Abstract
This review examines preclinical and clinical studies relevant to our understanding of how the bidirectional gut-brain axis influences the natural history of inflammatory bowel disease. Preclinical studies provide proof of concept that preexisting behavioral illness, such as depression, results in increased susceptibility to inflammatory stimuli and that commonly used classes of antidepressants protect against this vulnerability. However, clinical studies suggesting behavioral illness as a risk factor for IBD and a protective role for antidepressants have relied primarily on symptom-reporting rather than objective measurements of inflammation. In terms of gut-to-brain signaling, there is emerging evidence from preclinical and clinical observation that intestinal inflammation alters brain functions, including the induction of mood disorders, alteration of circadian rhythm both centrally and peripherally, and changes in appetitive behaviors. Furthermore, preclinical studies suggest that effective treatment of intestinal inflammation improves associated behavioral impairment. Taken together, the findings of this review encourage a holistic approach to the management of patients with IBD, accommodating lifestyle issues that include the avoidance of sleep deprivation, optimized nutrition, and the monitoring and appropriate management of behavioral disorders. The review also acknowledges the need for better-designed clinical studies evaluating the impact of behavioral disorders and their treatments on the natural history of IBD, utilizing hard end points to assess changes in the inflammatory process as opposed to reliance on symptom-based assessments. The findings of the review also encourage a better understanding of changes in brain function and circadian rhythm induced by intestinal inflammation.
Keywords: depression, antidepressants, circadian rhythm, inflammation, vagus nerve, cytokines
The review adopts a translational approach examining proof-of-concept from preclinical studies and clinical evidence supporting the role of the bidirectional gut-brain axis in IBD and describes implications for optimal management.
BACKGROUND
From a historical perspective, our understanding of the natures of ulcerative colitis and Crohn’s disease have followed quite different trajectories.1 Ulcerative colitis was recognized much earlier than Crohn's disease and was originally considered to be a psychosomatic disorder, or one in which behavioral issues played a significant etiological role. This line of thinking persisted until the latter half of the 20th century. In contrast, “regional ileitis” or Crohn’s disease was considered an organic disease, due in part to the fact that surgeons managed most cases in the early years. Nevertheless, as our understanding of the nature and extent of brain-gut communication has increased, further consideration has been given to the role of the psyche in the expression of Crohn’s disease. The first formal description of the inflammatory nature of ulcerative colitis was published in 19492 and was followed by the first demonstration of the efficacy of steroids in this condition.3 Consequently, ulcerative colitis and Crohn’s disease became recognized as primary chronic inflammatory diseases of the bowel. Interest in psychological factors in IBD subsequently declined, but the adoption of the biopsychosocial model of illness, first proposed by Engel in 1977,4 and discoveries of the impact of the bidirectional gut-brain axis on gut inflammation rekindled interest in evaluating the impact of the psyche on the natural history of IBD.
The gut-brain axis is a bidirectional communication system that involves neural, hormonal, metabolic, immunological, and microbial signals. Signaling from the gut allows the brain to monitor the physiological and inflammatory status of the gut and the luminal composition. The bidirectional nature of the axis enables the central nervous system to respond to changes in the status of the gut to maintain homeostasis. The efferent pathways of this axis transmit the impact of behavioral changes, such as depression or anxiety on gut functions that include inflammatory responses. A detailed review of the bidirectional pathways of the gut-brain axis is beyond the scope of this review, but the reader is directed to 2 recent excellent reviews.5, 6 Disruption of this axis has been implicated in the pathophysiology of chronic intestinal inflammation in animals and man and includes sympathetic-parasympathetic imbalance7 and loss of equilibrium between the HPA axis, the autonomic nervous system, and the limbic system.8 In the recent past, the intestinal microbiota has become incorporated into the bidirectional gut-brain axis9 and provides a pathway that links intestinal bacteria and the brain.10
Since the discovery of the inflammatory reflex by Tracy and colleagues,11 there has been considerable interest in the role of the vagus nerve in IBD. A preclinical study by Ghia and colleagues12 demonstrated the protective effect of the vagus nerve in a model of intestinal inflammation in the absence or presence of psychiatric comorbidity. In addition, preclinical studies of the efficacy of vagal nerve stimulation13 have shown therapeutic benefit in models of colitis.14, 15 The first pilot clinical trial has demonstrated symptomatic and endoscopic improvement in 5 of 7 patients with Crohn’s disease involving the terminal ileum; this was accompanied by a reduction in C-reactive protein and fecal calprotectin levels,13 and other studies are underway. Because the anti-inflammatory effect of vagal efferents are mediated by the α-7 subunit of the nicotinic cholinergic receptor, the latter provides a potential therapeutic target in the management of inflammatory bowel disease.16 Taken together, these findings provide an example of how the involvement of the gut-brain axis in IBD can be exploited for therapeutic gain.
Studies demonstrating that patients with IBD exhibit evidence of anxiety and/or depression more frequently than healthy controls have rekindled interest in the role of behavioral factors in these conditions. For example, the Manitoba IBD cohort study was the first population-based study to examine the prevalence of common psychiatric disorders among IBD patients and showed that the 12-month prevalence of major depression in a population-based cohort of subjects with IBD was 9.1% vs 5.5% in community controls. In addition, the same group identified that the lifetime prevalence of major depression in the subjects with IBD was 27.2% vs 12.3% in controls.17 A systematic review showed prevalence rates of 19.1% vs 9.6% for anxiety and 28.2% vs 13.4% for depression, and the rates were positively influenced by the activity of IBD.18 Other studies have found higher than expected prevalence rates for anxiety and depression in IBD patients, more commonly often in Crohn's disease than ulcerative colitis, and in patients with active disease.19–21 Placebo response rates average 19% in clinical trials evaluating a broad spectrum of treatments, including biological agents,22, 23 and this also prompts interest in the role of behavioral factors in IBD. The involvement of bidirectional immune regulatory vagal pathways11 and the putative role of inflammation in primary mood disorders24 provide additional grounds for examining brain-gut interactions in the natural history of IBD. As always, the question remains as to whether behavioral changes in IBD patients are simply a consequence of the physical morbidity associated with active disease or whether behavioral disposition plays a role in the development of these diseases.
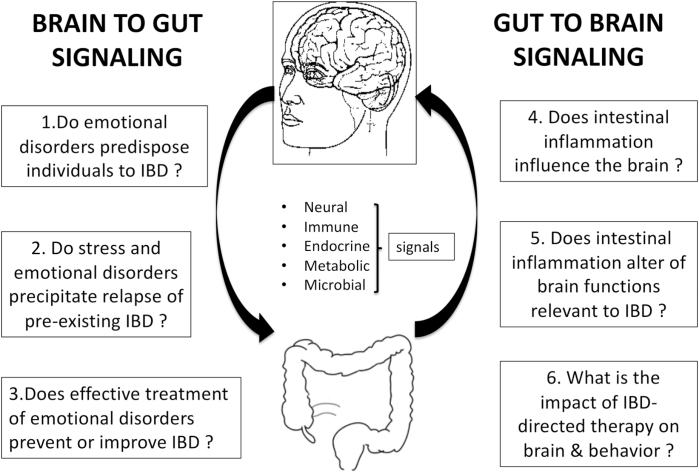
This review has been structured to address 6 questions pertaining to these and other issues; the questions are shown in Figure 1. Each question will be addressed first by examining evidence of biologic feasibility based on preclinical studies and by a review of the existing clinical literature pertinent to the question.
