Key Points
Question
How much do drug companies spend on research and development to bring a new medicine to market?
Findings
In this study, which included 63 of 355 new therapeutic drugs and biologic agents approved by the US Food and Drug Administration between 2009 and 2018, the estimated median capitalized research and development cost per product was $985 million, counting expenditures on failed trials. Data were mainly accessible for smaller firms, products in certain therapeutic areas, orphan drugs, first-in-class drugs, therapeutic agents that received accelerated approval, and products approved between 2014 and 2018.
Meaning
This study provides an estimate of research and development costs for new therapeutic agents based on publicly available data; differences from previous studies may reflect the spectrum of products analyzed and the restricted availability of data in the public domain.
Abstract
Importance
The mean cost of developing a new drug has been the subject of debate, with recent estimates ranging from $314 million to $2.8 billion.
Objective
To estimate the research and development investment required to bring a new therapeutic agent to market, using publicly available data.
Design and Setting
Data were analyzed on new therapeutic agents approved by the US Food and Drug Administration (FDA) between 2009 and 2018 to estimate the research and development expenditure required to bring a new medicine to market. Data were accessed from the US Securities and Exchange Commission, Drugs@FDA database, and ClinicalTrials.gov, alongside published data on clinical trial success rates.
Exposures
Conduct of preclinical and clinical studies of new therapeutic agents.
Main Outcomes and Measures
Median and mean research and development spending on new therapeutic agents approved by the FDA, capitalized at a real cost of capital rate (the required rate of return for an investor) of 10.5% per year, with bootstrapped CIs. All amounts were reported in 2018 US dollars.
Results
The FDA approved 355 new drugs and biologics over the study period. Research and development expenditures were available for 63 (18%) products, developed by 47 different companies. After accounting for the costs of failed trials, the median capitalized research and development investment to bring a new drug to market was estimated at $985.3 million (95% CI, $683.6 million-$1228.9 million), and the mean investment was estimated at $1335.9 million (95% CI, $1042.5 million-$1637.5 million) in the base case analysis. Median estimates by therapeutic area (for areas with ≥5 drugs) ranged from $765.9 million (95% CI, $323.0 million-$1473.5 million) for nervous system agents to $2771.6 million (95% CI, $2051.8 million-$5366.2 million) for antineoplastic and immunomodulating agents. Data were mainly accessible for smaller firms, orphan drugs, products in certain therapeutic areas, first-in-class drugs, therapeutic agents that received accelerated approval, and products approved between 2014 and 2018. Results varied in sensitivity analyses using different estimates of clinical trial success rates, preclinical expenditures, and cost of capital.
Conclusions and Relevance
This study provides an estimate of research and development costs for new therapeutic agents based on publicly available data. Differences from previous studies may reflect the spectrum of products analyzed, the restricted availability of data in the public domain, and differences in underlying assumptions in the cost calculations.
This study uses publicly available data to analyze research and development spending to win FDA approval and bring new drugs to market between 2009 and 2018.
Introduction
Rising drug prices have attracted public debate in the United States and abroad on fairness of drug pricing and revenues.1 Central to this debate is the scale of research and development investment by biopharmaceutical companies that is required to bring new medicines to market.2
The most widely cited studies of the cost of developing a new drug (DiMasi et al3,4) reported a sharp increase in the mean cost of developing a single new therapeutic agent from $1.1 billion in 2003 to $2.8 billion in 2013 (in 2018 US dollars), based on a real cost of capital rate of 11% per year in the former study3 and of 10.5% per year in the latter.4 Other studies in this period, most of which relied on confidential or proprietary data, reported figures from $314 million to $2.1 billion (in 2018 US dollars).5,6,7,8,9,10,11
In 2017, Prasad and Mailankody estimated the research and development costs of new cancer drugs using public data reported by pharmaceutical firms to the US Securities and Exchange Commission (SEC).12 They estimated the median research and development cost of bringing a single cancer drug to market to be $780 million (in 2018 US dollars), capitalized at a real cost of capital rate of 7% per year, based on a sample of 10 drugs.12
This present study estimates the research and development investment required to bring a new therapeutic agent to market using publicly available data for products approved by the US Food and Drug Administration (FDA) between 2009 and 2018.
