 While trigeminal neuralgia is the most common chronic neuropathic pain in the head and face, its pathogenesis is complex, and the current mainstream is divided into central and peripheral mechanism; the central theory of trigeminal neuralgia associated with voltage channel gating, and around the theory of trigeminal nerve, demyelination changes cause “short circuit” between adjacent nerves, and “short circuit” phenomenon of abnormal nerve impulses to the central cause sustained excitement. In order to simulate different pathogenesis, researchers have successfully established a variety of animal models of trigeminal neuralgia, including ION-CCI model, dental pulp perfusion model, and suborbital nerve injection model of cobra venom. This study is to replicate Liu et al.31 through the zygomatic line down to the road of ION-CCI model of trigeminal neuralgia. ION-CCI group rats showed a low response period one day after surgery, which may be due to axonal degeneration after nerve ligation, and the nerve impulse could not be transmitted through the ligation site, resulting in increased pain threshold. However, the specific mechanism remains to be further studied. The pain threshold was the lowest 15 days after surgery, which was considered to be the most severe myelin damage during this period. The pain threshold was slightly increased 30 days after surgery, this may be due to gradual absorption of the catgut, and the nerve was gradually repaired. This is consistent with the results reported in previous studies, indicating that the modeling is successful.
In this study, the Morris water maze experiment was conducted at three time points (1 day, 15 days, and 30 days after the surgery) from the acute stage, pain sensitive stage, and chronic stage after nerve injury in combination with the aforementioned changes in pain threshold, to verify that chronic trigeminal neuralgia would lead to spatial learning and memory dysfunction in rats. However, we found that at 30 days after surgery, the learning and memory ability of rats showed an opposite trend to the change of pain threshold, which may be due to the gradual recovery of the injury of the infraorbital nerve after catgut absorption, but the effect of pain on the central nervous system did not recover. Previous studies often choose to test the water maze behavior at a point in time, the data are relatively simple, and this experiment observed the three time points, more fully reflect the dynamic change of learning and memory ability of rats, so this study can reflect the trend of trigeminal neuralgia effect on cognitive function. However, whether the learning and memory abilities continue to decline or improve after 30 days remains to be further explored.
S100β protein is a specific protein of the nervous system. When the central nervous system is injured, S100β protein exudes from the damaged site to the cerebrospinal fluid and then enters the blood through the blood–brain barrier. In addition, studies have reported increased serum S100β protein concentration in AD patients32; therefore, serum S100β protein is often used as an indicator of the extent of reactive nerve injury and the prediction of neurodegeneration. The results of this study are consistent with previous studies showing that pain can lead to the upregulation of S100β protein levels.33–35 In particular, serum S100β protein levels were significantly upregulated 19 and 34 days after surgery, suggesting severe nerve damage in rats during this period. At the same time, the upregulation of S100β protein also suggested neurodegeneration in rats.
Excessive deposition of Aβ is an important pathological feature of AD; Aβ includes Aβ1-40 and Aβ1-42. Because Aβ1-42 is hydrophobic and it is much more likely to be the main ingredient in the SPs, this study aims to test the expression of Aβ1-42. Abnormal phosphorylation of tau protein can reduce the binding ability of tau protein to microtubules, the combination of tau protein and microtubule cytoskeleton damage, leading to the formation of NFTs, thus causing nerve degeneration; Ser404 is considered to be a reliable marker of the transition from mild cognitive impairment to AD; moreover, phosphorylation of this site was crucial for the formation of paired helical filaments, so this study selected Ser404 site to detect the tau protein phosphorylation level. In our study, Aβ1-42 and ser404p-tau level are raised after surgery, prompt degeneration of rat nerve may have happened when the pain threshold began to recover gradually, Aβ1-42 and ser404p-tau level is still on the rise, the tip of the central nervous system may be irreversibly damaged, and its mechanism and the in between is Aβ and p-tau synergies related to each other;36,37 further research is needed to confirm this.
S100β protein, Aβ protein, and p-tau protein are neurodegenerative protein-related proteins. The expression of all three was upregulated in the hippocampus of trigeminal neuralgia rats, in combination with the water maze rats behavior change, further confirmed the trigeminal neuralgia can cause nerve degeneration assumption; this is consistent with previous studies that neuropathic pain can lead to cognitive dysfunction; in this study, we further explore its possible mechanism.
