Abstract
Diclofenac (DF) is widely used in the treatment of pain and fever. Despite it therapeutic benefits, it triggered hepatorenal injury. Thus, the present study investigated the protective roles of kolaviron (KV) against DF-induced hepatic and renal toxicity in rats. The rats were allotted into groups: control group received propylene glycol and treatment groups received DF, which induced hepatorenal toxicity in rats and different doses of KV that prevented systemic toxicity of DF in rats. Twenty-four hours after the last treatment, all the rats were killed. Pro-inflammatory levels, markers of liver and kidney functions, oxidative stress, hematological indices, and histopathological alterations were evaluated. Diclofenac caused significant increase in the plasma levels of creatinine and urea and activities of liver enzymes, including bilirubin level, pro-inflammatory markers, and plasma prostaglandin E2 (PGE2). It also caused significant alteration in renal and hepatic PGE2, antioxidants, lipid peroxidation (malondialdehyde), and hematological indices. These toxic effects were confirmed by histological studies and levels of inflammatory infiltration (myeloperoxidase). However, KV significantly prevented or reduced the adverse effects of DF in the plasma, liver, and kidney of the rats pretreated with KV before DF administration. This study showed the efficacy of KV as hepatic and renal protector in DF-induced hepatorenal toxicity through reduction of oxidative stress and suppression of inflammation.
Keywords: anti-inflammatory, diclofenac, hepatorenal toxicity, kolaviron, prostaglandin
Introduction
The kidney and liver are important organs in the body. Kidney regulates body electrolytes, acid-base balance, and blood pressure. Both the kidney and liver serve the body as a natural filter of blood and remover of drugs or toxic waste products from the body.1 They produce hormones and maintaining the production and metabolism of prostaglandins via cyclooxygenase (COX), especially the kidney. Both organs also participated in the hematopoietic system functions. All these basic functions of the kidney and liver are necessary for homeostasis. Liver and kidney injuries often arise from their involvement in metabolism, detoxification, storage, and excretion of drugs and their metabolites, making them important target organs for drug-induced injuries.1-3 Nephrotoxicity and hepatotoxicity are serious health disorders that are caused by the usage of certain medication. Some therapeutic drug, such as nonsteroidal anti-inflammatory drugs (NSAIDs), are one of the drugs reported to induced kidney and liver toxicity.4-6
Nonsteroidal anti-inflammatory drugs are widely used drugs for the control of pain and inflammation. There normal therapeutic doses exhibit or produce mild side effect. However, overdose of these drugs exhibits severe toxicity. The majority of NSAIDs inhibit the COX enzymes7 and therefore alter prostaglandins synthesis,8 and as a result of that, kidney and liver cells are exposed to injury.9 Diclofenac (DF) is a phenylacetic acid NSAID, with anti-inflammatory, analgesic, antinociceptive, antipyretic, and antibacterial properties.10,11 It is widely used in the treatment of rheumatoid arthritis and pain. Despite the therapeutic benefits of DF, it has notable severe side effect. These include gastrointestinal toxicity and injury to the lungs, cardiac, hepatic, and renal tissues.5,6,12 Diclofenac has been confirmed as a non-threshold multitargeted drug that causes alterations in different organs of the body, including the lung, stomach, kidney, liver, and heart.4,5,12,13 Although the precise mechanism of kidney and liver toxicity caused by DF is not fully explicated, there is evidence that DF can cause mitochondrial injury by disrupting the immune-mediated protective mechanisms, generation of reactive oxygen metabolites, and inhibition of the activity of enzymatic and nonenzymatic antioxidants in the kidney and liver tissues.14-16 Reports from our previous study have demonstrated that DF metabolites (4′, 5-hydroxydiclofenac) are capable of causing neutrophil infiltration in hepatic cells and hepatocytes necrosis. These effects were shown to be associated with formation of reactive oxygen species (ROS).17 Therefore, antioxidant potential of any therapeutic agent that would attenuate ROS-mediated cellular damages can be the therapeutic approach to arrest or prevent DF cytotoxic effect.
Medicinal herbs play a significant role in protecting the body from hazardous chemical substances by restoring the antioxidant status and thereby inhibiting oxidative stress via ROS scavenging.18 Garcinia kola is an edible seed, which belong to family of Guttiferae, and it commonly known as bitter kola or male kola. Kolaviron (KV) is a fraction of the defatted acetone extract of Garcinia kola seeds, which was reported to contains Garcinia biflavonoids 1 (GB1), Garcinia biflavonoids 2 (GB2), and kolaflavone as its major components.19,20 It is known to have anti-ulcer, antioxidant, anti-inflammatory, analgesic, antidiabetic, and hepatoprotective activities.17,20-24 Kolaviron has been reported to improved antioxidant status by enhancing antioxidant gene expressions and scavenging ROS in atrazine-induced cytotoxicity of rat Leydig cells.25 Research studies indicated that KV exhibit wide range of medicinal values and play a significant role in protecting the body cells against oxidative stress induced by toxins in experimental model.17 Therefore, supplementation with KV, an active antioxidant component of Garcinia kola seed might exert beneficial effect against DF-induced systemic toxicity. The present study evaluated the therapeutic efficacy of KV against DF-induced hepatorenal toxicity in rats.
Materials and Methods
Drugs and Chemicals
Diclofenac was procured from Wuhan Grand Pharmaceutical Company (China); Propylene glycol and ketamine hydrochloride were obtained from Biovision (Milpitas, California) and Rotexmedica (Trittau, Germany), respectively; Petroleum ether, acetone, and ethyl acetate were purchased from Sigma (St Louis, Missouri), respectively. Other reagents used were of analytical grade.
Extraction of KV
Garcinia kola seeds were procured from Oja Oba, in Ikere Ekiti, Nigeria, and certified by a taxonomist at the herbarium of the department of Botany, Obafemi Awolowo University, with a voucher number of IFE 17540. Kolaviron was isolated from Garcinia kola according to the previous methods.24,26 The KV obtained was dissolved in propylene glycol (0.2 mL/administration) and given to rat according to the designed dosage.
Animal Care and Management
Twenty-five adult male Wistar rats weighing 110 to 150 g, procured from the Animal House of the College of Health Sciences, Obafemi Awolowo University, Ile-Ife, were used for this study. The rats were housed in plastic cages at a room temperature of about 32°C (using, Picer Multi Thermometer 408) and photoperiodicity of 12-hour light/12-hour dark. The animals were allowed to have access to standard rat chow (Ace Feed PLC Ibadan, Nigeria) and water ad libitum. The animal experimental procedures were conducted in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
Experimental Design
The rats were divided into 5 groups with each group consisting of 5 animals each.
Group 1—control received 0.2 mL/100 g of propylene glycol daily by oral route for 28 days.
Group 2 – received 100 mg/kg of KV daily by oral route for 28 days.
Group 3 – received 10 mg/kg intramuscularly (IM) injection of DF daily for 7 days.
Group 4 – received 100 mg/kg of KV daily by oral route for 28 days and thereafter received DF injection (10 mg/kg, IM) for 7 days.
Groups 5 – received 200 mg/kg of KV daily by oral route for 28 days and thereafter received DF injection (10 mg/kg, IM) for 7 days.
Blood and Tissues Collection
Twenty-four hours after the last treatment, the animals were killed under ketamine hydrochloride anesthesia (10 mg/kg/bw via intramuscular route). Blood of each animal was collected by cardiac puncture into separate EDTA tubes and lithium heparinized tubes. Blood dispensed into potassium EDTA tubes was used for hematological analysis while those collected in lithium heparinized tubes were centrifuged at 4000 revolutions per minute for 15 minutes at −4°C, using a cold centrifuge (Centurium Scientific, Model 8881) to separate the plasma. The plasma was collected into separate plain tubes for the assessment of some markers of both renal and liver functions and prostaglandin E2 (PGE2). Part of the liver lobe and the left kidney of each rat was homogenized for the assessment of PGE2, myeloperoxidase (MPO), antioxidant, and lipid peroxidation status. Part of the liver lobe and the right kidney of each rat was carefully excised, weighed, and fixed in 10% buffer formalin for histopathological studies using hematoxylin–eosin (H&E) stain.
Biochemical Assay
Plasma levels of creatinine, urea, activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total bilirubin levels were determined by the use of appropriate biochemical kits purchased from Randox Laboratories (Crumlin, Co, Antrim, United Kingdom).