FIGURE 1.
The structure of this review is illustrated. It will address 6 questions pertaining to the role of the bidirectional gut-brain axis in the natural history of IBD. Each question is addressed by first examining evidence of biologic feasibility based on preclinical studies, followed by a review of the existing clinical literature pertinent to the question.
BRAIN-TO-GUT SIGNALING
Question 1. Do Emotional Disorders Predispose Individuals to IBD?
Proof of concept from preclinical studies
Several models have demonstrated that preexisting depression increases vulnerability to intestinal inflammatory stimuli. Maternal separation among rodents is a well-established model in which the maternally deprived offspring exhibit depression-like and anxiety-like behaviors in adulthood and increased susceptibility to stress as a result of hyper-responsiveness of the Corticotrophin Releasing Factor (CRF)-mediated hypothalamaic pituitary axis.25 A study showed that maternally separated mice exhibited depression-like behavior at 8 weeks of age and developed increased intestinal permeability but no evidence of spontaneous inflammation in the gut. Colitis was then induced by dextran sodium sulphate (DSS), and maternally separated mice experienced a more severe inflammatory response than nonseparated control mice.26 In terms of underlying mechanisms, it is known from preclinical studies that maternal separation alters the composition of the intestinal microbiota and that this, along with host factors contribute to the model’s behavioral phenotype,27 but the effect of this altered microbiota on susceptibility to colitis has not yet been investigated. In a model of depression induced in mice by intracerebral ventricular injection of low-dose reserpine, depressed mice were more susceptible to the DSS-induced colitis.12 A study in rats has also demonstrated the vulnerability to experimental colitis in depressed rodents.28 Taken together, these results provide proof of concept that preexisting depression-like behavior increases vulnerability inflammatory stimuli and implicate alterations in intestinal permeability and attenuation of the vagal anti-inflammatory pathways among underlying mechanisms. If these results can be extrapolated to humans, they predict that depression is a risk factor for the development of intestinal inflammatory conditions.
Observations from clinical studies
The Manitoba IBD cohort study demonstrated that 64% of IBD patients reporting a lifetime anxiety or mood disorder received a psychiatric diagnosis at least 2 years before the onset of IBD; 54% of patients with a mood disorder experienced the first episode of depression at least 2 years before the diagnosis of IBD.17 A subsequent study utilizing the Manitoba Population Research Data Repository examined the risk of developing immune-mediated inflammatory disease (IMID), including IBD, following a previous diagnosis of a psychiatric disorder. In the IBD cohort of 3766 patients, the incidence of depression, anxiety, and bipolar disorder was increased compared with a matched cohort, and the onset of the psychiatric disorder preceded diagnosis of IBD by as much as 5 years.29 Taken together, these studies support the view that common psychiatric disorders increase the risk of developing IBD. A similar conclusion was drawn from a recent study investigating the incidence of IBD in 403,665 patients with a new onset diagnosis of depression established between 1986 and 2012. The study found that patients with a history of depression had an increased risk of developing Crohn's disease (adjusted hazard ratio [HR], 2.11; 95% Cl, 1.65–2.70) or ulcerative colitis (HR, 2.23; 95% Cl, 1.92–2.60).30
Do Stress or Mood Disorders Precipitate Relapse of Preexisting IBD?
Proof of concept from preclinical studies
Preclinical studies show that psychological stress reactivates quiescent inflammation in the gastrointestinal (GI) tract. In one study, restraint stress was applied to mice in histological remission after the induction of acute colitis with dintrobenzene sulfonic acid (DNBS). Stress alone failed to reactivate colitis. However, when stress was applied together with a subthreshold dose of DNBS, colitis was reactivated. Moreover, this susceptibility to colitis could be adaptively transferred into naïve mice via CD+ve lymphocytes from stressed mice. These findings suggest that stress alone is insufficient to induce relapse in the absence of an overt inflammatory stimulus.31 Other work has shown that stress increases intestinal permeability via pathways involving cholinergic nerves and the release of corticotropin releasing factor (CRF), and this may be a mechanism whereby stress increases susceptibility to inflammatory stimuli.32 Experimentally induced depression has been shown to reactivate quiescent colitis by attenuating vagally mediated inhibition of pro-inflammatory cytokine secretion from macrophages.12, 33, 34 In this study, acute colitis was induced by either DSS or DNBS and allowed to become quiescent. Colitis was reactivated by depression, induced by intra-cerebro-ventricular administration of reserpine, and was associated with increased secretion of pro-inflammatory cytokines from peritoneal macrophages mediated via the α-7 subunit of the nicotinic acetylcholine receptor.12, 33, 34 These findings invoke vagal dysfunction as a mechanism whereby depression influences intestinal inflammatory processes, and tricyclic antidepressants (TCAs) were shown to restore vagal function and reduce intestinal inflammation. Interestingly, vagal dysfunction is well recognized in depression35 and is the basis for the use of vagal stimulation in treatment-resistant depression.36
Do stress or mood disorders precipitate relapse of preexisting IBD? Observations from clinical studies
Depression has been associated with increased unplanned 30-day hospital readmission rates in ulcerative colitis,37 symptomatic exacerbations, and increased hospitalization38 and reduced responsiveness to anti-TNF therapy.39 A prospective observational longitudinal study involving 60 IBD patients followed for 18 months correlated the severity of baseline depression scores with the number of subsequent relapses and a shorter interval to the first clinical recurrence of IBD.40 A recent prospective cohort study found a significant association between symptoms of depression and clinical evidence of disease recurrence in patients with Crohn’s disease or ulcerative colitis.41 Symptoms of anxiety also correlated with clinical recurrence in that study. Depression has been associated with poor compliance with anti-inflammatory or immunosuppressive or biological therapies,42, 43 and this may be a contributing factor in these reports. Narula et al found that anxiety—but not depression—predicted increased hospitalizations, ER visits, and the need for corticosteroids.44 A more recent prospective study showed that high anxiety scores during documented remission predicted a more severe disease outcome in patients with ulcerative colitis or Crohn’s disease.45 The question arises as to whether these results simply reflect increased symptom reporting by patients with depression or anxiety or actual exacerbation of the underlying inflammatory disease. Gracie et al found a poor correlation between symptoms or the Harvey-Bradshaw Index and evidence of mucosal inflammation using fecal calprotectin as a biomarker of the inflammatory process in Crohn’s disease patients but not those with ulcerative colitis.46 Though preclinical studies link psychopathology with deterioration of IBD-associated symptoms, further prospective studies involving objective measures of inflammation are required to resolve the issue of whether depression or anxiety exacerbate intestinal inflammation or simply promote symptom reporting in IBD patients.
