Abstract
Background:
Allergic rhinitis (AR) is an immunoglobulin E (IgE)-mediated immune-inflammatory response mainly affecting nasal mucosa. Apigenin, a flavonoid, has been documented to possess promising anti-allergic potential.
Aim:
To determine the potential mechanism of action of apigenin against ovalbumin (OVA)-induced AR by assessing various behavioral, biochemical, molecular, and ultrastructural modifications.
Materials and Methods:
Allergic rhinitis was induced in BALB/c mice (18-22 grams) by sensitizing it with OVA (5%, 500 μL, intraperitoneal [IP] on each consecutive day, for 13 days) followed by intranasal challenge with OVA (5%, 5 μL per nostril on day 21). Animals were treated with either vehicle (distilled water, 10 mg/kg, IP) or apigenin (5, 10, and 20 mg/kg, IP).
Results:
Intranasal challenge of OVA resulted in significant induction (P < .05) of AR reflected by an increase in nasal symptoms (sneezing, rubbing, and discharge), which were ameliorated significantly (P < .05) by apigenin (10 and 20 mg/kg) treatment. It also significantly inhibited (P < .05) OVA-induced elevated serum histamine, OVA-specific IgE, total IgE, and IgG1 and β-hexosaminidase levels. Ovalbumin-induced increased levels of interleukin (IL)-4, IL-5, IL-13, and interferon (IFN)-γ in nasal lavage fluid were significantly decreased (P < .05) by apigenin. Ovalbumin-induced alterations in splenic GATA binding protein 3 (ie, erythroid transcription factor) (GATA3), T-box protein expressed in T cells (T-bet), signal transducer and activator of transcription-6 (STAT6), suppressor of cytokine signaling 1 (SOCS1), nuclear factor-kappa B (NF-κB), and nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-alpha messenger RNA, as well as protein expressions were significantly inhibited (P < .05) by apigenin. It also significantly ameliorated (P < .05) nasal and spleen histopathologic and ultrastructure aberration induced by OVA.
Conclusion:
Apigenin regulates Th1/Th2 balance via suppression in expressions of Th2 response (IgE, histamine, ILs, GATA3, STAT6, SOCS1, and NF-κB) and activation of Th1 response (IFN-γ and T-bet) to exert its anti-allergic potential in a murine model of OVA-induced AR.
Keywords: allergic rhinitis, apigenin, GATA-3, IFN-γ, IκBα, NF-κB, ovalbumin, SOCS1, STAT-6, T-bet
Introduction
Allergic rhinitis (AR) is a chronic, immune-inflammatory disease that affects the inner lining of nasal membrane resulting in characteristic symptoms including nasal rubbing, sneezing, itching, congestion, lacrimation, and rhinorrhea. Allergic rhinitis is a global health problem affecting almost 400 to 500 million individual’s quality of life and work productivity.1 Thus, AR is associated with substantial indirect costs.2 It has been suggested that the interaction of genetic and environmental factors may be the underlying cause for the development of allergic diseases.1,3 Chronic exposure to environmental allergens activates allergen-specific type-2T helper (Th2) cells, which result in elevated production of Th2 cytokines (such as interleukin [IL]-4 and IL-5), and antigen-specific immunoglobulin E (IgE) thus, inducing hyperresponsiveness in nasal mucosa.4
Researchers have reported that sensitized formation and expression of antigen-specific IgE by IL-4 from B lymphocytes is a characteristic feature of AR, which follows biphasic inflammatory response in the initial phase response, cross-linking of IgE with its high-affinity receptor Fc∊RI (immunoglobulin Fc epsilon receptor I) resulting in mast cell degranulation.3,5 Subsequently, these mast cells release inflammatory mediators, including histamine, β-hexosaminidase, chemokines, leukotrienes, and prostaglandins via cyclooxygenase (COX)-2 and 5-lipoxygenase pathways.6 Whereas, a late-phase response is associated with the aggravated response of granulocytes (such as eosinophils and lymphocytes), cytokines (such as tumor necrosis factor [TNF]-α and ILs), and adhesion molecules.7 Among various inflammatory cells, eosinophils, which are considered as “innate effector cells,” have an important contribution in induction and maintenance of allergic responses. Studies suggested that balance between Th1 cells and Th2 cells plays a vital role in the maintenance of the immune health, and its dysregulation leads to the induction of allergic responses.8,9
Current pharmacotherapy of AR is based on the available knowledge of various mediators involved in the induction of Type I allergic reactions. It includes antihistamines, leukotriene antagonists, cAMP stimulator, Th2 cytokine inhibitor, corticosteroids, and mast cell stabilizers.10 According to global guidelines of Allergic Rhinitis and its Impact on Asthma (ARIA), topical corticosteroids have been suggested as first-line therapy against the persistent forms of AR.2 However, this treatment regimen is not only associated with an economic burden but also bring various degrees of adverse reactions including nasal dryness, throat irritation, vision blurring, headache, sedation, tachycardia, and urinary retention.10 Consequently, the development of a safe, efficacious, and novel therapeutic strategy is necessary for the management of AR. Various animal models play important roles in the development of an array of therapeutic strategies, and murine models of ovalbumin (OVA) induced AR is one of them.7 It is a simple, widely used, reproducible, and noninvasive experimental model for investigation of IgE-mediated allergic airway diseases of the upper respiratory tract. This model has close resemblance with pathophysiological events and symptoms associated with clinical immunomodulation, including sneezing, rubbing, congestion, mucosa edema, and the elevated production of IgE.11-13
According to the National Asthma Campaign survey, allergic patients (11% of adults and 6% of children) preferred herbal medicine as a choice of medication to manage their allergic response.4 In Ayurveda, an array of therapeutic plants including basil (tulsi, Ocimum sanctum Linn) and chamomile (Babuna, Matricaria recutita) have been documented to treat various allergic diseases. Research carried out over past decades indicated the presence of flavonoids as responsible for its anti-allergic potential. Apigenin, one of the main active compounds, is accountable for its efficacy.14 Apigenin (4′,5,7-trihydroxyflavone, 5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) contain a wide range of beneficial pharmacological properties including antioxidant, anti-inflammatory, apoptotic potential, and several reports have cited its anti-allergic potential as well.15-19 Reports suggested that apigenin attenuated lipopolysaccharide-induced toxicity via inactivation of nuclear factor kappa-B (NF-κB) phosphorylation, thus inhibiting the expression of pro-inflammatory cytokines, including TNF-α and ILs.15 Furthermore, apigenin as a promising dietary flavone is shown to inhibit atopic dermatitis in NC/NGa mice via modulation of IgG1, IgE, IL-4, and interferon (IFN)- γ messenger RNA (mRNA) expressions.17 A recent study conducted by Ai et al suggested that apigenin suppresses the signal transducer and activator of transcription-3 (STAT3)-NF-κB signaling pathway, which inhibited colon tumors.18 Apigenin also balances Th1 and Th2 immune response via modulation of GATA-3 (GATA binding protein 3 [ie, erythroid transcription factor]), T-box protein expressed in T cells (T-bet), INF-γ, IgE, and Th1 cytokine production in asthmatic airways, which protects against OVA-induced asthma.16,19 However, the beneficial potential of apigenin has not been yet evaluated against AR. Thus, the present investigation was undertaken with the aim to determine the potential mechanism of action of apigenin against OVA-induced AR by assessing various behavioral, biochemical, molecular, and ultrastructural modifications.
Materials and Methods
Drugs and Chemicals
Apigenin (purity ≥95%), OVA (grade V), aluminum hydroxide, and histamine dihydrochloride were purchased from Sigma-Aldrich Co, St Louis, Missouri. Montelukast was obtained from Cipla Limited, Mumbai, India. Mouse OVA-specific IgE, total IgE, total IgG1, β-hexosaminidase, IL-4, IL-5, IL-13, IL-17, IFN-γ, and Leukotriene C4 enzyme-linked immunosorbent assay (ELISA) kit were obtained from Bethyl Laboratories Inc, Montgomery, Texas. The primary antibodies of GATA3, T-bet, NF-κB, IκBα, p-STAT6, suppressor of cytokine signaling 1 (SOCS1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Abcam, Cambridge, Massachusetts. Total RNA extraction kit and real time-polymerase chain reaction (RT-PCR) kit were purchased from MP Biomedicals India Private Limited, India.