Antioxidant and Malondialdehyde Analysis
The liver and kidney tissues of the rats were homogenized in 50 mM Tris-HCl buffer (pH 7.4) containing 1.15% potassium chloride, and the homogenate was centrifuged at 3000 revolution per minutes (rpm) for 15 minutes at 4°C. The supernatant was collected for the estimation of superoxide dismutase (SOD) and was assayed by the method described by Misra and Fridovich.27 Catalase (CAT) activity was estimated using hydrogen peroxide as substrate according to the method of Aebi.28 Reduced glutathione (GSH) was determined using the method described by Beutler et al.29 In addition, the hydrogen peroxide was determined by the method of Wolff,30 while lipid peroxidation was measured as malondialdehyde (MDA) according to the method described by Ohkawa et al31 and expressed as micromoles of MDA per gram tissue. Myeloperoxidase as marker of inflammation and oxidative stress was measured, according to the method of Xia and Zweier.32 The absorbance was read at 350 nm. One unit of MPO activity can be defined as the quantity of enzyme able to convert/degrade 1 μmol of H2O2 to water in 1 minute at room temperature.
Plasma Tumor Necrosis Factor-α and Interleukin-6 Estimations
Plasma tumor necrosis factor (TNF) α and interleukin (IL) 6 assays were estimated according to the manufacturer’s instructions using the enzyme-linked immunosorbent assay kits obtained from Wkea Med Supplies Corp (Changchun, Jilin, China).
Evaluation of Kidney and Liver PGE2
The plasma and part of the renal and hepatic tissues prostaglandin of each rat were measured. Part of the left renal and hepatic tissues were cut into small (approximately 1 mm2) pieces and washed twice in phosphate-buffered saline buffer. The cut tissue clusters were homogenized and centrifuged at 3000 rpm for 15 minutes at 4°C. The supernatant was collected for the estimation of prostaglandin. Prostaglandin E2 was then assessed using a high-sensitivity peptide enzyme immunoassay, enzyme immunoassay (R&D Systems, Minneapolis, USA), based on a competitive binding of the sample PGE2 and the fixed amount of horseradish peroxidase–labeled PGE2 to the sites of a specific monoclonal antibody, according to the manufacturer’s protocol.
Hematological Indices
Red blood cell (RBC), packed cell volume (PCV), hemoglobin (HB), mean corpuscular volume, mean corpuscular hemoglobin, white blood cell (WBC), lymphocyte (LYM), neutrophil (NEU), and platelet (PLT) counts were determined using an autoanalyzer (SFRI blood cell counter, H18 light; Sean-Jeand’Illac, France).
Histopathological Studies
The portions of the liver and kidney from all the experimental groups were fixed in 10% formol-saline, dehydrated in graded alcohol, cleared by xylene, and embedded in paraffin wax. The tissues were then cut into 3- to 4-μm-thick sections by a microtome, fixed on the slides, and stained with H&E. The slides were examined under a light microscope (Olympus CH; Olympus, Tokyo, Japan), and photomicrographs were taken with a Leica DM 750 camera at ×400 magnifications.
Statistical Analysis
All data were expressed as means ± standard errors of means (SEM). The statistical analysis was performed using 1-way analysis of variance followed by Neumann Keul post hoc test for comparison between groups. Differences were considered significant when P < .05. The data were analyzed using the statistical package, GraphPad Prism version 5 (GraphPad Software Inc, San Diego, California).
Results
Effects of KV on Kidney and Liver Functions
Relative to the normal control group, there was a significant (P < .05) increase in plasma urea and creatinine levels in DF control group (Table 1). Compared to the latter, there were significant (P < .05) decreases in plasma urea and creatinine levels in KV groups in a dose-dependent manner. The plasma activities of liver enzymes, such as AST, ALT, and ALP, were significantly (P < .05) increased in the DF group when compared with normal control group (Table 1). However, 100KV + DF and 200KV + DF groups significantly (P < .05) decreased plasma activities of AST, ALT, and ALP in a dose-dependent manner when compared with DF group. In addition, DF control group significantly (P < .05) increased plasma level of bilirubin when compared with the control. Kolaviron-treated groups had a significantly (P < .05) decreased in bilirubin level when compared with DF control group.
Table 1.
Effect of KV on Kidney and Liver Biochemical in DF-Induced Hepatorenal Toxicity.a
| Groups | Creatinine (mg/dL) | Urea (mg/dL) | AST (U/L) | ALT (U/L) | ALP (U/L) | Total Bilirubin (µmol/L) |
|---|---|---|---|---|---|---|
| Control | 0.76 ± 0.07 | 19.91 ± 1.02 | 50.66 ± 3.28 | 26.13 ± 5.73 | 99.28 ± 6.28 | 4.31 ± 0.85 |
| 100KV | 0.36 ± 0.03b | 15.07 ± 1.14c | 53.06 ± 4.21 | 27.76 ± 3.12 | 100.9 ± 6.18 | 4.89 ± 1.11 |
| DF | 1.77 ± 0.06d | 146.40 ± 15.60d | 110.4 ± 10.42b | 81.02 ± 7.95d | 200.1 ± 27.72d | 13.32 ± 1.24d |
| 100KV + DF | 0.88 ± 0.10e | 25.00 ± 2.34f | 60.08 ± 2.28e | 31.26 ± 3.06e | 118.6 ± 4.41e | 8.01 ± 1.00c,g |
| 200KV + DF | 0.79 ± 0.05e | 20.41 ± 1.30f | 56.78 ± 3.57e | 28.23 ± 2.16f | 110.6 ± 5.44e | 4.90 ± 0.91f |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DF, diclofenac; KV, kolaviron; SEM, standard error of mean; 100KV, 100 mg/kg of kolaviron; 200KV, 200 mg/kg of kolaviron.
a Data are expressed as means ± SEM.
b P < .01 is significantly different from control.
c P < .05 is significantly different from control.
d P < .001 is significantly different from control.
e P < .01 significantly different from DF.
f P < .001 significantly different from DF.
g P < .05 significantly different from DF.
Effects of KV on Kidney and Liver Antioxidant Activities and MDA Levels
Compared with normal control group, there were significant (P < .05) increases in kidney SOD and CAT in DF control group (Table 2). Also DF treated rats had a significantly increased in kidney MDA and H2O2 when compared with the control. However, KV administration significantly decreased the activities of SOD, CAT, MDA, and H2O2 in kidney toward control level in a dose-dependent manner (Table 2). Relative to the normal control group, there was a significant (P < .05) decrease in kidney GSH level in DF control group. Compared to the latter, there were significant (P < .05) increases in kidney GSH level in KV-treated groups in a dose-dependent manner.
Table 2.
Effect of KV on Kidney Antioxidant Activities and MDA Levels in DF-Induced Hepatorenal Toxicity.a
| Groups | SOD (µ/mg tissue) | CAT (µM/mg tissue) | GSH (µg/mg tissue) | MDA (nM/mg tissue) | H2O2 (nM/g tissue) |
|---|---|---|---|---|---|
| Control | 80.11 ± 1.29 | 2.24 ± 0.19 | 5.92 ± 0.47 | 10.92 ± 0.86 | 17.77 ± 1.04 |
| 100KV | 77.35 ± 1.62 | 2.11 ± 0.42 | 5.56 ± 0.40 | 11.86 ± 1.13 | 17.64 ± 1.53 |
| DF | 111.20 ± 3.36b | 4.31 ± 0.28c | 1.39 ± 0.15b | 25.15 ± 1.05b | 34.00 ± 2.03c |
| 100KV + DF | 78.65 ± 0.47d | 2.55 ± 0.58e | 5.08 ± 0.36d | 12.33 ± 1.06e | 20.21 ± 1.33e |
| 200KV + DF | 79.01 ± 1.25d | 2.20 ± 0.28e | 5.53 ± 0.54d | 12.55 ± 1.24e | 19.01 ± 0.45d |
Abbreviations: CAT, catalase; DF, diclofenac; GSH, reduced glutathione; KV, kolaviron; MDA, malondialdehyde; SEM, standard error of mean; SOD, superoxide dismutase; 100KV, 100 mg/kg of kolaviron; 200KV, 200 mg/kg of kolaviron.
a Data are expressed as means ± SEM.
b P < .001 is significantly different from control.
c P < .01 is significantly different from control.
d P < .001 is significantly different from DF.
e P < .01 is significantly different from DF.
