Abstract
This study aimed to investigate the mechanisms of Kai-Xin-San (KXS, a famous Chinese herbal decoction used to treat amnesia) on the degradation of Aβ and further elucidate the mechanism of KXS on Aβ-induced memory dysfunction. After pretreatment with KXS (1.08 g/kg/day) for two weeks, Aβ42 (2 μL, 200 μM) was injected into rat hippocampus to induce cognitive dysfunction. Morris water maze (MWM) test was developed to evaluate cognitive performance in rats. Hippocampal neurons were observed by histological staining using Hematoxylin-Eosin (HE) methods. Levels of exogenous Aβ42, which was injected into the hippocampus, were continually measured through a special Enzyme-linked immunoassay (ELISA) kit to observe the catabolic process of Aβ in the brain. Similarly, Aβ degradation in PC12 cells was also investigated using the ELISA kit. The expressions of Aβ degeneration enzymes, including neprilysin (NEP), angiotensin-converting enzyme (ACE), and endothelin-converting enzyme (ECE), were detected by western blotting to elucidate Aβ reduction mechanism. Our results showed that KXS prevented Aβ42-induced cognitive impairment and attenuated hippocampus neuronal damage caused by Aβ42. Moreover, KXS could accelerate Aβ42 degradation in Aβ42 injected rats. Furthermore, NEP, an Aβ degradation enzyme, was increased in the hippocampus while ECE and ACE, other two Aβ-degrading enzymes, were not changed. KXS accelerated Aβ degradation in PC12 cells. Our findings revealed that KXS facilitated the degradation of Aβ42 by increasing the expression of NEP in rat hippocampus. By reducing the Aβ burdens, KXS protected hippocampal neurons, leading to the improvement of cognitive function in rats.
1. Introduction
Alzheimer's disease (AD), a neurodegenerative disease associated with symptoms of cognitive dysfunction, is mainly characterized by the presence in the brain of senile plaques due to amyloid beta (Aβ) peptides deposits and neurofibrillary tangles [1]. An abnormal Aβ increase is considered the first sign indicating AD development [2]. Aβ homeostasis in the brain is governed by its production and clearance mechanisms. Under normal conditions, Aβ in the brain is constantly produced from an amyloid precursor protein (APP), which is sequentially cleaved by β- and γ-secretases, and mostly catabolized by Aβ-degrading enzymes (ADEs) including neprilysin (NEP), angiotensin-converting enzyme (ACE), and endothelin-converting enzyme (ECE). Aβ degradation by ADEs, the main modality to avoid cerebral Aβ accumulation, plays a central role in sustaining Aβ normal levels [3]. The imbalance between its production and clearance can lead to excessive Aβ accumulation in the brain, causing AD typical pathological cascade reactions. Studies have demonstrated that anabolic increase of Aβ was rarely observed in sporadic AD which accounts for the overwhelming percentage of AD [4]. Therefore, the importance of Aβ clearance in AD pathogenesis has been raised, and Aβ catabolic mechanism became the new therapeutic target.
Ding-Zhi-Wan, a famous herbal formula of traditional Chinese medicine that was formerly reported in Chinese ancient book (Bei Ji Qian Jin Yao Fang) by Sun Simiao, has another name as Kai-Xin-San (KXS) in (Tai Ping Hui Min He Ji Ju Fang) [5]. Kai-Xin-San (KXS), a famous herbal formula of traditional Chinese medicine, consists of Radix Ginseng (Radix Ginseng C.A. Meyer), Poria (Poria cocos F.A. Wolf), Polygalae (Polygala tenuifolia Wild) and Acorus (Acorus tatarinowii rhizome) with dosage proportion of 3 : 3 : 2 : 2, has been used to treat amnesia for thousands of years in China. Indeed, KXS improves learning and memory in experimental AD studies and the mechanisms were correlated with multiple effects [5, 6], including reduced Aβ toxicity, neuroprotection, and neurites regeneration improvement. Similarly, our previous studies demonstrated that KXS ameliorates neuron loss and cognitive dysfunction induced by Aβ in vivo [7].