Animals
Adult male BALB/c mice (18-22 grams) were purchased from the First Hospital of Jilin University Animal Centre, Pune, and kept in quarantine for 1-week in-house at the institute’s animal house under standard laboratory conditions, that is, a temperature of 24°C ± 1°C, relative humidity of 45% to 55% and 12:12 hours light/dark cycle. The experiments were carried out between 10:00 am and 5:00 pm. Animals had free access to standard chaw pelleted food and water ad libitum. The experimental protocol (2019452) was approved by the Animal Experimental Ethics Committee of the First Hospital of Jilin University, which is in line with animal protection, animal welfare, ethical principles, and is in line with the relevant regulations of the national experimental animal experiment ethics. Animals were brought to the testing laboratory 1 hour before the experiments for adaptation purposes.
Induction of AR and Treatment Schedule
Sensitization of BALB/c mice was done on days 1, 3, 5, 7, 9, 11, and 13 by intraperitoneal (IP) injection containing 500 μL of sensitization solution (50 mg of OVA and 1000 mg of aluminum hydroxide dissolved in 500 mL of saline).7 On day 14, mice were randomly divided into 6 treatment groups (n = 18 per group) and treated for the next 7 days (day 14 to day 21) as follows:
Group I Normal: Nonsensitized and received a suspension of aluminum hydroxide in saline followed by distilled water (10 mg/kg, IP)
Group II AR control: OVA-sensitized and received distilled water (10 mg/kg, IP)
Group III Montelukast (10): [MLT (10)]: OVA-sensitized and received standard drug treatment, that is, montelukast (10 mg/kg, orally)
Group IV Apigenin (5): [AP (5)]: OVA-sensitized and received apigenin (5 mg/kg, IP)
Group V Apigenin (10): [AP (10)]: OVA-sensitized and received apigenin (10 mg/kg, IP)
Group VI Apigenin (20): [AP (20)]: OVA-sensitized and received apigenin (20 mg/kg, IP)
Group VII Per se: Nonsensitized and received apigenin (20 mg/kg, IP)
The 3 different doses of apigenin (5, 10, and 20 mg/kg) were selected based on a previous study.16 Whereas dose of montelukast (10 mg/kg, orally) was based on the previously described report.4 The solutions of apigenin were freshly prepared daily and administered IP from day 14 to day 21 for biological evaluations. On day 21, 1 hour after the last dose of treatment, mice were challenged with intranasal administration of OVA (5%, 5 μL per nostril), and observations were recorded.
Nasal Symptoms in OVA-Induced AR Mice
On day 21, nasal symptoms were evaluated within a 10-minute period after the OVA challenge.7 The number of sneezes and nasal itching motions (nasal rubbing) were recorded. The nasal discharge was scored as 0 = no discharge, 1 = the discharge reaches the anterior nasal aperture, 2 = the discharge overshoots the anterior nasal aperture, and 3 = the discharge flows out.
Nasal Symptoms During Histamine-Induced Hypersensitivity in OVA-Induced AR Mice
To evaluate effects on histamine-induced hypersensitivity after interruption of the drugs, mice were challenged with histamine dihydrochloride (10 μL per nostril of a solution of 1 μmol/mL in physiological saline) on day 24 of study, and the number of instances of nasal rubbing and sneezing was counted during a 10-minute period after the challenge.7
Blood Sample Collection From OVA-Induced AR Mice
On day 21, 2 hours after OVA challenge, blood specimens were collected from the retro-orbital plexus, and serum was obtained by centrifugation at 8350 ×g for 10 minutes at 4°C. Samples were stored at −20°C until biochemical and hematological measurements.
Collection of Nasal Lavage Fluid
Nasal lavage fluid (NLF) collection was performed according to a previously described method.4 Mice underwent partial tracheotomy under deep anesthesia by IP injection of 1% sodium pentobarbital (50 mg/kg). A 22-gauge catheter was inserted into the posterior naris from the opening of the trachea and along the direction of the nostrils. Sterile saline solution (3 mL) was perfused gently into the nasal cavities, lavage fluid was collected from the anterior naris, centrifuged at 220 ×g and 4°C for 10 minutes, and the supernatant was stored at −20°C.
Biochemical Measurement in the Serum of OVA-Induced AR Mice
Ovalbumin-specific IgE, total IgE, total IgG1, and β-hexosaminidase in serum, while IL-4, IL-5, IL-13, IL-17, IFN-γ, and LTC-4 (Leukotriene C4) in NLF were evaluated using respective mouse ELISA quantitation kit (Bethyl Laboratories Inc) as per the manufacturer’s instructions. Results were evaluated by the positive/negative ratios value. The test was done in duplicate to avoid false-negative and false-positive results, and the average value was taken for the final calculation.
Determination of Histamine Level in Serum of OVA-Induced AR Mice
Histamine content of serum was measured by the o-phthalaldehyde spectrofluorometric procedure. The fluorescent intensity was measured at 460 nm (excitation at 355 nm) using a spectrofluorometer, and histamine contents were calculated.4
Real-Time PCR
The mRNA levels of GATA3, T-bet, signal transducer and activator of transcription-6 (STAT6), and SOCS1 in spleen were analyzed in spleen tissue (n = 4) using RT-PCR approach as per the manufacturer’s instructions. The intensity of mRNAs was standardized against that of the β-actin mRNA from each sample, and the results were expressed as the PCR-product/β-actin mRNA ratio.
Western Blot Assay
Spleen tissue was dissected and sonicated in tissue protein extraction reagent (Thermo Fisher Scientific, Inc., Bengaluru, Karnataka, India). The lysates were centrifuged at 10 000 × g for 10 minutes at 4°C. Protein concentration was determined using a Bicinchoninic acid assay kit (Beyotime Shanghai, China) on ice for 30 minutes. Equal amounts of extracted protein samples (50 μg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat dry milk at 37°C for 1 hour and incubated overnight at 4°C with the primary antibodies that recognized GATA3, T-bet, NF-κB, IκBα, p-STAT6, SOCS1, and GAPDH. Anti-rabbit horseradish-linked IgG was used as the secondary antibody, which was incubated at 37°C for 2 hours. Protein bands were visualized using the Chemiluminescent kit (Bio-Rad Laboratories, Inc., Bengaluru, Karnataka, India), and GAPDH served as the loading control.
Histological Examination
On day 21, after blood withdrawal, 3 mice from each group were sacrificed. The nasal mucosa and spleen tissues were dissected and stored for 24 hours in 10% formalin for histological examination. The specimens were dehydrated and placed in xylene for 1 hour (3 times) and later in ethyl alcohol (70%, 90%, and 100%) for 2 hours, respectively. Infiltration and impregnation were carried out by treating with paraffin wax twice, each time for 1 hour. For tissue slide preparation, specimens were cut into sections of 3 to 5 µm thickness and stained with hematoxylin and eosin. The specimens were mounted on slides by the use of Distrene phthalate xylene. Sections were examined under the light microscope (Olympus DP71, DP-BSW Version 03.03; Olympus Medical Systems India Private Limited, Bengaluru, Karnataka, India) to obtain a general impression of the histopathology features of specimen and infiltration of cells in epithelium and subepithelium. The intensity of histological aberrations in the nasal and spleen tissue was graded as grade 0 (not present or very slight); grade 1 (mild); grade 2 (moderate); and grade 3 (severe) as described in the literature study.
Electron Microscopic Analysis
For ultrastructural studies, nasal tissue samples were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, for 18 hours. The tissue samples were dissected into small pieces and postfixed for 1.5 hours in 1% osmium tetroxide dissolved in 0.1 M phosphate buffer (pH 7.4), then dehydrated through a series of graded ethanol solutions and embedded in Araldite (epoxy resin). Ultrathin sections were cut, stained with uranyl acetate and lead nitrate, mounted on copper grids, and examined under a transmission electron microscope (H-7000 Hitachi, Hitachi High-Technologies India Private Limited, Gurgaon, Haryana, India).