The liver activities of SOD and CAT were significantly (P < .05) decreased in DF control group when compared with control group (Table 3). The GSH levels in the liver of DF group were significantly lower when compared with the control group. DF control group exhibited a significant (P < .05) increase in liver level of MDA and H2O2 when compared with the control group. However, KV administration significantly restored the liver activities of SOD and CAT, levels of GSH, MDA, and H2O2 to normalcy in a dose-dependent manner (Table 3).
Table 3.
Effect of KV on Liver Antioxidant Activities and MDA Levels in DF-Induced Hepatorenal Toxicity.
| Groups | SOD (µ/mg protein) | CAT (µM/mg protein) | GSH (µg/mg protein) | MDA (nM/mg protein) | H2O2 (nM/g protein) |
|---|---|---|---|---|---|
| Control | 2.33 ± 0.17 | 4.99 ± 0.45 | 9.94 ± 0.24 | 12.74 ± 0.96 | 20.01 ± 1.21 |
| 100KV | 2.30 ± 1.18 | 5.01 ± 0.32 | 9.40 ± 1.01 | 13.96 ± 1.32 | 20.64 ± 1.11 |
| DF | 1.19 ± 0.07b | 2.98 ± 1.06c | 6.33 ± 0.49b | 25.92 ± 2.17d | 40.00 ± 2.10d |
| 100KV + DF | 1.99 ± 0.43e | 4.45 ± 0.39e | 8.99 ± 0.56e | 15.01 ± 1.06f | 25.21 ± 1.86f |
| 200KV + DF | 2.25 ± 0.23f | 4.88 ± 0.18e | 9.23 ± 1.54e | 14.55 ± 1.44f | 22.01 ± 0.23g |
Abbreviations: CAT, catalase; DF, Diclofenac; GSH, reduced glutathione; KV, kolaviron; MDA, malondialdehyde; SEM, standard error of mean; SOD, superoxide dismutase; 100 KV, 100mg/kg of kolaviron; 200KV, 200 mg/kg of kolaviron.
a Data are expressed as means ± SEM.
b P < .01 is significantly different from control
c P < .05 is significantly different from control
d P < .001 is significantly different from control
e P < .05 is significantly different from DF.
f P < .01 is significantly different from DF.
g P < .001 is significantly different from DF.
Effect of KV on MPO, TNF-α, IL-6, PLT/LYM, and NEU/LYM
A significant (P < .05) increase in liver and kidney MPO activities was observed in DF control group compared to the normal control group (Table 4). Relative to the former, there were significant (P < .05) decreases in both liver and kidney MPO activities in KV-treated groups in a dose-dependent manner.
Table 4.
Effect of KV on MPO, TNF-α, IL-6, PLT/LYM, and NEU/LYM in DF-Induced Hepatorenal Toxicity.a
| Groups | Kidney MPO (U/mg protein) | Liver MPO (U/mg protein) | TNF-α (pg/ml) | IL-6 (pg/ml) | PLT/LYM | NEU/LYM |
|---|---|---|---|---|---|---|
| Control | 7.64 ± 3.04 | 5.94 ± 2.04 | 6.32 ± 1.17 | 13.27 ± 1.93 | 4.89 ± 0.09 | 0.15 ± 0.00 |
| 100KV | 7.92 ± 2.46 | 7.04 ± 3.20 | 7.01 ± 1.66 | 12.91 ± 1.09 | 5.32 ± 0.15 | 0.19 ± 0.01 |
| DF | 30.01 ± 5.21b | 24.94 ± 6.04b | 25.83 ± 3.09b | 28.43 ± 3.79c | 10.89 ± 0.98c | 0.53 ± 0.02c |
| 100KV + DF | 15.98 ± 5.98d,e | 10.94 ± 3.44d,f | 14.23 ± 1.91d,f | 13.93 ± 1.64f | 5.26 ± 0.82g | 0.21 ± 0.00g |
| 200KV + DF | 10.08 ± 4.35e | 10.06 ± 3.10d,f | 13.65 ± 1.36d,f | 12.99 ± 1.31f | 4.59 ± 0.10f | 0.16 ± 0.01f |
Abbreviations: DF, diclofenac; IL-6, interleukin 6; KV, kolaviron; MPO, myeloperoxidase; NEU/LYM, neutrophil/lymphocyte; PLT/LYM, platelet/lymphocyte; SEM, standard error of mean; TNF-α, tumor necrosis factor α; 100KV, 100 mg/kg of kolaviron; 200KV, 200 mg/kg of kolaviron.
a Data are expressed as means ± SEM.
b P < .001 significantly different from control.
c P < .01 significantly different from control.
d P < .05 significantly different from control.
e P < .001 significantly different from DF.
f P < .01 significantly different from DF.
g P < .05 significantly different from DF.
Relative to the normal control group, there was a significant (P < .05) increase in TNF-α and IL-6 levels in DF control group (Table 4). Compared to the latter, there were significantly (P < .05) decreased in TNF-α and IL-6 levels in KV-treated groups in a dose-dependent manner.
Compared to the control group, there was a significant (P < .05) increase in platelet/lymphocyte (PLT/LYM) ratio in the DF control group (Table 4). Relative to the latter, a significant (P < .05) decrease in PLT/LYM ratio was documented in KV groups in a dose-dependent manner.
A significant (P < .05) increases in neutrophil/lymphocyte (NEU/LYM) ratio were recorded in DF group when compared with the normal control group (Table 4). Relative to the latter, a significant (P < .05) decrease in NEU/LYM ratio was observed in KV-treated groups in a dose-dependent manner.
Effect of KV on Hematological Indices
There was a significantly (P < .05) decreased RBC count in DF control group compared to the normal control group (Table 5). Relative to the DF control group, significant (P < .05) increases in RBC were observed in KV groups in a dose-dependent manner.
Table 5.
Effect of KV on Hematological Indices in DF-Induced Hepatorenal Toxicity.a
| Parameters | Control | 100KV | DF | 100KV + DF | 200KV + DF |
|---|---|---|---|---|---|
| WBC (×103/µL) | 7.98 ± 0.62 | 6.86 ± 0.90 | 3.54 ± 0.63b | 4.86 ± 0.99 | 6.24 ± 1.17c |
| LYM % | 85.74 ± 0.79 | 84.40 ± 4.75 | 70.98 ± 4.97d | 83.44 ± 3.17c | 95.24 ± 3.91e |
| NEUT % | 12.42 ± 1.09 | 15.32 ± 8.42 | 32.14 ± 7.62b | 17.36 ± 5.21c | 14.06 ± 1.81e |
| RBC (×106/µL) | 7.48 ± 0.24 | 7.82 ± 0.26 | 4.08 ± 0.35d | 6.99 ± 0.36c | 7.32 ± 0.37c |
| HGB (g/dL) | 15.52 ± 0.61 | 15.08 ± 0.53 | 10.10 ± 0.65d | 14.21 ± 0.95c,d | 15.04 ± 0.70c |
| PCV (%) | 47.76 ± 2.48 | 47.30 ± 1.44 | 21.28 ± 3.65d | 36.26 ± 2.38c,d | 43.62 ± 1.91c |
| MCV (fL) | 60.02 ± 0.99 | 60.56 ± 0.47 | 60.38 ± 0.87 | 60.48 ± 0.75 | 59.42 ± 0.73 |
| MCH (pg) | 19.54 ± 0.19 | 19.60 ± 0.08 | 20.02 ± 2.23 | 18.96 ± 0.51 | 19.38 ± 0.11 |
| MCHC (g/dL) | 32.30 ± 0.31 | 32.46 ± 0.18 | 31.64 ± 0.56 | 31.40 ± 0.56 | 32.84 ± 0.76 |
| PLT (×103/µL) | 410.40 ± 58.63 | 424.00 ± 34.06 | 700.00 ± 53.93b | 438.40 ± 147.01e | 430.60 ± 209.23e |
Abbreviations: DF, diclofenac; HB, hemoglobin; KV, kolaviron; LYM, lymphocyte; MCHC, mean corpuscular hemoglobin; MCV, mean corpuscular volume; NEU, neutrophil; PCV, packed cell volume; PLT, platelet counts; RBC, red blood cell; SEM, standard error of mean; WBC, white blood cell; 100KV, 100 mg/kg of kolaviron; 200KV, 200 mg/kg of kolaviron.
a Data are expressed as means ± SEM.
b P < .01 significantly different from control.
c P < .05 significantly different from DF.
d P < .05 significantly different from control.
e P < .01 significantly different from DF.
