Abstract
Spatiotemporal control of integrin-mediated cell adhesion to the extracellular matrix (ECM) is critical for physiological and pathological events in multicellular organisms, such as embryonic development, angiogenesis, platelet aggregation, leukocytes extravasation, and cancer cell metastatic dissemination. Regulation of integrin adhesive function and signaling relies on the modulation of both conformation and traffic. Indeed, integrins exist in a dynamic equilibrium between a bent/closed (inactive) and an extended/open (active) conformation, respectively endowed with low and high affinity for ECM ligands. Increasing evidence proves that, differently to what hypothesized in the past, detachment from the ECM and conformational inactivation are not mandatory for integrin to get endocytosed and trafficked. Specific transmembrane and cytosolic proteins involved in the control of ECM proteolytic fragment-bound active integrin internalization and recycling exist. In the complex masterplan that governs cell behavior, active integrin traffic is key to the turnover of ECM polymers and adhesion sites, the polarized secretion of endogenous ECM proteins and modifying enzymes, the propagation of motility and survival endosomal signals, and the control of cell metabolism.
Keywords: cell adhesion, extracellular matrix , integrins, protein conformation, trafficking
Introduction
Metazoan cells attach to extracellular matrix (ECM) proteins via integrin αβ heterodimers that, through a series of cytosolic adaptor proteins, physically connect to the actin cytoskeleton and modulate the enzymatic activity of kinases, phosphatases, and small GTPases [1–3]. Overall, the complex protein network associated with integrin-based adhesion sites is known as the adhesome [2,3]. Twenty-four integrin heterodimers exist which promiscuously allow the interaction with hundreds of ECM proteins in different tissues and organs [4]. On the cellular surface, integrin receptors are in an allosteric equilibrium between a bent/inactive and an extended/active conformation that respectively interact at a low and high affinity with ligands [1]. The percentage of surface integrins adopting the extended/active conformation vary significantly depending on the adhesion and spreading degree of cell types, being for example ∼0.2% in poorly adherent K562 leukemia cells [5] or 10% in widely spread endothelial cells (ECs) [6]. Active integrin conformers are stabilized by the four-point-one, ezrin, radixin, moesin (FERM) domain of talin [7,8] and kindlin [8,9] adaptor proteins with a membrane proximal and distal Asn-Pro-X-Tyr (NPXY) motif in the cytodomain of integrin β subunits, respectively. Both talins and kindlins are required for integrin conformational activation, to which they seem to contribute differently by allowing the vinculin-mediated perception of mechanical forces (talins) and triggering biochemical signaling pathways (kindlins) [8], e.g. through paxillin and focal adhesion kinase (FAK) [10–12]. It has long been thought that the appearance of integrins and associated proteins allowed the evolutionary transition from unicellular to multicellular organisms [13]. Yet, over the last decade, independent studies proved that genes encoding for integrins [14,15] and most adhesome proteins, such as talins [15], but not kindlins [16], were already present in unicellular ancestors of animals that exploited them in the aggregation phase of their life cycle [13]. Hence, it is conceivable that the emergence of kindlins had been important for the development of multicellular organisms [16].
The dynamic control of integrin conformational activation is central in different physiological and pathological settings, such as tissue and organ development, platelet aggregation, leukocyte extravasation, autoimmune diseases, fibrosis, and cancer [1,17–19]. In these contexts, the small GTPase Rap1 is a main driver of integrin conformational activation. Indeed, talin exists in an autoinhibited state that is relieved by its direct [20–24] or indirect (e.g. through Rap1-interacting adapter molecule — RIAM) [25] binding to Rap1-GTP, allowing the FERM domain of talin to bind and promote integrin activation. Both the fulfillment of complex morphogenetic programs [26,27] as well as the normal functioning of platelets and leukocytes [1,25,28] rely on a fine spatiotemporal modulation of integrin activation by Rap1 and talin. In this scenario, chemoattractant ligands, e.g. C-X-C motif chemokine 12 (CXCL12) [29] or vascular endothelial growth factor-A (VEGF-A) [30], promote Rap1 activation via G protein coupled receptors (GPCRs) or tyrosine kinase receptors (TKRs) coupled to downstream Rap1 guanine nucleotide exchange factors (GEFs) [31]. Conversely, chemorepulsive ligands such as semaphorins (SEMAs) signal through the cytosolic GTPase activating protein (GAP) of Plexin receptors to inhibit Rap1 GTP loading [32–34] and integrin activation [17,18]. The evidence that both talin autoinhibition [27] and Plexin-mediated SEMA inhibition of Rap1 [17,34,35] are required for morphogenesis, in diverse experimental systems, supports the concept that conformational integrin inactivation is as crucial as activation for the accomplishment of complex shaping programs in animal tissues, organs and systems [17,36–39].
In addition to integrin conformational activation, cell-to-ECM adhesion contact dynamics critically relies on integrin traffic [19,40–43]. While it has long been thought that detachment from ECM ligands [44,45] and conformational inactivation [46] were necessary to allow integrin internalization, in the last decade, thanks to the availability of monoclonal antibodies detecting activation dependent epitopes [47], different laboratories showed that conformationally active integrins can also be endocytosed and trafficked under the control of molecular machineries and signaling pathways distinct from those regulating inactive integrin traffic. Here, we will review our current understanding of the rationale and the mechanisms by which cells traffic conformationally active integrins in both normal and cancer cells.
Active integrin traffic controls ECM adhesion site turnover and cell metabolism
Integrin binding to soluble monomeric ECM proteins, such as fibronectin (FN), results in traction force-dependent unmasking the otherwise cryptic protein–protein interaction sites that promote ECM polymerization in insoluble protein networks [48,49]. Polymerized FN act as a scaffold on which collagen I molecules are deposited to form fibrils and fibers via repeated cycles of integrin-mediated contraction and relaxation [50]. Subsequently, matrix metalloproteinases (MMPs) cleave ECM fibrils into fragments that get endocytosed and degraded, thus requiring the replenishment with freshly synthesized ECM molecules (Figure 1). Thus, ECM meshworks are unstable objects whose dynamic remodeling is crucial for tissue morphogenesis and healing as well as cancer cell invasion and dissemination [51]. Additionally, it was found, in keratinocytes, that microtubules anchored by cytoplasmic linker associated proteins (CLASPs) in proximity of ECM adhesions allow the targeted transport of secretory vesicles, which locally deliver membrane-type 1 MMP (MT1-MMP) [52] to degrade the ECM [53]. Moreover, it has been shown, in fibroblasts, that, upon MT1-MMP dependent cleavage [54], FN fragments bound to active α5β1 get endocytosed, trafficked and degraded into lysosomes [55]. As a result, in the absence of such constant FN synthesis, secretion and polymerization, FN fibrils disappear, being degraded [56]. Integrins have been reported to mediate the internalization and turnover of other ECM proteins, e.g. type I collagen [57] in fibroblasts, vitronectin [58] and laminin 111 [59] in cancer cells. Therefore, active integrins participate to the control of ECM turnover on the cell surface by mediating the endocytosis of MMP-cleaved ECM fragments in different cell types.
