Abstract
Intra-arterial administration of an adenovirus serotype 5 (Ad5) vector in a gene therapy trial caused lethal, systemic inflammation in subject 019 with ornithine transcarbamylase deficiency. This unanticipated inflammatory response was absent in another subject receiving the same vector dose and in 16 subjects receiving lower vector doses. We hypothesized that an immune memory to a previous natural adenovirus infection enhanced the immune response to high-dose systemic Ad5 vector, causing the exaggerated immune response in subject 019. To investigate this, we found that rabbit polyclonal sera to Ad5 and pooled human immunoglobulin (Ig) inhibited Ad5 vector transduction of non-immune cells in vitro, but enhanced transduction and activation of human dendritic cells (DCs). Sera from approximately 7% of normal human subjects and 50% of patients treated topically with Ad5 vectors enhanced Ad5 transduction and activation of DCs, apparently from formation of Ig-Ad5 immune complexes and binding to DCs through FcγR. Subject 019’s blood substantially increased Ad5-vector activation of human DC primary cultures at levels exceeding those from normal subjects. Although this study is based on one event in a single subject, the results implicate a pre-existing humoral immune response to Ad5 in the lethal systemic inflammatory response that occurred in subject 019.
Keywords: gene therapy, adenovirus, ornithine transcarbamylase deficiency, OTCD, antibody mediated enhancement, lethal systemic inflammatory response, cytokine, safety, dendritic cell, transduction/activation, innate immune response, gene therapy trial
In order to understand the mechanism underlying a lethal systemic inflammation in an adenoviral vector gene therapy trial, we explored the hypothesis that immune memory to the vector may be a contributing factor. We show that an antibody to the vector capsid from a natural infection enhanced activation of antigen-presenting cells.
Introduction
Adenoviral vectors are being evaluated in applications of gene therapy and genetic vaccines in a wide array of diseases. A consistent theme is the critical role that host immune responses play in vector performance. Initial gene therapy studies demonstrated the generation of cytotoxic T cells (CTLs) to antigenic transgene products that complicated their use for gene therapy,1 but encouraged their use as genetic vaccines.2 The problem of humoral immunity initially focused on the role of neutralizing antibodies (NAbs) in diminishing vector efficacy3 and, more recently, has been implicated in enhancing the acquisition of HIV infection in subjects who received an adenovirus serotype 5 (Ad5)-based HIV vaccine.4
Following the report of lethal systemic inflammation as the result of intravascular administration of an Ad5 vector in a gene therapy clinical trial of ornithine transcarbamylase deficiency (OTCD), studies focused on the relationship between vector and activation of innate immunity.5, 6, 7 Interactions between capsid proteins and Toll-like receptors (TLRs) appeared to drive inflammatory responses.8 What remained unexplained, however, was why subject 019 (previously disclosed as Jesse Gelsinger in Raper et al.5) responded to systemic Ad5 vector with such intensity, whereas another patient at this dose and 16 others in this trial did not. We hypothesized that an aspect of immune memory to a natural Ad5 infection in subject 019 enhanced the innate response to the vector. In this study, we show that immune complexes, which formed from the Ad5 vector and a unique population of pre-existing Ad5 antibodies (Abs), substantially enhanced innate immune responses, and that subject 019 had high levels of these enhancing Abs.
Results
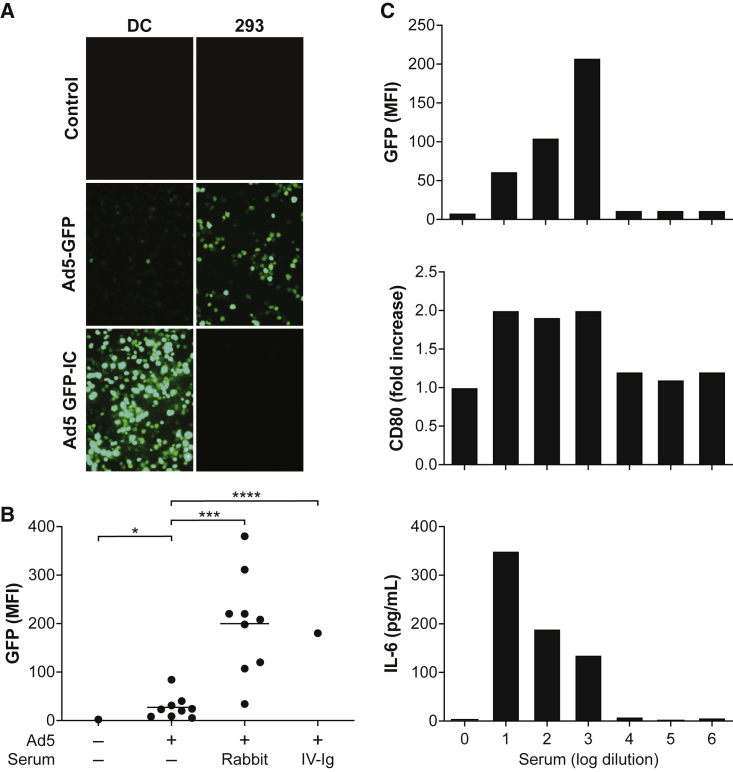
We first studied the impact of rabbit polyclonal Abs to Ad5 (rabbit antiserum) on vector transduction in a variety of cell types. As expected, this serum substantially inhibited transduction of 293 cells by Ad5-expressing green fluorescent protein (GFP; Figure 1A). We next evaluated the impact of Ad5-Ab complexes on human innate immune responses by incubating mixtures of Ad5-GFP and rabbit antiserum on immature monocyte-derived dendritic cells (DCs) established from nine different human donors. Using flow cytometry, we analyzed the effect of these complexes on GFP expression (Figure 1B). Unexpectedly, a mixture of Ad5 and rabbit antiserum that inhibited transduction of 293 cells increased Ad5-GFP transduction 8-fold over that observed with Ad5 alone (p < 0.001). A similar enhancement of transduction was observed when human intravenous immunoglobulin (IV-Ig) was incubated with Ad5. Human IV-Ig is an Ab mixture pooled from 10,000 human blood donors to be used for i.v. administration; in this preparation, IV-Ig had an Ad5 NAb titer of 1/2,560. Polyclonal rabbit serum against a serologically distinct simian Ad (SAdV-249) failed to enhance transduction of Ad5-GFP on human DCs (mean fluorescence intensity [MFI] = 8.5). Our studies are consistent with others that reported Ab-enhanced transduction of adenoviral vectors on DCs.10,11
Figure 1.