There was a significantly (P < .05) decreased HB level in DF control and 100KV + DF groups, relative to the normal control group (Table 5). Compared to the DF control group, significant (P < .05) increases in HB level were noted in 100KV + DF and 200KV + DF groups in a dose-dependent manner.
Relative to the normal control group, there were significant (P < .05) decreases in the PCV in DF control and 100KV + DF (Table 5). Compared to the DF control group, there were significant (P < .05) increases in PCV in 100KV + DF and 200KV + DF groups in a dose-dependent manner.
A significant (P < .05) increase in PLT count was noted in the DF group, compared to the normal control (Table 5). Relative to the former, significant (P < .05) decreases in PLT count were documented in KV-treated groups in a dose-dependent manner.
Relative to the normal control group, there were significant (P < .05) decreases in WBC count in DF control group (Table 5). Compared to the DF control group, significant (P < .05) increases in WBC count were observed in 200KV + DF groups.
There were significantly (P < .05) decreased in LYM count in DF control group, relative to the normal control group (Table 5). Compared to the DF control group, significant (P < .05) increases in LYM count were noted in 100KV + DF and 200KV + DF groups in a dose-dependent manner.
Relative to the normal control group, there were significant (P < .05) increases in the NEU in DF control group (Table 5). Compared to the DF control group, there were significant (P < .05) decreases in NEU in 100KV + DF and 200KV + DF groups in a dose-dependent manner.
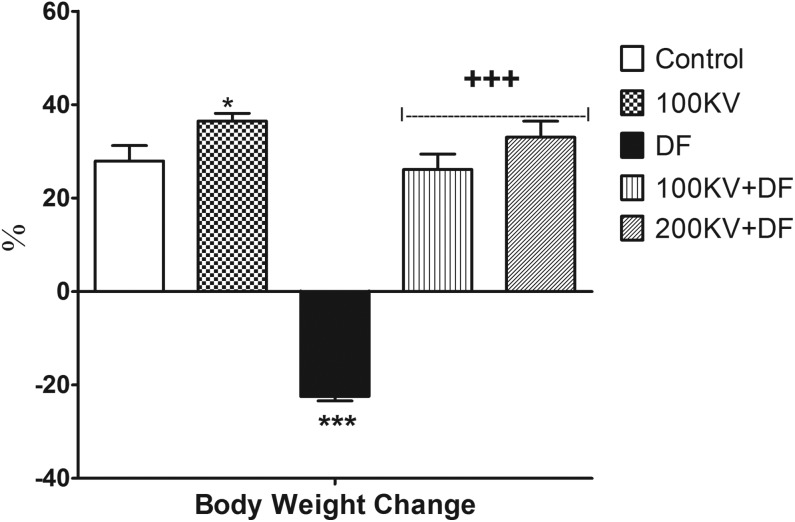
Effect of KV on Body Weight Change
There were significantly (P < .05) decreased in body weight in DF-treated rats, relative to the normal control rats (Figure 1). Compared to the DF control group, significant (P < .05) increases in body weight were noted in 100KV + DF and 200KV + DF groups in a dose-dependent manner. Kolaviron-treated only (100KV) had significant (P < .05) increase in body weight when compared with the control group.
Figure 1.
Effect of KV on body weight change in DF-induced hepatorenal toxicity. Data are expressed as means ± SEM. *P < .05 and ***P < .001 significantly different from control; +++P <.001, significantly different from DF. DF indicates diclofenac; KV, kolaviron; SEM, standard error of mean; 100KV, 100 mg/kg of kolaviron; 200KV, 200 mg/kg of kolaviron.
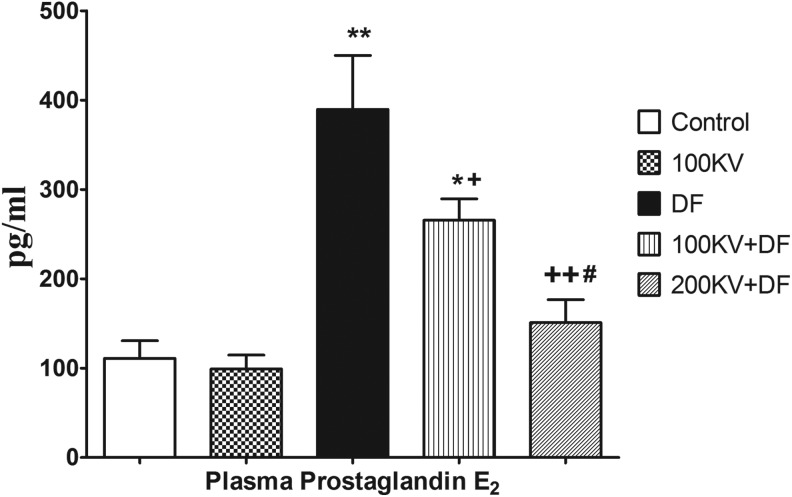
Effect of KV on Plasma PGE2 Level
Diclofenac-induced inflammation was associated with increased in plasma PGE2 level. There were significant (P < .05) increases in plasma PGE2 level in DF and 100KV + DF-treated groups, compared with the normal control (Figure 2). Compared to the DF control group, there were significant (P < .05) decreases in plasma PGE2 level in 100KV + DF and 200KV + DF groups in a dose-dependent manner.
Figure 2.
Effect of KV on renal tissue prostaglandin E2 in DF-induced hepatorenal toxicity. Data are expressed as means ± SEM. *P < .05, **P < .01, and ***P < .001 significantly different from control; +P < .05, ++P < .01, and +++P < .001 significantly different from DF. DF indicates diclofenac; KV, kolaviron; SEM, standard error of mean; 100KV, 100 mg/kg of kolaviron; 200KV, 200 mg/kg of kolaviron.
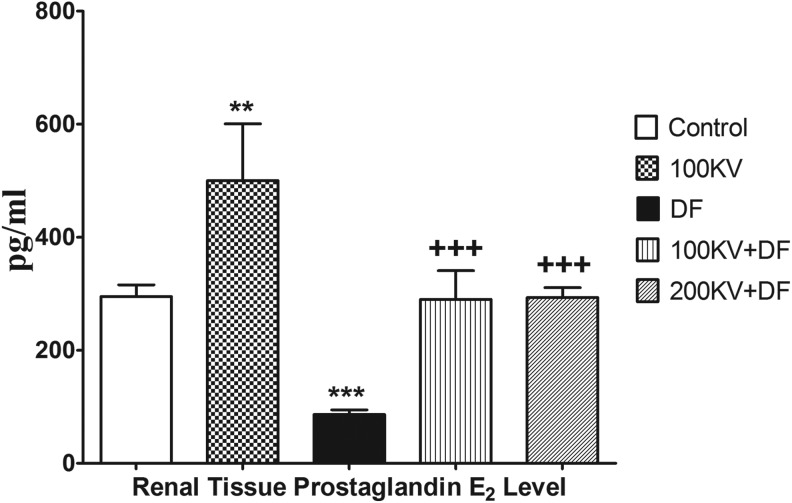
Effect of KV on Renal and Hepatic Tissue PGE2 Level
A significant (P < .05) decrease in renal PGE2 level was observed in DF group when compared with the normal control group (Figure 3). Relative to the latter, a significant increase in renal tissue PGE2 level toward basal level was observed in KV-treated groups in a dose-dependent manner.
Figure 3.
Effect of KV on hepatic tissue prostaglandin E2 in DF-induced hepatorenal toxicity. Data are expressed as means ± SEM. *P < .05, **P < .01, and ***P < .001 significantly different from control; +P < .05, ++P < .01, and +++P < .001 significantly different from DF. #P < .05 significantly different from 200 KV. DF indicates diclofenac; KV, kolaviron; SEM, standard error of mean; 100KV, 100 mg/kg of kolaviron; 200KV, 200 mg/kg of kolaviron.