In other words, those studies usually focus on the symptom-alleviating effects of KXS on a neuropathological cascade of events caused by deposition of Aβ. In addition, our previous study showed that KXS could upregulate insulin-degrading enzyme (IDE); however, whether KXS could involve other ADEs mediated Aβ catabolic pathway has not been exhaustively illuminated. Therefore, our study aimed to further evaluate the mechanisms from the catabolic pathway of KXS ability on ameliorating AD.
2. Materials and Methods
2.1. Materials
Herbs of Ginseng (Renshen; the root of Panax ginseng C. A. Mey.), Poria (Fuling; sclerotium of Poria cocos (Schw.) Wolf), Polygalae (Yuanzhi; the root of Polygala tenuifolia Willd. or Polygala sibirica L.), and Acorus (Shichangpu; the root of Acorus tatarinowii Schott.) were purchased from Harbin Tongrentang Drug Company (Harbin, China) in Heilongjiang Province and were authenticated by Dr. Shuming Huang. Thiorphan was purchased from Abcam Company. Primary antibodies against rabbit NEP, ECE, and ACE were purchased from Santa Cruz Technology (California, USA). Monoclonal antibody against mouse β-actin and the enzyme horseradish peroxidase (HRP)-linked secondary antibodies were purchased from Beyotime Institute of Biotechnology (Beijing, China). Other reagents and solvents were of analytical grade and were commercially available.
2.2. Animals and Experimental Protocol
A total of 150 male Wistar rats (180–220 g) were provided by the Laboratory Animal Center of Heilongjiang University of Chinese Medicine (Harbin, China). Animals were housed in an animal laboratory with a temperature of 22 ± 2°C, 60 ± 3% humidity, and 12 h dark/light cycles with ad libitum access to rat chow and tap water. The animal facilities and protocols were approved by the Institutional Animal Care and Use Committee of the Heilongjiang University of Chinese Medicine. All procedures were in accordance with the National Institute of Health's guidelines regarding the principles of animal care [3].
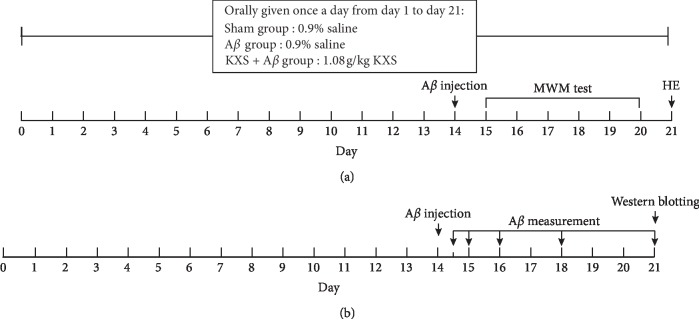
Two independent experiments were carried out in the present study. In the first experiment (Figure 1(a)), 30 rats were randomly divided into 3 groups for MWM test and HE as follows: sham group (10 rats, given orally 0.9% saline and injected 0.9% saline into brain), Aβ group (10 rats, given orally 0.9% saline and injected Aβ42 into brain), and Aβ + KXS group (10 rats, given orally KXS and injected Aβ42 into brain). On the 14th day, Aβ42 or 0.9% saline were injected into rat brain; HE was developed at day 21 after MWM test from day 15 to day 20.
Figure 1.
Two experimental protocols. (a) was developed for the MWM test and HE staining. At day 14, Aβ was injected into rat hippocampus in the Aβ group as well as the KXS + Aβ group, whereas 0.9% saline was injected in the sham group. After the MWM test from day 15 to day 20, HE was developed on day 21. (b) was developed for Aβ measurement and western blot for NEP, ACE, and ECE. ELISA for Aβ measurement was developed from day 14.5 to day 21 (12 h, 24 h, 48 h, 96 h, and 168 h after Aβ injection). Rat brains were removed for western blotting on day 21.