Statistical Analysis
Data were expressed as means ± standard error of means, and analyses were performed using GraphPad Prism 5.0 software (GraphPad, San Diego, California). Statistical comparisons were made between drug-treated groups and AR control animals. Data of biochemical parameters were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple range test for post hoc analysis. Scores of nasal rubbing, sneezing, and discharge were analyzed by nonparametric Kruskal-Wallis ANOVA followed by Mann-Whitney’s multiple comparison tests. A value of P <.05 was considered to be statistically significant.
Results
Effect of Apigenin on Body Weight and Relative Spleen Weight in OVA-Induced AR Mice
There was a significant increase (P < .05) in body weight and a significant decrease (P < .05) in relative spleen weight in the AR control group as compared to the normal group. Administration of montelukast (10 mg/kg) significantly attenuated (P < .05) OVA-induced alterations in body weight and relative spleen weight as compared to the AR control group. Mice treated with apigenin (10 and 20 mg/kg) also significantly decreased (P < .05) body weight and significantly increased (P < .05) relative spleen weight when compared with the AR control group. However, treatment with montelukast (10 mg/kg) showed more significant attenuation (P < .05) in OVA-induced alterations in body weight and relative spleen weight as compared to apigenin treatment (Table 1).
Table 1.
Effect of Apigenin Treatment on OVA-Induced Alterations in Body Weight, Relative Spleen Weight, OVA Challenge-Induced Nasal Rubbing, Sneezing, and Nasal Discharge as Well as Histamine Challenge-Induced Nasal Rubbing and Sneezing in AR Mice.a
| Parameters | Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Normal | AR Control | MLT (10) | AP (5) | AP (10) | AP (20) | Per se | |
| Body weight (g) on day 21 | 31.17 ± 1.25 | 23.50 ± 1.06b | 29.83 ± 1.54c,d | 26.50 ± 1.82 | 28.00 ± 1.21c,d | 29.67 ± 0.71c,d | 30.33 ± 1.31 |
| Spleen wt/body wt (mg/g) (×10−3) on day 21 | 3.37 ± 0.13 | 6.68 ± 0.35b | 3.92 ± 0.23c,d | 5.96 ± 0.35 | 4.80 ± 0.34c,d | 3.73 ± 0.09c,d | 3.34 ± 0.29 |
| OVA challenge on day 21 | |||||||
| Rubbing (number) | 15.17 ± 2.3 | 71.33 ± 2.16b | 23.67 ± 1.33c,d | 66.17 ± 2.04 | 48.83 ± 1.28c,d | 27.00 ± 1.63c,d | 15.83 ± 1.38 |
| Sneezing (number) | 10.83 ± 1.08 | 41.33 ± 0.76b | 13.17 ± 0.95c,d | 35.17 ± 1.42 | 28.67 ± 0.80c,d | 18.50 ± 0.99c,d | 12.50 ± 0.99 |
| Discharge (score) | 0.33 ± 0.21 | 2.50 ± 0.22b | 0.50 ± 0.22c,d | 2.33 ± 0.21 | 1.33 ± 0.21c,d | 0.50 ± 0.22c,d | 0.33 ± 0.21 |
| Histamine challenge on day 24 | |||||||
| Rubbing (number) | 17.83 ± 3.33 | 71.5 ± 2.59b | 24.00 ± 2.53c,d | 66.83 ± 2.68 | 44.33 ± 1.65c,d | 32.33 ± 3.65c,d | 21.00 ± 3.16 |
| Sneezing (number) | 8.67 ± 2.08 | 52.33 ± 2.45b | 16.00 ± 1.55c,d | 50.33 ± 1.43 | 31.00 ± 3.13c,d | 21.33 ± 2.53c,d | 10.67 ± 1.12 |
Abbreviations: ANOVA, analysis of variance; AP (5), apigenin (5 mg/kg) treated; AP (10), apigenin (10 mg/kg) treated; AP (20), apigenin (20 mg/kg) treated; AR, allergic rhinitis; MLT (10), montelukast (10 mg/kg) treated; OVA, ovalbumin; SEM, standard error of the mean.
a Data are represented as mean ± SEM (n = 4-6). Data for body weight and relative spleen weight were analyzed by one-way ANOVA followed by Tukey multiple range test, whereas data of OVA and histamine challenge number and score were analyzed by nonparametric Kruskal-Wallis test ANOVA followed by Mann-Whitney’s multiple comparison tests. Figures in parentheses indicate a dose in mg/kg.
b P < .05 as compared with normal group.
c P < .05 as compared with AR control group.
d P < .05 as compared montelukast with apigenin.
Effect of Apigenin on Nasal Symptoms in OVA-Induced AR Mice
When compared with the normal group, the intranasal OVA challenge resulted in a significant increase (P < .05) in nasal rubbing, sneezing, and nasal discharge in the AR control group. However, montelukast (10 mg/kg) treatment significantly decreased (P < .05) OVA-induced nasal rubbing, sneezing, and nasal discharge as compared to the AR control group. When compared with the AR control group, treatment with apigenin (10 and 20 mg/kg) also significantly inhibited (P < .05) nasal symptoms induced by OVA. However, OVA challenge induced nasal rubbing and sneezing was more significantly decreased (P < .05) by treatment with montelukast (10 mg/kg) as compared to apigenin treatment. Per se treated mice did not show any induction of nasal rubbing, sneezing, and nasal discharge after intranasal OVA challenge (Table 1).
Effect of Apigenin on Histamine-Induced Nasal Hypersensitivity in OVA-Induced AR Mice
Intranasal challenge with histamine resulted in a significant increase (P < .05) in nasal hypersensitivity reflected by increased nasal rubbing and sneezing in the AR control group as compared to the normal group. Treatment with montelukast (10 mg/kg) significantly attenuated (P < .05) histamine-induced nasal rubbing and sneezing as compared to the AR control group. Apigenin (10 and 20 mg/kg) administration also significantly decreased (P < .05) nasal rubbing and sneezing induced after histamine challenge as compared to the AR control group. However, attenuation of histamine-induced hypersensitivity was more significant (P < .05) in montelukast (10 mg/kg) treatment as compared to apigenin treatment (Table 1).
Effect of Apigenin on Serum Histamine, IgE, IgG1, and β-Hexosaminidase Levels in Serum of OVA-Induced AR Mice
The levels of serum histamine, IgE, IgG1, and β-hexosaminidase increased significantly (P < .05) in the AR control group as compared to the normal group. However, administration of montelukast (10 mg/kg) and apigenin (10 and 20 mg/kg) significantly attenuated (P < .05) elevated levels of serum histamine, IgE, IgG1, and β-hexosaminidase as compared to AR control group. Montelukast (10 mg/kg) treatment showed more significant attenuation (P < .05) of serum histamine, IgE, IgG1, and β-hexosaminidase as compared to apigenin treatment. However, serum histamine, IgE, IgG1, and β-hexosaminidase did not alter in per se treated mice (Table 2).
Table 2.
Effect of Apigenin Treatment on OVA-Induced Alterations in Serum Histamine, OVA-Specific IgE, Total IgE and IgG1, and β-Hexosaminidase Levels in AR Mice.a
| Parameters | Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Normal | AR Control | MLT (10) | AP (5) | AP (10) | AP (20) | Per se | |
| Histamine (µg/mL) | 70.94 ± 8.47 | 368.40 ± 5.27b | 100.20 ± 10.02c,d | 340.80 ± 11.10 | 248.60 ± 7.56c,d | 136.80 ± 8.63c,d | 82.41 ± 10.23 |
| OVA-specific IgE (ng/mL) | 12.69 ± 2.16 | 61.75 ± 1.62b | 21.54 ± 1.03c,d | 59.35 ± 2.38 | 43.99 ± 2.14c,d | 28.18 ± 2.22c,d | 14.75 ± 1.72 |
| Total IgE (ng/mL) | 92.78 ± 12.82 | 442.50 ± 13.13b | 148.60 ± 16.45c,d | 401.00 ± 10.24 | 324.70 ± 11.77c,d | 185.80 ± 13.92c,d | 121.70 ± 13.77 |
| Total IgG1 level (ng/mL) | 0.26 ± 0.06 | 0.72 ± 0.05b | 0.41 ± 0.05c,d | 0.68 ± 0.05 | 0.65 ± 0.03 | 0.49 ± 0.06c,d | 0.39 ± 0.05 |
| β-hexosaminidase (ng/mL) | 13.28 ± 1.60 | 44.43 ± 1.75b | 18.88 ± 1.71c,d | 42.32 ± 1.41 | 33.10 ± 1.48c,d | 26.21 ± 1.69c,d | 19.08 ± 1.58 |
Abbreviations: ANOVA, analysis of variance; AP (5), apigenin (5 mg/kg) treated; AP (10), apigenin (10 mg/kg) treated; AP (20), apigenin (20 mg/kg) treated; AR, allergic rhinitis; Ig, immunoglobulin; MLT (10), montelukast (10 mg/kg) treated; OVA, ovalbumin; SEM, standard error of the mean.
a Data are represented as mean ± SEM (n = 4-6) and analyzed by one-way ANOVA followed by Tukey multiple range test. Figures in parentheses indicate dose in mg/kg.
b P < .05 as compared with normal group.
c P < .05 as compared with AR control group.
d P < .05 as compared montelukast with apigenin.