Ad5-Immune Complexes Enhance Transduction and Activation of Human DCs
(A) DCs and 293 cells were transduced with Ad5 vector expressing enhanced GFP or Ad5 pre-incubated with rabbit antiserum (NAb titer, 81920) for 15 min at 37°C. Cells were analyzed 48 h later by fluorescent microscopy. Representative fluorescent microscopy images of DCs (first column) and 293 cells (second column) are presented. Cells were incubated with no vector and no antibody (control, first row), Ad5-GFP alone (Ad5-GFP, second row), and the complex of Ad5-GFP and rabbit antiserum (Ad5-GFP-IC, third row). (B) Analysis of DC cultures by flow cytometry for GFP expression (mean fluorescence intensity [MFI]) following incubation with various combinations of Ad5 and antibodies. DC cultures from nine different human donors were incubated with Ad5 alone and Ad5 mixed with rabbit antiserum. DC cultures from a single source were also analyzed in the absence of vector or antibody and in the presence of Ad5 and human pooled serum (IV-Ig). Statistical relevance was determined using paired t test for Ad5 versus Ad5 mixed with rabbit serum. The one-sample t test was used for comparison of Ad5 alone versus cells alone, and Ad5 alone versus Ad5 mixed with IV-Ig. *p < 0.05; **p < 0.001; ****p < 0.0001. (C) Effect of increasing serum rabbit antiserum dilutions on the transduction and activation of DCs. Ad5 vectors were pre-incubated with increasing dilutions of rabbit antiserum before being added to human DCs (multiplicity of infection [MOI], 104). Cells were analyzed 48 h later for GFP expression by flow cytometry (MFI), fold increase in CD80 expression over background (i.e., Ad5 but no antiserum), and total secretion of IL-6 (pg/mL). Reciprocal log serum dilutions are plotted on the x axis. “0” log dilution indicates that the sample was not diluted. This experiment was repeated using cells from another donor and showed a similar dose response on transduction and upregulation of CD80; there were insufficient cells to measure IL-6 production (data not shown).
We then evaluated the impact of rabbit antiserum complexed with Ad5 on the activation state of human DCs (Figure 1C). In this experiment, we used increasing dilutions of rabbit antiserum mixed with a fixed quantity of Ad5-GFP, which were then incubated on human DCs derived from a single donor. Flow cytometry was used to measure the increase in cell-surface expression of the DC activation marker, CD80, and an enzyme-linked immunosorbent assay (ELISA) was used to measure secretion of the pro-inflammatory cytokine, interleukin-6 (IL-6). At lower dilutions of rabbit antiserum (dilutions of 101–103), we observed enhanced transduction along with an increase in both CD80 expression and secretion of IL-6. Transduction precipitously declined to baseline levels, along with a return of CD80 and IL-6 to the levels seen with vector alone when serum was diluted an additional 10-fold to 104, suggesting that enhanced transduction can be used as a surrogate of DC activation.
We then assessed the impact of Ad5 gene therapy on the generation of Abs capable of enhancing DC transduction/activation. Sera were available from research subjects who received topical applications of Ad5-expressing platelet-derived growth factor (PDGF-β) onto non-healing wounds.12 Table 1 summarizes the outcome of experiments from a single source of DCs incubated with Ad5 complexed with serum before and after gene therapy (day 28), serum from a normal subject, and rabbit antiserum. We evaluated cells for transduction (GFP MFI), fold induction of CD80, and secretion of IL-6 (pg/mL). Three of the four subjects showed an increase in Ad5 NAb, although only two subjects demonstrated increases in DC transduction and activation. The fourth subject did not demonstrate an increase in NAb, and as expected, the DCs did not show increases in transduction or activation.
Table 1.
Enhancement of DC Transduction by Serum from Human Subjects
| Samples | NAb Titer (Reciprocal Dilution) | GFP (MFI) | CD80 (Fold) | IL-6 (pg/mL) |
|---|---|---|---|---|
| Gene Therapy | ||||
| Control serum | 20 | 31 | 1 | 3 |
| Ad5 serum | 81,920 | 220 | 2.68 | 320 |
| GT 1 | 20/320 (16-fold) | 30/26 (1-fold) | 1/1 (1-fold) | 18/6 (0.3-fold) |
| GT 2 | 2,560/81,920 (32-fold) | 32/210 (7-fold) | 1/2.5 (2.5-fold) | 10/271 (27-fold) |
| GT 3 | 20/10,240 (512-fold) | 30/265 (9-fold) | 1/3.9 (3.9-fold) | 5/302 (60-fold) |
| GT 4 | 20/20 (1-fold) | 28/29 (1-fold) | 1/1 (1-fold) | 15/9 (0.6-fold) |
| Normal Subjects | ||||
| Control serum | 20 | 84 | 1 | 0 |
| Ad5 serum | 81,920 | 311 | 3.35 | 290 |
| N298 | 2,560 | 644 | 8.12 | 53 |
| N50 | 20,480 | 616 | 9.65 | 210 |
| N291 | 320 | 145 | 1.24 | 0 |
| N67 | 20,480 | 142 | 1.35 | 0 |
| N169 | 163,840 | 90 | 1 | 0 |
| N17 | 20 | 58 | 1.18 | 0 |
| N292 | 20 | 444 | 3 | 125 |
Analysis of serum from human subjects before and after gene therapy or from normal donors. We examined the effect of serum from pre- and post-Ad5 vector administration (pre/day 28) from subjects enrolled in a phase I clinical trial for PDGF-β for enhancement of Ad5 transduction and activation of DCs. Sera obtained from four gene therapy subjects (GT 1–4) was diluted 100-fold prior to analysis and compared with data obtained with vector incubated with control, non-immune rabbit serum (control serum), or anti-Ad5 rabbit serum (Ad5 serum; also diluted 100-fold for analysis). Cells were analyzed 48 h after transduction. Titers of neutralizing antibodies (NAbs) are shown as reciprocal serum dilutions required for inhibiting Ad5 transduction in HEK293 cells. We also evaluated cells for GFP expression (mean fluorescence intensity [MFI]) and fold increase in CD80 expression (fold) over nontransduced cells. We analyzed supernatants for the presence of IL-6 by ELISA (pg/mL). Sera obtained from normal subjects naive to Ad5 vector (N298, N50, N291, N67, N17, and dN292) were similarly evaluated. We have shown the pre- and post-vector administration values (pre/post) and the fold change for each GT subject. A single value is shown for normal subjects.