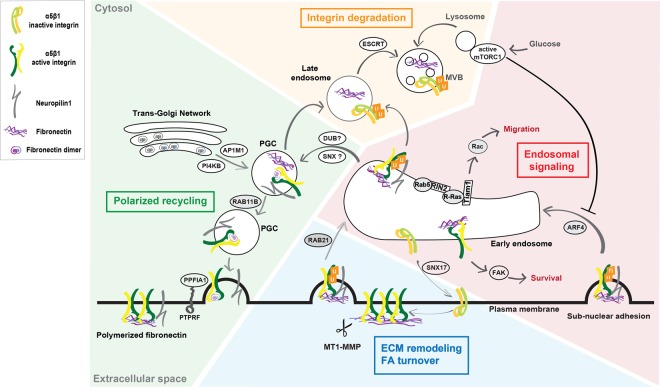
Figure 1. Active integrin internalization and trafficking pathways.
Upon MMP-dependent cleavage of polymerized FN fibrils, the endocytic receptor NRP1 interacts on the cell surface with FN fragment-bound ubiquitinated α5β1 active-integrin that is internalized within early endosomes (EEs) through either Rab21, or Rab5, or Afr4 small GTPase, depending on the internalization site and cell type (see text and Figure 2). In EEs, active α5β1 integrin may activate different signaling pathways such as: (i) Rac1 to promote integrin-dependent cell spreading and migration on FN; (ii) FAK to suppress anoikis in normal cells or promote cancer cell anchorage-independent growth. Moreover, in Rab25 overexpressing ovarian cancer cells Arf4-dependent active α5β1 integrin is required for correct lysosome positioning and activation of mTORC1, whose kinase activity is also regulated by glucose levels. From the EE compartment, inactive integrins can move back to the plasma membrane via sorting nexin 17 (SNX17) and the retriever complex [124]. A fraction of FN fragment-bound active α5β1 integrins is sorted into multivesicular bodies (MVBs) and degraded into lysosomes; this degradative fate depends on α5β1 integrin ubiquitination and on endosomal sorting complexes required for transport (ESCRT) protein complex. Another fraction of FN fragment-bound active α5β1 integrin instead traffics to PGCs; it is still unclear if this latter step relies on deubiquitinase (DUB) and/or SNX activities. It is posited that, in PGCs, endocytosed old FN may be separated from active α5β1 integrin that would then be free and able to bind freshly synthesized FN originating from TGN cisternae in a PI4KB and AP-1A-dependent manner. Through a RAB11B-dependent endosomal recycling pathway, vesicles containing newly synthesized FN-bound active α5β1 integrins are next directed to the basolateral side of the cell surface, where the PTPRF/PPFIA1 complex support their docking, likely via PPFIA1-mediated interaction with the β1 integrin subunit cytodomain.
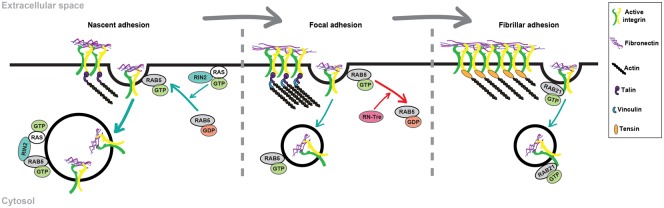
In agreement with the fact that active integrins act as receptors for ECM internalization, several key regulators of endocytosis were found to localize at adhesion sites and regulate the rate at which their molecular components are renewed both in vitro and in vivo. The small GTPase Rab5 concentrates at myotendinous junctions (MTJs) of Drosophila embryos, where it promotes β position-specific (βPS) integrin turnover allowing MTJ remodeling in developing skeletal muscles [60]. Of note, internalization rather than lateral diffusion in the plasma membrane is the main mechanism responsible of βPS integrin dynamics in Drosophila MTJs [60]. In adhesion sites, integrins withstand retrograde actin flow-driven traction [61], exist in stationary ECM-bound subpopulations [62], and active integrins form tightly ordered nanoclusters [63]. MMP-assisted endocytosis may hence represent an efficient strategy to allow the turnover of ECM-bound active integrins at adhesion sites. The Rab5 GEF Ras and Rab interactor 2 (RIN2) [64] and the Rab5 GAP USP6NL (also known as RN-Tre) [65,66] respectively localize in nascent adhesions and focal adhesions to promote or inhibit active β1 integrin endocytosis and motility of ECs and fibroblasts (Figure 2). In migrating cells, small, round, and peripheral nascent adhesions initially form at leading edge lamellipodium and later either disassemble or, due to actomyosin contractility, mature into elongated and stable focal adhesions [67]. It is tempting to speculate that, by exerting opposite effects on Rab5 GTP-loading and active β1 integrin internalization, RIN2 [64] and RN-Tre [65,66] may co-operate in funneling the conversion of nascent adhesions into focal adhesions in migrating cells. In fibroblasts, FN-bound α5β1 integrin slides outside focal adhesions and translocates along stress fibers giving rise to elongated fibrillar adhesions and FN fibrils [67] that are not influenced by Rab5 activity [65]. At least in ECs [6], active α5β1 integrin endocytosis at fibrillar adhesions and FN fibril turnover are promoted instead by Rab21, which localizes to adhesion sites [68,69] and was previously reported to stimulate integrin internalization [68,70,71] (Figure 2).
Figure 2. Working model for Rab5/Rab21 small GTPase interplay in active α5β1 integrin endocytosis in non-cancer cells.
The Rab5 GEF RIN2 and Rab5 GAP RN-Tre/USP6NL are respectively located in nascent and focal adhesions. Due to their enzymatic antagonistic activity on Rab5 GTP-loading and active α5β1 integrin internalization, RIN2 and RN-Tre might co-operate to foster the evolution of nascent into focal adhesions. Moving along stress fibers, active α5β1 integrins generate fibrillar adhesions and FN fibrils, which are not influenced by Rab5. Indeed, in ECs active α5β1 integrin endocytosis at fibrillar adhesions and FN fibril turnover are promoted instead by Rab21.
Differently from ECs [6], Arf4, rather than Rab21, was implicated in the endocytosis of FN-bound active α5β1 integrin from FN fibrils specifically localized in the subnuclear of area of A2780 ovarian cancer cells overexpressing Rab25 (A2780-Rab25) [72] (Figure 1), which was previously found to co-operate with chloride intracellular channel protein 3 (CLIC3) to allow the recycling from late endosomes/lysosomes of endocytosed FN-bound active α5β1 integrin in this cell line [73]. Interestingly, in A2780-Rab25 ovarian cancer cells, upon endocytosis of FN-bound active α5β1 integrin and its delivery to late endosomes/lysosomes, likely due to the increased lysosomal concentration of amino acids caused by FN degradation, the master regulator of cell metabolism and growth mechanistic target of rapamycin complex 1 (mTORC1) gets activated [72] (Figure 1). Similarly, in starving conditions, mammary epithelial cells up-regulate α6β4 integrin-mediated laminin endocytosis and lysosomal degradation thus resulting in mTORC1 activation [74]. In addition, pancreatic ductal adenocarcinoma cells internalize and degrade type I and type IV collagens as source of proline that fuels tricarboxylic acid cycle metabolism under nutrient limited conditions [75]. Finally, the metabolic sensor adenosine monophosphate activated protein kinase (AMPK), which is activated when AMP level raises during energy stresses, was discovered to inhibit the transcription of the α5β1 integrin-specific adaptors and activators tensin1 and 3, thus impairing α5β1 integrin activation and FN fibrillogenesis [76].