Effect of Apigenin on NLFIL-4, IL-5, IL-13, IL-17, IFN-γ, and LTC-4 Levels of OVA-Induced AR Mice
There was a significant increase (P < .05) in levels of IL-4, IL-5, IL-13, IL-17, and LTC-4, whereas a significant decrease (P < 0.05) in the level of IFN-γ in NLF of AR control group as compared to the normal group. Administration of montelukast (10 mg/kg) significantly attenuated (P < .05) alterations in levels of IL-4, IL-5, IL-13, IL-17, IFN-γ, and LTC-4 as compared to AR control group. Treatment with apigenin (10 and 20 mg/kg) also significantly inhibited (P < .05) OVA-induced alterations in levels of IL-4, IL-5, IL-13, and IFN-γ in NLF when compared with AR control group. However, apigenin treatment failed to produce any significant reduction in NLF LTC-4 levels as compared to the AR control group (Table 3).
Table 3.
Effect of Apigenin Treatment on OVA-Induced Alterations in IL-4, IL-5, IL-13, IL-17, IFN-γ, and LTC-4 Levels in NLF in AR Mice.a
| Parameters | Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Normal | AR Control | MLT (10) | AP (5) | AP (10) | AP (20) | Per se | |
| IL-4 (pg/mL) | 58.09 ± 5.73 | 150.60 ± 6.51 b | 75.66 ± 5.28c,d | 136.90 ± 6.67 | 105.40 ± 4.71c,d | 86.57 ± 6.12 c, d | 62.60 ± 6.22 |
| IL-5 (pg/mL) | 45.81 ± 4.22 | 101.00 ± 4.49 b | 52.29 ± 2.98 c,d | 94.59 ± 3.81 | 78.05 ± 3.43 c,d | 54.17 ± 3.90 c,d | 36.84 ± 2.80 |
| IL-13 (pg/mL) | 77.49 ± 15.82 | 196.80 ± 13.85b | 103.90 ± 11.50c,d | 187.30 ± 13.26 | 156.40 ± 10.72c,d | 124.20 ± 13.32c,d | 87.02 ± 7.66 |
| IL-17 (pg/mL) | 2.79 ± 1.49 | 38.03 ± 2.51b | 7.54 ± 0.66 c, d | 33.23 ± 2.43 | 32.36 ± 2.33 | 32.10 ± 2.16 | 7.16 ± 0.69 |
| IFN-γ (pg/mL) | 71.28 ± 1.29 | 40.73 ± 1.88b | 65.09 ± 1.34 c,d | 44.40 ± 1.76 | 54.00 ± 2.64 c,d | 62.86 ± 3.52 c,d | 64.08 ± 1.30 |
| IL-4/IFN-γ ratio | 0.82 ± 0.10 | 3.75 ± 0.29 b | 1.16 ± 0.07 c, d | 3.10 ± 0.18 c | 2.00 ± 0.19 c,d | 1.38 ± 0.04 c,d | 0.98 ± 0.11 |
| LTC-4 | 18.38 ± 3.75 | 95.11 ± 3.53 b | 41.09 ± 4.37 c,d | 93.41 ± 1.61 | 86.48 ± 2.83 | 87.93 ± 3.08 | 22.35 ± 1.00 |
Abbreviations: ANOVA, analysis of variance; AR, allergic rhinitis; AP (5), apigenin (5 mg/kg) treated; AP (10), apigenin (10 mg/kg) treated; AP (20), apigenin (20 mg/kg) treated; IFN-γ, interferon gamma; IL, interleukin; LTC-4, Leukotriene C4; MLT (10), montelukast (10 mg/kg) treated; NLF, nasal lavage fluid; OVA, ovalbumin; SEM, standard error of the mean.
a Data are represented as Mean ± SEM (n = 4-6) and analyzed by one-way ANOVA followed by Tukey multiple range test. Figures in parentheses indicate dose in mg/kg.
b P < .05 as compared with normal group.
c P < .05 as compared with AR control group.
d P < .05 as compared montelukast with apigenin.
Effect of Apigenin on Splenic GATA3, T-bet, STAT6, and SOCS1 mRNA Expressions of OVA-Induced AR Mice
The splenic mRNA expressions of GATA3, STAT6, and SOCS1 were significantly upregulated (P < .05), whereas T-bet mRNA expression was significantly downregulated (P < .05) in AR control group as compared to the normal group. Montelukast (10 mg/kg) significantly upregulated (P < .05) T-bet mRNA expression and significantly downregulated (P < .05) GATA3, STAT6, and SOCS1 mRNA expressions in spleen as compared to AR control group. Administration of apigenin (10 and 20 mg/kg) also significantly attenuated (P < .05) OVA-induced alterations in splenic GATA3, T-bet, STAT6, and SOCS1 mRNA expressions as compared to AR control group. However, when compared with montelukast (10 mg/kg) treatment, apigenin (20 mg/kg) treatment more significantly downregulated (P < .05) splenic GATA3 mRNA expression (Table 4).
Table 4.
Effect of Apigenin Treatment on OVA-Induced Alterations in Spleen GATA3, T-bet, STAT6, and SOCS1 mRNA Expression in AR Mice.a
| Parameters | Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Normal | AR Control | MLT (10) | AP (5) | AP (10) | AP (20) | Per se | |
| GATA3/β-actin ratio | 1.09 ± 0.08 | 2.07 ± 0.19b | 1.50 ± 0.07c,d | 2.03 ± 0.07 | 1.70 ± 0.13c,d | 1.48 ± 0.11c,d | 1.09 ± 0.12 |
| T-bet/β-actin ratio | 0.89 ± 0.10 | 0.43 ± 0.12b | 0.86 ± 0.06c,d | 0.43 ± 0.09 | 0.71 ± 0.13c,d | 0.78 ± 0.09c,d | 0.90 ± 0.12 |
| p-STAT6/β-actin Ratio | 1.05 ± 0.14 | 2.21 ± 0.09b | 1.32 ± 0.09c,d | 2.07 ± 0.13 | 1.54 ± 0.10c,d | 1.36 ± 0.15c,d | 1.37 ± 0.08 |
| SOCS1/β-actin ratio | 0.44 ± 0.12 | 1.28 ± 0.09b | 0.57 ± 0.09c,d | 1.17 ± 0.10 | 0.88 ± 0.10c,d | 0.64 ± 0.11c,d | 0.51 ± 0.11 |
Abbreviations: ANOVA, analysis of variance; AP (5), apigenin (5 mg/kg) treated; AP (10), apigenin (10 mg/kg) treated; AP (20), apigenin (20 mg/kg) treated; AR, allergic rhinitis; GATA-3, GATA binding protein 3; OVA, ovalbumin; MLT (10), montelukast (10 mg/kg) treated; mRNA, messenger RNA; SEM, standard error of the mean; STAT6, signal transducer and activator of transcription 6; SOCS1, silencing of the suppressor of the cytokine signaling-1; T-bet, T-box protein expressed in T cells. Figures in parentheses indicate a dose in mg/kg.
a Data are represented as mean ± SEM (n = 4-6) and analyzed by one-way ANOVA followed by Tukey multiple range test.
b P < .05 as compared with normal group.
c P < .05 as compared with AR control group.
d P < 0.05 as compared montelukast with apigenin.