Although clinical experience strongly suggests an association between stressful life events and exacerbations of IBD, support for this association from existing clinical studies is weak. A study by Bernstein et al47 demonstrated that perceived stress, but not major stressful life events or low mood, were significantly associated with symptomatic flare of IBD. A subsequent study demonstrated that the association between perceived stress and symptomatic activity did not reflect worsening inflammation as reflected by fecal calprotectin levels of >250 ug/g.48 Thus, although the association of stress with exacerbation of the inflammatory process in IBD remains clinically plausible and biologically feasible,49 further studies involving objective measurements of the inflammatory process are much needed.
Does Effective Treatment of Emotional Disorders Prevent or Improve IBD?
Proof of concept from preclinical studies
Preclinical studies have focused on models of depression-like behavior and on the ability of antidepressants to influence the inflammatory process. Varghese et al used a model of maternal separation to induce depression-like behavior in mice. Maternally separated mice exhibited increased intestinal permeability and developed a more severe colitis induced by DSS compared with nonmaternally separated mice. The antidepressant desipramine attenuated depression-like behavior in maternally separated mice, and colitis was less severe in these mice. Importantly, desipramine had no effect on the severity of colitis in nonmaternally separated mice.26 These results are consistent with the view that desipramine attenuated the severity of colitis by reducing depression-like behavior, rather than via a direct effect of the drug on the colonic inflammatory process. Desmethylimipramine, a closely related tricyclic antidepressant, was also shown to reduce depression-induced relapses of quiescent colitis induced by previous exposure to DSS. Depression was induced by intracerebro-ventricular administration of reserpine once the DSS colitis became quiescent, and this was accompanied by reactivation of the colitis. Treatment with the antidepressant attenuated both the depression-like behavior and reactivation of colitis. The protective effect of the antidepressant was dependent on the integrity of the vagus nerve, and thus the proposed mechanism of action was the restoration of vagally mediated suppression on cytokine release by macrophages.12, 33 Thus, although at least 1 mechanism of action underlying the ability of tricyclic antidepressants to attenuate depression-induced exacerbation of inflammation is via the restoration of vagal modulation of cytokine release from macrophages, other studies also reveal a direct anti-inflammatory effect. In a study involving rodents without induced depression, the tricyclic antidepressant amitriptyline stimulated production of interleukin (IL)-10 and directly attenuated inflammatory responses to abdominal sepsis induced by cecal ligation or lipopolysaccharide (LPS).50 Studies on the ability serotonin-modulating antidepressants to attenuate depression-induced exacerbation of experimental colitis in rodents suggest a direct inhibitory effect on the intestinal inflammatory process. Studies using fluvoxamine (a selective serotonin reuptake inhibitor [SSRI]) or ventafaxine (a serotonin-norepinephrine reuptake inhibitor [SNRI]) showed that although these agents attenuated exacerbation of acetic acid–induced colitis in rats depressed by peripheral administration of reserpine, the antidepressants also reduced inflammation in rats not treated with nonreserpine.51 Under the same conditions, these investigators also demonstrated that the tricyclic amitriptyline reduced colitis in nonreserpine-treated rodents.28 These results differ from others in which TCAs failed to attenuate colitis in the absence of depression26
Taken together, these preclinical results indicate that several classes of antidepressants are able to attenuate the increased severity of de novo colitis or exacerbations of preexisting colitis induced by depression, but mechanisms of action differ depending on the model under study and may include a direct anti-inflammatory effect.
Clinical observations on the impact of treatment of behavioral disorders on IBD
Up to 30% of IBD patients receive antidepressant therapy, with selective serotonin reuptake inhibitors and tricyclic antidepressants being the most commonly prescribed treatments.52 Indeed, the use of antidepressants is more common in patients suffering from IBD compared with a control population, and this is seen in both adolescents and adults.53, 54 Despite this usage of antidepressants in IBD, surveyed gastroenterologists expressed skepticism regarding the impact of this drug category on the natural history of the disease.55 It is not surprising, therefore, to find few studies that examine this relationship. A retrospective review of the records of 29 IBD patients 1 year before and 1 year after commencing antidepressant therapy showed that there were fewer steroid courses, endoscopies, and relapses during antidepressant therapy compared with the year prior.56 A review of records of 42,890 IBD patients in the Danish national database found that the incident rate of disease activity was lower among patients on antidepressants, and this was prominent in those patients with no history of antidepressant use before the onset of IBD.57 Interestingly, a showing an increased risk of IBD in over 400,000 patients with recent onset found that the risk was reduced in those patients receiving antidepressant treatment. Specifically, selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants appeared protective against the risk of developing Crohn's disease, whereas selective serotonin and norepinephrine reuptake inhibitors (SNSRIs) in addition to SSRIs and TCAs seemed protective against ulcerative colitis.30 Three systematic reviews evaluating the impact of antidepressants on the course of IBD were unable to draw firm conclusions regarding the benefit of antidepressants in this context, primarily because of the lack of prospective and randomized trials and particularly those with inflammation-based objective end points.58–60 Each of the previously cited papers identified this as the major obstacle to determine whether antidepressants impact the natural history of these chronic intestinal inflammatory diseases or simply attenuate symptom reporting.
There have been 3 randomized controlled trials of cognitive behavioral therapy (CBT) in IBD patients. One study demonstrated an improvement in quality of life,61 but another study failed to confirm this or an effect on the course of IBD over 24 months.62 A more recent study found no effect of CBT on disease activity in young patients with mild anxiety or depression.63 At this time, one cannot conclude that CBT influences the natural history of IBD. There is a limited number of studies on the benefit of hypnosis in the management of IBD. These studies are limited by small sample size but suggest that courses of hypnotherapy can induce remission lasting from 2.5 months to up to 5 years.64 Interestingly, reduction in the expression of inflammatory cytokines including TNF-α have been observed in the intestine after a single session of hypnotherapy.65 Many IBD patients use complementary and alternative approaches to manage their condition, and these include herbal therapy, acupuncture, exercise, and mind-body therapy, but the efficacy of these approaches has yet to be determined in well-designed clinical studies.66
GUT-TO-BRAIN SIGNALING
The influence of intestinal inflammation of the brain has received relatively little clinical attention in comparison with the brain-to-gut communication in IBD. The interrogation of this axis will, therefore, rely more on an emerging body of preclinical studies rather than clinical observations.
Question 4: Does Intestinal Inflammation Influence Brain Function and Promote Psychiatric Morbidity?