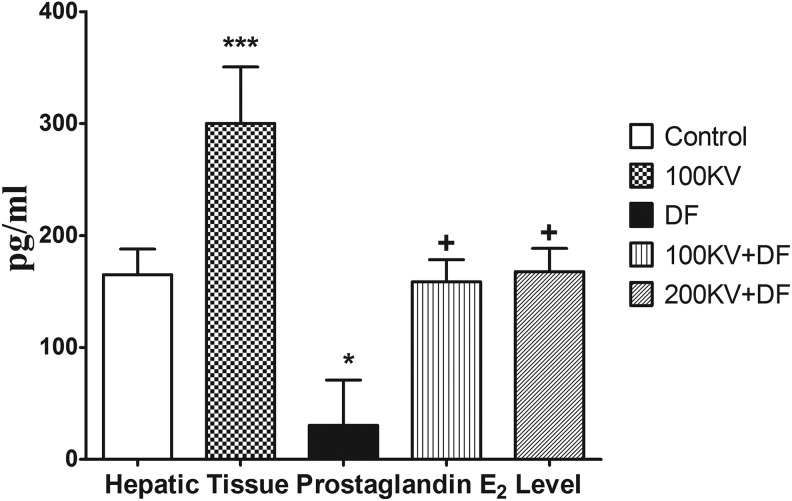
Relative to the normal control group, there were significant (P < .05) decreases in the hepatic tissue PGE2 level in DF control (Figure 4). Compared to the DF control group, there were significant increases in hepatic tissue PGE2 toward basal level in KV-treated groups in a dose-dependent manner.
Figure 4.
Effect of KV on plasma prostaglandin E2 in DF-induced hepatorenal toxicity. Data are expressed as means ± SEM. *P < .05, **P < .01, and ***P < .001 significantly different from control; +P < .05, ++P < .01, and +++P < .001 significantly different from DF. DF indicates diclofenac; KV, kolaviron; SEM, standard error of mean; 100KV, 100 mg/kg of kolaviron; 200KV, 200 mg/kg of kolaviron.
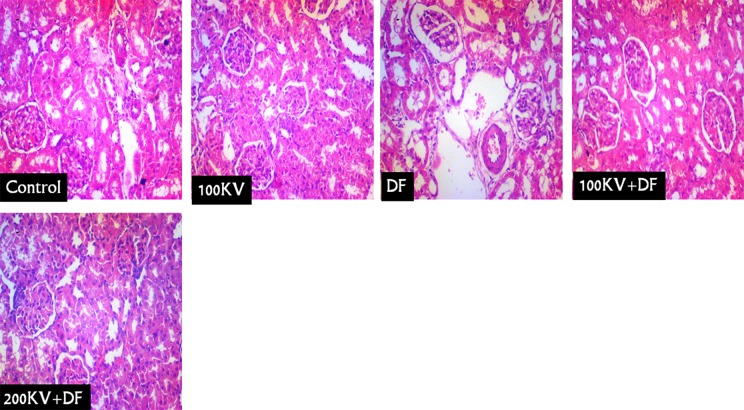
Effects of KV on Renal and Hepatic Histopathological Examination
Kidney sections from normal control and KV-treated only (KV 100 mg/kg/b.w.) rats showed normal glomerulus and tubules with usual morphology (CN and 100KV; Figure 5). However, histological analysis of the kidneys from DF-treated rats showed distorted renal corpuscles with hyper-infiltration of the glomerulus and increased mesangial matrix as well as severe and widespread of necrosis of the renal tubules (particularly proximal tubules), which lead to loss of tubular cellular constituents, with dilatation of renal vessels and tubular cell desquamation and intraluminal cast formation and infiltration of inflammatory leukocytes (DF; Figure 5). Histological analysis of the kidneys from DF-treated rats pretreated with KV showed less histopathological renal alteration (100KV + DF and 200KV + DF).
Figure 5.
The histoarchitecture of the normal control and kolaviron treated only (KV 100 mg/kg/bw) rats kidneys showed normal glomerulus and tubules with usual morphology (Figure 5 (CN) and (100 KV)). However, histological analysis of the kidneys from DF-treated rats showed distorted renal corpuscles with hyper-infiltration of the glomerulus and increased mesangial matrix as well as severe and widespread of necrosis of the renal tubules (particularly proximal tubules), which lead to loss of tubular cellular constituents, with dilatation of renal vessels and tubular cell desquamation and intraluminal cast formation and infiltration of inflammatory leukocytes (Figure 5 (DF)). Histological analysis of the kidneys from DF-treated rats pretreated with kolaviron showed less histopathological renal alteration (100 KV + DF and 200 KV + DF). DF indicates Diclofenac; KV, kolaviron; 100 KV, 100 mg/kg of kolaviron; 200 KV, 200 mg/kg of kolaviron.
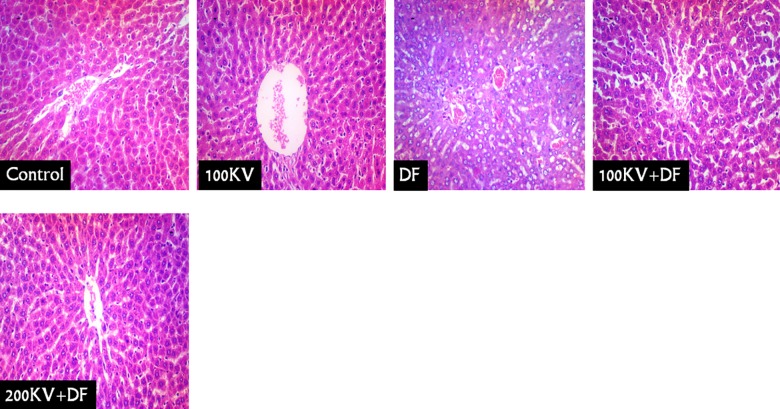
Liver sections of the normal control and KV-treated only (KV 100 mg/kg/b.w.) rats showed normal hepatic cells with usual morphology (CN and 100KV; Figure 6). However, the liver of DF-treated rats had severe periportal congestion, bile duct proliferation in portal area, portal cellular infiltration, and diffused hydropic degeneration of hepatocytes. Histological analysis of the livers of 100KV + DF and 200KV + DF showed less histopathological hepatic alteration (100KV + DF and 200KV + DF; Figure 6).
Figure 6.
Liver sections of the normal control and kolaviron-treated only (KV 100 mg/kg/b.w.) rats showed normal hepatocyte cells with usual morphology (Figure 6 (CN) and (100 KV)). However, the liver of DF treated rats showed dilated central vein, mild paracentral, hepatocyte cell loss, prominent cell degeneration, and inflammation in the centrilobular areas. Severe periportal congestion, bile duct proliferation in portal area, portal cellular infiltration, and diffused hydropic degeneration of hepatocytes were also seen in DF-treated group. Histological analysis of the livers of 100 KV + DF and 200 KV + DF showed less histopathological hepatic alteration (Figure 6 (100 KV + DF and 200 KV + DF)). DF indicates Diclofenac; KV, kolaviron; 100 KV, 100 mg/kg of kolaviron; 200 KV, 200 mg/kg of kolaviron.
Discussion
In the present study, DF treatment caused an alteration in the pro-inflammatory response, antioxidant status, lipid peroxidation, hematological indices, plasma, and tissues prostaglandin synthesis and significantly increases the plasma activities of ALT, AST and ALP, creatinine, urea, and bilirubin level. However, KV pretreatment prevented these toxic effects of DF.
Diclofenac treatment in this study resulted to significant decrease in body weight of rats. The decrease in the body weight of rats treated with DF alone could be correlated with incidence of diarrhea in the treated rats. The increase in body weight of rats pretreated with KV prior to DF treatment prevents reduction in body mass and improved fitness of the rats after DF treatment. These attribute showed beneficial health effect of KV during DF treatment in rats.
Researches confirmed that DF treatment caused generation of ROS, which increased oxidative stress as a result of reduction of the antioxidant system activities.15 In this study, the significant increases in the level of lipid peroxidation marker—MDA and hydrogen peroxide (H2O2)—were indication of reduction in the body antioxidant system and decrease body defense mechanism to scavenging the free radicals. In the present study, the kidney tissues antioxidant enzymes, SOD and CAT, were increased along with MDA and H2O2 in DF-treated rats, while the liver tissues antioxidant enzymes (SOD and CAT) were found to be decreases following DF treatment. The increased in SOD and CAT observed in the kidney of DF-treated group may be due to an increase in the renal tissues H2O2 and OH− caused by inhibition of peroxidases.33,34 The reduced activities of liver tissue SOD and CAT were result of elevated level of MDA and H2O2, which caused pathological damages to the liver.17 There was a marked reduction in renal and hepatic nonenzymatic antioxidant (GSH) levels in the DF-treated group compared with the control. This observed decline may confirm an impaired antioxidant defense and thus increased the susceptibility of both the kidney and liver to oxidative stress. Kolaviron treatment shows antioxidant property by inhibiting DF exposure threat, which is a pro-oxidative agents. Researchers have suggested that KV is a good source of antioxidants in the biological system and its therapeutic effects have been attributed to its ROS scavenging ability.17,35 Kolaviron administered groups prior to DF treatment normalized kidney and liver antioxidant enzymes (SOD and CAT) and stabilize nonenzymatic antioxidant GSH level. Thus, the normalization of SOD, CAT, and GSH in KV-treated groups therefore suggested a protective effect of KV against ROS overproduction induced by DF in the kidney and liver of the rats.