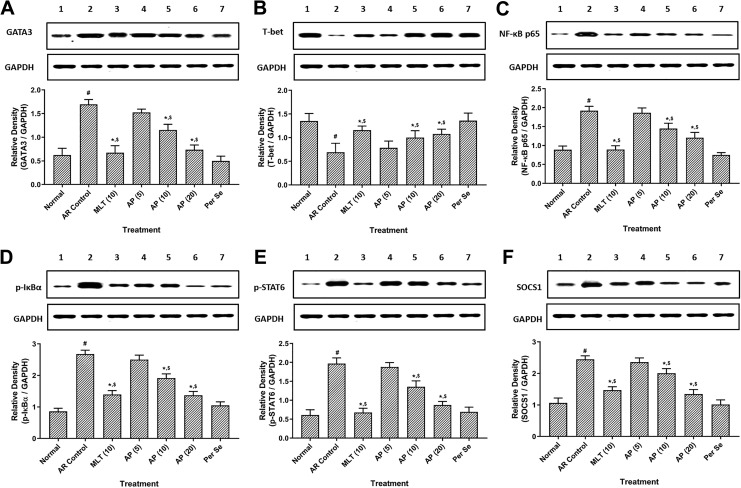
Effect of Apigenin on Splenic GATA3, T-bet, NF-κB, IκBα, p-STAT6, and SOCS1 Protein Expressions of OVA-Induced AR Mice
There was significant upregulation (P < .05) in splenic protein expressions of GATA3, NF-κB, IκBα, p-STAT6, and SOCS1, whereas significant downregulation (P < .05) in splenic T-bet protein expression in AR control group as compared to the normal group. Treatment with montelukast (10 mg/kg) and apigenin (10 and 20 mg/kg) significantly attenuated (P < .05) OVA-induced alterations in splenic GATA3, T-bet, NF-κB, IκBα, p-STAT6, and SOCS1 protein expressions as compared to AR control group. However, OVA-induced upregulation in splenic GATA3, NF-κB, IκBα, and p-STAT6 protein expressions were more significant (P < .05) in montelukast (10 mg/kg) treatment as compared to apigenin treatment (Figure 1).
Figure 1.
Effect of apigenin treatment on OVA-induced alterations in spleen GATA3 (A), T-bet (B), NF-κB (C), IκBα (D), p-STAT6 (E), and SOCS1 (F) protein expression in AR mice. Data were represented as mean ± SEM (n = 4) and analyzed by one-way ANOVA followed by Tukey multiple range test. # P < .05 as compared with normal group, *P < .05 as compared with AR control group, and $ P < .05 as compared montelukast with apigenin. Figures in parentheses indicate a dose in mg/kg. Lane 1: normal, Lane 2: AR control, Lane 3: montelukast (10 mg/kg) treated, Lane 4: apigenin (5 mg/kg) treated, Lane 5: apigenin (10 mg/kg) treated, Lane 6: apigenin (20 mg/kg) treated, and Lane 7: Per se. AP (5) indicates apigenin (5 mg/kg) treated; AP (10), apigenin (10 mg/kg) treated; AP (20), apigenin (20 mg/kg) treated; ANOVA, analysis of variance; AR, allergic rhinitis; GATA-3, GATA binding protein 3; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-alpha; MLT (10), montelukast (10 mg/kg) treated; NF-κB, nuclear factor-kappa B; OVA, ovalbumin; SEM, standard error of the mean; STAT6, signal transducer and activator of transcription 6; SOCS1, silencing of the suppressor of the cytokine signaling-1; T-bet, T-box protein expressed in T cells.
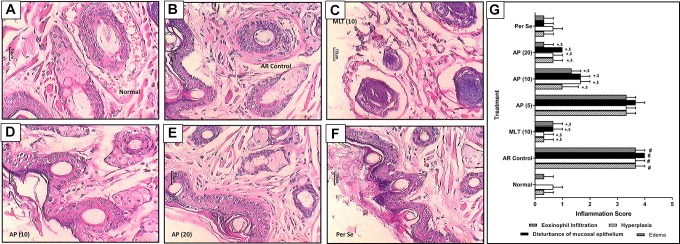
Effect of Apigenin on Histopathology of the Nasal Mucosa of OVA-Induced AR Mice
Intranasal OVA challenge resulted in a significant increase (P < .05) of inflammatory infiltration in the nasal mucosa of AR control mice (Figure 2B). Eosinophil count increased significantly (P < .05) in the nasal mucosa of AR control mice as compared to normal mice. Histological analysis of nasal mucosa of normal mice showed normal architecture devoid of any disturbance in the nasal epithelium; however, it showed mild inflammatory infiltration and edema (Figure 2A). Whereas, treatment with montelukast (10 mg/kg) significantly attenuated (P < .05) OVA-induced histopathology alterations in the nasal mucosa of mice reflected by decreased inflammatory infiltration, hyperplasia of nasal epithelial cells, and edema (Figure 2C) as compared to AR control group. Administration of apigenin (10 and 20 mg/kg) also significantly decreased (P < .05) inflammatory infiltration, disturbances in nasal mucosal epithelial, and edema (Figure 2D and E) when compared with AR control group. Furthermore, histological analysis of nasal mucosa from per se treated mice did not show any significant histological aberrations (Figure 2F) when compared with nasal mucosa from normal mice (Figure 2G).
Figure 2.
Effect of apigenin treatment on OVA-induced alteration in nasal histopathology in AR mice. Photomicrograph of sections of nasal tissue from normal (A), AR control (B), montelukast (10 mg/kg) treated (C), apigenin (5 mg/kg) treated (D), apigenin (10 mg/kg) treated (E), and apigenin (20 mg/kg) treated (F) mice (H&E stain). The quantitative representation of histological score (G). Data were expressed as mean ± SEM (n = 3), and one-way ANOVA followed by the Kruskal-Wallis test was applied for post hoc analysis. # P < .05 as compared with normal group, *P < .05 as compared with AR control group, and $ P < .05 as compared montelukast with apigenin. AP (5) indicates apigenin (5 mg/kg) treated; AP (10), apigenin (10 mg/kg) treated; AP (20), apigenin (20 mg/kg) treated mice; ANOVA, analysis of variance; AR, allergic rhinitis; H&E, hematoxylin and eosin; MLT (10), montelukast (10 mg/kg) treated; OVA, ovalbumin; SEM, standard error of the mean.
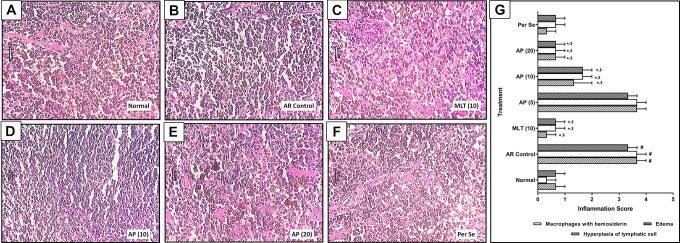
Effect of Apigenin on Histopathology of Spleen of OVA-Induced AR Mice
There was a significant increase (P < .05) in the hyperplasia of lymphatic cells, macrophages with hemosiderin, and edema in the spleen tissue of AR control mice (Figure 3B) as compared to normal mice (Figure 3A). However, administration of montelukast (10 mg/kg) significantly attenuated (P < .05) OVA-induced elevated hyperplasia of lymphatic cells, macrophages with hemosiderin, and edema (Figure 3C) as compared to AR control group. Spleen tissue from apigenin (10 and 20 mg/kg) treated mice also significantly decreased (P < .05) histological aberration such as hyperplasia of lymphatic cells, macrophages with hemosiderin, and edema (Figure 3D and E) when compared with AR control group. Spleen tissue from per se treated mice showed the presence of mild hyperplasia of lymphatic cells, macrophages with hemosiderin, and edema (Figure 3F and G).
Figure 3.