In the second experiment (Figure 1(b)), 120 rats were randomly divided into 3 groups for Enzyme-linked immunosorbent assay (ELISA) and western blot analysis. The groups, the treatment of drug-given, and Aβ injection were as same as that in the first experiment. However, Aβ measurement using ELISA was developed from day 14.5 to day 21 (12 h, 24 h, 48 h, 96 h, and 168 h after Aβ injection, 8 rats for each time point) to observe Aβ catabolic process in the brain of each rat, while western blotting was developed for protein expressions including NEP, ECE, and ACE at day 21. Moreover, from day 1 to day 21, the rats in the sham group and the Aβ group were orally given with 0.9% saline once a day, while the rats in the KXS group were orally given to KXS with a dosage of 1.08 g/kg.
2.3. KXS Extraction
KXS composition was the following: Ginseng, Poria, Polygalae, and Acorus. The four dried raw herbs were mixed together in a weight ratio of 3 : 3 : 2 : 2 (Ginseng = 60 g, Poria = 60 g, Polygalae = 40 g, and Acorus = 40 g), and decocted/extracted by refluxing for 1.5 h in 2000 ml boiling 60% ethanol (1 : 10, w/v). Then, the extracts were filtered, dried under vacuum, and stored at −80°C and the yield of KXS extracts was 20%.
2.4. Aβ Injection
Aβ injection was performed as follows: Aβ42 (Wako Pure Chemical Industries, Ltd., Japan) was dissolved in DMSO, diluted with 0.9% saline, and injected into rat hippocampus as previously described [8]. Briefly, rats were anesthetized and fixed on a stereotaxic instrument. Aβ42 (200 μM, 1 μL each side for a total of 2 μL per rat) was accurately injected bilaterally into the skull above the dentate gyrus area of the hippocampus in 10 min. Five minutes after injection, the needle was slowly withdrawn from the brain, the surgical incision was sutured, and penicillin sodium was applied to prevent infection.
2.5. Morris Water Maze Test
The Morris water maze (MWM) test was used to confirm the effects of KXS on the cognitive function of the rats as previously described [9]. Briefly, MWM consisted of a black circular pool (diameter 120 cm, depth 40 cm) filled with water (25 ± 1°C). The pool was divided into four quadrants and a platform (12 cm in diameter) was placed in a quadrant of the pool, submerged 1 cm below the water surface. The rats were trained to find the platform in the pool, 5 trials per day (1 block, 90 s/trial) for 5 consecutive days. The time needed by the rats to search and reach the escape platform was counted as escape latency. On the 6th day, the platform was removed and the rats have to search for the platform in the pool within 60 s; the swimming times across the platform area were recorded. The escape latency and the swimming times across the platform area were used to evaluate the memory function of each rat.
2.6. Histological Staining
After the behavioral experiment, the brain tissues of the rats in each group were removed and fixed in 4 % formalin for histological analysis. Rat brain was dehydrated and embedded in paraffin as previously described [10]. Histopathological changes were evaluated in 4 μm thick deparaffinized brain tissue sections stained with Hematoxylin-Eosin (HE). Furthermore, the pathological neuronal injury was evaluated by neurons irregular shape, neurons arrangement, and neuronal death in different fields.
2.7. ELISA Study
At each time point (Figure 1(b)), the brain of each rat was removed and quickly dissected on ice and (left hemisphere of the brain was used for ELISA assay while the right hemisphere was used for western blot analysis) and left hemisphere of the brain was homogenized for 1 h in 70% formic acid buffer containing protease inhibitor cocktail (Roche, Switzerland). After the lysates were centrifuged at 100,000 ×g for 1 h, the supernatants were collected and neutralized with 1 M Tris-based solution. Aβ42 concentration was measured using a specific and sensitive Sandwich ELISA kit purchased from Wako Company (Japan) [8]. The bicinchoninic acid assay (BAC) method was used to measure total protein concentration. Aβ42 “Quantity-Time” curve was made on the basis of Aβ42 measurement results.
2.8. Aβ Degradation in PC12 Cells
PC12 cells were plated on 96-well dishes at a density of 1 × 104 cells/well. PC12 cells were treated with 10% rat serum containing KXS (rat serum was collected after daily oral administration of KXS (1.08 g/kg) for three days) in the culture medium, thiorphan (10 μM), or KXS plus thiorphan for 24 h, respectively. The control group was treated with 10% rat serum without KXS (rat serum was collected after daily oral administration of normal saline for three days). The culture medium was removed on the day of the experiment, and the monolayers were preincubated with Aβ (1 μM) in the culture medium for 3 hours. The culture medium (100 μL) contained in each well was then transferred to a plastic vial for quantitation by ELISA (BlueGene Biotech).