Preclinical studies
Preclinical studies support the view that intestinal inflammation produces changes in brain functions and behavior. Colitis induced by DNBS induced anxiety and depression-like behaviors, and this was associated with increased hippocampal expression of genes related to inflammation and innate immunity, in addition to a reduction in mitochondrial function.67 Dextran sodium sulphate–induced colitis resulted in reduced brain excitability and seizure thresholds, anxiety-like behavior in all mice, and hyperalgesia to painful somatic stimuli in male mice.68 In a study of chronic low-grade colitis induced by a noninvasive nematode infection, anxiety-like behavior was associated with a reduction in brain-derived neurotrophic factor (BDNF) and increased circulating levels of TNF-α and kynurenine, a tryptophan metabolite. Administration of anti-TNF-α normalized behavior but did not normalize BDNF expression. Anxiety induced by colitis was evident in vagotomised mice, suggesting that behavioral changes were a result of circulating TNF-α rather than activation of afferent vagal pathways.69 Interestingly, interferon-α induced changes in behavior also persisted in vagotomised mice.70 Other cytokines including interleukin-6 and interferon-γ have also been implicated in inflammation-induced depression or anxiety.
A relative newcomer to the bidirectional brain-gut axis is the intestinal microbiota,9 and experimentally induced changes in microbiota composition have been shown to alter brain function.71 The introduction of a bacterial pathogen, Campylobacter jejuni, into the rodent gut was shown to activate the vagus and induce anxiety-like behavior before a local inflammatory response was established.10 A causal link between anxiety and the microbiota has been suggested in a recent paper in which colonization of germ-free mice with the microbiota of IBS patients with high levels of anxiety induced anxiety-like behavior in the recipient mice, and this was associated with innate immune activation.27 Similar studies involving the microbiota of IBD patients with high levels of anxiety or depression have not yet been published. Taken together, these preclinical studies demonstrate that intestinal inflammation induces changes in brain function and behavior.
Clinical observations
Changes in T cell activation, circulating cytokines, acute phase reactants, oxidative and nitrosative stress, and the serotonin-depleting catabolism of tryptophan have been implicated in the pathogenesis of depression; similar changes have also been documented in IBD.72 Thus, depression and IBD may share a common inflammatory-based pathophysiology to account for the increased coexistence of these conditions. This is supported by the observations that (1) depression may precede the onset of IBD and (2) clinical disease activity was the only independent risk factor for depression in Crohn’s disease patients.20
Several changes have been described in the brains of IBD patients73, 74 and include structural changes in white matter and functional changes on fMRI. For example, young patients with IBD showed increased activation in brain regions associated with pain perception compared with healthy controls or patients with irritable bowel syndrome.75 In addition, an MRI study of the brain in patients with stable Crohn’s disease and no psychiatric comorbidity demonstrated changes in brain connectivity compared with healthy controls; this included the region involved in processing of emotion and visceral stimuli including pain.76 However, the relationship between brain changes and the activity of the intestinal inflammatory process has not yet been established.
Question 5: Does Intestinal Inflammation Alter Other Behaviors Relevant to IBD?
Disruption of circadian rhythm and IBD
Preclinical studies.
Circadian rhythm governs both behavior and physiology and is determined by a central oscillator in the suprachiasmatic nucleus (SCN) of the hypothalamus where it is cued by exposure to light. The central oscillator synchronizes peripheral clocks via neurohumeral pathways.77 Several mammalian clock genes have been identified that govern positive and negative transcriptional feedback control loops.78 There is increasing interest in the circadian control of immune function and inflammatory responses in IBD.79 Genetic ablation of circadian clock function or environmental circadian rhythm disruption (CRD) in mice disrupted intestinal barrier function and increased susceptibility to DSS-induced colitis.80 In a recent study, C57BL/6J mice were exposed to circadian shifts for 3 months before the induction of DSS colitis. Circadian shifts induced changes in intestinal permeability aggravated by DSS-induced colitis; this was accompanied and increased by circulating TNF-α , interleukin-1β, interleukin 6, C-reactive protein, and fecal calprotectin.81 Conversely, marked changes in circadian gene profiles were observed in mice after induction of experimental colitis induced by DSS or TNBS.82 Thus, results of preclinical studies reveal a vicious cycle in which primary changes in circadian rhythm worsen intestinal inflammatory responses that, in turn, alter the expression of circadian genes.82The circadian clock governs other functions that impact IBD patients, and these include sleep83 and feeding behavior.84
Clinical observations
Circadian clock gene expression in IBD.
A study in young, recently diagnosed, untreated patients with IBD demonstrated reductions in expression levels of all clock genes, except for PER2, in noninflamed intestinal mucosal tissues from patients compared with controls (P < 0.05). The expression of clock genes (eg, CLOCK, BMAL1, CRY1, CRY2, PER1, and PER2) were lower in white blood cells from recently diagnosed young patients with IBD compared with controls,85 suggesting that circadian clock gene disruption is a very early feature of IBD. Furthermore, a cohort study suggested that sleep deprivation is a risk factor for the development of ulcerative colitis.86 Liu et al found that the expression of circadian genes was downregulated in peripheral blood monocytes and in intestinal biopsies from IBD patients, and the findings were more marked in ulcerative colitis patients. Circadian gene expression was inversely related with assessments of disease activity and acute phase reactants.82 Another study linked polymorphism of the PERIOD3 (PER3) clock gene with a more aggressive form of Crohn’s disease; the rs2797685 variant of the PER3 gene was associated with early onset of disease, higher use of immunosuppressants, and more frequent stricturing and fistulizing disease.87 Taken together, these observations suggest that circadian disruption is a risk factor for IBD. However, it is also clear from preclinical studies that inflammatory mediators can disrupt circadian function. It is possible that a vicious cycle exists in some IBD patients in whom disruption of circadian clock function increases susceptibility to or enhances intestinal inflammation, and the products of the inflammatory process further disrupt circadian function both centrally and in the periphery, as illustrated in Figure 2.
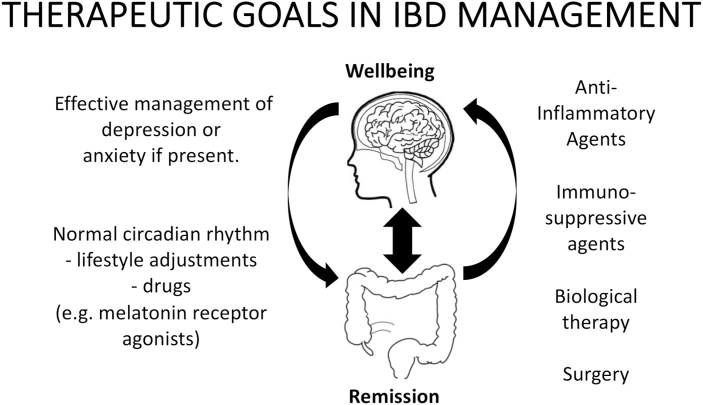
FIGURE 2.
Bidirectional gut-brain interactions in IBD may lead to a vicious cycle that promotes intestinal inflammation and reduces well-being. Thus, a holistic approach to the management of IBD is encouraged, involving both gut-to-brain and brain-to-gut signaling.
Sleep deprivation and IBD
Preclinical studies.