Effect of apigenin treatment on OVA-induced alteration in spleen histopathology in AR mice. Photomicrograph of sections of spleen tissue from normal (A), AR control (B), montelukast (10 mg/kg) treated (C), apigenin (5 mg/kg) treated (D), apigenin (10 mg/kg) treated (E), and apigenin (20 mg/kg) treated (F) mice: spleen H&E stain. The quantitative representation of histological score (G). Data were expressed as mean ± SEM (n = 3), and one-way ANOVA followed by the Kruskal-Wallis test was applied for post hoc analysis. # P < .05 as compared with normal group, *P < .05 as compared with AR control group, and $ P < .05 as compared montelukast with apigenin. AP (10) indicates apigenin (10 mg/kg) treated; AP (20), apigenin (20 mg/kg) treated mice; ANOVA, analysis of variance; AR, allergic rhinitis; MLT (10), montelukast (10 mg/kg) treated; AP (5), apigenin (5 mg/kg) treated; OVA, ovalbumin; SEM, standard error of the mean.
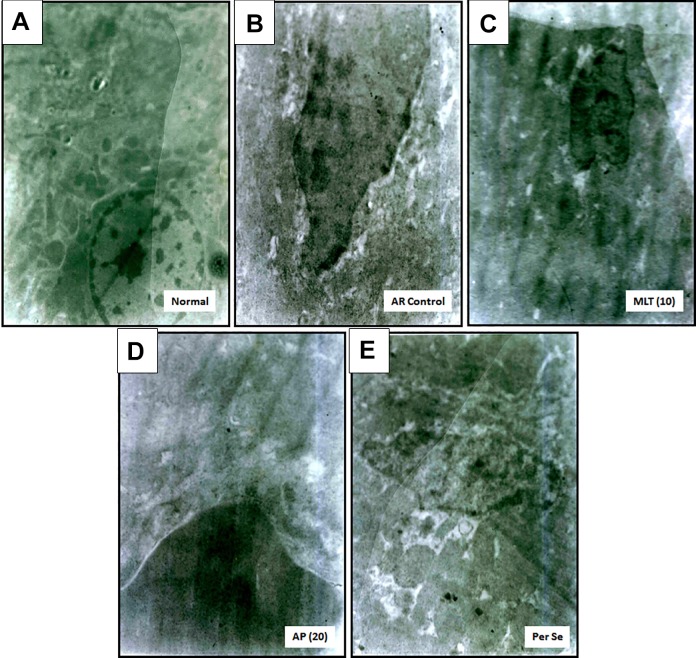
Effect of Apigenin on Hematological Parameters of OVA-Induced AR Mice
Figure 4A and E depicts the normal architecture of nasal mucosa from normal mice and per se treated mice, respectively. Nasal mucosa showed well-preserved epithelium with nuclei and cytoplasm along with the presence of normal structure of rough endoplasmic reticulum devoid of any edema. Intranasal OVA challenge caused nasal mucosal aberrations evident from the presence of swollen mitochondria, electron-dense mitochondria, tissue edema, inconsistent density of granules, eminent cytoplasmic vacuolization, and damaged endoplasmic reticulum (Figure 4B). However, administration of montelukast (10 mg/kg) and apigenin (20 mg/kg) attenuated OVA-induced ultrastructural damage induced in nasal mucosa (Figure 4C and D).
Figure 4.
Effect of apigenin treatment on OVA-induced alteration in nasal mucosal ultrastructure in AR mice (n = 2). Photomicrographs of sections of nasal mucosa from normal (7160 X) (A), AR control (14320 X) (B), montelukast (10 mg/kg) treated (12530 X) (C), apigenin (20 mg/kg) treated (14320 X) (D), and per se treated (14320 X) (E) mice. AR indicates allergic rhinitis; OVA, ovalbumin.
Discussion
Allergic rhinitis is a common chronic immune-inflammatory disorder mediated by IgE-associated processes, mainly affecting nasal mucosa. During the biphasic allergic reaction, in early stage, the elevated IgE levels by activated mast cells release inflammatory mediators, including histamine, leukotrienes, prostaglandins, and cytokines that induce nasal sneezing, itching, rubbing, and discharge.3 In the late phase, the accumulated eosinophils, mast cells, and basophils in lamina propria of nasal epithelial further release pro-inflammatory cytokines (TNF-α and ILs), chemokines, LTC-4, and COX-2 that sustain the allergic response.3 In the present investigation, sensitization of mice via IP administration of OVA followed by its intranasal challenge induced IgE-mediated allergic immune response, which was attenuated by administration of apigenin. The findings of the present investigation demonstrated that apigenin exerts its anti-allergic potential via balancing the Th1/Th2 response by modulating the expression of IgE, histamine, ILs, IFN-γ, GATA3, T-bet, STAT6, SOCS1, and NF-κB in a murine model of AR.
Researchers have documented that dendritic cells are the most affected antigen-presenting cells during the induction and regulation of primary immune response by various allergens.3 These dendritic cells are abundantly present over nasal mucosa surrounding the basal epithelial cells and have a vital role in the uptake of an antigen by 3 different mechanisms.20 Primarily, it acquires antigen through receptor-mediated endocytosis where these immature dendritic cells are released as a consequence of chemical mediators (such as histamine and leukotrienes) via specialized cell receptors such as C-type lectin carbohydrate receptors. Secondly, antigen uptake can occur via macropinocytosis, where the ruffling membrane of dendritic cells engulfs fluid and solutes in large amounts. In the third mechanism, phagocytosis occurs in which antigen materials such as latex beads, whole bacteria, and apoptotic cells can be taken up by dendritic cells.3 Activation of mast cells by IgE results in infiltration of dendritic cells, which lead to allergic response reflected by elevated clinical symptoms including sneezing, rubbing, and discharge.3 In the present investigation, OVA-challenged mice also exhibited similar nasal symptoms, which were significantly attenuated by the administration of apigenin. This inhibitory potential of apigenin may be due to the inhibition of the release of histamine and β-hexosaminidase from immature dendritic cells. Previous researchers have also documented the structure-activity relationship between flavonoids and its anti-allergic potential via inhibition of release of β-hexosaminidase from activated mast cells.21
Overexpression of IgE to the exposed allergens is one of the characteristic features of allergic diseases. It has been reported that mast cells, basophils, and Th2 cells have the ability to induce signal transaction in B cells via interaction of CD40 with its ligand as well as through activation of IL-4 or IL-13 response.8,9 This induction of signal transaction results in the formation of IgE producing cells from B cells.11,22 It is also responsible for the infiltration of eosinophil in the nasal mucosa.13 Clinically, it has been demonstrated that patients with AR exhibited elevated levels of eosinophils, basophils, and mast cells.23 Thus, the production of IgE is considered as an important step during the induction of allergic response. Hence, cross-linking of IgE to the cell surface of mast cell and basophils through its high-affinity IgE receptor immunoglobulin Fc epsilon receptor I (Fc∊RI) leads to degranulation of these cells and subsequent release of inflammatory cytokines including ILs.6 These inflammatory cytokines play a central role in the induction of innate immunity. Furthermore, allergen-specific IgG has been suggested as another important contributor in the Th2-mediated allergic response. Activation of Fc-gamma receptor (FcγR) signaling present on antigen-presenting cells by IgG results in the induction of secondary Th2 responses.6 In the present study, intranasal OVA challenge resulted in elevated IgE and IgG1 levels, which were attenuated by the administration of apigenin. The ameliorative effect of apigenin against OVA-induced allergic reaction may be ascribed to its inhibitory potential against IgE and IgG1, which corroborates with the findings of previous researchers.17