Sera selected from a larger pool of naive normal human subjects (i.e., not previously exposed to Ad5 vector) spanned a range of pre-existing Ad5 NAbs from undetectable (<1/20) to 1/163,840. We screened these sera for Abs capable of enhancing DC transduction and activation (see Figure S1 for sera with a wide range of NAbs and Figure S2 for sera with no detectable NAbs). We observed significantly enhanced transduction from the sera of 3 out of 46 samples: subject N292, NAb <1/20 (Figure S2); subject N298, NAb 1/2560 (Figure S1); and subject N50, NAb 1/20,480 (Figure S1). All other samples, including some with the same NAb titers as subject N298 and subject N50, failed to show significant enhancement (Figure S1). The three sera demonstrating enhanced transduction (N298, N50, and N292), along with several that did not show enhanced transduction (N17, N67, N169, and N291), were also analyzed for induction of CD80 cell-surface expression and enhanced secretion of IL-6 using the same DC culture from the analysis of sera from the gene therapy study (Table 1). Enhanced activation of DCs, as measured by CD80 upregulation and IL-6 selection, was limited to samples that showed enhanced transduction, thus reinforcing the usefulness of the DC-transduction assay as a surrogate for DC activation.
We performed western blot on some of the naive human sera characterized for DC transduction/activation (Figures S1 and S2) to evaluate the profile of Ad-binding Abs in this group (Figure S3). Virtually all subjects (21/23) had detectable binding Abs to Ad5; penton and fiber bands had approximately equal intensities that were stronger than the hexon bands. There was no correlation between band intensity and levels of NAb, suggesting the presence of non-NAbs in some samples, as has been previously described.13 Interesting exceptions to the Ab-binding profiles to Ad5 proteins came from samples N50 and N292, both of which exhibited enhanced transduction/activation. These sera showed more binding to fiber, suggesting that the quality of the Ab response rather than the quantity of the response may be important.
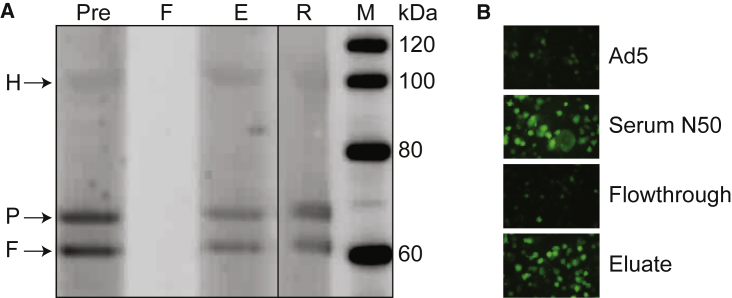
Our data suggest a relationship between Ab to Ad5 and enhanced transduction/activation of human DCs, but there is no quantitative correlation with NAb to Ad5. Because binding Ad5 Abs exist in more than 90% of humans, establishing a correlation is difficult. This begs the question of whether Abs contribute to the observed effect of serum on Ad5-induced activation of DCs. We therefore conducted experiments to confirm that Abs within sera from subjects N50, N292, and N298 are directly involved in the enhancement of Ad5-mediated DC transduction and activation. We conducted the initial studies with serum from sample N50, which was run over a protein G column to bind all Ig and eluted to specifically release bound Ig. The original serum sample, the flowthrough, and the eluate were evaluated for the presence of Ad5 Abs by western blot (Figure 2A) and enhanced Ad5 transduction (Figure 2B). These studies used the mouse monocyte cell line RAW264.7, which in separate experiments was shown to recapitulate the phenomenon of Ab-enhanced transduction shown for primary cultures of human DCs (data not shown). The flowthrough was depleted of all binding Abs to Ad5 based on western blots and failed to enhance Ad5-GFP transduction. Ad5-binding Abs and enhanced transduction were present in the samples eluted from the column and were not diminished when the flowthrough was mixed with the eluate (data not shown). Similar results were obtained with serum from the two additional subjects that showed enhanced transduction/activation (N292 and N298; data not shown). These data are most consistent with a component of the Ig fraction of serum that contributes to the increased transduction/activation of DCs.
Figure 2.
Purified IgG Enhances Ad5 Transduction of DCs
(A) A protein G column was used to purify IgG from serum N50, and the different fractions were used to probe a western blot of denatured Ad5 vector particles. Following transfer, narrow strips of the PVDF membrane were cut and incubated with serum N50 (Pre), unbound flowthrough (F), bound eluate (E), and rabbit antiserum (R). The migration of protein standards, along with their respective molecular weights, is shown in the right lane (M). The Ad5 capsid proteins hexon (H), penton (P), and fiber (F) are indicated by arrows. (B) Serum N50 fractions were further analyzed for enhanced transduction of RAW264.7 cells, a murine macrophage cell line, by incubating them with Ad5-GFP before being added to cells. Panels show GFP expression in cells at 48 h following pre-incubation of vector (MOI, 104) with different fractions.
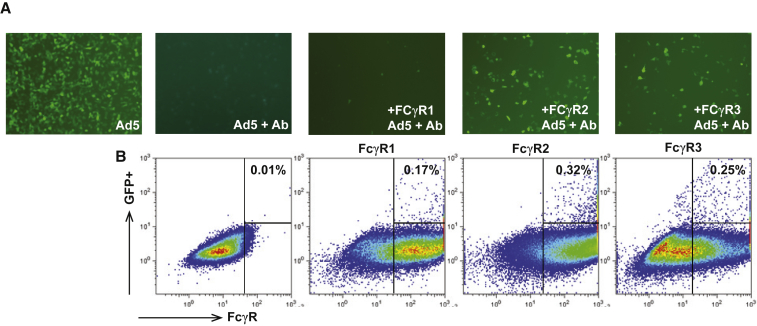
Because both human and mouse DCs are known to selectively express activating FcγR1, FcγR2, and FcγR3,14 we conducted additional in vitro studies to determine whether IgG Fc receptors (FcγR) mediated the enhancement of DC transduction/activation by Ad5-Ab complexes. We transiently transfected 293 cells with plasmids expressing several isoforms of FcγR (i.e., FcγR1, FcγR2, or FcγR3) prior to incubation with Ad5-Ab complexes. Microscopic evaluation of 293 cultures revealed transduction in cells expressing FcγR2 or FcγR3, but not in cells expressing FcγR1 or in mock-transfected cells (Figure 3A). Flow cytometry confirmed cell-surface expression of the individual FcγRs and quantified the number of FcγR-expressing cells that were also expressing GFP (Figure 3B). These results confirmed that FcγR2 or FcγR3 and, to a lesser extent, FcγR1, mediated enhanced transduction by Ad5-Ab complexes.