2.9. Western Blot Analysis
To detect NEP, ACE, and ECE, the hippocampi were dissociated and lysed in radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitor for 20 minutes. Next, 10 μL protein samples (2 μg/μL) were resolved on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked by 5% skim milk powder for 2 hours and subsequently incubated with primary antibodies against NEP (1 : 100), ACE (1 : 100), and ECE (1 : 400) overnight at 4°C. Immunoreactive bands were incubated with the HRP-linked secondary antibody (1 : 800) for 2 hours. After washing with tris-buffered saline (TBS) and Tween 20 (TBST), enhanced chemiluminescence (ECL) detection system (Beyotime) was used to quantify the optical densities of immunoreactive bands using Gel Imaging System (BIO-RAD, USA). All the protein bands were analyzed by comparison with the internal reference protein (β-actin).
2.10. Statistical Analysis
All data were expressed as mean ± SD. ANOVA was used for comparisons of multiple groups and LSD-t was used between two groups. Ap value less than 0.05 was considered statistically significant.
3. Results
3.1. KXS Improved Rat Cognitive Deficit Induced by Aβ42
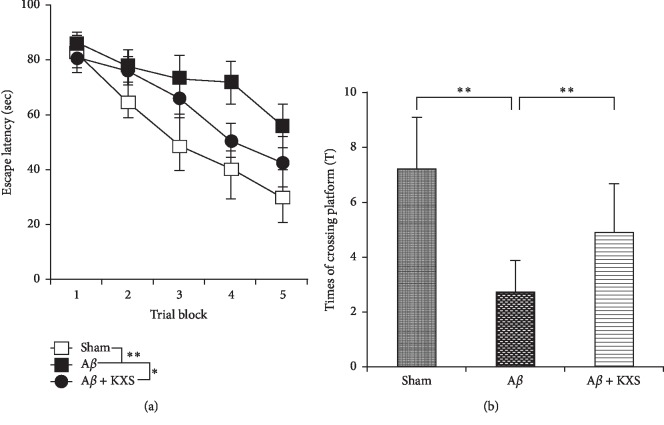
MWM test was used to confirm the effects of KXS on cognitive function. Significant differences were found in escape latency and the time of crossing platform area between the Aβ group and the sham group (P < 0.01), indicating that Aβ injection into the hippocampus caused cognitive deficits in the rats. In the animals that underwent KXS administration, however, memory dysfunction induced by Aβ was dramatically reduced. Indeed, in the place navigation test (Figure 2(a)), the escape latency of rats was in the Aβ + KXS group significantly shorter than that in the Aβ group (P < 0.01). In the spatial probe test (Figure 2(b)), the times of crossing the platform area increased significantly in the Aβ + KXS group compared to that in the Aβ group (P < 0.05). These results indicated that KXS improved rats' cognitive deficit induced by Aβ.
Figure 2.
KXS prevented the cognitive deficit induced by Aβ42 injection. (a) In the navigation test, the average escape latency of each group changed gradually and became shorter following training during the 5 days. From the 3rd to 5th day, the average escape latency of the Aβ group was significantly longer than that in the sham group and Aβ + KXS group (∗∗P < 0.01, ∗P < 0.05; n = 10). (b) In the spatial probe test, the average times of the animals swimming across the platform area of the Aβ group were reduced significantly compared to that of the sham group. After the oral administration of KXS, the average times were significantly increased (∗∗P < 0.01, ∗P < 0.05; n = 10).