Forced acute or chronic intermittent sleep deprivation results in worsening of colitis induced in mice by DSS and also delays recovery from the colitis.88 Sleep deprivation using a water-immersion technique also aggravated DSS colitis in mice. This was accompanied by downregulation of adiponectin and aquaporin 8 and upregulation of of E2F transcription factor (E2F2) mRNA, histocompatibility class II antigen A, and beta 1 (H2-Ab1) mRNA in the colon. Administration of melatonin revered these microRNA profiles and attenuated the severity of colitis in sleep-deprived mice.89 Another study confirmed the ability of melatonin to attenuate the severe colitis induced by sleep deprivation, and this was associated with restoration of the normal expression of adiponectin and the cytokines IL-6 and IL-17.90
Clinical observations.
Results of surveys have indicated that IBD patients often experience fragmentation of sleep, prolonged sleep latency, and poor overall sleep quality that correlates with low scores using the IBD Quality of Life (QoL) questionnaire.91 Psychiatric comorbidity is associated with poor quality of sleep in IBD, and patients with depression had a 2-fold higher risk of poor quality sleep compared with nondepressed patients.86The question arises as to the extent to which poor quality sleep is a consequence of a primary sleep disorder or whether it is secondary to immune activation associated with chronic intestinal inflammation and its attendant morbidities. Graff et al found that poor quality of sleep and subsequent fatigue were highly prevalent in not only IBD patients with active disease but also those in remission.92 Quantitative and qualitative impairment of sleep is therefore associated with poor quality of life and worse disease outcomes and may be a risk factor for IBD.
Altered feeding behavior in IBD
Preclinical studies.
It is well established in animal studies that administration of pro-inflammatory cytokines including TNF-α and IL-1β results in reduced food intake and weight loss in animals.93 Induction of experimental colitis using acetic acid resulted in anorexia and weight loss that was prevented by centrally administered interleukin-1β antagonist.94, 95 Interleukin-1β also mediated anorexia in a model of TNBS-colitis. The action is mediated, in part, by IL-1β-induced production of 5-HT in the hypothalamus.96 In another study in TNBS-induced colitis, the severity of colonic inflammation correlated with plasma leptin concentrations. As the colitis resolved, food intake normalized and plasma leptin levels fell below normal values, suggesting that the adipokine contributed to the anorexia.97
Clinical studies.
Reduced appetite is common in IBD, particularly in Crohn’s disease, and is likely multifactorial.98 It is an important factor in weight loss and growth retardation in young patients.99 According to a systematized review, there may also be a link between eating disorders, particularly anorexia nervosa and Crohn’s disease, associated with a poor prognosis due in part to noncompliance with therapy.100 Mechanisms underlying inflammation-associated anorexia are poorly understood, but interest has focused on the anorexigenic properties of pro-inflammatory cytokines and adipokines such as leptin, produced in part by mesenteric white adipose tissue. For example, it is postulated, primarily on the basis of preclinical studies, that pro-inflammatory cytokines release leptin from adipose tissue. Circulating leptin would then signal the hypothalamus to reduce appetite. Although data regarding leptin levels and food intake in IBD have been conflicting, recent attention has focused on the ability of adipokines to modulate the inflammatory process in IBD.101, 102
Question 6: What Is the Impact of Anti-Inflammatory Therapy on Brain and Behavior?
Preclinical studies
In a model of chronic colitis induced by nematode infection, administration of anti-TNF by intraperitoneal injection resulted in an improvement in colitis-associated anxiety-like behavior.69 In a model of depression induced by chronic restraint stress, peripheral administration of anti-TNF improved depression scores but, interestingly, not the attendant weight loss.103
Clinical observations
A retrospective analysis of 69 patients with active IBD receiving anti-TNF-α therapy showed a significant improvement in depression-like symptoms as assessed by the Patient Health Questionnaire (PHQ-9).104 In a prospective cohort study, IBD patients with moderate to severe disease starting biologic therapy with vedolizumab or anti-TNF reported improvement in sleep and elevation of mood105 by 6 weeks. A recent study evaluated cognitive and affective biases before and after anti-TNF-α treatment in 9 patients with Crohn’s disease. Treatment was associated with an improved sense of well-being and postprandial fullness. Importantly, these subjective improvements were associated with post-treatment changes in the limbic system as assessed by fMRI.106
SUMMARY AND CONCLUSIONS
Preclinical data indicate that preexisting behavioral disturbance increases susceptibility to inflammatory stimuli or exacerbates quiescent inflammation and that effective treatment of altered behavior mitigates this vulnerability. Studies also indicate that antidepressants may have direct anti-inflammatory effects. From clinical studies, it is evident from epidemiological studies that psychiatric comorbidity, particularly depression or anxiety, is more common in IBD patients compared with non-IBD controls, may precede the onset of IBD, and is associated with clinical relapse of IBD. However, there is no good evidence at this time that effective treatment of psychiatric comorbidity attenuates the inflammatory process; virtually all studies rely on symptom-based evaluations of IBD disease activity. Absence of evidence does not, of course, imply absence of an effect, and there is general agreement that well-designed randomized controlled studies of antidepressants are urgently needed and must utilize objective measurements of inflammatory disease activity. If psychotropic drugs are shown to impact disease activity, their use in depressed IBD patients should improve response rates of existing immunomodulatory therapies and further improve quality of life.
In terms of gut-to-brain signaling in IBD, it is evident from preclinical models that mediators generated by intestinal inflammation alter brain function and modify behavior. Clinical studies suggest that effective counterinflammatory therapy directed at the gut can improve mood, a general sense of well-being, and sleep quality. This may, in part, reflect the reduction in intestinal symptomatology. It may also reflect changes in brain function. Investigation of the impact of disrupted circadian rhythm in the brain and the periphery on the natural history of IBD is in its infancy and may be subject to novel therapeutic approaches; recent preclinical studies suggest that activation of melatonin receptors may restore disrupted circadian function in the intestine and improve inflammatory responses.82, 107
The findings summarized in this review prompt consideration for a holistic approach to the management and perhaps the prevention of IBD by optimizing bidirectional gut-brain interactions (see Fig. 2). Such an approach may involve the appropriate use of antidepressant therapy, chronotherapy, or other approaches to restore disrupted circadian rhythm, in conjunction with pharmacotherapeutic or biologic anti-inflammatory therapies.
Supported by : This work was supported by funds made available by The Farncombe Family Chair in IBD Research.