Recent studies have demonstrated the importance of the CD95/CD95L pathway to the nervous system, which is involved in the occurrence and development of a variety of neurological diseases such as AD,38–41 but whether it is associated with neuropathic pain is not clear. The main function of CD95/CD95L is to participate in the immune response, etc. And a number of studies suggest that pain may affect the immune response of the body.42-44 In this study, we found that trigeminal neuralgia resulted in upregulation of CD95/CD95L expression in the hippocampus of rats. The processing of pain information by the nervous system needs to be completed through a complex neural network. The upregulation of CD95/CD95L induced by trigeminal neuralgia may be related to the upward conduction of pain information. When pain information from the periphery is transmitted to the central nervous system, it may activate the hippocampus to participate in the processing of pain information, but the exact mechanism remains to be determined by further research.
The prefrontal cortex is also involved in the regulation of spatial learning and memory abilities, but in our study, no upregulation of these proteins was found in the prefrontal cortex, which suggests that CD95/CD95L pathway may be one of the molecules specifically expressed in the hippocampus in cognitive impairment caused by trigeminal neuralgia. Although hippocampus and prefrontal cortex have many similar functions, their specific molecules may not be consistent. Studies have shown that in inbred Roman High-Avoidance (RHA-I) strain rats with poor learning and memory ability and cognitive impairment, some synaptic-related molecules show different trends in hippocampus and prefrontal cortex.45 Of course, whether there are also molecular pathways related to cognitive impairment caused by trigeminal neuralgia in prefrontal cortex can be the direction of our future research.
Upregulation of CD95/CD95L may affect the occurrence of neurodegeneration through multiple pathways. It can promote the secretion of cytokines such as IL-6, IL-8, and IL-1β or the migration of immune cells such as neutrophils to cause neuroinflammatory reactions, which are caused by long-term chronic neuroinflammatory reactions. At the same time, CD95 is also a signaling molecule upstream of the caspase apoptosis pathway, which activates the cascade reaction of the caspase family after binding to CD95L, among which the activation shearing of caspase-3 is the most critical, and eventually leads to the apoptosis of cells due to the degradation of DNA. Cleaved caspase-3 is an iconic protein of cell apoptosis. The increased expression of cleaved caspase-3 protein after ION-CCI surgery suggested that trigeminal neuralgia increased the apoptosis of hippocampal cells, which was consistent with the results of previous animal studies that pathological pain increased the apoptosis of hippocampal cells in rats.8,19 Abnormal increase of apoptosis leads to excessive loss of neurons, which can lead to neurodegeneration. This study found that the expression of cleaved caspase-3 protein was positively correlated with the expression of CD95/CD95L, suggesting that the CD95/CD95L apoptosis pathway might be involved in the apoptosis of hippocampal cells in rats with trigeminal neuralgia. In addition, CD95/CD95L can affect Aβ and tau proteins by stimulating the activation of downstream caspase-3, and studies have found that caspase-3 can regulate the production of Aβ;46 The specific mechanism is: γ-secretase hydrolyzes amyloid precursor protein (APP) to produce Aβ, γ-secretase activating protein (GSAP) is the key protein that regulates this process, and GASP is regulated by Caspase3, so the activation of Caspase3 can promote the production of Aβ, and the activated Caspase3 can also cleave Tau protein and make Tau protein more likely to aggregate by exposing phosphorylation sites.
Nissl bodies exist in neuron bodies or dendrites and are basophilic granules. It can reflect the changes of morphological structure and function of neurons.47 In this study, the obvious histological changes of neurons in rats with trigeminal neuralgia were observed by Nissl staining, which is another direct and strong evidence that trigeminal neuralgia causes neurodegeneration in rats.
In this study, the above experiments indicated that trigeminal neuralgia may lead to increased neuroinflammation and neuronal apoptosis by upregulating the expression of CD95/CD95L, thus causing neurodegeneration. However, further studies are needed to determine the exact mechanism of upregulation of CD95/CD95L.
Conclusions
This study verified that trigeminal neuralgia can cause learning and memory dysfunction in rats and revealed that the upregulation of the hippocampal CD95/CD95L pathway may be involved in relevant mechanisms, providing a theoretical basis for the prevention and intervention of cognitive dysfunction in patients with trigeminal neuralgia.
Limitations
Trigeminal neuralgia causes neurodegeneration in rats associated with upregulation of the CD95/CD95L pathway, but the upstream impact factors of this pathway remains to be elucidated. The exact mechanism of the CD95/CD95L pathway needs to be further determined using knockout rats.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from the National Science Foundation of China (No. 81873930), partly by grants from research project of Sichuan Provincial Health Department (16PJ567), and the joint foundation of Luzhou Government and Southwest Medical University (2015LZCYD-S06(4/11), 2018LZXNYD-ZK02 and 2019LZXNYDJ23), and Sichuan Provincial Science and Technology (2019YJ0692).