3.2. KXS Attenuated Hippocampal Neurons Injury
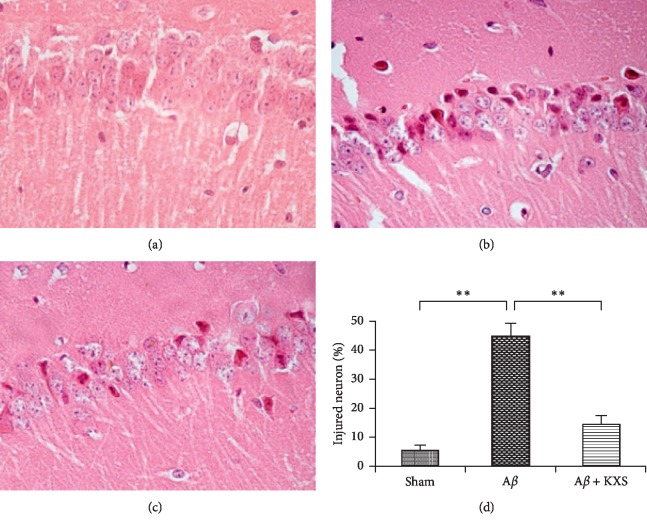
HE stain was performed to analyze histological evidence explaining the above memory function changes (Figure 3). The results showed pathological neuronal injury such as irregular neuronal shape and arrangement, as well as neuronal death in the hippocampus after Aβ injection (Figure 3(b)). However, the damage was remarkably reduced in the Aβ + KXS group (Figures 3(c) and 3(d)), and the number of surviving neurons increased significantly compared to the Aβ group (P < 0.01, Figure 3(b)), suggesting that KXS could prevent neuronal injury induced by Aβ42.
Figure 3.
KXS prevented the neuronal pathological injury by Aβ42 injection. (a) Few damaged neurons in the sham group. (b) A neuronal pathological injury was induced by injected Aβ42. (c) The Aβ induced neuronal injury was ameliorated after treatment with KXS. (d) Statistic analysis of injured neurons. Light microscope slices in each of the 5 randomly selected fields, the number of injured neurons (irregular neuronal shape and arrangement) and dead neurons (disappearance of nucleolus) were recorded. The average proportion of injured neurons in the Aβ + KXS group was significantly reduced compared with that in the Aβ group (∗∗P < 0.01, n = 10).
3.3. KXS Decreased Aβ42 Concentration
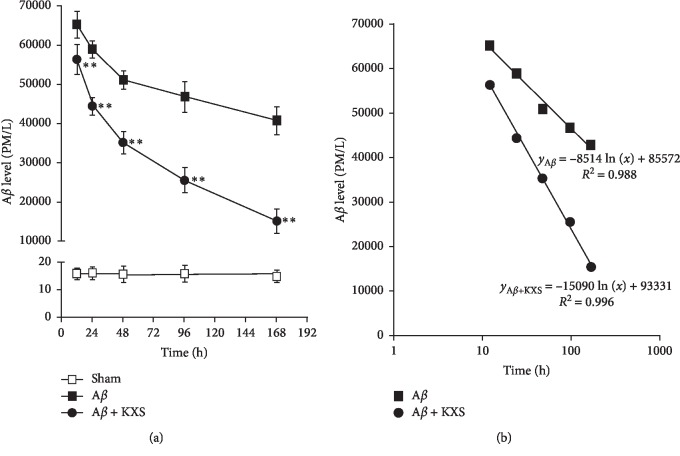
The next step was to explore the mechanism conferring to the ability of KXS to reduce Aβ induced injury. Aβ levels in the hippocampus were gradually reduced after Aβ injection. Aβ levels were significantly lower in the Aβ + KXS group compared to that in the Aβ group at each point (P < 0.01, Figure 4(a)). Moreover, the experimental data were fitted well with the logarithm model to analyze the degradation ratio of Aβ (Figure 4(b)). By comparing the regression coefficients of two regression equations (15090 > 8514), we suggested that KXS decreased Aβ42 concentration.
Figure 4.
KXS decreased Aβ42 concentration after injection in the brain (a) Aβ levels were assessed by specific ELISA with the same time interval. At t = 0, rats were injected with Aβ42 directly into the hippocampus and the brains were sampled for Aβ measurement at t = 12, 24, 48, 96, 168 hours. As time went on, the injected Aβ42 gradually declined in each group (∗∗P < 0.01 vs. Aβ on identical time points; n = 8). (b) The curves of the logarithm model were used to fit the trend of Aβ42 degradation. By analysis of the parameters of the two regression equations (i.e., Aβ + KXS of 15090 > Aβ of 8514), which represent the ratio of Aβ42 degradation, we demonstrated that the Aβ concentration was accelerated after treatment of KXS.