In sum, the turnover of the different types of cell adhesion structures relies on distinct pro-endocytic small GTPases and regulatory proteins that differentially modulate in space and time the internalization of ECM-bound active integrins. Furthermore, a direct link between ECM-bound active integrin traffic and nutrient signaling exists. To support their proliferation rate, cancer cells exploit active integrin-mediated ECM endocytosis as an effective strategy to directly acquire nutrients from the extracellular environment.
Active integrin traffic role in establishing cell polarity
Polarized epithelial cells, neurons, vascular ECs, and directional migrating cells are characterized by spatially and functionally distinct plasma membrane areas defined by PAR proteins, the CRB complex, and phosphatidylinositol-phosphates (PIPs) [77]. β1 integrin-mediated adhesion to the ECM elicits biochemical signals aimed at establishing and maintaining apico-basal polarity both in epithelial cells [78] and ECs [79]. Specifically, β1 integrin functions upstream of PAR polarity proteins in the signaling cascade that defines EC apico-basal axis, driving vascular morphogenesis and lumen formation [79] in response to FN [80,81]. On the contrary, basement membrane proteins, such as laminin, are alternative β1 integrin ligands that inhibit vascular morphogenesis, while maintaining the stability of mature blood vessels [80,81]. During blood vessel formation, once apico-basal axis is defined, FN-bound active α5β1 integrin signals to keep directing the secretion of freshly synthesized endogenous FN towards the abluminal basolateral plasma membrane of ECs [82], giving rise to a self-sustaining polarity signaling cascade.
In ECs, apart from its ability to extracellularly interact with SEMA3A or VEGF-A [83], the transmembrane glycoprotein neuropilin 1 (NRP1) localizes at adhesion sites [66,69,84–86], where it promotes FN-bound active α5β1 integrin endocytosis [84] (Figure 1). The binding of extracellular NRP1 b1 domain to the C-terminal basic motif of SEMA3A [34,83] or C-end rule (CendR) peptides [87] fosters the internalization of NRP1 [88] and associated membrane receptor cargos, such as active α5β1 integrin [34,84]. NRP1-dependent endocytosis largely relies on its short cytodomain [84,88] that, via its C-terminal Ser-Glu-Ala (SEA) motif, binds the PSD95-DLG1-ZO1 (PDZ) domain of the endocytic adaptor GAIP interacting protein C terminus, member 1 (GIPC1, also known as synectin) that associates to myosin VI (MYO6) motor to allow the transport of early endosomes through the cortical actin network [89]. Arterial branching morphogenesis is substantially impaired in knock-in mice lacking NRP1 cytodomain [90] and, albeit at lower extent, in GIPC1 [91] and MYO6 [92] knock-out animals. In contrast, knock-in mice expressing a b1 domain NRP1 mutant unable to bind VEGF-A only do not display any vascular defect [93]. Altogether, these findings support a model in which, VEGF-A-independent NRP1/GIPC1/MYO6-driven internalization of transmembrane cargos, such as integrins, promote FN-dependent branching [94] and vascular [95] morphogenesis.
Along with the fact that, upon NRP1-driven internalization, active α5β1 integrins are returned back to the EC surface [84], the observation that, via its cytodomain, NRP1 also promotes endothelial FN fibrillogenesis [84] suggests that these integrins may, either by signaling or by direct binding or both, favor the exocytosis of newly synthesized FN from perinuclear trans-Golgi network (TGN) to replace MMP-cleaved FN fibrils. This hypothesis is also in agreement with the observation that endocytosed active α5 [6] and β1 [96] integrins are recycled, likely from vesicular compartments laid closer to the nucleus and farther from the plasma membrane [97], with considerably slower kinetics compared with their inactive counterparts in different cell types. Indeed, in ECs, Rab21, which may interact with NRP1 via adaptor protein containing a PH domain, PTB domain, and leucine zipper motif 1 (APPL1) and GIPC1 [42], promotes the internalization in early endosomes of FN fragment-bound active α5β1 integrins, which subsequently traffic to post-Golgi carrier (PGC) vesicles stemmed from the TGN in a phosphatidylinositol 4-kinase, catalytic, beta (PI4KB) and clathrin adaptor protein complex-1A (AP-1A)-dependent manner and containing freshly synthesized FN [6] (Figure 1). Albeit further studies are needed to elucidate this step, once in PGCs, active α5β1 integrins might exchange exhausted and to-be-degraded FN fragments for newly synthesized FN dimers (Figure 1). Whether internalized NRP1 reaches the TGN/PGC compartment and participates in the recycling of active α5β1 integrins is still open issue. Next, RAB11B routes PGCs containing newly synthesized FN-bound active α5β1 towards basolateral side of ECs, where a complex formed by protein tyrosine phosphatase receptor type f polypeptide (PTPRF, otherwise identified as leukocyte common antigen related — LAR protein) and PTPRF interacting protein a1 (PPFIA1), also known as liprin-α1, concentrates in close proximity of fibrillar adhesions [6] (Figure 1). Intriguingly, in neuronal pre-synapses PTPRF and PPFIA1/liprin-α1 synchronize endo-exocytic traffic and mediate the docking of neurotransmitter vesicles at the plasma membrane [98,99]. In addition, similarly to kindlins, the emergence of liprins has also been implicated in the rise of multicellular organisms [16]. Analogously to its neuronal function and due to its binding to the β1 cytodomain of active α5β1 integrin, in ECs PPFIA1/liprin-α1 promotes the docking of PGCs containing newly synthesized FN and active α5β1 integrins [6]. The fusion of these PGCs with the plasma membrane may bolster the local release of newly synthesized FN and active α5β1 integrins and the exchange of old for new fibrillar FN at the abluminal side of endothelium [6]. Of note, PPFIA1/liprin-α1 silencing also affects subendothelial FN deposition and vascular morphogenesis in developing Zebrafish embryo [6]. Supporting its role in defining EC polarity [6], PPFIA1/liprin-α1 was discovered to control the basolateral secretion of at least three additional ECM components that are pivotal regulators of vascular ECM organization and remodeling, namely lysyl oxidase, multimerin 2, and dystroglycan-1 [100]. Thus, ECs employ a synaptic-like machinery to control the coupling of active α5β1 integrin endo-exocytosis and the replenishment of degraded FN fibrils with the basolateral secretion of TGN-derived newly synthesized FN [6], along with proteins involved in FN rehandling [100]. It has been for long known that, during embryogenesis, dynamically remodeling angiogenic blood vessels develop in a FN-rich ECM, which, during the subsequent vascular maturation and stabilization phase, is substituted with a laminin-rich basement membrane [81,101]. To this matter, the discovery that PPFIA1/liprin-α1 also controls the basolateral secretion of the basement membrane organizing protein dystroglycan-1 [100] suggests that PPFIA1/liprin-α1 may participate in orchestrating the transition from angiogenic to quiescent blood vessels [81].