REFERENCES
- 1. Mulder DJ, Noble AJ, Justinich CJ, et al. . A tale of two diseases: the history of inflammatory bowel disease. J Crohns Colitis. 2014;8:341–348. [DOI] [PubMed] [Google Scholar]
- 2. Warren S, Sommers SC. Pathogenesis of ulcerative colitis. Am J Pathol. 1949;25:657–679. [PMC free article] [PubMed] [Google Scholar]
- 3. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–136. [DOI] [PubMed] [Google Scholar]
- 5. Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144:36–49. [DOI] [PubMed] [Google Scholar]
- 6. Abautret-Daly Á, Dempsey E, Parra-Blanco A, et al. . Gut-brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease. Acta Neuropsychiatr. 2018;30:275–296. [DOI] [PubMed] [Google Scholar]
- 7. Mogilevski T, Burgell R, Aziz Q, et al. . Review article: the role of the autonomic nervous system in the pathogenesis and therapy of IBD. Aliment Pharmacol Ther. 2019;50:720–737. [DOI] [PubMed] [Google Scholar]
- 8. Bonaz B. Inflammatory bowel diseases: a dysfunction of brain-gut interactions? Minerva Gastroenterol Dietol. 2013;59:241–259. [PubMed] [Google Scholar]
- 9. Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. [DOI] [PubMed] [Google Scholar]
- 10. Goehler LE, Gaykema RP, Opitz N, et al. . Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005;19:334–344. [DOI] [PubMed] [Google Scholar]
- 11. Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. [DOI] [PubMed] [Google Scholar]
- 12. Ghia JE, Blennerhassett P, Collins SM. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest. 2008;118:2209–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonaz B, Sinniger V, Hoffmann D, et al. . Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol Motil. 2016;28:948–953. [DOI] [PubMed] [Google Scholar]
- 14. Meregnani J, Clarençon D, Vivier M, et al. . Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton Neurosci. 2011;160:82–89. [DOI] [PubMed] [Google Scholar]
- 15. Sun P, Zhou K, Wang S, et al. . Involvement of MAPK/NF-κB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS One. 2013;8:e69424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walker JR, Ediger JP, Graff LA, et al. . The Manitoba IBD cohort study: a population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am J Gastroenterol. 2008;103:1989–1997. [DOI] [PubMed] [Google Scholar]
- 18. Mikocka-Walus A, Knowles SR, Keefer L, et al. . Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:752–762. [DOI] [PubMed] [Google Scholar]
- 19. Byrne G, Rosenfeld G, Leung Y, et al. . Prevalence of anxiety and depression in patients with inflammatory bowel disease. Can J Gastroenterol Hepatol. 2017;2017:6496727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geiss T, Schaefert RM, Berens S, et al. . Risk of depression in patients with inflammatory bowel disease. J Dig Dis. 2018;19:456–467. [DOI] [PubMed] [Google Scholar]
- 21. Neuendorf R, Harding A, Stello N, et al. . Depression and anxiety in patients with inflammatory bowel disease: a systematic review. J Psychosom Res. 2016;87:70–80. [DOI] [PubMed] [Google Scholar]
- 22. Jairath V, Zou G, Parker CE, et al. . Systematic review with meta-analysis: placebo rates in induction and maintenance trials of Crohn’s disease. Aliment Pharmacol Ther. 2017;45:1021–1042. [DOI] [PubMed] [Google Scholar]
- 23. Jairath V, Zou GY, Parker CE, et al. . Placebo response and remission rates in randomised trials of induction and maintenance therapy for ulcerative colitis. Cochrane Database Syst Rev. 2017;9:CD011572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodes GE, Kana V, Menard C, et al. . Neuroimmune mechanisms of depression. Nat Neurosci. 2015;18:1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vetulani J. Early maternal separation: a rodent model of depression and a prevailing human condition. Pharmacol Rep. 2013;65:1451–1461. [DOI] [PubMed] [Google Scholar]
- 26. Varghese AK, Verdú EF, Bercik P, et al. . Antidepressants attenuate increased susceptibility to colitis in a murine model of depression. Gastroenterology. 2006;130:1743–1753. [DOI] [PubMed] [Google Scholar]
- 27. De Palma G, Lynch MD, Lu J, et al. . Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017;9:eaaf6397. [DOI] [PubMed] [Google Scholar]
- 28. Fattahian E, Hajhashemi V, Rabbani M, et al. . Anti-inflammatory effect of amitriptyline on ulcerative colitis in normal and reserpine-induced depressed rats. Iran J Pharm Res. 2016;15:125–137. [PMC free article] [PubMed] [Google Scholar]
- 29. Marrie RA, Walld R, Bolton JM, et al. ; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease Rising incidence of psychiatric disorders before diagnosis of immune-mediated inflammatory disease. Epidemiol Psychiatr Sci. 2019;28:333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frolkis AD, Vallerand IA, Shaheen AA, et al. . Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut. 2019;68:1606–1612. [DOI] [PubMed] [Google Scholar]
- 31. Qiu BS, Vallance BA, Blennerhassett PA, et al. . The role of CD4+ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat Med. 1999;5:1178–1182. [DOI] [PubMed] [Google Scholar]
- 32. Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol. 2007;293:G198–G203. [DOI] [PubMed] [Google Scholar]
- 33. Ghia JE, Blennerhassett P, Deng Y, et al. . Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. 2009;136:2280–2288.e1. [DOI] [PubMed] [Google Scholar]
- 34. Ghia JE, Park AJ, Blennerhassett P, et al. . Adoptive transfer of macrophage from mice with depression-like behavior enhances susceptibility to colitis. Inflamm Bowel Dis. 2011;17:1474–1489. [DOI] [PubMed] [Google Scholar]
- 35. Sgoifo A, Carnevali L, Alfonso Mde L, et al. . Autonomic dysfunction and heart rate variability in depression. Stress. 2015;18:343–352. [DOI] [PubMed] [Google Scholar]
- 36. Carreno FR, Frazer A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics. 2017;14:716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Poojary P, Saha A, Chauhan K, et al. . Predictors of hospital readmissions for ulcerative colitis in the United States: a national database study. Inflamm Bowel Dis. 2017;23:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gaines LS, Slaughter JC, Horst SN, et al. . Association between affective-cognitive symptoms of depression and exacerbation of Crohn’s disease. Am J Gastroenterol. 2016;111:864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Persoons P, Vermeire S, Demyttenaere K, et al. . The impact of major depressive disorder on the short- and long-term outcome of Crohn’s disease treatment with infliximab. Aliment Pharmacol Ther. 2005;22:101–110. [DOI] [PubMed] [Google Scholar]
- 40. Mittermaier C, Dejaco C, Waldhoer T, et al. . Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18-month follow-up study. Psychosom Med. 2004;66:79–84. [DOI] [PubMed] [Google Scholar]
- 41. Mikocka-Walus A, Pittet V, Rossel JB, et al. ; Swiss IBD Cohort Study Group. Symptoms of depression and anxiety are independently associated with clinical recurrence of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016;14:829–835.e1. [DOI] [PubMed] [Google Scholar]