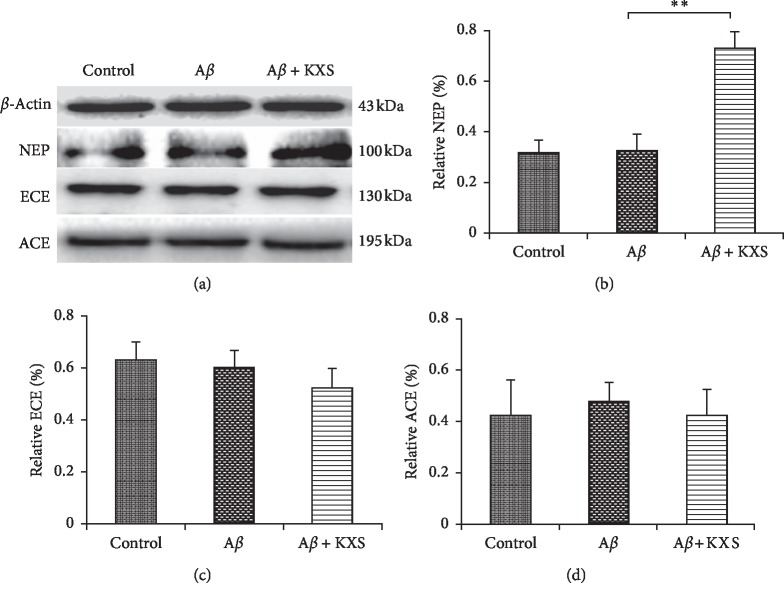
3.4. KXS Upregulated the Expression of NEP but Not ACE and ECE
To further explore the Aβ reduction mechanism due to KXS, three main ADEs protein expressions such as NEP, ACE, and ECE were investigated in hippocampal tissue by western blotting. NEP expression was significantly higher in the Aβ + KXS groups than that in the Aβ group (P < 0.01, Figures 5(a) and 5(b)). However, ACE and ECE protein levels were not changed in the KXS group and Aβ group (Figures 5(a), 5(c), and 5(d)). These results indicated that KXS could upregulate NEP expression in the brain.
Figure 5.
KXS upregulated the expression of NEP, but not ACE and ECE. (a) Protein expression of NEP, ACE, and ECE measured from the samples of the hippocampus of rats. (b) Compared to the Aβ group, the relative expression of NEP increased significantly in the Aβ + KXS group. No significant changes were found in the relative expression of ECE (c) and ACE (d), respectively (∗∗P < 0.01, n = 10).
3.5. KXS Decreased Aβ Concentration in PC12 Cells
PC12 cells were employed to evaluate the effect of KXS on NEP activity in vitro. We examined the accumulation of Aβ. When pretreated with KXS in PC12 cells, extracellular concentration of Aβ was decreased by KXS in PC12 cell medium, while it was elevated by thiorphan (known NEP inhibitor, NEPi) (Figure 6). The in vitro results confirmed that KXS could elevate NEP activity, accelerating Aβ degradation.
Figure 6.
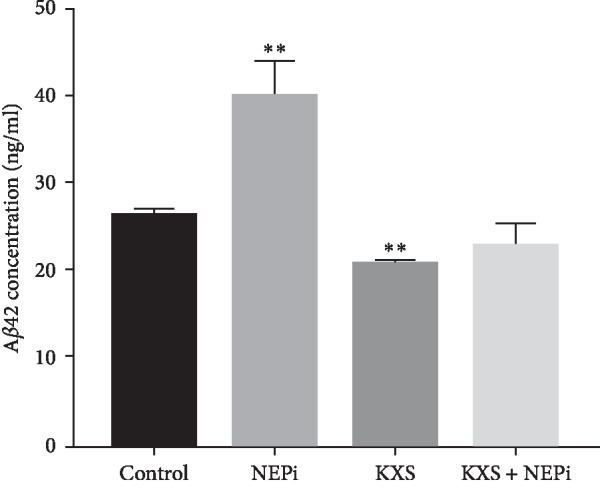
Effect of KXS on NEP activity in PC12 cells. PC12 cells were treated with 10% rat serum containing KXS in the culture medium, thiorphan (NEP inhibitor, 10 μM), or KXS plus thiorphan for 24 h, respectively. Then PC12 cells were incubated with Aβ (1 μM) for 3 h in the culture medium and extracellular Aβ concentration was investigated using the ELISA kit. Data were expressed as mean ± S.D (∗∗P < 0.01 vs. control; n = 3).