ORCID iD
Maohua Wang https://orcid.org/0000-0002-1723-8088
References
- 1.Bianchini KJ, Aguerrevere LE, Guise BJ, Ord JS, Etherton JL, Meyers JE, Soignier RD, Greve KW, Curtis KL, Bui J. Accuracy of the modified somatic perception questionnaire and pain disability index in the detection of malingered pain-related disability in chronic pain. Clin Neuropsychol 2014; 28: 1376–1379. [DOI] [PubMed] [Google Scholar]
- 2.Landro NI, Fors EA, Vapenstad LL, Holthe O, Stiles TC, Borchgrevink PC. The extent of neurocognitive dysfunction in a multidisciplinary pain centre population. Is there a relation between reported and tested neuropsychological functioning? Pain 2013; 154: 972–977. [DOI] [PubMed] [Google Scholar]
- 3.Schneiderhan J, Clauw D, Schwenk TL. Primary care of patients with chronic pain. JAMA 2017; 317: 2367–2368. [DOI] [PubMed] [Google Scholar]
- 4.Moriarty O, Finn DP. Cognition and pain. Curr Opin Support Palliat Care 2014; 8: 130–136. [DOI] [PubMed] [Google Scholar]
- 5.Meskal I, Rutten GJ, Beute GN, Salden ME, Sitskoorn MM. Cognitive deficits in patients with trigeminal neuralgia: opportunities to improve care and quality of life. Acta Neurochir (Wien) 2014; 156: 1565–1566. [DOI] [PubMed] [Google Scholar]
- 6.Wu Z, Qian XY, An JX, Liu CC, Tian M, Cope DK, Williams JP. Trigeminal neuralgia induced by cobra venom in the rat leads to deficits in abilities of spatial learning and memory. Pain Physician 2015; 18: E207–E216. [PubMed] [Google Scholar]
- 7.Zhang L, Ding X, Wu Z, Qian X, An J, Tian M. Trigeminal neuralgia induced by cobra venom leads to cognitive deficits associated with downregulation of CREB/BDNF pathway. Pain Physician 2017; 20: 53–68. [PubMed] [Google Scholar]
- 8.Teles-Grilo Ruivo LM, Baker KL, Conway MW, Kinsley PJ, Gilmour G, Phillips KG, Isaac JTR, Lowry JP, Mellor JR. Coordinated acetylcholine release in prefrontal cortex and hippocampus is associated with arousal and reward on distinct timescales. Cell Rep 2017; 18: 905–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castilla-Ortega E, Serrano A, Blanco E, Araos P, Suárez J, Pavón FJ, Rodríguez de Fonseca F, Santín LJ. A place for the hippocampus in the cocaine addiction circuit: potential roles for adult hippocampal neurogenesis. Neurosci Biobehav Rev 2016; 66: 15–32. [DOI] [PubMed] [Google Scholar]
- 10.Chen LJ, Wang YJ, Chen JR, Tseng GF. Hydrocephalus compacted cortex and hippocampus and altered their output neurons in association with spatial learning and memory deficits in rats. Brain Pathol 2017; 27: 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasick V, Spengler RN, Samankan S, Nader ND, Ignatowski TA. The hippocampus and TNF: common links between chronic pain and depression. Neurosci Biobehav Rev 2015; 53: 139–159. [DOI] [PubMed] [Google Scholar]
- 12.Tu W, Wang W, Xi H, He R, Gao L, Jiang S. Regulation of neurotrophin-3 and interleukin-1βand inhibition of spinal glial activation contribute to the analgesic effect of electroacupuncture in chronic neuropathic pain states of rats. Evid Based Complement Alternat Med 2015; 2015: 642081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerard E, Spengler RN, Bonoiu AC, Mahajan SD, Davidson BA, Ding H, Kumar R, Prasad PN, Knight PR, Ignatowski TA. Chronic constriction injury-induced nociception is relieved by nanomedicine-mediated decrease of rat hippocampal tumor necrosis factor. Pain 2015; 156: 1320–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyrtyshnaia AA, Manzhulo IV, Sultanov RM, Ermolenko EV. Adult hippocampal neurogenesis in neuropathic pain and alkyl glycerol ethers treatment. Acta Histochem 2017; 119: 812–821. [DOI] [PubMed] [Google Scholar]