Diclofenac has been reported to cause kidney and liver injuries in rats.5,17 In this study, DF treatment significantly elevated plasma creatinine and urea level, indicating compromised of renal function. Similarly, the plasma activities of liver enzymes, such as AST, ALT, ALP, and including total bilirubin level, were significantly increased in the rats treated with DF. These results were in agreement with previous studies which indicated that DF caused significant elevation of all these markers when compared with control group.8,17 Elevation of creatinine and urea in the blood suggested a compromised integrity of the glomerular filtration rate barrier, leading to impaired renal function. It has been reported that administration of DF caused a dramatic elevation in serum AST and ALT, indicated hepatotoxicity with severe damage to hepatic tissue membrane and the leakage of these enzymes into circulation.17,18 The increase in the activity of plasma ALP and bilirubin level in DF-treated rats indicated injury to the biliary duct of the liver.36 However, treatment with KV before DF injection resulted in marked decreased in plasma levels of creatinine and urea and decreased in the plasma activities of AST, ALT, ALP, and bilirubin level, shown improvement in both kidney and liver functions. These results are in accordance with the previous researches.17,26,37 Ateşşahín et al38 and Abdel-Daim et al39 have confirmed ROS over generation in kidney and liver tissues as a result of increase in MDA concentrations in the renal and hepatic tissues, which resulted to necrosis and back leak of liver enzymes and kidney function makers into circulation. Interestingly, the protective effect observed for KV in both the kidneys and liver of rats treated with DF indicated that KV may be involved in the elimination of ROS or other reactive bye products generated by DF toxic metabolites in both the kidney and liver tissues.
Researches have confirmed that prostaglandins use different physiologic effects from protective to cytotoxic and their modes of action are cell and organ precise.40,41 In this study, DF-treated rats had a significantly lower kidney and liver PGE2 level, indicating inhibition of prostaglandin synthesis. It has been reported that normal renal tissue contains sufficient amount of arachidonic acid and COX.26 Alabi et al26 confirmed in their previous study that conversion of arachidonic acid to PGE2 in the kidneys of DF-treated rats was probably repressed at the phase of its disintegration to PGG2. As a result of this blocking effect of DF, less PGG2 will be available for conversion to PGH2 and subsequently to PGE2.42 Hence, the inhibited renal prostaglandin in rats treated with DF could result to disruption of renal physiology by reducing kidney blood flow and glomerular filtration rate43 and altering electrolytes homeostasis.44 Additionally, the inhibited liver prostaglandin could result to inability of the liver to regenerate. Researches have confirmed that treatment with COX-2 inhibitor inhibits hepatic prostaglandin synthesis and impaired hepatic tissue regeneration as a result of low level of prostaglandin in binding to G protein receptors available for cyclic adenosine monophosphate signaling necessary for liver regeneration.45 However, KV administration prevented alteration caused by DF in both renal and hepatic PGE2 synthesis. Kolaviron has been previously reported to increase the concentrations of GSH molecule in biological system,46 hence facilitated the process of PGE2 synthesis in the renal and hepatic cells. Thus, beneficial activity of KV against DF toxicity in liver and kidney could be due to the ability of KV in enhancing the concentrations of GSH, which was reported to serve as electron donor in the process of conversion of PGG2 to PGH2 and subsequently to PGE2.41,47
Plasma PGE2 was observed to increase in rats treated with DF when compared with the control group. This was as a result of inflammatory effects of DF on the systems of the rats. It has been confirmed that COX was overexpressed as a result of pro-inflammatory cytokines induction which trigger prostaglandins released at the site of inflammation.48 Pretreatment of rats with KV alleviates the increase in plasma level of PGE2 induced by DF toxic metabolites.
Inflammation is considered as one of the consequences of oxidative stress, the pathways that activate the production of inflammatory mediators.49 Researches confirmed that over production of free radicals initiate production of nuclear factor-kappa B (NF-κB) and other intracellular signaling cascade that enhances the expression of pro-inflammatory gene such as IL-1β, IL-6, TNF-α, and COX-2.50,51 Tumor necrosis factor α, IL-6, decreased WBC count, increased PLT/LYM, and NEU/LYM ratios have been considered as systemic inflammation index.48,52-54 Our results showed an elevation in the plasma levels of TNF-α and IL-6 on DF-treated rats, indicating the formation of oxidative stress and inflammation, which could ultimately lead to the activation of NF-κB. In addition to pro-oxidative events, the administration of DF also elicited pro-inflammatory reactions. This was confirmed by the significant decrease in the total WBC count, increase in PLT/LYM, and NEU/LYM ratios in the DF-treated rats. However, KV administration prevented the expression of pro-inflammatory cytokines (IL-6 and TNF-α) in DF-treated rats. This study further supports our previous published article on the ability of KV to suppress the expression of inflammatory mediators.17 This study indicated that the anti-inflammatory property of KV could be related to its ability to downregulate the production of IL-6 and TNF-α and normalize the PLT/LYM and NEU/LYM ratios to physiological state.
Myeloperoxidase is a hemoprotein enzyme that is stored in azurophilic granules of polymorphonuclear neutrophils and macrophages. In this study, we found significant increase activity of MPO in both liver and kidney tissues of DF-treated rats, indicating renal and hepatic tissues damage as result of granulocyte infiltration. Myeloperoxidase plays a key role in tissue damage by catalyzing the formation of hypochlorous acid (HOCl), from chloride (Cl−) and hydrogen peroxide (H2O2), producing other reactive molecules such as cross-links proteins and tyrosyl radical from oxidation of tyrosine using H2O2 as an oxidizing agent.55 The resultant events of MPO are cytotoxic and result to oxidative damage of lipids, proteins, and DNA, thereby causing both the inflammation and oxidative stress in both the liver and kidney tissues. Kolaviron administration led to reduced MPO activity in both the liver and kidney tissues, indicating an inhibitory effect of the treatment on liver and kidney tissues granulocyte infiltration and inflammation. Thus, we suggest that the anti-inflammatory activity presented by KV could be mediated, in part, through reduced activity of MPO.
In this study, the toxic effect of DF on blood parameters was demonstrated by the significant decreases in RBCs, HB, and PCV. Previous studies suggested that there was an etiological relationship between anemia and DF treatment.56 These relationship could be due to increase in osmotic fragility of RBCs. The accompanied increase in the production of free radicals in DF could promote the osmotic fragility of erythrocytes.57 Thus, DF intoxication might lead to anemia as a result of suppression of the activities of endogenous enzymatic and nonenzymatic antioxidants, and the probable increased peroxidation of lipids in the membrane of RBCs. This process results to accelerated RBCs destruction because of the altered RBCs membrane permeability, increased RBCs mechanical fragility, and/or defective iron metabolism. In addition, the reduction of HB, RBCs, and PCV in DF-treated rats could be associated with upregulation of pro-inflammatory cytokines, including IL-6. It has been reported that the increase in production of IL-6 in inflammation triggers the development of anemia via induction of hepcidin.58 Over production of hepcidin has been linked to systemic inflammation.59 Abnormal increased of hepcidin caused significant serum reduction in iron by preventing iron recycles from senescent RBCs60 and/or inhibiting iron export from the gut enterocytes, macrophages, and hepatocytes by binding to ferroportin—a transmembrane iron transporter protein that located on the basolateral surface of the gut enterocytes and the plasma membrane of macrophages.60 This event typically leads to anemia as a result of lower amount of serum iron available for developing RBCs. The antioxidant capacity of KV seemed to be responsible for the significant increases in RBC, HB, and PCV count in the treated groups. Our results are consistent with the reports of Adaramoye and Akinloye61 which confirmed that KV prevented RBC deformity caused by carbon tetrachloride by acting as a free radical scavenger and binding to the plasma membrane of the erythrocytes to protect erythrocyte membranes from free radical attack on both lipids and proteins of the membrane.