More recently, it has been reported that the other TGN-associated clathrin adaptor protein, known to participate together with AP-1 [102] in the secretory pathway, namely Golgi-localized gamma ear-containing Arf-binding protein 2 (GGA2) [102], is also involved in active β1 integrin recycling in human MDA-MB-231 breast cancer cells [103]. The association of GGA2 with active β1 integrin is stabilized by the small GTPase RAB13, which also supports the return of internalized active β1 integrin to the cell surface. Moreover, both GGA2 and RAB13 are required for efficient cancer cell migration and invasion [103]. Of note, along with RAB13, proximity biotinylation analyses also identified PTPRF as a GGA2 interactor [103]. In addition, since PTPRF was previously reported to drive active α5β1 integrin recycling in ECs [6,104], it may hence function as a multipurpose docking receptor that, localizing at ECM adhesion sites [105], allows the polarized and targeted recycling of active β1 integrins via different TGN-connected trafficking pathways. Indeed, GGAs and AP-1 TGN-associated clathrin adaptors may have overlapping [102], as well as distinct functions, GGAs, but not AP-1, transporting ubiquitinated protein cargos [106,107]
Internalized active integrins elicit endosomal signaling pathways
Rac1-stimulated actin branched polymerization drives the formation of plasma membrane extensions, known as lamellipodia, which need to be confined at the leading edge of migrating cells to effectively allow directional motility [108]. In this context, integrin-ECM engagement at the cell front triggers a self-sustaining Rac1-activating positive feedback loop that supports lamellipodium-driven cell motility [109]. In addition, Rac1 endocytosis, from and recycling to, the plasma membrane represents a strategy to selectively restrain and polarize, in space and time, the signaling of this small GTPase [110]. Early endosomes have been identified as further subcellular sites for Rac1 activation in addition to the plasma membrane [110]. Indeed, the major Rac1 GEF T-lymphoma invasion and metastasis-inducing protein 1 (TIAM1) was found to reside on early endosomes of HeLa cancer cells [110], likely because, via its pleckstrin homology (PH) domain [111], TIAM1 binds PI3P [112], a phospholipid produced by the key Rab5 effector PI3KC3, also known as VPS34 [113] (Figure 1). The small GTPase R-Ras, one of the master regulators of integrin function [114], is highly expressed in vascular ECs [64], localizes in lamellipodia-associated nascent ECM-adhesions, where it recruits RIN2 as a result of the interaction with its Ras association (RA) domain [64]. In ECs, the binding to R-Ras converts RIN2 from a Rab5 GEF to an adaptor that first interacts at high affinity with Rab5-GTP to selectively promote ECM-bound/active β1 integrin endocytosis and next causes R-Ras repositioning on early endosomes [64]. After the active β1 integrin/Rab5/RIN2-dependent transfer on early endosomes, R-Ras contacts the RA domain of TIAM1, thus promoting the GTP loading of Rac1 [64] (Figure 1) followed by its polarized relocation to the plasma membrane, likely via the small GTPase Arf6 [110,115]. In sum, it appears that, at the leading edge of migrating ECs, the endocytosis of active β1 integrins co-ordinates the RIN2-dependent translocation of R-Ras on early endosomes, where it triggers, via TIAM1, a self-sustaining Rac1-activating positive feedback loop that drives directed cell motility. Along this line, it was previously reported that in cancer cells Rab5 gets activated upon integrin-mediated cell spreading on FN [116] that, indeed, depends on a focal adhesion kinase (FAK)-Rab5-Rac1 signaling pathway that critically acts downstream of the mechanosensing protein vinculin [117]. More recently, it has been proposed that FAK may favor Rab5 GTP loading by binding and inhibiting the Rab GAP activity [118] of the p85α subunit of PI3K [119]. Furthermore, upon GTP loading, Rac1 elicits additional phosphorylation of FAK that in turn promotes further Rac1 activation, giving rise to a positive feedback mechanism [117]. Altogether these data support a model in which active integrin endocytosis is key in promoting the GTP loading of Rac1 to enable cell spreading.
FAK activation at the plasma membrane regulates cell proliferation, survival, migration, and invasion [120]. However, it has been proposed that endosomal FAK signaling may also support the resistance of normal cells to anoikis as well as breast cancer cell anchorage independent growth and metastatic dissemination [121]. In the cytoplasm, FAK exists in an autoinhibited conformation and the binding of its FERM domain to PI(4,5)P2, which at the plasma membrane is generated in close proximity to ECM-bound integrins, elicits FAK conformational activation [120]. Consistently, the FERM domain is sufficient to recruit FAK to active integrin containing endosomes through still unknown mechanisms [121] (Figure 1). While conformational activation is not required for vesicular targeting, FAK endosomal signaling strictly depends on its tyrosine kinase activity. Similarly to what observed at the plasma membrane [122], FAK, once activated, may promote talin recruitment and tension-independent activation on endosomes. Mechanistically, it has been proposed that FAK phosphorylates and activates type I phosphatidylinositol phosphate kinase (PIPKIγi2), generating PI(4,5)P2 promoting talin recruitment [123]. The presence of talin on endosomes may facilitate the maintenance of integrin active conformation during its recycling to the plasma membrane, even in the absence of ligands [123].
Perspective
Differently from what previously hypothesized, integrins do not need to be conformationally inactivated to be endocytosed. Dedicated transmembrane and cytosolic proteins contribute to ECM-bound active integrin internalization and recycling to the cell surface, along with the secretion of newly synthesized ECM.
Active integrin endocytosis and traffic control ECM and adhesion site turnover, metabolism, polarity, and endosomal signaling supporting motility and survival both of normal and cancer cells.
It will be crucial to identify and thoroughly dissect the molecular mechanisms responsible for those signaling aspects of active integrin traffic still poorly understood, such as those involved in the control of ECM-containing PGCs from the TGN or in the targeting of FAK on endosomes.
Acknowledgements
The authors thank Giulia Villari for critical reading of the manuscript and discussion.
Abbreviations
- AMPK
adenosine monophosphate activated protein kinase
- AP-1A
adaptor protein complex-1A
- CLASPs
cytoplasmic linker associated proteins
- CLIC3
chloride intracellular channel protein 3
- CXCL12
C-X-C motif chemokine 12
- ECM
extracellular matrix
- ECs
endothelial cells
- FAK
focal adhesion kinase
- FERM
four-point-one, ezrin, radixin, moesin
- FN
fibronectin
- GAP
GTPase activating protein
- GEFs
guanine nucleotide exchange factors
- GGA2
Golgi-localized gamma ear-containing Arf-binding protein 2
- MMPs
matrix metalloproteinases
- MT1-MMP
membrane-type 1 MMP
- MTJs
myotendinous junctions
- MYO6
myosin VI
- NRP1
neuropilin 1
- PGC
post-Golgi carrier
- PI4KB
phosphatidylinositol 4-kinase, catalytic, beta
- PPFIA1
PTPRF interacting protein a1
- RA
Ras association
- TGN
trans-Golgi network
- TIAM1
T-lymphoma invasion and metastasis-inducing protein 1
- VEGF-A
vascular endothelial growth factor-A
- βPS
β position-specific
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
Supported by Fondazione AIRC (IG grants #16702 and 21315 to G.S.; #20366 to D.V.); FPRC-ONLUS Grant ‘FPRC - 5 per mille 2014 Ministero Salute' (to G.S.); Associazione ‘Augusto per la Vita’ (to G.S.); Fondazione Telethon [grant n. GGP15102] (to G.S.).
Author Contribution
All authors contributed substantially to the conception and design of work leading to this review, provided content and wrote the article. All authors have approved the final version for publication.