- 42. Kochar B, Barnes EL, Long MD, et al. . Depression is associated with more aggressive inflammatory bowel disease. Am J Gastroenterol. 2018;113:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calloway A, Dalal R, Beaulieu DB, et al. . Depressive symptoms predict anti-tumor necrosis factor therapy noncompliance in patients with inflammatory bowel disease. Dig Dis Sci. 2017;62:3563–3567. [DOI] [PubMed] [Google Scholar]
- 44. Narula N, Cooray M, Anglin R, et al. . Impact of high-dose vitamin d3 supplementation in patients with Crohn’s disease in remission: a pilot randomized double-blind controlled study. Dig Dis Sci. 2017;62:448–455. [DOI] [PubMed] [Google Scholar]
- 45. Gracie DJ, Guthrie EA, Hamlin PJ, et al. . Bidirectionality of brain-gut interactions in patients with inflammatory bowel disease. Gastroenterology. 2018;154:1635–1646.e3. [DOI] [PubMed] [Google Scholar]
- 46. Gracie DJ, Williams CJ, Sood R, et al. . Poor correlation between clinical disease activity and mucosal inflammation, and the role of psychological comorbidity, in inflammatory bowel disease. Am J Gastroenterol. 2016;111:541–551. [DOI] [PubMed] [Google Scholar]
- 47. Bernstein CN, Singh S, Graff LA, et al. . A prospective population-based study of triggers of symptomatic flares in IBD. Am J Gastroenterol. 2010;105:1994–2002. [DOI] [PubMed] [Google Scholar]
- 48. Targownik LE, Sexton KA, Bernstein MT, et al. . The relationship among perceived stress, symptoms, and inflammation in persons with inflammatory bowel disease. Am J Gastroenterol. 2015;110:1001–1012; quiz 13. [DOI] [PubMed] [Google Scholar]
- 49. Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54:1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xia BT, Beckmann N, Winer LK, et al. . Amitriptyline treatment mitigates sepsis-induced tumor necrosis factor expression and coagulopathy. Shock. 2019;51:356–363. [DOI] [PubMed] [Google Scholar]
- 51. Minaiyan M, Hajhashemi V, Rabbani M, et al. . Evaluation of anti-colitic effect of fluvoxamine against acetic acid-induced colitis in normal and reserpinized depressed rats. Eur J Pharmacol. 2015;746:293–300. [DOI] [PubMed] [Google Scholar]
- 52. Thorkelson G, Bielefeldt K, Szigethy E. empirically supported use of psychiatric medications in adolescents and adults with IBD. Inflamm Bowel Dis. 2016;22:1509–1522. [DOI] [PubMed] [Google Scholar]
- 53. Haapamäki J, Tanskanen A, Roine RP, et al. . Medication use among inflammatory bowel disease patients: excessive consumption of antidepressants and analgesics. Scand J Gastroenterol. 2013;48:42–50. [DOI] [PubMed] [Google Scholar]
- 54. Virta LJ, Kolho KL. Antidepressant use among paediatric patients with recent-onset inflammatory bowel disease: a nationwide case control study in Finland. J Paediatr Child Health. 2014;50:562–565. [DOI] [PubMed] [Google Scholar]
- 55. Mikocka-Walus AA, Turnbull DA, Moulding NT, et al. . “It doesn’t do any harm, but patients feel better”: a qualitative exploratory study on gastroenterologists’ perspectives on the role of antidepressants in inflammatory bowel disease. BMC Gastroenterol. 2007;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goodhand JR, Greig FI, Koodun Y, et al. . Do antidepressants influence the disease course in inflammatory bowel disease? A retrospective case-matched observational study. Inflamm Bowel Dis. 2012;18:1232–1239. [DOI] [PubMed] [Google Scholar]
- 57. Kristensen MS, Kjærulff TM, Ersbøll AK, et al. . The influence of antidepressants on the disease course among patients with Crohn’s disease and ulcerative colitis-a Danish nationwide register-based cohort study. Inflamm Bowel Dis. 2019;25:886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fiest KM, Bernstein CN, Walker JR, et al. ; CIHR Team “Defining the burden and managing the effects of psychiatric comorbidity in chronic immunoinflammatory disease.” Systematic review of interventions for depression and anxiety in persons with inflammatory bowel disease. BMC Res Notes. 2016;9:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mikocka-Walus A, Prady SL, Pollok J, et al. . Adjuvant therapy with antidepressants for the management of inflammatory bowel disease. Cochrane Database Syst Rev. 2019;4:CD012680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Macer BJ, Prady SL, Mikocka-Walus A. Antidepressants in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2017;23:534–550. [DOI] [PubMed] [Google Scholar]
- 61. Bennebroek Evertsz’ F, Sprangers MAG, Sitnikova K, et al. . Effectiveness of cognitive-behavioral therapy on quality of life, anxiety, and depressive symptoms among patients with inflammatory bowel disease: a multicenter randomized controlled trial. J Consult Clin Psychol. 2017;85:918–925. [DOI] [PubMed] [Google Scholar]
- 62. Mikocka-Walus A, Bampton P, Hetzel D, et al. . Cognitive-behavioral therapy for inflammatory bowel disease: 24-month data from a randomised controlled trial. Int J Behav Med. 2017;24:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van den Brink G, Stapersma L, Bom AS, et al. . Effect of cognitive behavioral therapy on clinical disease course in adolescents and young adults with inflammatory bowel disease and subclinical anxiety and/or depression: results of a randomized trial. Inflamm Bowel Dis. 2019;25:1945–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Taft TH, Ballou S, Bedell A, et al. . Psychological considerations and interventions in inflammatory bowel disease patient care. Gastroenterol Clin North Am. 2017;46:847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mawdsley JE, Jenkins DG, Macey MG, et al. . The effect of hypnosis on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Am J Gastroenterol. 2008;103:1460–1469. [DOI] [PubMed] [Google Scholar]
- 66. Cheifetz AS, Gianotti R, Luber R, et al. . Complementary and alternative medicines used by patients with inflammatory bowel diseases. Gastroenterology. 2017;152:415–429.e15. [DOI] [PubMed] [Google Scholar]
- 67. Haj-Mirzaian A, Amiri S, Amini-Khoei H, et al. . Anxiety- and depressive-like behaviors are associated with altered hippocampal energy and inflammatory status in a mouse model of Crohn’s disease. Neuroscience. 2017;366:124–137. [DOI] [PubMed] [Google Scholar]
- 68. Nyuyki KD, Cluny NL, Swain MG, et al. . Altered brain excitability and increased anxiety in mice with experimental colitis: consideration of hyperalgesia and sex differences. Front Behav Neurosci. 2018;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bercik P, Verdu EF, Foster JA, et al. . Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112.e1. [DOI] [PubMed] [Google Scholar]
- 70. Friebe A, Brünahl C, Karimi K, et al. . Effects of complete vagotomy and blockage of cell adhesion molecules on interferon-α induced behavioral changes in mice. Behav Brain Res. 2013;240:1–10. [DOI] [PubMed] [Google Scholar]
- 71. Bercik P, Denou E, Collins J, et al. . The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609, 609.e1. [DOI] [PubMed] [Google Scholar]