4. Discussion
Lines of evidence supported that Aβ, especially its soluble oligomeric forms, causes a series of pathological changes including the dysfunction and loss of synapses [7], loss and impairment of neurons, cholinergic dysfunction, and aberrant neural network activity [1, 11]. Besides, the inflammatory and oxidative injury usually takes place after the formation of senile plaque. The copathogenic interactions among diverse factors were usually responsible for the cognitive disorder in AD development [1]. Thus, the excessive Aβ accumulation has been considered as the upstream factor in the cascade of events that characterized AD development [12]. Correlated with the toxicity of Aβ, many studies have shown that multiple forms of Aβ cause experimental dementia in animals and induce cell injury in vitro [13]. In the present study, behavioral tests by MWZ showed cognitive disorders induced by Aβ injection, while cognitive disorders were improved after KXS treatment. Furthermore, HE staining results also revealed the protective effect of KXS on hippocampal neurons. As a consequence of that, hippocampal neurons whose dysfunction and death due to Aβ was responsible for learning and memory impairment were protected by KXS, preventing the loss of memory. These results indicated that KXS prevented cognitive disorder and inhibited the injury of hippocampal neurons. Moreover, Aβ consecutive measurement showed that the injected Aβ42 was gradually reduced in the brain of both the Aβ group and Aβ + KXS group (Figure 4). “Quantity-Time” of Aβ42 showed that Aβ was reduced faster after KXS treatment, which can be analyzed by the parameters of two regression equations. The results also indicated that decreased Aβ concentration is not due to diffusion but due to Aβ degradation. However, many pathways participated in Aβ clearance through a combination of transport across vessel walls into the blood stream, diffusion along perivascular extracellular matrix and ADEs [14]. In our studies, we focused on the effect of KXS on ADEs mediated Aβ degradation, and western blotting was performed to detect ADEs expression. Whether KXS could affect other pathways of Aβ clearance, we will further explore in our further studies.
Studies have demonstrated that the normal content of Aβ in the brain mainly depends on the metabolic balance between its anabolism and catabolism [2]. The anabolic Aβ increase is rarely observed in the sporadic AD [4]. Little evidence is available to support that the increase of Aβ upon aging is preceding the Aβ deposition [2]. The reduction in the catabolic activity of ADEs might be a possible reason for the increasing concentration of Aβ in the brain. Therefore, it might be reasonable to hypothesize the upregulation of ADEs to explain Aβ reduction. Our previous study showed that IDE protein expression was upregulated in rats treated with KXS without affecting the IDE mRNA level. However, other ADEs expressions were not further investigated [8]. In this study, KXS increased NEP expression in the hippocampus but had no effect on ACE and ECE expressions. NEP, a type II membrane-associated peptidase, is exclusively expressed in neurons and more evidence proved that NEP is the predominant ADE for degrading both monomeric and oligomeric forms of Aβ [15]. It has been reported that NEP plays a critical role in inhibiting Aβ accumulation in animals [16]. Furthermore, NEP activity was activated by KXS in PC12 cells, leading to accelerating Aβ degradation. Due to the effect of NEP on degrading Aβ, we concluded that the upregulation of NEP expression after treatment of KXS in our study was one important factor for Aβ42 reduction.
5. Conclusion
In conclusion, KXS facilitated Aβ42 degradation through increasing NEP expression in the hippocampus. By reducing the Aβ burden, KXS protected hippocampal neurons and prevented the loss of cognitive function in the rats. Our findings not only clarified KXS molecular mechanism on protecting brain neurons from Aβ toxicity but also provided a new strategy for AD treatment.