References
- 1.Bachmann M., Kukkurainen S., Hytönen V.P. and Wehrle-Haller B. (2019) Cell adhesion by integrins. Physiol. Rev. 99, 1655–1699 10.1152/physrev.00036.2018 [DOI] [PubMed] [Google Scholar]
- 2.Byron A. and Frame M.C. (2016) Adhesion protein networks reveal functions proximal and distal to cell-matrix contacts. Curr. Opin. Cell Biol. 39, 93–100 10.1016/j.ceb.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphries J.D., Chastney M.R., Askari J.A. and Humphries M.J. (2019) Signal transduction via integrin adhesion complexes. Curr. Opin. Cell Biol. 56, 14–21 10.1016/j.ceb.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 4.Hynes R.O. (2012) Evolution: the evolution of metazoan extracellular matrix. J. Cell Biol. 196, 671–679 10.1083/jcb.201109041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J., Su Y., Xia W., Qin Y., Humphries M.J., Vestweber D. et al. (2017) Conformational equilibria and intrinsic affinities define integrin activation. EMBO J. 36, 629–645 10.15252/embj.201695803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mana G., Clapero F., Panieri E., Panero V., Böttcher R.T., Tseng H.Y. et al. (2016) PPFIA1 drives active α5β1 integrin recycling and controls fibronectin fibrillogenesis and vascular morphogenesis. Nat. Commun. 7, 13546 10.1038/ncomms13546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goult B.T., Yan J. and Schwartz M.A. (2018) Talin as a mechanosensitive signaling hub. J. Cell Biol. 217, 3776–3784 10.1083/jcb.201808061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Z., Costell M. and Fässler R. (2019) Integrin activation by talin, kindlin and mechanical forces. Nat. Cell Biol. 21, 25–31 10.1038/s41556-018-0234-9 [DOI] [PubMed] [Google Scholar]
- 9.Rognoni E., Ruppert R. and Fässler R. (2016) The kindlin family: functions, signaling properties and implications for human disease. J. Cell Sci. 129, 17–27 10.1242/jcs.161190 [DOI] [PubMed] [Google Scholar]
- 10.Theodosiou M., Widmaier M., Böttcher R.T., Rognoni E., Veelders M., Bharadwaj M. et al. (2016) Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. eLife 5, e10130 10.7554/eLife.10130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klapproth S., Bromberger T., Türk C., Krüger M. and Moser M. (2019) A kindlin-3-leupaxin-paxillin signaling pathway regulates podosome stability. J. Cell Biol. 218, 3436–3454 10.1083/jcb.201903109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu L., Liu H., Lu F., Yang J., Byzova T.V. and Qin J. (2019) Structural basis of paxillin recruitment by kindlin-2 in regulating cell adhesion. Structure 27, 1686–1697.e5 10.1016/j.str.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sebé-Pedrós A., Degnan B.M. and Ruiz-Trillo I. (2017) The origin of metazoa: a unicellular perspective. Nat. Rev. Genet. 18, 498–512 10.1038/nrg.2017.21 [DOI] [PubMed] [Google Scholar]
- 14.King N., Westbrook M.J., Young S.L., Kuo A., Abedin M., Chapman J. et al. (2008) The genome of the choanoflagellate monosiga brevicollis and the origin of metazoans. Nature 451, 783–788 10.1038/nature06617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sebé-Pedrós A., Roger A.J., Lang F.B., King N. and Ruiz-Trillo I. (2010) Ancient origin of the integrin-mediated adhesion and signaling machinery. Proc. Natl Acad. Sci. U.S.A. 107, 10142–7 10.1073/pnas.1002257107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paps J. and Holland P.W.H. (2018) Reconstruction of the ancestral metazoan genome reveals an increase in genomic novelty. Nat. Commun. 9, 1730 10.1038/s41467-018-04136-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serini G., Valdembri D., Zanivan S., Morterra G., Burkhardt C., Caccavari F. et al. (2003) Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424, 391–397 10.1038/nature01784 [DOI] [PubMed] [Google Scholar]
- 18.Valdembri D., Regano D., Maione F., Giraudo E. and Serini G. (2016) Class 3 semaphorins in cardiovascular development. Cell Adh. Migr. 10, 641–651 10.1080/19336918.2016.1212805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno-Layseca P., Icha J., Hamidi H. and Ivaska J. (2019) Integrin trafficking in cells and tissues. Nat. Cell Biol. 21, 122–132 10.1038/s41556-018-0223-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu L., Yang J., Bromberger T., Holly A., Lu F., Liu H. et al. (2017) Structure of Rap1b bound to talin reveals a pathway for triggering integrin activation. Nat. Commun. 8, 1744 10.1038/s41467-017-01822-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camp D., Haage A., Solianova V., Castle W.M., Xu Q.A., Lostchuck E. et al. (2018) Direct binding of talin to Rap1 is required for cell-ECM adhesion in drosophila. J. Cell Sci. 131, jcs225144 10.1242/jcs.225144 [DOI] [PubMed] [Google Scholar]
- 22.Bromberger T., Klapproth S., Rohwedder I., Zhu L., Mittmann L., Reichel C.A. et al. (2018) Direct Rap1/Talin1 interaction regulates platelet and neutrophil integrin activity in mice. Blood 132, 2754–2762 10.1182/blood-2018-04-846766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gingras A.R., Lagarrigue F., Cuevas M.N., Valadez A.J., Zorovich M., McLaughlin W. et al. (2019) Rap1 binding and a lipid-dependent helix in talin F1 domain promote integrin activation in tandem. J. Cell Biol. 218, 1799–1809 10.1083/jcb.201810061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bromberger T., Zhu L., Klapproth S., Qin J. and Moser M. (2019) Rap1 and membrane lipids cooperatively recruit talin to trigger integrin activation. J. Cell Sci. 132, jcs235531 10.1242/jcs.235531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagarrigue F., Kim C. and Ginsberg M.H. (2016) The Rap1-RIAM-talin axis of integrin activation and blood cell function. Blood 128, 479–487 10.1182/blood-2015-12-638700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chrzanowska-Wodnicka M. (2013) Distinct functions for Rap1 signaling in vascular morphogenesis and dysfunction. Exp. Cell Res. 319, 2350–2359 10.1016/j.yexcr.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis S.J., Goult B.T., Fairchild M.J., Harris N.J., Long J., Lobo P. et al. (2013) Talin autoinhibition is required for morphogenesis. Curr. Biol. 23, 1825–1833 10.1016/j.cub.2013.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogg N., Patzak I. and Willenbrock F. (2011) The insider's guide to leukocyte integrin signalling and function. Nat. Rev. Immunol. 11, 416–426 10.1038/nri2986 [DOI] [PubMed] [Google Scholar]
- 29.Strazza M., Azoulay-Alfaguter I., Peled M., Smrcka A.V., Skolnik E.Y., Srivastava S. et al. (2017) PLCε1 regulates SDF-1α-induced lymphocyte adhesion and migration to sites of inflammation. Proc. Natl Acad. Sci. U.S.A. 114, 2693–2698 10.1073/pnas.1612900114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chrzanowska-Wodnicka M. (2017) Rap1 in endothelial biology. Curr. Opin. Hematol. 24, 248–255 10.1097/MOH.0000000000000332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gloerich M. and Bos J.L. (2011) Regulating Rap small G-proteins in time and space. Trends Cell Biol. 21, 615–623 10.1016/j.tcb.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., He H., Srivastava N., Vikarunnessa S., Chen Y.B., Jiang J. et al. (2012) Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Sci. Signal. 5, ra6 10.1126/scisignal.2002636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bos J.L. and Pannekoek W.J. (2012) Semaphorin signaling meets rap. Sci. Signal. 5, pe6 10.1126/scisignal.2002913 [DOI] [PubMed] [Google Scholar]