Studies documented that ILs are associated with transcription of various signaling pathways in allergic airway disease.9,13 During the process of class-switching of IgE-producing B cells from IgM-producing B cells, IL-4 and IL-13 play a vital role.5,19 Interleukin-4 serves as a mast cell growth factor along with its chemoattractant.24 Interleukin-13 also plays a vital role in the regulation of allergic illness pathogenesis via amplification of immediate hypersensitivity.8,11 Interleukin-5 is responsible for infiltration of eosinophilia in the airway, which leads to late-phase allergic response.22,25 Furthermore, clinically, it has been documented that patients with AR are associated with elevated levels of IL-17 in nasal mucosa.26 Additionally, researcher suggested that mice with AR deficient in IL-17 showed a significant reduction in nasal symptoms as well as levels of serum IgE and eosinophilis as compared to mice with AR of wild-type.27 Thus, elevated expression of IL-4, IL-5, IL-13, and IL-17 suggests the development of allergic reactions in the murine models. On the other hand, Th1 cytokine such as IFN-γ plays an important role in the suppression of Th2 immune response in nasal mucosa via inhibition of mast cell-mediated IgE production.8,11 Thus, elevation in the ratio of IL-4 to IFN-γ determines the production of IgE after allergen exposure. In the present investigation, IgE-mediated allergic reactions were evaluated by the determination of alterations in these ILs expressions in NLF. Results demonstrate that apigenin induces Th1 type response via activation of IFN-γ and suppress Th2 type response via inhibition of IL-4, IL-5, IL-13, and IL-17. These findings indicated that apigenin inhibited mast cell-mediated IgE production via regulation of IL-4/IFN-γ ratio in allergic responses depicting its anti-allergic potential. A previous study has described that flavonoids such as apigenin significantly suppressed the expression of both IL-4 and IL-13, thus considered as potential natural IgE inhibitors21 and results of the present investigation are in line with findings of the previous investigator.21
Numerous evidence suggest that T lymphocytes, especially Th1 and Th2, are the key immune cells, playing a crucial role in inflammatory infiltration and its release during an allergic reaction.3 T-bet is a T cell-associated transcription factor expressed during the development of Th1 cytokines such as IFN-γ and participates in class-switching of B lymphocytes.11 T-bet has the ability to form direct binding with IFN-γ at its regulatory element locus at the time of Th1 differentiation resulting in IFN-γ transcription.28 Thus, downregulation in the expression of T-bet causes impaired Th1 immunity. Conversely, GATA3, a transcription factor expressed specifically during STAT6, depends on Th2 differentiation.29 Binding of GATA3 to the various proximal and distal sites of IL-4, IL-5, and IL-13 results in the activation of expressions of these cytokines.29,30 Furthermore, T-bet has an ability to bind with GATA3 at its Th1-specific sites, thus inhibiting GATA3 binding with ILs promoter site and serving as regulatory elements for GATA3.28 In the present study, OVA-challenged AR mice showed overexpression of GATA-3 along with suppressed expression of T-bet in spleen tissue, which was markedly inhibited following treatment with apigenin depicting its role in the regulation of Th1/Th2 differentiation to ameliorate allergen-induced allergic reactions. The results of the present investigation corroborate the findings of the previous investigator.19
Signal transducer and activator of transcription-6 is a nuclear transcription factor involved in the mediation of Th2-type immunity. The study reported that IL-4 or IL-13 plays an important role in the phosphorylation of STAT6 via JAK1 and JAK3 kinases, which results in the formation of homodimer and its nuclear translocation.24,31 Thus, STAT6 is thought to be a vital regulator during the development of Th2 response, class switching of IgE, and during the expression of endothelial P-selectin.22 Furthermore, IL-4 or IL-13 induced production of eotaxin from fibroblasts is mediated by STAT6.5,22 The crucial role of STAT6 in nasal hyperreactivity is further supported by the findings of Akei et al, where STAT6-deficient mice exhibited decreased number of nasal sneezing and rubbing along with reduced infiltration of eosinophil in nasal mucosa.32 On another hand, SOCS1 are negative regulators of cytokine signaling, which play vital roles in the differentiation of T helper cell and thus contribute to the development of Th2-mediated allergic responses.8 The SOCS1 regulates the cross talk between the complicated cytokine signal networks, including IL-4 and IFN-γ, thus regulates Th1/Th2 balance.29 In the present investigation, OVA-challenged mice showed elevated Th2 allergic responses depicted by upregulated STAT6 and SOCS1 mRNA expressions, whereas apigenin treatment significantly inhibited these OVA-induced elevated mRNA expressions, thus balancing Th1/Th2 response. Previous researchers also documented the inhibitory potential of apigenin against STAT6 and SOCS1 mRNA expressions to ameliorate allergen-induced hyperreactivity, and findings of the present study are in line with this researcher.17
An array of evidence suggests that NF-κB plays a pivotal role in the modulation of allergic inflammatory reactions.33 Inflammatory stimuli cause NF-κB to translocate into the nucleus in the form of NF-κB p65 by phosphorylating and degrading IκBα, which rapidly upregulates the expression of pro-inflammatory cytokines (such as TNF-α and ILs) and other pro-inflammatory substances.34,35 Additionally, ILs also play an important role in the direct activation and amplification of NF-κB that in turn aggravates inflammatory response.25 Previously, a researcher reported the diminished GATA-3 and inflammatory response in mice with a lack of NF-kB subunit.22 Indeed, activated NF-κB response was documented in the nasal mucosa of patients having AR.20 Thus, researchers have attempted to develop a therapeutic moiety that exhibits NF-κB inhibitory potential for the treatment of AR.25 In the present investigation also, administration of apigenin in OVA-challenged mice showed a significant reduction in nasal symptoms, which might be attributed to its NF-κB and IκBα inhibitory potential.
Chronic immune inflammatory disease such as AR is characterized by a prominent influx of inflammatory cells, including eosinophils and lymphocytes. The spleen is an important organ of the immune system, and massive enlargement of the spleen is an indication of immune-inflammatory disease condition like AR.7,17 The murine model of AR is associated with the presence of an abundant number of neoplastic cells and centroblastic centrocytic lymphoid in the spleen.17,25,29 Histological analysis of spleen tissue from AR control mice showed dark-stained lymphocytes containing coarse chromatin pattern with thick fibrous capsule. Additionally, eosinophil infiltration was increased significantly in the nasal mucosa after OVA challenge. However, this spleen atrophy as well as the presence of inflammatory cells in spleen and nasal mucosa were significantly attenuated by the treatment of apigenin depicting its anti-inflammatory potential.
Several investigations have reported the potential of montelukast in the treatment of AR.12,36 It is the only drug from class CysLT1 receptor antagonists that have been approved for the symptomatic relief of AR, including rhinorrhea, sneezing, and day-time congestion.37 However, its prolonged use is associated with various psychiatric adverse events, including aggression, agitation, depression, irritability, anxiousness, and restlessness.37 On the other hand, plant moieties of natural origin provide a promising therapeutic alternative for the avoidance of AR.38 In this regard, researchers have well investigated the safety and efficacy of Cinnamonum zylanicum, Panax ginseng, Zingiber officinale, Nigella sativa, Tinospora cordifolia, and grape seed extract for the treatment of AR.23,39 Apigenin has also been documented for its promising anti-allergic potential.16,17,19 Thus findings from the present investigation suggest that apigenin might be a useful therapeutic alternative for the management of AR clinically.