- 72. Martin-Subero M, Anderson G, Kanchanatawan B, et al. . Comorbidity between depression and inflammatory bowel disease explained by immune-inflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gut-brain pathways. CNS Spectr. 2016;21:184–198. [DOI] [PubMed] [Google Scholar]
- 73. Agostini A, Ballotta D, Righi S, et al. . Stress and brain functional changes in patients with Crohn’s disease: a functional magnetic resonance imaging study. Neurogastroenterol Motil. 2017;29:1–10. [DOI] [PubMed] [Google Scholar]
- 74. Bao CH, Liu P, Liu HR, et al. . Alterations in brain grey matter structures in patients with crohn’s disease and their correlation with psychological distress. J Crohns Colitis. 2015;9:532–540. [DOI] [PubMed] [Google Scholar]
- 75. Huang JS, Terrones L, Simmons AN, et al. . Pilot study of functional magnetic resonance imaging responses to somatic pain stimuli in youth with functional and inflammatory gastrointestinal disease. J Pediatr Gastroenterol Nutr. 2016;63:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thomann AK, Griebe M, Thomann PA, et al. . Intrinsic neural network dysfunction in quiescent Crohn’s Disease. Sci Rep. 2017;7:11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. [DOI] [PubMed] [Google Scholar]
- 78. Yu W, Hardin PE. Circadian oscillators of Drosophila and mammals. J Cell Sci. 2006;119:4793–4795. [DOI] [PubMed] [Google Scholar]
- 79. Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pagel R, Bär F, Schröder T, et al. . Circadian rhythm disruption impairs tissue homeostasis and exacerbates chronic inflammation in the intestine. Faseb J. 2017;31:4707–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Amara J, Saliba Y, Hajal J, et al. . Circadian rhythm disruption aggravates DSS-induced colitis in mice with fecal calprotectin as a marker of colitis severity. Dig Dis Sci. 2019;64:3122–3133. [DOI] [PubMed] [Google Scholar]
- 82. Liu X, Yu R, Zhu L, et al. . Bidirectional regulation of circadian disturbance and inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:1741–1751. [DOI] [PubMed] [Google Scholar]
- 83. Albrecht U, Ripperger JA. Circadian clocks and sleep: impact of rhythmic metabolism and waste clearance on the brain. Trends Neurosci. 2018;41:677–688. [DOI] [PubMed] [Google Scholar]
- 84. Johnston JD. Physiological links between circadian rhythms, metabolism and nutrition. Exp Physiol. 2014;99:1133–1137. [DOI] [PubMed] [Google Scholar]
- 85. Weintraub Y, Cohen S, Chapnik N, et al. . Clock gene disruption is an initial manifestation of inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020;18:115–122.e1. [DOI] [PubMed] [Google Scholar]
- 86. Ananthakrishnan AN, Khalili H, Konijeti GG, et al. . Sleep duration affects risk for ulcerative colitis: a prospective cohort study. Clin Gastroenterol Hepatol. 2014;12:1879–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mazzoccoli G, Palmieri O, Corritore G, et al. . Association study of a polymorphism in clock gene PERIOD3 and risk of inflammatory bowel disease. Chronobiol Int. 2012;29:994–1003. [DOI] [PubMed] [Google Scholar]
- 88. Tang Y, Preuss F, Turek FW, et al. . Sleep deprivation worsens inflammation and delays recovery in a mouse model of colitis. Sleep Med. 2009;10:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chung SH, Park YS, Kim OS, et al. . Melatonin attenuates dextran sodium sulfate induced colitis with sleep deprivation: possible mechanism by microarray analysis. Dig Dis Sci. 2014;59:1134–1141. [DOI] [PubMed] [Google Scholar]
- 90. Kim TK, Park YS, Baik HW, et al. . Melatonin modulates adiponectin expression on murine colitis with sleep deprivation. World J Gastroenterol. 2016;22:7559–7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hood MM, Wilson R, Gorenz A, et al. . Sleep quality in ulcerative colitis: associations with inflammation, psychological distress, and quality of life. Int J Behav Med. 2018;25:517–525. [DOI] [PubMed] [Google Scholar]
- 92. Graff LA, Vincent N, Walker JR, et al. . A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1882–1889. [DOI] [PubMed] [Google Scholar]
- 93. Tracey KJ, Wei H, Manogue KR, et al. . Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988;167:1211–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. McHugh K, Castonguay TW, Collins SM, et al. . Characterization of suppression of food intake following acute colon inflammation in the rat. Am J Physiol. 1993;265:R1001–R1005. [DOI] [PubMed] [Google Scholar]
- 95. McHugh KJ, Collins SM, Weingarten HP. Central interleukin-1 receptors contribute to suppression of feeding after acute colitis in the rat. Am J Physiol. 1994;266:R1659–R1663. [DOI] [PubMed] [Google Scholar]
- 96. El-Haj T, Poole S, Farthing MJ, et al. . Anorexia in a rat model of colitis: interaction of interleukin-1 and hypothalamic serotonin. Brain Res. 2002;927:1–7. [DOI] [PubMed] [Google Scholar]
- 97. Barbier M, Cherbut C, Aubé AC, et al. . Elevated plasma leptin concentrations in early stages of experimental intestinal inflammation in rats. Gut. 1998;43:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Singh S, Blanchard A, Walker JR, et al. . Common symptoms and stressors among individuals with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2011;9:769–775. [DOI] [PubMed] [Google Scholar]
- 99. Hartman C, Marderfeld L, Davidson K, et al. . Food intake adequacy in children and adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2016;63:437–444. [DOI] [PubMed] [Google Scholar]
- 100. Ilzarbe L, Fàbrega M, Quintero R, et al. . Inflammatory bowel disease and eating disorders: a systematized review of comorbidity. J Psychosom Res. 2017;102:47–53. [DOI] [PubMed] [Google Scholar]
- 101. Ballinger A, Kelly P, Hallyburton E, et al. . Plasma leptin in chronic inflammatory bowel disease and HIV: implications for the pathogenesis of anorexia and weight loss. Clin Sci (Lond). 1998;94:479–483. [DOI] [PubMed] [Google Scholar]
- 102. Hoppin AG, Kaplan LM, Zurakowski D, et al. . Serum leptin in children and young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1998;26:500–505. [DOI] [PubMed] [Google Scholar]
- 103. Krügel U, Fischer J, Radicke S, et al. . Antidepressant effects of TNF-α blockade in an animal model of depression. J Psychiatr Res. 2013;47:611–616. [DOI] [PubMed] [Google Scholar]
- 104. Horst S, Chao A, Rosen M, et al. . Treatment with immunosuppressive therapy may improve depressive symptoms in patients with inflammatory bowel disease. Dig Dis Sci. 2015;60:465–470. [DOI] [PubMed] [Google Scholar]
- 105. Stevens BW, Borren NZ, Velonias G, et al. . Vedolizumab therapy is associated with an improvement in sleep quality and mood in inflammatory bowel diseases. Dig Dis Sci. 2017;62:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gray MA, Chao CY, Staudacher HM, et al. . Anti-TNFα therapy in IBD alters brain activity reflecting visceral sensory function and cognitive-affective biases. PLoS One. 2018;13:e0193542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mannino G, Caradonna F, Cruciata I, et al. . Melatonin reduces inflammatory response in human intestinal epithelial cells stimulated by interleukin-1β. J Pineal Res. 2019;67:e12598. [DOI] [PubMed] [Google Scholar]