- 34.Gioelli N., Maione F., Camillo C., Ghitti M., Valdembri D., Morello N. et al. (2018) A rationally designed NRP1-independent superagonist SEMA3A mutant is an effective anticancer agent. Sci. Transl. Med. 10, eaah4807 10.1126/scitranslmed.aah4807 [DOI] [PubMed] [Google Scholar]
- 35.Worzfeld T., Swiercz J.M., Sentürk A., Genz B., Korostylev A., Deng S. et al. (2014) Genetic dissection of plexin signaling in vivo. Proc. Natl Acad. Sci. U.S.A. 111, 2194–2199 10.1073/pnas.1308418111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serini G. and Bussolino F. (2004) Common cues in vascular and axon guidance. Physiology (Bethesda) 19, 348–354 10.1152/physiol.00021.2004 [DOI] [PubMed] [Google Scholar]
- 37.Bussolino F., Valdembri D., Caccavari F. and Serini G. (2006) Semaphoring vascular morphogenesis. Endothelium 13, 81–91 10.1080/10623320600698003 [DOI] [PubMed] [Google Scholar]
- 38.Bouvard D., Pouwels J., De Franceschi N. and Ivaska J. (2013) Integrin inactivators: balancing cellular functions in vitro and in vivo. Nat. Rev. Mol. Cell Biol. 14, 430–442 10.1038/nrm3599 [DOI] [PubMed] [Google Scholar]
- 39.Bussolino F., Caccavari F., Valdembri D. and Serini G. (2009) Angiogenesis: a balancing act between integrin activation and inhibition? Eur. Cytokine Netw. 20, 191–196 10.1684/ecn.2009.0168 [DOI] [PubMed] [Google Scholar]
- 40.Bridgewater R.E., Norman J.C. and Caswell P.T. (2012) Integrin trafficking at a glance. J. Cell Sci. 125 (Pt 16), 3695–3701 10.1242/jcs.095810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valdembri D. and Serini G. (2012) Regulation of adhesion site dynamics by integrin traffic. Curr. Opin. Cell Biol. 24, 582–591 10.1016/j.ceb.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 42.Valdembri D., Sandri C., Santambrogio M. and Serini G. (2011) Regulation of integrins by conformation and traffic: it takes two to tango. Mol. Biosyst. 7, 2539–2546 10.1039/c1mb05066d [DOI] [PubMed] [Google Scholar]
- 43.Santambrogio M., Valdembri D. and Serini G. (2011) Increasing traffic on vascular routes. Mol. Aspects Med. 32, 112–122 10.1016/j.mam.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 44.Bretscher M.S. (1989) Endocytosis and recycling of the fibronectin receptor in CHO cells. EMBO J. 8, 1341–1348 10.1002/j.1460-2075.1989.tb03514.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawson M.A. and Maxfield F.R. (1995) Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature 377, 75–79 10.1038/377075a0 [DOI] [PubMed] [Google Scholar]
- 46.Puklin-Faucher E. and Sheetz M.P. (2009) The mechanical integrin cycle. J. Cell Sci. 122 (Pt 2), 179–186 10.1242/jcs.042127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Byron A., Humphries J.D., Askari J.A., Craig S.E., Mould A.P. and Humphries M.J. (2009) Anti-integrin monoclonal antibodies. J. Cell Sci. 122 (Pt 22), 4009–4011 10.1242/jcs.056770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwarzbauer J.E. and DeSimone D.W. (2011) Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb. Perspect. Biol. 3, a005041 10.1101/cshperspect.a005041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogel V. (2018) Unraveling the mechanobiology of extracellular matrix. Annu. Rev. Physiol. 80, 353–387 10.1146/annurev-physiol-021317-121312 [DOI] [PubMed] [Google Scholar]
- 50.Humphrey J.D., Dufresne E.R. and Schwartz M.A. (2014) Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15, 802–812 10.1038/nrm3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonnans C., Chou J. and Werb Z. (2014) Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stehbens S.J., Paszek M., Pemble H., Ettinger A., Gierke S. and Wittmann T. (2014) CLASPs link focal-adhesion-associated microtubule capture to localized exocytosis and adhesion site turnover. Nat. Cell Biol. 16, 561–573 10.1038/ncb2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y. and McNiven M.A. (2012) Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J. Cell Biol. 196, 375–385 10.1083/jcb.201105153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi F. and Sottile J. (2011) MT1-MMP regulates the turnover and endocytosis of extracellular matrix fibronectin. J. Cell Sci. 124 (Pt 23), 4039–4050 10.1242/jcs.087858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi F. and Sottile J. (2008) Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. J. Cell Sci. 121, 2360–2371 10.1242/jcs.014977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh P., Carraher C. and Schwarzbauer J.E. (2010) Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 26, 397–419 10.1146/annurev-cellbio-100109-104020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi F., Harman J., Fujiwara K. and Sottile J. (2010) Collagen I matrix turnover is regulated by fibronectin polymerization. Am. J. Physiol. Cell Physiol. 298, C1265–C1275 10.1152/ajpcell.00341.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Memmo L.M. and McKeown-Longo P. (1998) The alphavbeta5 integrin functions as an endocytic receptor for vitronectin. J. Cell Sci. 111 (Pt 4), 425–433 PMID: [DOI] [PubMed] [Google Scholar]
- 59.Leonoudakis D., Huang G., Akhavan A., Fata J.E., Singh M., Gray J.W. et al. (2014) Endocytic trafficking of laminin is controlled by dystroglycan and is disrupted in cancers. J. Cell Sci. 127 (Pt 22), 4894–4903 10.1242/jcs.152728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan L., Fairchild M.J., Perkins A.D. and Tanentzapf G. (2010) Analysis of integrin turnover in fly myotendinous junctions. J. Cell Sci. 123 (Pt 6), 939–946 10.1242/jcs.063040 [DOI] [PubMed] [Google Scholar]
- 61.Hu K., Ji L., Applegate K.T., Danuser G. and Waterman-Storer C.M. (2007) Differential transmission of actin motion within focal adhesions. Science 315, 111–115 10.1126/science.1135085 [DOI] [PubMed] [Google Scholar]
- 62.Rossier O., Octeau V., Sibarita J.B., Leduc C., Tessier B., Nair D. et al. (2012) Integrins β1 and β3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat. Cell Biol. 14, 1057–1067 10.1038/ncb2588 [DOI] [PubMed] [Google Scholar]
- 63.Spiess M., Hernandez-Varas P., Oddone A., Olofsson H., Blom H., Waithe D. et al. (2018) Active and inactive β1 integrins segregate into distinct nanoclusters in focal adhesions. J. Cell Biol. 217, 1929–1940 10.1083/jcb.201707075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandri C., Caccavari F., Valdembri D., Camillo C., Veltel S., Santambrogio M. et al. (2012) The R-Ras/RIN2/Rab5 complex controls endothelial cell adhesion and morphogenesis via active integrin endocytosis and Rac signaling. Cell Res. 22, 1479–1501 10.1038/cr.2012.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palamidessi A., Frittoli E., Ducano N., Offenhauser N., Sigismund S., Kajiho H. et al. (2013) The GTPase-activating protein RN-tre controls focal adhesion turnover and cell migration. Curr. Biol. 23, 2355–2364 10.1016/j.cub.2013.09.060 [DOI] [PubMed] [Google Scholar]