Figure 3.
FcγR-Dependent Enhancement of Ad-Ab Complex Transduction
293 cells were transiently transfected with cDNAs encoding human FcγR1, FcγR2, or FcγR3 constructs expressed by a CMV promoter. The next day, cells were transduced with Ad5-GFP complexed with pooled human IV-Ig. (A) Cells were imaged 24 h later using an inverted Nikon microscope for GFP expression: Ad5 (no antibody, no FcγR); Ad5+IV-Ig and no FcγR; Ad5+Ab and FcγR1; Ad5+Ab and FcγR2; and Ad5+Ab and FcγR3. (B) Flow cytometric analysis of FcγR-expressing cells transduced with Ad5-GFP vector. Transfected cells were stained using antibodies against individual FcγRs, followed by gating on the transfected cells for GFP expression.
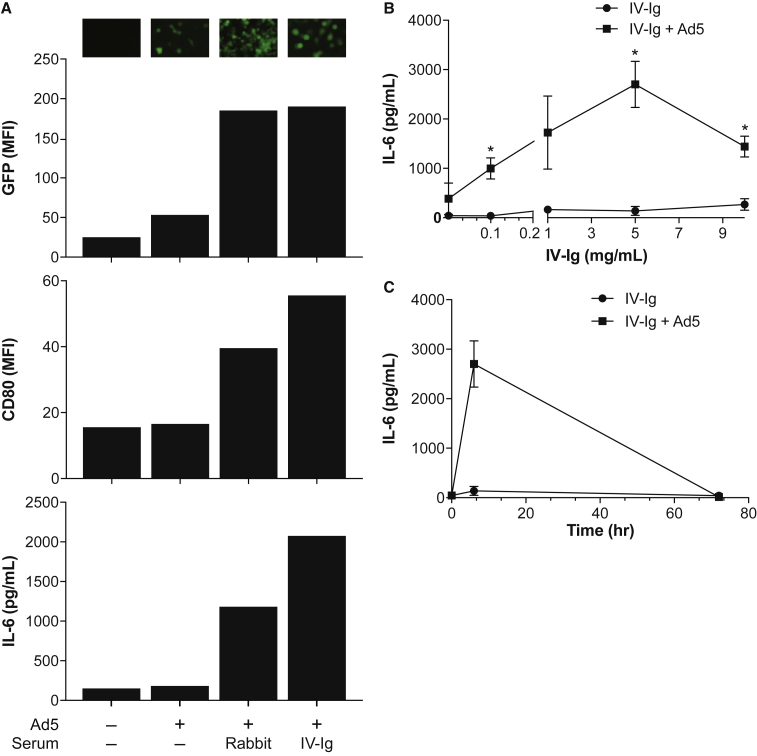
The adverse event in the human OTCD trial was characterized by an immediate release of IL-6 in the serum that peaked at 6 h, followed by a rapid and intractable course of systemic inflammatory response syndrome.5 Systemic administration of high-dose Ad5 showed similar increases in serum IL-6 in both naive mice, which showed few clinical sequelae, and in macaques, which exhibited a sepsis-like syndrome.6,7 Our previous studies in mice and monkeys that received high-dose systemic Ad5 vectors in the presence of pre-existing Abs to Ad515,16 have demonstrated that some inflammatory cytokines were higher in immunized mice and macaques compared with naive animals. Systemic vector in pre-immunized animals was associated with limited mortality in mice and a more severe sepsis-like syndrome in macaques that included hematologic abnormalities. To validate our hypothesis, we investigated whether there was a correlation between the in vitro observation of an Ab-dependent increase in DC activation and an increase in systemic inflammation in animals receiving Ad5 vector in the setting of pre-existing Ad5 Ab. Using C57BL/6 mice, we harvested bone marrow (BM)-derived DCs that were then cultured and exposed to Ad5 complexed with IV-Ig or rabbit antiserum. Both sources of Ab to Ad5 enhanced transduction of mouse DCs over that seen with Ad5 alone (Figure 4A; see micrographs and quantification of GFP as measured by flow cytometry). Mouse DCs exposed to Ad5 with rabbit antiserum or IV-Ig also showed increased expression of CD80 and secretion of IL-6 (Figure 4A), similar to that observed in human DCs (Figures 2A and 2B). Next, we passively transferred increasing doses of IV-Ig into mice, followed by systemic delivery of Ad5 vector, and for each dose, we examined IL-6 secretion into the serum at 6 h. Ad5 vector alone did not increase IL-6 over non-injected animals (Figure 4B; see data at 0 IV-Ig). However, we observed statistically significant elevations in IL-6 (p < 0.05) at three of the four IV-Ig doses compared with serum IL-6 in animals that received only IV-Ig. A limited time course of IL-6 secretion in passively transferred mice showed very high levels at 6 h after Ad5 vector delivery, which returned to baseline some time before 72 h (Figure 4C). These findings are consistent with the time course of IL-6 secretion in OTCD research subjects.5
Figure 4.
Activation of Murine DCs and Enhanced In Vivo Inflammatory Responses to Ad5 Immune Complexes
(A) Bone marrow DCs (106) from C57BL/6 mice were transduced with Ad5-GFP (MOI, 104) particles, Ad5 pre-complexed with rabbit antiserum, or Ad5-pre-complexed with IV-Ig. Cells were imaged 48 h later using an inverted fluorescent microscope, and GFP and CD80 expression were quantified using flow cytometry. IL-6 release in supernatants was determined using a mouse Luminex multi-analyte assay. (B) Ad5-GFP (1011 vp) was injected intravenously into the tail vein of C57BL/6 mice that previously received IV-Ig. IL-6 (pg/mL) is plotted against the expected final IV-Ig concentration (mg/mL). We used a t test to compare IL-6 levels at a given final IV-Ig concentration with and without Ad5 vector. *p < 0.05. (C) Serum IL-6 levels were measured 6 and 72 h after vector administration in animals that received IV-Ig + Ad5 or IV-Ig alone. (B and C) Each data point represents IL-6 levels in three mice (mean ± SD) for IV-Ig alone (circles) and IV-Ig + Ad5 (squares).