Acknowledgments
The authors thank the financial support of the Natural Science Foundation of China (nos. 81803869 and 81303248) and the Natural Science Foundation of Heilongjiang Province of China (no. H2015028). This research was partly supported by the Nursing Program for Young Scholars of Heilongjiang Province of China (nos. UNPYSCT-2018032 and UNPYSCT-2016116).
Contributor Information
Shuming Huang, Email: huangsm1958@126.com.
Xuewei Liu, Email: lxw_qmu@126.com.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Na Wang and Yongming Jia contributed equally to this work.
References
- 1.Huang Y., Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saido T. C. Metabolism of amyloid β; peptide and pathogenesis of alzheimer’s disease. Proceedings of the Japan Academy, Series B. 2013;89(7):321–339. doi: 10.2183/pjab.89.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leissring M. A. Aβ-degrading proteases: therapeutic potential in alzheimer disease. CNS Drugs. 2016;30(8):667–675. doi: 10.1007/s40263-016-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campion D., Dumanchin C., Hannequin D., et al. Early-onset autosomal dominant alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. The American Journal of Human Genetics. 1999;65(3):664–670. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Y., Chao C., Duan X., et al. Kai-Xin-San series formulae alleviate depressive-like behaviors on chronic mild stressed mice via regulating neurotrophic factor system on hippocampus. Scientific Reports. 2017;7(1):p. 1467. doi: 10.1038/s41598-017-01561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y., Cao Y., Liu M., Liu P., Cui H., Dai-Hong G. Behavioral and biochemical effects of a formulation of the traditional Chinese medicine, Kai-Xin-San, in fatigued rats. Experimental and Therapeutic Medicine. 2013;6(4):973–976. doi: 10.3892/etm.2013.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang B., Li Y., Liu J.-W., et al. Postsynaptic GluR2 involved in amelioration of aβ-induced memory dysfunction by KAIXIN-san through rescuing hippocampal LTP in mice. Rejuvenation Research. 2019;22(2):131–137. doi: 10.1089/rej.2018.2080. [DOI] [PubMed] [Google Scholar]
- 8.Wang N., Jia Y. M., Zhang B., et al. Neuroprotective mechanism of Kai Xin San: upregulation of hippocampal insulin-degrading enzyme protein expression and acceleration of amyloid-beta degradation. Neural Regeneration Research. 2017;12:654–659. doi: 10.4103/1673-5374.205107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunez J. Morris water maze experiment. Journal of Visualized Experiments. 2008;19:897–902. doi: 10.3791/897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar H. B., Kumar D. B., Diwan P. V. Hesperidin, a citrus flavonoid, protects against l-methionine-induced hyperhomocysteinemia by abrogation of oxidative stress, endothelial dysfunction and neurotoxicity in wistar rats. Pharmaceutical Biology. 2017;55:146–155. doi: 10.1080/13880209.2016.1231695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohamed T., Shakeri A., Rao P. P. N. Amyloid cascade in alzheimer’s disease: recent advances in medicinal chemistry. European Journal of Medicinal Chemistry. 2016;113:258–272. doi: 10.1016/j.ejmech.2016.02.049. [DOI] [PubMed] [Google Scholar]
- 12.Benilova I., Karran E., De Strooper B. The toxic Aβ oligomer and alzheimer’s disease: an emperor in need of clothes. Nature Neuroscience. 2012;15(3):349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 13.Parikh V., Bernard C. S., Naughton S. X., Yegla B. Interactions between Aβ oligomers and presynaptic cholinergic signaling: age-dependent effects on attentional capacities. Behavioural Brain Research. 2014;274:30–42. doi: 10.1016/j.bbr.2014.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deane R., Bell R., Sagare A., Zlokovic B. Clearance of Amyloid-β; peptide across the blood-brain barrier: implication for therapies in alzheimers disease. CNS & Neurological Disorders—Drug Targets. 2009;8(1):16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ries M., Sastre M. Mechanisms of aβ clearance and degradation by glial cells. Frontiers in Aging Neuroscience. 2016;8:160–166. doi: 10.3389/fnagi.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marr R. A., Rockenstein E., Mukherjee A., et al. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. The Journal of Neuroscience. 2003;23(6):1992–1996. doi: 10.1523/jneurosci.23-06-01992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.