- 66.Dong J.M., Tay F.P., Swa H.L., Gunaratne J., Leung T., Burke B. et al. (2016) Proximity biotinylation provides insight into the molecular composition of focal adhesions at the nanometer scale. Sci. Signal. 9, rs4 10.1126/scisignal.aaf3572 [DOI] [PubMed] [Google Scholar]
- 67.Kechagia J.Z., Ivaska J. and Roca-Cusachs P. (2019) Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 20, 457–473 10.1038/s41580-019-0134-2 [DOI] [PubMed] [Google Scholar]
- 68.Pellinen T., Arjonen A., Vuoriluoto K., Kallio K., Fransen J.A. and Ivaska J. (2006) Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J. Cell Biol. 173, 767–780 10.1083/jcb.200509019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuo J.C., Han X., Hsiao C.T., Yates Iii J.R. and Waterman C.M. (2011) Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 13, 383–393 10.1038/ncb2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mai A., Veltel S., Pellinen T., Padzik A., Coffey E., Marjomäki V. et al. (2011) Competitive binding of Rab21 and p120RasGAP to integrins regulates receptor traffic and migration. J. Cell Biol. 194, 291–306 10.1083/jcb.201012126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pellinen T., Tuomi S., Arjonen A., Wolf M., Edgren H., Meyer H. et al. (2008) Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev. Cell 15, 371–385 10.1016/j.devcel.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 72.Rainero E., Howe J.D., Caswell P.T., Jamieson N.B., Anderson K., Critchley D.R. et al. (2015) Ligand-occupied integrin internalization links nutrient signaling to invasive migration. Cell Rep. 10, 398–413 10.1016/j.celrep.2014.12.037 [DOI] [PubMed] [Google Scholar]
- 73.Dozynkiewicz M.A., Jamieson N.B., Macpherson I., Grindlay J., van den Berghe P.V., von Thun A. et al. (2012) Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev. Cell 22, 131–145 10.1016/j.devcel.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muranen T., Iwanicki M.P., Curry N.L., Hwang J., DuBois C.D., Coloff J.L. et al. (2017) Starved epithelial cells uptake extracellular matrix for survival. Nat. Commun. 8, 13989 10.1038/ncomms13989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olivares O., Mayers J.R., Gouirand V., Torrence M.E., Gicquel T., Borge L. et al. (2017) Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat. Commun. 8, 16031 10.1038/ncomms16031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Georgiadou M., Lilja J., Jacquemet G., Guzmán C., Rafaeva M., Alibert C. et al. (2017) AMPK negatively regulates tensin-dependent integrin activity. J. Cell Biol. 216, 1107–1121 10.1083/jcb.201609066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rodriguez-Boulan E. and Macara I.G. (2014) Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 15, 225–242 10.1038/nrm3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Datta A., Bryant D.M. and Mostov K.E. (2011) Molecular regulation of lumen morphogenesis. Curr. Biol. 21, R126–R136 10.1016/j.cub.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zovein A.C., Luque A., Turlo K.A., Hofmann J.J., Yee K.M., Becker M.S. et al. (2010) Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev. Cell 18, 39–51 10.1016/j.devcel.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iruela-Arispe M.L. and Davis G.E. (2009) Cellular and molecular mechanisms of vascular lumen formation. Dev. Cell 16, 222–231 10.1016/j.devcel.2009.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Senger D.R. and Davis G.E. (2011) Angiogenesis. Cold Spring Harb. Perspect. Biol. 3, a005090 10.1101/cshperspect.a005090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turner C.J., Badu-Nkansah K. and Hynes R.O. (2017) Endothelium-derived fibronectin regulates neonatal vascular morphogenesis in an autocrine fashion. Angiogenesis 20, 519–531 10.1007/s10456-017-9563-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo H.F. and Vander Kooi C.W. (2015) Neuropilin functions as an essential cell surface receptor. J. Biol. Chem. 290, 29120–6 10.1074/jbc.R115.687327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valdembri D., Caswell P.T., Anderson K.I., Schwarz J.P., König I., Astanina E. et al. (2009) Neuropilin-1/GIPC1 signaling regulates α5β1 integrin traffic and function in endothelial cells. PLoS Biol. 7, e1000025 10.1371/journal.pbio [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ellison T.S., Atkinson S.J., Steri V., Kirkup B.M., Preedy M.E., Johnson R.T. et al. (2015) Suppression of β3-integrin in mice triggers a neuropilin-1-dependent change in focal adhesion remodelling that can be targeted to block pathological angiogenesis. Dis. Model. Mech. 8, 1105–1119 10.1242/dmm.019927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schiller H.B., Friedel C.C., Boulegue C. and Fässler R. (2011) Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 12, 259–266 10.1038/embor.2011.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Teesalu T., Sugahara K.N., Kotamraju V.R. and Ruoslahti E. (2009) C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl Acad. Sci. U.S.A. 106, 16157–16162 10.1073/pnas.0908201106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pang H.B., Braun G.B., Friman T., Aza-Blanc P., Ruidiaz M.E., Sugahara K.N. et al. (2014) An endocytosis pathway initiated through neuropilin-1 and regulated by nutrient availability. Nat. Commun. 5, 4904 10.1038/ncomms5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O'Loughlin T., Masters T.A. and Buss F. (2018) The MYO6 interactome reveals adaptor complexes coordinating early endosome and cytoskeletal dynamics. EMBO Rep. 19, e44884 10.15252/embr.201744884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lanahan A., Zhang X., Fantin A., Zhuang Z., Rivera-Molina F., Speichinger K. et al. (2013) The neuropilin 1 cytoplasmic domain is required for VEGF-A-dependent arteriogenesis. Dev. Cell 25, 156–168 10.1016/j.devcel.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chittenden T.W., Claes F., Lanahan A.A., Autiero M., Palac R.T., Tkachenko E.V. et al. (2006) Selective regulation of arterial branching morphogenesis by synectin. Dev. Cell 10, 783–795 10.1016/j.devcel.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 92.Lanahan A.A., Hermans K., Claes F., Kerley-Hamilton J.S., Zhuang Z.W., Giordano F.J. et al. (2010) VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev. Cell 18, 713–724 10.1016/j.devcel.2010.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gelfand M.V., Hagan N., Tata A., Oh W.J., Lacoste B., Kang K.T. et al. (2014) Neuropilin-1 functions as a VEGFR2 co-receptor to guide developmental angiogenesis independent of ligand binding. eLife 3, e03720 10.7554/eLife.03720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang S., Sekiguchi R., Daley W.P. and Yamada K.M. (2017) Patterned cell and matrix dynamics in branching morphogenesis. J. Cell Biol. 216, 559–570 10.1083/jcb.201610048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Astrof S. and Hynes R.O. (2009) Fibronectins in vascular morphogenesis. Angiogenesis 12, 165–175 10.1007/s10456-009-9136-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arjonen A., Alanko J., Veltel S. and Ivaska J. (2012) Distinct recycling of active and inactive β1 integrins. Traffic 13, 610–625 10.1111/j.1600-0854.2012.01327.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Neefjes J., Jongsma M.M.L. and Berlin I. (2017) Stop or go? endosome positioning in the establishment of compartment architecture, dynamics, and function. Trends Cell Biol. 27, 580–594 10.1016/j.tcb.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 98.Haucke V., Neher E. and Sigrist S.J. (2011) Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat. Rev. Neurosci. 12, 127–138 10.1038/nrn2948 [DOI] [PubMed] [Google Scholar]