Aside the reduction in RBCs and HB, chronic treatment of DF induced an increase in the numbers of platelets and decrease in WBCs in the blood of rats. The increase in platelets count might be due to tissue injury and inflammatory response caused by DF toxic metabolites.62 However, the decrease in the WBCs number could be the consequence of inflammation during DF treatment. The rats pretreated with KV revealed significant restoration in most of the hematological indices changed in DF-treated rats. Therefore, hematological indices protective capability observed in 100KV and 200KV were similar to what we observed in our previous study.63
The histopathological evident from both the liver and kidney tissues in this study corroborated the biochemical results. Histopathologic examinations of both the liver and kidney showed severe lesions following DF treatment. However, the KV-treated groups showed intact renal and hepatic histoarchitecture. Thus, the ability of KV to maintain the structural and functional integrity of both the liver and kidney in DF-treated groups almost to the same extent as that of the control groups was evident of its chemoprotective potential in the liver and kidney of the rats.
Conclusion
This study revealed that KV protected the kidney and liver of Wistar rats against toxicity induced by DF via upregulating the antioxidant defenses, inhibition of plasma PGE2 and pro-inflammatory markers, and restoration of both renal and hepatic tissues PGE2 release back to the basal levels. Also KV prevented toxic effects of DF on hematopoietic system and renal and hepatic tissues. The hepatorenal protection of KV could be attributed in part to its ability in enhancing liver and kidney PGE2 synthesis.
Footnotes
Authors’ Note: Q.K.A. made significant contributions to conception, design, experimentation, acquisition, and interpretation of data and writing of manuscript. R.O.A. made substantial contribution in revising the manuscript for intellectual content. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Quadri K. Alabi  https://orcid.org/0000-0002-9984-0565
https://orcid.org/0000-0002-9984-0565
References
- 1. Shitara Y, Sato H, Sugiyama Y. Evaluation of drug-drug interaction in the hepatobiliary and renal transport of drugs. Annu Rev Pharmacol Toxicol. 2005;45:689–723. [DOI] [PubMed] [Google Scholar]
- 2. Nair SS, Manalil JJ, Ramavarma SK, Suseela IM, Thekkepatt A, Raghavamenon AC. Virgin coconut oil supplementation ameliorates cyclophosphamide-induced systemic toxicity in mice. Hum Exp Toxicol. 2016;35(2):205–212. [DOI] [PubMed] [Google Scholar]
- 3. Abdel-Daim MM, Abdeen A. Protective effects of rosuvastatin and vitamin E against fipronil-mediated oxidative damage and apoptosis in rat liver and kidney. Food Chem Toxicol. 2018;114:69–77. [DOI] [PubMed] [Google Scholar]
- 4. Brater DC. Renal effects of cyclooxygyenase-2-selective inhibitors. J Pain Symptom Manage. 2002;23(4):S15–S23. [DOI] [PubMed] [Google Scholar]
- 5. Tomic Z, Milijasevic B, Sabo A, et al. Diclofenac and ketoprofen liver toxicity in rat. Eur J Drug Metab Pharmacokinet. 2008;33(4):253–260. [DOI] [PubMed] [Google Scholar]
- 6. Harirforoosh S, West KO, Murrell DE, Denham JW, Panus PC, Hanley GA. Examination of the pharmacodynamics and pharmacokinetics of a diclofenac poly(lactic-co-glycolic) acid nanoparticle formulation in the rat. Eur Rev Med Pharmacol Sci. 2016;20(23):5021–5031. [PubMed] [Google Scholar]
- 7. Besen A, Kose F, Paydas S, et al. The effects of the nonsteroidal anti-inflammatory drug diclofenac sodium on the rat kidney, and alteration by furosemide. Int Urol Nephrol. 2009;41(4):919–926. [DOI] [PubMed] [Google Scholar]
- 8. Chandrasekharan NV, Dai H, Roos KL, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99(21):13926–13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wood RC, III, Wyatt JE, Bullins KW, et al. Effects of rebamipide on nephrotoxicity associated with selected NSAIDs in rats. Eur J Pharmacol. 2013;720(1-3):138–146. [DOI] [PubMed] [Google Scholar]
- 10. Mazumdar K, Dutta NK, Dastidar SG, Motohashi N, Shirataki Y. Diclofenac in the management of E. coli urinary tract infections. In Vivo. 2006;20(5):613–619. [PubMed] [Google Scholar]
- 11. Novartis. Voltaren-XR (Diclofenac Sodium Extended-Release Tablets) Prescribing Information. East Hanover, NJ: Novartis; 2006. [Google Scholar]
- 12. Abdel-Daim MM, Eltaysh R, Hassan A, Mousa SA. Lycopene attenuates tulathromycin and diclofenac sodium-induced cardiotoxicity in mice. Int J Mol Sci. 2018;19(2):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Batlouni M. Nonsteroidal anti-inflammatory drugs: cardiovascular, cerebrovascular and renal effects [in Portuguese]. Arq Bras Cardiol. 2010;94(4):556–563. [DOI] [PubMed] [Google Scholar]
- 14. Gil ML, Ramirez MC, Terencio MC, Castell JV. Immunochemical detection of protein adducts in cultured human hepatocytes exposed to diclofenac. Bioch Biophysic Acta. 1995;1272(3):140–146. [DOI] [PubMed] [Google Scholar]
- 15. Galati G, Tafazoli S, Sabzevari O, Chan TS, O’Brien PJ. Idiosyncratic NSAID drug induced oxidative stress. Chem Biol Interact. 2002;142(1-2):25–41. [DOI] [PubMed] [Google Scholar]
- 16. Masubuchi Y, Nakayama S, Horie T. Role of mitochondrial permeability transition in diclofenac-induced hepatocyte injury in rats. Hepatology. 2002;35(3):544–551. [DOI] [PubMed] [Google Scholar]
- 17. Alabi QK, Akomolafe RO, Olukiran OS, et al. The Garcinia kola biflavonoid kolaviron attenuates experimental hepatotoxicity induced by diclofenac. Pathophysiology. 2017;24(4):281–290. [DOI] [PubMed] [Google Scholar]
- 18. Iwu MM, Igboko OA, Okunji CO, Tempesta MS. Antidiabetic and aldose reductase 248 activities of biflavanones of Garcinia kola. J Pharm Pharmacol. 1990;42(4):290–292. [DOI] [PubMed] [Google Scholar]
- 19. Maalej A, Mahmoudi A, Bouallagui Z, Fki I, Marrekchi R, Sayadi S. Olive phenolic compounds attenuate deltamethrin-induced liver and kidney toxicity through regulating oxidative stress, inflammation and apoptosis. Food Chem Toxicol. 2017;106(pt A):455–465. [DOI] [PubMed] [Google Scholar]
- 20. Farombi EO, Alabi MC, Akuru TO. Kolaviron modulates cellular redox status and impairment of membrane protein activities induced by potassium bromate (kbro3) in rats. Pharmacol Res. 2002;45(1):63–68. [DOI] [PubMed] [Google Scholar]
- 21. Iwu MM. Handbook of African Medicinal Plants. London, United Kingdom: CRC Press; 1993, 183–184. [Google Scholar]
- 22. Olaleye SB, Farombi EO, Adewoye EA, Owoyele BV, Onasanwo SA, Elegbe RA. Analgesic and anti-inflammatory effects of kolaviron (a Garcinia kola seed extract). Afr J Biomed Res. 2000;3(3):171–174. [Google Scholar]
- 23. Terashima K, Takaya Y, Niwa M. Powerful antioxidative agents based on Garcinoic acid from Garcinia kola. Bioorg Med Chem. 2002;10(5):1619–1625. [DOI] [PubMed] [Google Scholar]
- 24. Farombi EO, Adepoju BF, Ola-Davies OE, Emerole GO. Chemoprevention of aflatoxin B1-induced genotoxicity and hepatic oxidative damage in rats by kolaviron, a natural biflavonoid of Garcinia kola seeds. Eur J Cancer Prev. 2005;14(3):207–214. [DOI] [PubMed] [Google Scholar]
- 25. Abarikwu SO, Farombi EO, Pant AB. Kolaviron biflavonoids of Garcinia kola seeds protect Atrazine-induced cytotoxicity in primary cultures of rat Leydig cells. Int J Toxicol. 2012;31(4):407–415. [DOI] [PubMed] [Google Scholar]