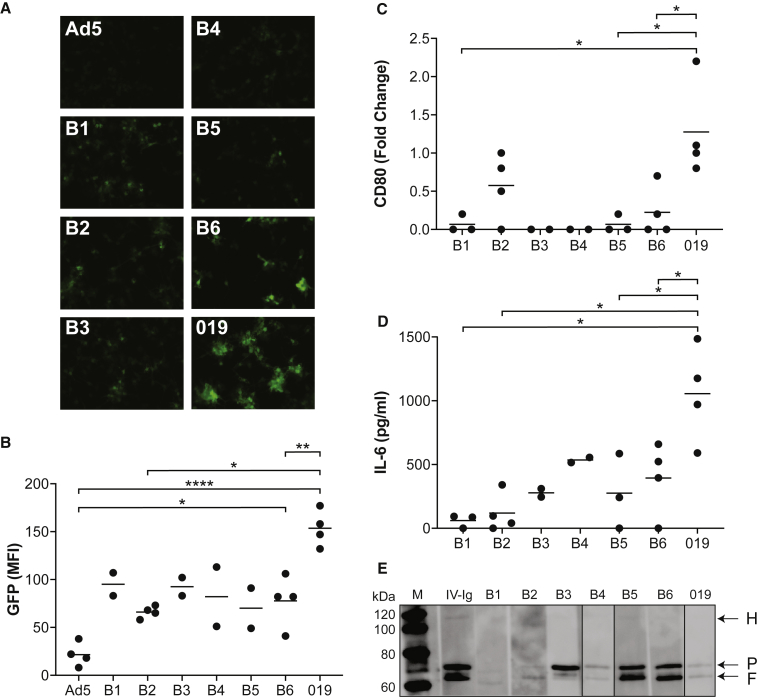
Having confirmed our hypothesis that some normal subjects have Ad5 Abs capable of enhancing the activation of human DCs, we analyzed blood from subject 019 in the OTCD trial to determine whether this finding could have contributed to the lethal outcome. The only remaining samples from this patient allowing us to test this hypothesis were frozen whole blood obtained prior to vector administration; hence comparisons with normal subjects also utilized frozen whole blood rather than serum. Blood samples were prepared from six normal subjects, labeled B1–B6. A western blot of these blood samples, including that of subject 019, demonstrated binding to penton and fiber in all samples, with substantial differences in band intensities between samples (Figure 5E). We assessed the impact of Abs in these blood samples (B1–B6 and subject 019) on transduction and activation in monocyte-derived DC cultures obtained from four different donors. Limited DC numbers from two of the donors restricted analysis of some normal subjects to less than four. Normal subject samples demonstrated modest increases in transduction over that achieved with Ad5 alone (e.g., Ad5 versus Ad5/B6, p < 0.05; see Figure 5B). The enhancement was higher and much more significant (p < 0.0001) with blood from subject 019 (Figure 5B). Direct comparisons between blood samples revealed statistically significant differences between subject 019 and the two normal subject samples B2 (p < 0.05) and B6 (p < 0.01). We also evaluated blood samples for activation of DCs in these same cultures. Variations in measurements in the presence of Ad5 alone led us to evaluate the data across cell isolates after subtracting the baseline levels. The sample from subject 019 demonstrated the highest fold increase in CD80 that was significantly elevated beyond that observed with B1, B5, and B6 (p < 0.05; see Figure 5C). Of more relevance to the findings in the OTCD trial were the measurements of IL-6. Compared with samples treated with Ad5 alone, IL-6 was increased in all samples studied; however, IL-6 was much higher in blood from subject 019. This difference reached statistical significance (p < 0.05) when subject 019 was compared with control samples that were analyzed in at least three cell lines (B1, B2, B5, and B6).
Figure 5.
Enhanced Transduction and Activation of Human DCs from Ad5 Complexed with Blood from Subject 019
(A–D) Monocyte-derived DCs were established from four different human sources and analyzed for enhanced Ad5 transduction and activation in the presence of whole blood from six normal subjects (B1–B6) and subject 019. Analyses included GFP transduction (A and B), CD80 expression (C), and IL-6 secretion (D). All assays were conducted in all four cell preparations with Ad5 alone and Ad5 mixed with samples from subjects B2, B6, and 019, respectively. Only partial datasets are available for the other samples due to insufficient DC numbers. We used a two-sample t test, limited to those groups that had data for more than two DC preparations. Absolute data are presented for GFP, whereas background from Ad5 alone is subtracted from the data generated with blood for CD80 and IL-6. *p < 0.05, **p < 0.01, ****p < 0.0001. (E) Immunoblot of Ad5 capsid proteins, electrophoresed on a 4%–12% denaturing gradient gel and probed with whole blood from human subjects. The positions of hexon, penton, and fiber (H, P, and F) bands are indicated by arrows. A blot probed with pooled human IV-Ig is shown in the first lane.
Discussion
The impetus for our studies was to understand why subject 019 mounted such an exaggerated inflammatory response to systemic Ad5 vector. Our experiments show that approximately 5%–10% of naive, normal subjects harbor Abs to Ad5. Previous reports have demonstrated these Abs increased Ad-IC binding and uptake by immune cells.17,18 Furthermore, Ad5-IC activation of a DC-T cell axis may have caused increased HIV acquisition in the STEP trial.4 In our studies, Abs generated from natural infections were also capable of eliciting very high inflammatory responses from cultured human primary DCs when complexed with Ad5 vector.
Blood from subject 019, harvested before gene therapy, demonstrated very high activation of DCs, consistent with observations in 7% of highly sensitive, normal subjects. Our data suggest that the combination of the high-dose Ad5 vector that subject 019 received as a member of the highest-dose cohort, together with the presence of enhancing, pre-existing Abs to Ad5, may have contributed to the lethal inflammatory response that ensued. Although our studies suggest that pre-existing immunity to adenovirus (Ad) influenced this serious adverse event, we cannot draw any firm conclusions regarding causation because our data are limited to an association of in vitro experiments with a complex clinical event that occurred in a single research subject.