- 99.Südhof T.C. (2012) The presynaptic active zone. Neuron 75, 11–25 10.1016/j.neuron.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wei H., Sundararaman A., Dickson E., Rennie-Campbell L., Cross E., Heesom K.J. et al. (2019) Characterization of the polarized endothelial secretome. FASEB J. 33, 12277–12287 10.1096/fj.201900262R [DOI] [PubMed] [Google Scholar]
- 101.Risau W. and Lemmon V. (1988) Changes in the vascular extracellular matrix during embryonic vasculogenesis and angiogenesis. Dev. Biol 125, 441–450 10.1016/0012-1606(88)90225-4 [DOI] [PubMed] [Google Scholar]
- 102.Hirst J., Borner G.H., Antrobus R., Peden A.A., Hodson N.A., Sahlender D.A. et al. (2012) Distinct and overlapping roles for AP-1 and GGAs revealed by the “knocksideways” system. Curr. Biol. 22, 1711–1716 10.1016/j.cub.2012.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sahgal P., Alanko J., Icha J., Paatero I., Hamidi H., Arjonen A. et al. (2019) GGA2 and RAB13 promote activity-dependent β1-integrin recycling. J. Cell Sci. 132, jcs233387 10.1242/jcs.233387 [DOI] [PubMed] [Google Scholar]
- 104.Hamidi H. and Ivaska J. (2017) Vascular morphogenesis: an integrin and fibronectin highway. Curr Biol. 27, R158–R161 10.1016/j.cub.2016.12.036 [DOI] [PubMed] [Google Scholar]
- 105.Serra-Pagès C., Kedersha N.L., Fazikas L., Medley Q., Debant A. and Streuli M. (1995) The LAR transmembrane protein tyrosine phosphatase and a coiled-coil LAR-interacting protein co-localize at focal adhesions. EMBO J. 14, 2827–2838 10.1002/j.1460-2075.1995.tb07282.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Puertollano R. and Bonifacino J.S. (2004) Interactions of GGA3 with the ubiquitin sorting machinery. Nat. Cell Biol. 6, 244–251 10.1038/ncb1106 [DOI] [PubMed] [Google Scholar]
- 107.Scott P.M., Bilodeau P.S., Zhdankina O., Winistorfer S.C., Hauglund M.J., Allaman M.M. et al. (2004) GGA proteins bind ubiquitin to facilitate sorting at the trans-Golgi network. Nat. Cell Biol. 6, 252–259 10.1038/ncb1107 [DOI] [PubMed] [Google Scholar]
- 108.Krause M. and Gautreau A. (2014) Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat. Rev. Mol. Cell Biol. 15, 577–590 10.1038/nrm3861 [DOI] [PubMed] [Google Scholar]
- 109.Ridley A.J. (2015) Rho GTPase signalling in cell migration. Curr. Opin. Cell Biol. 36, 103–112 10.1016/j.ceb.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Palamidessi A., Frittoli E., Garré M., Faretta M., Mione M., Testa I. et al. (2008) Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell 134, 135–147 10.1016/j.cell.2008.05.034 [DOI] [PubMed] [Google Scholar]
- 111.Snyder J.T., Rossman K.L., Baumeister M.A., Pruitt W.M., Siderovski D.P., Der C.J. et al. (2001) Quantitative analysis of the effect of phosphoinositide interactions on the function of Dbl family proteins. J. Biol. Chem. 276, 45868–45875 10.1074/jbc.M106731200 [DOI] [PubMed] [Google Scholar]
- 112.Huotari J. and Helenius A. (2011) Endosome maturation. EMBO J. 30, 3481–3500 10.1038/emboj.2011.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bilanges B., Posor Y. and Vanhaesebroeck B. (2019) PI3K isoforms in cell signalling and vesicle trafficking. Nat. Rev. Mol. Cell Biol. 20, 515–534 10.1038/s41580-019-0129-z [DOI] [PubMed] [Google Scholar]
- 114.Lilja J., Zacharchenko T., Georgiadou M., Jacquemet G., De Franceschi N., Peuhu E. et al. (2017) SHANK proteins limit integrin activation by directly interacting with Rap1 and R-Ras. Nat. Cell Biol. 19, 292–305 10.1038/ncb3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goldfinger L.E., Ptak C., Jeffery E.D., Shabanowitz J., Hunt D.F. and Ginsberg M.H. (2006) RLIP76 (RalBP1) is an R-Ras effector that mediates adhesion-dependent Rac activation and cell migration. J. Cell Biol. 174, 877–888 10.1083/jcb.200603111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Torres V.A., Mielgo A., Barbero S., Hsiao R., Wilkins J.A. and Stupack D.G. (2010) Rab5 mediates caspase-8-promoted cell motility and metastasis. Mol. Biol. Cell 21, 369–376 10.1091/mbc.e09-09-0769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Atherton P., Lausecker F., Harrison A. and Ballestrem C. (2017) Low-intensity pulsed ultrasound promotes cell motility through vinculin-controlled Rac1 GTPase activity. J. Cell Sci. 130, 2277–2291 10.1242/jcs.192781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arriagada C., Silva P., Millet M., Solano L., Moraga C. and Torres V.A. (2019) Focal adhesion kinase-dependent activation of the early endocytic protein Rab5 is associated with cell migration. J. Biol. Chem. 294, 12836–12845 10.1074/jbc.RA119.008667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mellor P., Marshall J.D.S., Ruan X., Whitecross D.E., Ross R.L., Knowles M.A. et al. (2018) Patient-derived mutations within the N-terminal domains of p85α impact PTEN or Rab5 binding and regulation. Sci. Rep. 8, 7108 10.1038/s41598-018-25487-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kleinschmidt E.G. and Schlaepfer D.D. (2017) Focal adhesion kinase signaling in unexpected places. Curr. Opin. Cell Biol. 45, 24–30 10.1016/j.ceb.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alanko J., Mai A., Jacquemet G., Schauer K., Kaukonen R., Saari M. et al. (2015) Integrin endosomal signalling suppresses anoikis. Nat. Cell Biol. 17, 1412–1421 10.1038/ncb3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lawson C., Lim S.T., Uryu S., Chen X.L., Calderwood D.A. and Schlaepfer D.D. (2012) FAK promotes recruitment of talin to nascent adhesions to control cell motility. J. Cell Biol. 196, 223–232 10.1083/jcb.201108078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nader G.P., Ezratty E.J. and Gundersen G.G. (2016) Talin and PIPKIγ regulate endocytosed integrin activation to polarize focal adhesion assembly. Nat. Cell Biol. 18, 491–503 10.1038/ncb3333 [DOI] [PubMed] [Google Scholar]
- 124.Cullen P.J. and Steinberg F. (2018) To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 19, 679–696 10.1038/s41580-018-0053-7 [DOI] [PubMed] [Google Scholar]