Conclusion
Observations of the present investigation suggest that apigenin has potent anti-allergic properties for the management of rhinitis symptoms. Apigenin regulates Th1/Th2 balance via suppression in expressions of Th2 response (IgE, histamine, ILs, GATA3, STAT6, SOCS1, and NF-κB) and activation Th1 response (IFN-γ and T-bet) to exert its anti-allergic potential in a murine model of OVA-induced AR.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Bailing Yan  https://orcid.org/0000-0002-1068-0711
https://orcid.org/0000-0002-1068-0711
References
- 1. Frohlich M, Pinart M, Keller T, et al. Is there a sex-shift in prevalence of allergic rhinitis and comorbid asthma from childhood to adulthood? A meta-analysis. Clin Transl Allergy. 2017;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brożek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126(3):466–476. [DOI] [PubMed] [Google Scholar]
- 3. Eifan AO, Durham SR. Pathogenesis of rhinitis. Clin Exp Allergy. 2016;46(9):1139–1151. [DOI] [PubMed] [Google Scholar]
- 4. Liang K, Kandhare AD, Mukherjee-Kandhare AA, Bodhankar SL, Xu D. Morin ameliorates ovalbumin-induced allergic rhinitis via inhibition of STAT6/SOCS1 and GATA3/T-bet signaling pathway in BALB/c mice. J Funct Foods. 2019;55:391–401. [Google Scholar]
- 5. Li LJ, Ma N, Zeng L, et al. Flagellin modulates IgE expression in B cells to initiate food allergy in mice. Am J Transl Res. 2016;8(6):2748–2757. [PMC free article] [PubMed] [Google Scholar]
- 6. Manikandan J, Kothandaraman N, Hande MP, Pushparaj PN. Deciphering the structure and function of FcepsilonRI/mast cell axis in the regulation of allergy and anaphylaxis: a functional genomics paradigm. Cell Mol Life Sci. 2012;69(12):1917–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aswar UM, Kandhare AD, Mohan V, Thakurdesai PA. Anti-allergic effect of intranasal administration of type-A procyanidin polyphenols based standardized extract of cinnamon bark in ovalbumin sensitized BALB/c mice. Phytother Res. 2015;29(3):423–433. [DOI] [PubMed] [Google Scholar]
- 8. Kim TH, Kim K, Park SJ, et al. Expression of SOCS1 and SOCS3 is altered in the nasal mucosa of patients with mild and moderate/severe persistent allergic rhinitis. Int Arch Allergy Immunol. 2012;158(4):387–396. [DOI] [PubMed] [Google Scholar]
- 9. Ma Y, Ge A, Zhu W, et al. Morin attenuates ovalbumin-induced airway inflammation by modulating oxidative stress-responsive MAPK signaling. Oxid Med Cell Longev. 2016;2016:5843672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoyte FCL, Nelson HS. Recent advances in allergic rhinitis. F1000Res. 2018;7:F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen B, Qu S, Li M, et al. Effects of 1,25-dihydroxyvitamin D3 in an ovalbumin-induced allergic rhinitis model. Int Immunopharmacol. 2017;47:182–189. [DOI] [PubMed] [Google Scholar]
- 12. Mukherjee AA, Kandhare AD, Rojatkar SR, Bodhankar SL. Ameliorative effects of Artemisia pallens in a murine model of ovalbumin-induced allergic asthma via modulation of biochemical perturbations. Biomed Pharmacother. 2017;94:880–889. [DOI] [PubMed] [Google Scholar]
- 13. Mlcek J, Jurikova T, Skrovankova S, Sochor J. Quercetin and its anti-allergic immune response. Molecules. 2016;21(5):E623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanaka T, Iuchi A, Harada H, Hashimoto S. Potential beneficial effects of wine flavonoids on allergic diseases. Diseases. 2019;7(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nicholas C, Batra S, Vargo MA, et al. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J Immunol. 2007;179(10):7121–7127. [DOI] [PubMed] [Google Scholar]
- 16. Choi JR, Lee CM, Jung ID, et al. Apigenin protects ovalbumin-induced asthma through the regulation of GATA-3 gene. Int Immunopharmacol. 2009;9(7-8):918–924. [DOI] [PubMed] [Google Scholar]
- 17. Yano S, Umeda D, Yamashita S, Yamada K, Tachibana H. Dietary apigenin attenuates the development of atopic dermatitis-like skin lesions in NC/Nga mice. J Nutr Biochem. 2009;20(11):876–881. [DOI] [PubMed] [Google Scholar]
- 18. Ai XY, Qin Y, Liu HJ, et al. Apigenin inhibits colonic inflammation and tumorigenesis by suppressing STAT3-NF-kappaB signaling. Oncotarget. 2017;8(59):100216–100226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li RR, Pang LL, Du Q, Shi Y, Dai WJ, Yin KS. Apigenin inhibits allergen-induced airway inflammation and switches immune response in a murine model of asthma. Immunopharmacol Immunotoxicol. 2010;32(3):364–370. [DOI] [PubMed] [Google Scholar]
- 20. Watts AM, Cripps AW, West NP, Cox AJ. Modulation of allergic inflammation in the nasal mucosa of allergic rhinitis sufferers with topical pharmaceutical agents. Front Pharmacol. 2019;10(294):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirano T, Higa S, Arimitsu J, et al. Flavonoids such as luteolin, fisetin and apigenin are inhibitors of interleukin-4 and interleukin-13 production by activated human basophils. Int Arch Allergy Immunol. 2004;134(2):135–140. [DOI] [PubMed] [Google Scholar]
- 22. Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat Immunol. 2001;2(1):45–50. [DOI] [PubMed] [Google Scholar]
- 23. Walanj S, Walanj A, Mohan V, Thakurdesai PA. Efficacy and safety of the topical use of intranasal cinnamon bark extract in seasonal allergic rhinitis patients: a double-blind placebo-controlled pilot study. J Herb Med. 2014;4(1):37–47. [Google Scholar]
- 24. Bloodworth MH, Newcomb DC, Dulek DE, et al. STAT6 signaling attenuates interleukin-17-producing gamma delta T cells during acute Klebsiella pneumoniae infection. Infect Immun. 2016;84(5):1548–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wee JH, Zhang YL, Rhee CS, Kim DY. Inhibition of allergic response by intranasal selective NF-kappaB decoy oligodeoxynucleotides in a murine model of allergic rhinitis. Allergy Asthma Immunol Res. 2017;9(1):61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carr VM, Robinson AM, Kern RC. Tissue-specific effects of allergic rhinitis in mouse nasal epithelia. Chem Senses. 2012;37(7):655–668. [DOI] [PubMed] [Google Scholar]
- 27. Matsuyama M, Ishii Y, Yageta Y, et al. Role of Th1/Th17 balance regulated by T-bet in a mouse model of Mycobacterium avium complex disease. J Immunol. 2014;192(4):1707–1717. [DOI] [PubMed] [Google Scholar]
- 28. Kanhere A, Hertweck A, Bhatia U, et al. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun. 2012;3:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shao YY, Zhou YM, Hu M, et al. The anti-allergic rhinitis effect of traditional Chinese medicine of Shenqi by regulating mast cell degranulation and Th1/Th2 cytokine balance. Molecules. 2017;22(3):E504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li B, Jin X, Meng H, et al. Morin promotes prostate cancer cells chemosensitivity to paclitaxel through miR-155/GATA3 axis. Oncotarget. 2017;8(29):47849–47860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hosoya K, Satoh T, Yamamoto Y, et al. Gene silencing of STAT6 with siRNA ameliorates contact hypersensitivity and allergic rhinitis. Allergy. 2011;66(1):124–131. [DOI] [PubMed] [Google Scholar]
- 32. Akei HS, Brandt EB, Mishra A, et al. Epicutaneous aeroallergen exposure induces systemic TH2 immunity that predisposes to allergic nasal responses. J Allergy Clin Immunol. 2006;118(1):62–69. [DOI] [PubMed] [Google Scholar]
- 33. Liu T, Zhang L, Joo D, Sun SC. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kandhare AD, Liu Z, Mukherjee AA, Bodhankar SL. Therapeutic potential of morin in ovalbumin-induced allergic asthma via modulation of SUMF2/IL-13 and BLT2/NF-kB signaling pathway. Curr Mol Pharmacol. 2019;12(2):122–138. [DOI] [PubMed] [Google Scholar]
- 35. Zhou Z, Kandhare AD, Kandhare AA, Bodhankar SL. Hesperidin ameliorates bleomycin-induced experimental pulmonary fibrosis via inhibition of TGF-β1/Smad3/AMPK and IκBα/NF-κB pathways. EXCLI J. 2019;18:723–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bozkurt MK, Tülek B, Bozkurt B, Akyürek N, Öz M, Kiyici A. Comparison of the efficacy of prednisolone, montelukast, and omalizumab in an experimental allergic rhinitis model. Turk J Med Sci. 2014;44(3):439–447. [DOI] [PubMed] [Google Scholar]
- 37. Mandhane SN, Shah JH, Thennati R. Allergic rhinitis: an update on disease, present treatments and future prospects. Int Immunopharmacol. 2011;11(11):1646–1662. [DOI] [PubMed] [Google Scholar]
- 38. Wang S, Tang Q, Qian W, Fan Y. Meta-analysis of clinical trials on traditional Chinese herbal medicine for treatment of persistent allergic rhinitis. Allergy. 2012;67(5):583–592. [DOI] [PubMed] [Google Scholar]
- 39. Nikakhlagh S, Rahim F, Aryani FH, Syahpoush A, Brougerdnya MG, Saki N. Herbal treatment of allergic rhinitis: the use of Nigella sativa. Am J Otolaryngol. 2011;32(5):402–407. [DOI] [PubMed] [Google Scholar]