- 26. Alabi QK, Akomolafe RO, Adefisayo MA, et al. Kolaviron attenuates diclofenac-induced nephrotoxicity in male Wistar rats. Appl Physiol Nutr Metab. 2018;43(9):956–968. doi:10.1139/apnm-2017-0788. [DOI] [PubMed] [Google Scholar]
- 27. Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- 28. Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. [DOI] [PubMed] [Google Scholar]
- 29. Beutler E, Durgun O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 30. Wolff SP. Ferrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;233:182–189. [Google Scholar]
- 31. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxide in animal tissues by thiobarbutiric acid reaction. Anal Biochem. 1979;95(2):351–358. [DOI] [PubMed] [Google Scholar]
- 32. Xia Y, Zweier JL. Measurement of myeloperoxidase in leukocyte-containing tissues. Anal Biochem. 1997;245(1):93–96. [DOI] [PubMed] [Google Scholar]
- 33. Rohrdanz E, Kahl R. Alterations of antioxidant enzyme expression in response to hydrogen peroxide. Free Radic Biol Med. 1997;24(1):27–38. [DOI] [PubMed] [Google Scholar]
- 34. Hickey EJ, Raje RR, Reid VE, Gross SM, Ray SD. Diclofenac induced in vivo nephrotoxicity may involve oxidative stress-mediated massive genomic DNA fragmentation and apoptotic cell death. Free Radic Biol Med. 2001;31(2):139–152. [DOI] [PubMed] [Google Scholar]
- 35. Farombi EO, Nwaokeafor IA. Anti-oxidant mechanisms of kolaviron: studies on serum lipoprotein oxidation, metal chelation and oxidative membrane damage in rats. Clin Exp Pharmacol Physiol. 2005;32(8):667–674. [DOI] [PubMed] [Google Scholar]
- 36. Brandoni A, Hazelhoff MH, Bulacio RP, Torres AM. Expression and function of renal and hepatic organic anion transporters in extrahepatic cholestasis. World J Gastroenterol. 2012;18(44):6387–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Adedara IA, Daramola YM, Dagunduro JO, Aiyegbusi MA, Farombi EO. Renoprotection of Kolaviron against benzo (A) pyrene-induced renal toxicity in rats. Ren Fail. 2015;37(3):497–504. [DOI] [PubMed] [Google Scholar]
- 38. Ateşşahín A, Ceríbaşi AO, Yuce A, Bulmus O, Cikim G. Role of ellegic acid against cisplatin induced nephrotoxicity and oxidative stress in rats. Basic Clin Pharmacol Toxicol. 2007;100(2):121–126. [DOI] [PubMed] [Google Scholar]
- 39. Abdel-Daim MM, Abuzead SMM, Halawa SM. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One. 2013;8(9):e72991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paller M, Manivel J. Prostaglandins protect kidneys against ischemic and toxic injury by a cellular effect. Kidney Int. 1992;42(6):1345–1354. [DOI] [PubMed] [Google Scholar]
- 41. Weber TJ, Monks TJ, Lau SS. PGE2 mediated cytoprotection in renal epithelial cells: evidence for a pharmacologically distinct receptor. Am J Physiol. 1997;273(4):F507–F515. [DOI] [PubMed] [Google Scholar]
- 42. Efrati S, Berman S, Siman-Tov Y, et al. N -acetylcysteine attenuates NSAID induced rat renal failure by restoring intrarenal prostaglandin synthesis. Nephrol Dial Transplant. 2007;22(7):1873–1881. [DOI] [PubMed] [Google Scholar]
- 43. Brater DC. Effects of nonsteroidal anti-inflammatory drugs on renal function: focus on cycloogenase-2-selective inhibition. Am J Med. 1999;107(6A):65S–70S. [DOI] [PubMed] [Google Scholar]
- 44. Harirforoosh S, Jamali F. Effect of nonsteroidal anti-inflammatory drugs with varying extent of COX-2-COX-1 selectivity on urinary sodium and potassium excretion in the rat. Can J Physiol Pharmacol. 2005;83(1):85–90. [DOI] [PubMed] [Google Scholar]
- 45. Rudnick DA, Perlmutter DH, Muglia LJ. Prostaglandins are required for CREB activation and cellular proliferation during liver regeneration. Proc Natl Acad Sci U S A. 2001;98(15):8885–8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Omole JG, Ayoka OA, Alabi QK, et al. Protective effect of kolaviron on cyclophosphamide-induced cardiac toxicity in rats. J Evid Based Integr Med. 2018;23:1–11. doi:10.1177/2156587218757649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Asbóth G, Gimes G, Hertelendy F, Tóth M. The relation between thromboxane and prostaglandin synthesis in human deciduas tissue: a comparison of eicosanoid synthesis in minced tissue with that in a cell-free preparation. Biochim Biophys Acta. 1989;1002(1):101–108. [DOI] [PubMed] [Google Scholar]
- 48. Fonseca LC, Dadarkar SS, Lobo AS, et al. 7-hydroxyfrullanolide, a sesquiterpene lactone, inhibits pro-inflammatory cytokine production from immune cells and is orally efficacious in animal models of inflammation. Eur J Pharmacol. 2010;644(1-3):220–229. [DOI] [PubMed] [Google Scholar]
- 49. Haddad JJ. Oxygen-sensitive pro-inflammatory cytokines, apoptosis signaling and redox-responsive transcription factors in development and pathophysiology. Cytokines Cell Mol Ther. 2002;7(1):1–14. [DOI] [PubMed] [Google Scholar]
- 50. Anderson MT, Staal FJ, Gitler C, Herzenberg LA, Herzenberg LA. Separation of oxidant-initiated and redox-regulated steps in the NF-κB signal transduction pathway. Proc Natl Acad Sci U S A. 1994;91(24):11527–11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Flohé L, Brigelius-Flohé R, Saliou C, Traber MG, Packer L. Redox regulation of NF-κB activation. Free Radic Biol Med. 1997;22(6):1115–1126. [DOI] [PubMed] [Google Scholar]
- 52. Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97(7):993–996. [DOI] [PubMed] [Google Scholar]
- 53. Mustafa KD, Adülkerim B. Evaluation of preoperative neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients undergoing major vascular surgery. Turkish J Thoracic Cardiovasc Surg. 2013;21(4):930–935. [Google Scholar]
- 54. Bayrakci N, Ozkayar N, Akyel F, Ates I, Akyel S, Dede F. The platelet-to-lymphocyte ratio as an inflammation marker in non-dipper hypertensive patients. Hippokratia. 2015;19(2):114–118. [PMC free article] [PubMed] [Google Scholar]
- 55. Heinecke JW. Mechanisms of oxidative damage by myeloperoxidase in atherosclerosis and other inflammatory disorders. J Lab Clin Med. 1999;133(4):321–325. [DOI] [PubMed] [Google Scholar]
- 56. El-Maddawy ZK, El-Ashmawy IM. Hepato-renal and hematological effects of diclofenac sodium in rats. Glob J Pharmacol. 2013;7(2):123–132. [Google Scholar]
- 57. Brzezinzka-Slebodzinska E. Erythrocytes osmotic fragility test as the measure of defence against free radicals in rabbits of different age. Acta Vet Hung. 2001;49(4):413–419. [DOI] [PubMed] [Google Scholar]
- 58. Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. [DOI] [PubMed] [Google Scholar]
- 59. Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci U S A. 2005;102(5):1324–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Adaramoye OA, Akinloye O. Possible protective effect of kolaviron on CCl4 induced erythrocyte damage in rats. Biosci Rep. 2000;20(4):259–264. [DOI] [PubMed] [Google Scholar]
- 62. Basavraj ST, Fefar DT, Prajapati KS, et al. Haemato-biochemical alterations induced by diclofenac sodium toxicity in Swiss albino mice. Vet World. 2012;5(7):417–419. [Google Scholar]
- 63. Alabi QK, Akomolafe RO, Olukiran OS, et al. Assessment of haematological and biochemical effects of kolaviron in male Wistar rats. Br J Pharma Res. 2017. b;16(3):1–14. [Google Scholar]