Another question we addressed was the impact of gene therapy in sensitizing a recipient to exaggerated toxicity following a second administration of vector. We have shown previously that rhesus macaques immunized with Ad5 have a broader spectrum of toxicity and increased serum inflammatory cytokines following high-dose systemic Ad5 vector compared to naive macaques.16 In this study, we show that topically applied Ad5 vectors in research subjects caused the formation of Abs that enhance DC transduction/activation.
Although we are confident that Abs to Ad5 that arise from natural infections and gene therapy can enhance vector-induced inflammation, we do not understand which species of Abs within polyclonal populations are actually causative. There is no correlation with neutralizing activity or total binding to Ad5 antigens. Work with Dengue vaccines showed worse clinical outcomes when patients were exposed to viruses of a different serotype than that used in the vaccine. The mechanism is believed to be caused by vaccine-induced Abs with low affinity to the infecting agent; when complexed with the virus, the Abs can enhance activation of DCs without neutralizing the pathogen.19 A similar situation could explain our results: a repertoire of binding Abs to the vector that can facilitate an update in DCs without functionally neutralizing it in vivo. In this scenario, the current in vitro NAb assay would not predict in vivo neutralizing or enhancing activity. A better understanding of these Abs may enable a biochemical test for pre-screening patients. Studies with cloned human anti-Ad Abs may help better define the relevant Ab population.
What impact does this study have on the current practice of gene therapy?20 Researchers are actively pursuing systemically administered Ad5 vectors as a novel therapeutic approach to treat cancers or as in vivo delivery carriers to hematopoietic stem cells.21, 22, 23 It is possible that a subset of vaccine or gene therapy recipients may demonstrate an exaggerated immune response to Ad5 vectors, which could confound the interpretation of safety. Our studies suggest that a cell line-based assay evaluating enhanced transduction of antigen-presenting cells may be useful in identifying subjects at higher risk. A cell line is preferred over primary human DC cultures to ensure the supply of a single source of cells without introducing donor-to-donor variability. Of more importance would be the possible relevance of these findings to the acute toxicity that is being observed following high systemic doses of adeno-associated virus (AAV) vectors in rhesus macaque and in clinical trials for Duchenne muscular dystrophy (DMD).24, 25, 26, 27 Toxicity from both high-dose AAV and Ad5 vectors occurs early, is not linearly related to dose, and results in acute liver damage and a drop in platelets that can evolve into a severe coagulopathy in monkeys.5,7,16,24, 25, 26, 27 As we observed in the Ad5 OTCD trial, there is substantial subject-to-subject variation in the magnitude of the acute response when AAV has been delivered at high doses to human and non-human primates.24, 25, 26, 27 However, the kinetics and nature of the acute toxicity observed with Ad5 in macaques and in the OTCD trial are different from what we observed with AAV in macaques and what has been reported in DMD patients.5,7,16,24, 25, 26, 27 The response to high-dose systemic Ad5 occurs within hours and is associated with very high production of inflammatory cytokines that are probably increased by the enhancing Abs described in this paper. In contrast, the response to high-dose systemic AAV appears several days after infusion and seems to be associated with the activation of complement rather than the production of inflammatory cytokines. What we have shown in this study, however, is that pre-existing vector Abs have a possible confounding effect on toxicity that may not be unique to Ad5 vectors. Although the early data with high-dose AAV for neuromuscular diseases is impressive, there appears to be a narrow therapeutic window with substantial variation between patients. As we learned from the OTCD trial, we need to understand those who exhibit amplified responses in order to assure a product that is both safe and effective.
Materials and Methods
Vectors
Replication-defective E1/E3-deleted human Ad5 expressing enhanced GFP was obtained from the Penn Vector Core at the University of Pennsylvania. Vector preparations were validated by restriction endonuclease analysis, devoid of endotoxin contamination (<20 endotoxin units [EU]/mL), and had very low replication-competent Ad (less than 1 viral particle [vp] in 107).
Serum Samples
Polyclonal serum against Ad5 was generated by repeatedly immunizing rabbits with the respective vectors.9 In brief, New Zealand white rabbits received intramuscular injections of 1013 vp of recombinant Ad5.CMV.GFP, followed by a boost with an equal dose with Freund’s incomplete adjuvant 34 days later. The animals were terminally bled 14 days after the boost to collect antiserum that had an NAb titer of 81,920 (reciprocal of serum dilution). Polyclonal rabbit serum against SAdV-24, a chimpanzee-derived adenoviral vector, does not cross-react with Ad5.9 Serum from human subjects pre- and day 28 post-Ad5 vector administration was kindly provided by Dr. David Margolis (Department of Dermatology, University of Pennsylvania). The samples were obtained from subjects enrolled in a phase I clinical trial (ClinicalTrials.gov: NCT01858272) to evaluate the safety of Ad5-expressing PDGF-β. Additional serum samples from healthy donors and pooled human IV-Ig administration were obtained from the blood bank at the Hospital at University of Pennsylvania, Philadelphia, PA, USA. Pooled human Ig had an anti-Ad5 NAb titer of 2,560; the NAb titers of serum samples against individual serum samples are indicated in the figures. All serum samples were heat-inactivated at 56°C for 30 min prior to their use.
NAb Assay
Recombinant Ad5.CMV.LacZ (107 vp/well) was diluted in serum-free DMEM (Cellgro, Corning, NY, USA) and incubated with 2-fold serial dilutions (initial dilution, 1:20) of heat-inactivated serum samples in DMEM for 1 h at 37°C. Subsequently, the serum-vector mixture was added to 96-well plates seeded with 1 × 105 293 cells/well. After 1 h, each well was supplemented with an equal volume of DMEM with 20% fetal bovine serum (FBS; Thermo Fisher Scientific, Hampton, NH, USA) and incubated for 18–22 h at 37°C and 5% CO2. Then cells were washed twice in phosphate-buffered saline (PBS). Cells were lysed and analyzed with a mammalian β-galactosidase assay kit for bioluminescence (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol and measured in a microplate luminometer (Clarity, BioTek, Winooski, VT, USA). The NAb titer is defined as the highest serum dilution that inhibited Ad5.CMV.LacZ transduction (β-galactosidase expression) by ≥50%, compared with the naive mouse serum control (S3509; Sigma-Aldrich, St. Louis, MO, USA). NAb assays were repeated with the Ad5-GFP vector, and NAb titers were found to be similar to titers obtained using vector encoding LacZ.