- 15.Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res 2013; 6: 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics 2010; 7: 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuda M. Modulation of pain and itch by spinal glia. Neurosci Bull 2018; 34: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang YY, Zhang Y, Cui S, Liu FY, Yi M, Wan Y. Cholinergic neurons in medial septum maintain anxiety-like behaviors induced by chronic inflammatory pain. Neurosci Lett 2018; 671: 7–12. [DOI] [PubMed] [Google Scholar]
- 19.Del Pino J, Moyano P, Díaz GG, Anadon MJ, Diaz MJ, García JM, Lobo M, Pelayo A, Sola E, Frejo MT. Primary hippocampal neuronal cell death induction after acute and repeated paraquat exposures mediated by AChE variants alteration and cholinergic and glutamatergic transmission disruption. Toxicology 2017; 390: 88–99. [DOI] [PubMed] [Google Scholar]
- 20.de la Monte SM, Sohn YK, Wands JR. Correlates of p53- and Fas (CD95)-mediated apoptosis in Alzheimer’s disease. J Neurol Sci 1997; 152: 73–83. [DOI] [PubMed] [Google Scholar]
- 21.Ramos-Miguel A, García-Sevilla JA, Barr AM, Bayer TA, Falkai P, Leurgans SE, Schneider JA, Bennett DA, Honer WG, García-Fuster M. Decreased cortical FADD protein is associated with clinical dementia and cognitive decline in an elderly community sample. Mol Neurodegener 2017; 12: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Tisch N, Kegel M, Yerbes R, Hermann R, Hudalla H, Zuliani C, Gülcüler G, Zwadlo K, von Engelhardt J, Ruiz de Almodóvar C, Martin-Villalba A. CNS macrophages control neurovascular development via CD95L. Cell Rep 2017; 19: 1378–1393. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Tang M. Dysfunction of various organelles provokes multiple cell death after quantum dot exposure. Int J Nanomedicine 2018; 13: 2729–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams JW, Ferreira CM, Blaine KM, Rayon C, Velázquez F, Tong J, Peter ME, Sperling AI. Non-apoptotic Fas (CD95) signaling on T cells regulates the resolution of Th2-mediated inflammation. Front Immunol 2018; 9: 2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchiyama R, Yonehara S, Taniguchi S, Ishido S, Ishii KJ, Tsutsui H. Inflammasome and Fas-mediated IL-1β contributes to Th17/Th1 cell induction in pathogenic bacterial infection in vivo. J Immunol 2017; 199: 1122–1130. [DOI] [PubMed] [Google Scholar]
- 26.Kooshki R, Abbasnejad M, Esmaeili-Mahani S, Raoof M. The role of trigeminal nucleus caudalis orexin 1 receptors in orofacial pain transmission and in orofacial pain-induced learning and memory impairment in rats. Physiol Behav 2016; 157: 20–27. [DOI] [PubMed] [Google Scholar]
- 27.Parise M, Kubo TT, Doring TM, Tukamoto G, Vincent M, Gasparetto EL. Cuneus and fusiform cortices thickness is reduced in trigeminal neuralgia. J Headache Pain 2014; 15: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raoof R, Esmaeili-Mahani S, Abbasnejad M, Raoof M, Sheibani V, Kooshki R, Amirkhosravi L, Rafie F. Changes in hippocampal orexin 1 receptor expression involved in tooth pain-induced learning and memory impairment in rats. Neuropeptides 2015; 50: 9–16. [DOI] [PubMed] [Google Scholar]
- 29.Uritani D, Nishida T, Sakaguchi N, Kawakami T, Jones LE, Kirita T. Difference in response to a motor imagery task: a comparison between individuals with and without painful temporomandibular disorders. Pain Res Manag 2018; 2018: 6810412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao YR, Drew PJ. Effects of voluntary locomotion and calcitonin gene-related peptide on the dynamics of single dural vessels in awake mice. J Neurosci 2016; 36: 2503–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CY, Lu ZY, Li N, Yu LH, Zhao YF, Ma B. The role of large-conductance, calcium-activated potassium channels in a rat model of trigeminal neuropathic pain. Cephalalgia 2015; 35: 16–35. [DOI] [PubMed] [Google Scholar]
- 32.Yao JJ, He SR, Chen L, Yang L, Qiao XB, Zhang W, Du J, Liu DG. Expression of cytokine IL-1αand S100βin different types of plaques in Alzheimer’s disease. Chin J Pathol 2011; 40: 581–584. [PubMed] [Google Scholar]