Human and Mouse DCs
Human immature DCs were derived by differentiating plastic-adherent monocytes obtained from the Human Immunology Core, Center for AIDS Research at the University of Pennsylvania, for 7 days in serum-free medium (AIM-V; Invitrogen, Carlsbad, CA, USA) supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF; Berlex, Cedar Knolls, NJ, USA) and IL-4 (R&D Systems, Minneapolis, MN, USA). Cells were phenotyped on day 7 using CD11c, human leukocyte antigen - DR isotype (HLA-DR), CD80, CD86, and DC-SIGN (BD Biosciences, San Jose, CA, USA); the resulting purity was >90%. Day 7 DCs were transduced with Ad5 or Ad5-Ab immune complexes, and 48 h later, the cells were analyzed by flow cytometry for GFP expression and upregulation of CD80. Mouse BM cells were isolated by flushing the femurs and tibias with PBS supplemented with 2% heat-inactivated FBS. Mouse immature DCs were derived by culturing mouse BM cells in RPMI medium supplemented with 20 ng/mL murine GM-CSF (R&D Systems, Minneapolis, MN, USA). After 5 days of culture, BM-DCs were phenotyped and used in experiments.
Ab-Dependent Enhancement Assay
Ad5 vectors (5 × 1010 genome copies) were diluted in 100 μL serum-free DMEM and incubated with an equal volume of heat-inactivated serum, also diluted in DMEM, for 15 min at 37°C. At the end of the incubation period, Ad-Ab complexes were added to cells at a MOI of 104 and incubated for 48 h at 37°C and 5% CO2. A MOI of 102 was used for transduction assays of 293 cells.
Western Blotting and IgG Purification
Equal amounts of denatured Ad5 vectors were electrophoresed on a 4%–12% denaturing polyacrylamide gel (Invitrogen, Carlsbad, CA, USA), transferred to a polyvinylidene fluoride (PVDF) membrane (Invitrogen, Carlsbad, CA, USA), and probed using various serum samples as indicated in the text. Following incubation with an anti-human polyclonal Ab (Santa Cruz Biotechnology, Dallas, TX, USA) or anti-rabbit Ab (Pierce/Thermo Fisher Scientific, Hampton, NH, USA), blots were developed using a Dura West Extended Substrate detection kit (Pierce/Thermo Fisher Scientific, Hampton, NH, USA) and imaged using a Syngene fluorescent gel imager (Syngene, Frederick, MD, USA). Total IgG was isolated from the serum samples using an agarose-protein G spin column (Pierce/Thermo Fisher Scientific, Hampton, NH, USA). IgG samples were further desalted using a spin column (Pierce/Thermo Fisher Scientific, Hampton, NH, USA), and the amount of IgG or IgM in the isolated fractions was quantitated by ELISA (Bethyl Laboratories, Montgomery, TX, USA).
Animal Studies
All animal procedures were performed in accordance with protocols approved by the institutional animal care and use committee of University of Pennsylvania, Philadelphia, PA, USA. Six- to eight-week-old C57BL/6 mice were adoptively transferred (i.v.) with increasing amounts of pooled human IV-Ig as indicated in the text. Human IV-Ig was administered to mice (n = 3) in two doses, 24 and 2 h before vector injection. Ad5.GFP vectors (1011 vp/mouse) were injected via tail vein, and serum was collected from animals 6 h after vector administration for cytokine analysis. Serum from animals that received either vector or human IV-Ig was used as controls for the experiment.
ELISA and Luminex Assays
Supernatants from human DC cultures were analyzed for the presence of IL-6 by ELISA. Levels of IL-6 in murine samples were analyzed using a Luminex-100 (Luminex, Austin, TX, USA) using a mouse Milliplex multi-analyte cytokine kit (Millipore Sigma, Temecula, CA, USA).
Flow Cytometry and Microscopy
Co-stimulatory molecule upregulation and GFP expression in DCs were quantified using an FC500 flow cytometer (Beckman Coulter, Brea, CA, USA), and data were analyzed using FlowJo software (Tree Star/Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Images of GFP expression in cultured cells were collected using an inverted Nikon fluorescent microscope fitted with a Nikon digital camera.
Author Contributions
S.S. designed and conducted all studies, interpreted the data, and wrote parts of the manuscript. S.S. and R.C. conducted and analyzed the flow cytometry studies. J.M.W. and S.S. conceived the studies. J.M.W. was responsible for research coordination, strategy, and writing the manuscript. All authors discussed the results and commented on the manuscript.
Conflicts of Interest
J.M.W. is a paid advisor to and holds equity in Scout Bio and Passage Bio; he holds equity in Surmount Bio; he also has a sponsored research agreement with Ultragenyx, Biogen, Janssen, Precision Biosciences, Moderna Therapeutics, Scout Bio, Passage Bio, Amicus Therapeutics, and Surmount Bio, which are licensees of Penn technology. J.M.W. is an inventor on patents that have been licensed to various biopharmaceutical companies and for which he may receive payments.
Acknowledgments
This research was supported by grants P30-DK-47757 (J.M.W.) and P01-HL-059407 (J.M.W.) from the National Institutes of Health and a grant from GlaxoSmithKline, Inc. We thank Dr. David J. Margolis, Department of Dermatology, University of Pennsylvania, for providing serum samples from subjects enrolled in a phase I adenoviral gene therapy trial for PDGF-β. We thank Dr. Donald L. Siegel, Department of Pathology and Laboratory Medicine, University of Pennsylvania, for providing serum samples from normal subjects. We also thank the Vector Core at the University of Pennsylvania for providing the vectors used in this study.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2020.01.006.