- 33.Arroyo Hernández M, Rodríguez Suárez J, Álvarez Menéndez F. Serum S100β protein reference values in a paediatric population. An Pediatr (Barc) 2016; 84: 254–259. [DOI] [PubMed] [Google Scholar]
- 34.Carniglia L, Ramírez D, Durand D, Saba J, Turati J, Caruso C, Scimonelli TN, Lasaga M. Neuropeptides and microglial activation in inflammation, pain, and neurodegenerative diseases. Mediators Inflamm 2017; 2017: 5048616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blaszczyk L, Maître M, Lesté-Lasserre T, Clark S, Cota D, Oliet SHR, Fénelon VS. Sequential alteration of microglia and astrocytes in the rat thalamus following spinal nerve ligation. J Neuroinflammation 2018; 15: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray ME, Kouri N, Lin WL, Jack CR, Jr, Dickson DW, Vemuri P. Clinicopathologic assessment and imaging of tauopathies in neurodegenerative dementias. Alzheimers Res Ther 2014; 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vossel KA, Xu JC, Fomenko V, Miyamoto T, Suberbielle E, Knox JA, Ho K, Kim DH, Yu GQ, Mucke L. Tau reduction prevents Abeta-induced axonal transport deficits by blocking activation of GSK3beta. J Cell Biol 2015; 209: 419–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pérez AR, Lambertucci F, González FB, Roggero EA, Bottasso OA, de Meis J, Ronco MT, Villar SR. Death of adrenocortical cells during murine acute T. cruzi infection is not associated with TNF-R1 signaling but mostly with the type II pathway of Fas-mediated apoptosis. Brain Behav Immun 2017; 65: 284–295. [DOI] [PubMed] [Google Scholar]
- 39.Al-Saeedi M, Steinebrunner N, Kudsi H, Halama N, Mogler C, Büchler MW, Krammer PH, Schemmer P, Müller M. Neutralization of CD95 ligand protects the liver against ischemia-reperfusion injury and prevents acute liver failure. Cell Death Dis 2018; 9: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva-Gómez AB, Bravo-Duran DA, Eguibar JR, Cortes C. Juvenile Taiep rats have shorter dendritic trees in the dorsal field of the hippocampus without spatial learning disabilities. Synapse 2018; 72: e22024. [DOI] [PubMed] [Google Scholar]
- 41.Chi H, Chang HY, Sang TK. Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci 2018; 19: pii: E3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beyer K, Menges P, Keßler W, Heidecke CD. Pathophysiology of peritonitis. Chirurg 2016; 87: 5–12. [DOI] [PubMed] [Google Scholar]
- 43.Papathanassoglou E, Mpouzika MD, Giannakopoulou M, Bozas E, Middleton N, Tsiaousis G, Karabinis A. Association between lymphocyte expression of the apoptotic receptor Fas and pain in critically ill patients. J Pain Res 2017; 10: 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakaue S, Sunagawa M, Tanigawa H, Saito Y, Guo SY, Okada M, Nakamura A, Arai K, Hisamitsu T. A single administration of morphine suppresses the reduction of the systemic immune activity caused by acute inflammatory pain in rats. Masui the Masui 2011; 60: 336–342. [PubMed] [Google Scholar]
- 45.Elfving B, Müller H K, Oliveras I, Østerbøg T B, Rio-Alamos C, Sanchez-Gonzalez A, Tobeña A, Fernandez-Teruel A, Aznar S. Differential expression of synaptic markers regulated during neurodevelopment in a rat model of schizophrenia-like behavior. Prog Neuropsychopharmacol Biol Psychiatry 2019; 95:109669. [DOI] [PubMed] [Google Scholar]
- 46.Hu HY, Chen ZY, Xu DM, Zhang YH, Wang YR, Wang WH, Zhang XY. Study on the mechanism of three kinds extracts of Qingxin kaiqiao recipe in improving learning and memory capabilities of AD rats. Chin J Integr Tradit West Med 2015; 35: 595–602. [PubMed] [Google Scholar]
- 47.Ghumatkar PJ, Patil SP, Peshattiwar V, Vijaykumar T, Dighe V, Vanage G, Sathaye S. The modulatory role of phloretin in Aβ25-35 induced sporadic Alzheimer’s disease in rat model. Naunyn Schmiedebergs Arch Pharmacol 2019; 392: 327–339. [DOI] [PubMed] [Google Scholar]