Supplemental Information
References
- 1.Yang Y., Nunes F.A., Berencsi K., Furth E.E., Gönczöl E., Wilson J.M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiang Z.Q., Yang Y., Wilson J.M., Ertl H.C. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology. 1996;219:220–227. doi: 10.1006/viro.1996.0239. [DOI] [PubMed] [Google Scholar]
- 3.Kozarsky K., Grossman M., Wilson J.M. Adenovirus-mediated correction of the genetic defect in hepatocytes from patients with familial hypercholesterolemia. Somat. Cell Mol. Genet. 1993;19:449–458. doi: 10.1007/BF01233250. [DOI] [PubMed] [Google Scholar]
- 4.Perreau M., Pantaleo G., Kremer E.J. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J. Exp. Med. 2008;205:2717–2725. doi: 10.1084/jem.20081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raper S.E., Chirmule N., Lee F.S., Wivel N.A., Bagg A., Gao G.P., Wilson J.M., Batshaw M.L. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y., Chirmule N., Gao G.P., Qian R., Croyle M., Joshi B., Tazelaar J., Wilson J.M. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- 7.Schnell M.A., Zhang Y., Tazelaar J., Gao G.P., Yu Q.C., Qian R., Chen S.J., Varnavski A.N., LeClair C., Raper S.E., Wilson J.M. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- 8.Huang X., Yang Y. Innate immune recognition of viruses and viral vectors. Hum. Gene Ther. 2009;20:293–301. doi: 10.1089/hum.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy S., Gao G., Lu Y., Zhou X., Lock M., Calcedo R., Wilson J.M. Characterization of a family of chimpanzee adenoviruses and development of molecular clones for gene transfer vectors. Hum. Gene Ther. 2004;15:519–530. doi: 10.1089/10430340460745838. [DOI] [PubMed] [Google Scholar]
- 10.Sapinoro R., Maguire C.A., Burgess A., Dewhurst S. Enhanced transduction of dendritic cells by FcgammaRI-targeted adenovirus vectors. J. Gene Med. 2007;9:1033–1045. doi: 10.1002/jgm.1112. [DOI] [PubMed] [Google Scholar]
- 11.Taylor A., Foo S.S., Bruzzone R., Dinh L.V., King N.J., Mahalingam S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 2015;268:340–364. doi: 10.1111/imr.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margolis D.J., Morris L.M., Papadopoulos M., Weinberg L., Filip J.C., Lang S.A., Vaikunth S.S., Crombleholme T.M. Phase I study of H5.020CMV.PDGF-beta to treat venous leg ulcer disease. Mol. Ther. 2009;17:1822–1829. doi: 10.1038/mt.2009.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chirmule N., Propert K., Magosin S., Qian Y., Qian R., Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 14.Guilliams M., Bruhns P., Saeys Y., Hammad H., Lambrecht B.N. The function of Fcγ receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 2014;14:94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- 15.Varnavski A.N., Calcedo R., Bove M., Gao G., Wilson J.M. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther. 2005;12:427–436. doi: 10.1038/sj.gt.3302347. [DOI] [PubMed] [Google Scholar]
- 16.Varnavski A.N., Zhang Y., Schnell M., Tazelaar J., Louboutin J.P., Yu Q.C., Bagg A., Gao G.P., Wilson J.M. Preexisting immunity to adenovirus in rhesus monkeys fails to prevent vector-induced toxicity. J. Virol. 2002;76:5711–5719. doi: 10.1128/JVI.76.11.5711-5719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu Y., Sperber K., Mayer L., Hsu M.T. Persistent infection of human adenovirus type 5 in human monocyte cell lines. Virology. 1992;188:793–800. doi: 10.1016/0042-6822(92)90534-v. [DOI] [PubMed] [Google Scholar]
- 18.Lyons M., Onion D., Green N.K., Aslan K., Rajaratnam R., Bazan-Peregrino M., Phipps S., Hale S., Mautner V., Seymour L.W., Fisher K.D. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol. Ther. 2006;14:118–128. doi: 10.1016/j.ymthe.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Dengue vaccine: WHO position paper, September 2018 - Recommendations. Vaccine. 2019;37:4848–4849. doi: 10.1016/j.vaccine.2018.09.063. [DOI] [PubMed] [Google Scholar]
- 20.Jönsson F., Kreppel F. Barriers to systemic application of virus-based vectors in gene therapy: lessons from adenovirus type 5. Virus Genes. 2017;53:692–699. doi: 10.1007/s11262-017-1498-z. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Vallvé S., Romeu A., Palau J. Horizontal gene transfer in bacterial and archaeal complete genomes. Genome Res. 2000;10:1719–1725. doi: 10.1101/gr.130000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machiels J.P., Salazar R., Rottey S., Duran I., Dirix L., Geboes K., Wilkinson-Blanc C., Pover G., Alvis S., Champion B. A phase 1 dose escalation study of the oncolytic adenovirus enadenotucirev, administered intravenously to patients with epithelial solid tumors (EVOLVE) J. Immunother. Cancer. 2019;7:20. doi: 10.1186/s40425-019-0510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter M., Stone D., Miao C., Humbert O., Kiem H.P., Papayannopoulou T., Lieber A. In Vivo Hematopoietic Stem Cell Transduction. Hematol. Oncol. Clin. North Am. 2017;31:771–785. doi: 10.1016/j.hoc.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hordeaux J., Wang Q., Katz N., Buza E.L., Bell P., Wilson J.M. The Neurotropic Properties of AAV-PHP.B Are Limited to C57BL/6J Mice. Mol. Ther. 2018;26:664–668. doi: 10.1016/j.ymthe.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinderer C., Katz N., Buza E.L., Dyer C., Goode T., Bell P., Richman L.K., Wilson J.M. Severe Toxicity in Nonhuman Primates and Piglets Following High-Dose Intravenous Administration of an Adeno-Associated Virus Vector Expressing Human SMN. Hum. Gene Ther. 2018;29:285–298. doi: 10.1089/hum.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solid Biosciences . 2019. Solid Biosciences reports first quarter 2019 financial results and provides business update.https://www.solidbio.com/about/media/press-releases/solid-biosciences-reports-first-quarter-2019-financial-results-and-provides-business-update [Google Scholar]
- 27.Pfizer . 2019. Pfizer presents initial clinical data on phase 1b gene therapy study for Duchenne Muscular Dystrophy (DMD)https://www.pfizer.com/news/press-release/press-release-detail/pfizer_presents_initial_clinical_data_on_phase_1b_gene_therapy_study_for_duchenne_muscular_dystrophy_dmd [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.