Methods
Sample Identification and Characteristics
We identified all new therapeutic agents, ie, new drug applications and biologics license applications approved by the FDA between 2009 and 2018, in the Drugs@FDA database.13 For each, we extracted the date of approval, date of submission of investigational new drug application, date of submission of new drug application or biologics license application, indication, type (pharmacologic or biologic), expedited programs (priority review, accelerated approval, fast track, or breakthrough), orphan status, route of administration (oral, injection, intravenous, or other), and manufacturer (eTable 1 in the Supplement). To capture innovation, we determined whether an agent was first in class using publications by FDA officials.14,15 We checked the data for consistency with published reports.15,16
Therapeutic areas were obtained from the anatomical therapeutic chemical classification system database.17 For agents that were not yet classified, we based our decision on the approved indication.
For each agent, we identified start and end dates of clinical studies (phases 1, 2, and 3 for the FDA-approved indication) from ClinicalTrials.gov (search conducted on April 4, 2019). If there were multiple studies in the same phase, the earliest start date was selected. We verified these dates with reports in SEC filings and used the dates from SEC filings if there were discrepancies. We classified combined phase 1 and 2 trials as phase 2 and combined phase 2 and 3 trials as phase 3, consistent with other studies.18,19,20 Dates of submission of investigational new drug applications were used to approximate the end of preclinical testing; these dates were checked for consistency with filings to ensure clinical testing had not already begun outside the United States.
No data were collected from human participants, and all data in this study were publicly available.
Research and Development Data Extraction
Publicly traded US companies are legally required by the SEC to file annual 10-K and quarterly 10-Q forms, which are reports of key financial performance indicators that include audited financial statements and data on research and development expenditures. For every agent in our sample, we searched the SEC website for reports from the firm that received FDA approval for it.21
Exclusions
As reports for private US drug firms and foreign companies listed on non-US stock exchanges were unavailable, their products were excluded. For firms with available reports, we screened 10-K and 10-Q filings for data on research and development expenditures on individual drug candidates. We excluded products developed by companies that only reported total research and development expenditures across all drug candidates or across therapeutic areas.
For excluded products, we searched the 10-K and 10-Q forms and online press releases of manufacturers at the time that agents were approved to see if any were developed in collaboration with other firms via licensing deals. If so, we searched for 10-K and 10-Q forms from those firms in case there were research and development data for the product in question.
Inclusions
For each therapeutic agent with available data, we extracted direct and indirect research and development expenditures in each year of development. Drugs were tracked across years in SEC filings using the brand, generic, or compound names of agents, as appropriate.
Direct research and development expenses included all resources directly allocated to a particular agent. Indirect research and development expenses, which included personnel and overhead costs, were sometimes reported as a lump sum across all drug development programs. If so, we applied the same percentage of direct research and development costs attributable to a particular agent to estimate indirect costs for the same agent. The proportional allocation of personnel and overhead expenses is common practice in costing studies.22
Costs were tracked from the year a company started reporting costs for a particular drug candidate in their financial statements until the quarter of approval, which often included 1 or more years of preclinical costs. In some cases, at the first mention of the candidate in SEC filings, companies reported the costs incurred since inception of the drug development program. Certain companies only started tracking costs at late stages of preclinical development or at the start of phase 1 of development, resulting in an underreporting of preclinical costs.
Some drugs were initially developed by companies that subsequently licensed out their drug candidates to other firms, which then brought these products to market. In these cases, it was assumed that any preclinical and clinical costs incurred during initial development was included in licensing fees and milestone payments. Hence, where these fees and payments were recorded as research and development expenses for the agent in question, these costs were extracted. Data on costs incurred by the originator firms were not collected.
If SEC filings were missing for 3 or fewer years since the inception of the drug development program (eg, if a company was privately held during early years of development) and the product did not move between development phases (ie, either from 1 to 2 or 2 to 3), we extrapolated costs from the closest available year. Products were excluded if more than 3 years of SEC filings were missing.
Three investigators independently extracted all research and development data used in this study. Discrepancies were resolved through discussions. Where disagreements existed, we assumed the higher estimate of research and development expenditures.
Quality Assessments
Consistency and completeness of company reporting in SEC filings varied over time. Many reported detailed research and development costs, which allowed us to track outlays over time for individual candidates. Others reported costs inconsistently or with missing data for some years, requiring various assumptions, for example on timings of transitions between phases and extrapolations when SEC filings were missing.
To aid interpretation, we categorized each estimate as high, medium, or low quality, depending on the availability and consistency of reported data. The categorization was developed through discussion between all authors.
High-quality estimates comprised drugs discovered internally, allowing tracking of costs back to inception of the development program, and products licensed at preclinical or phase 1 stages with minimal up-front fees or milestone payments captured in SEC filings. Late commercialization deals related to marketing of products in non-US markets were also deemed high-quality estimates, as they would have had little or no effect on research and development expenses incurred on trials required for FDA approval.
Low-quality estimates comprised all acquisitions, licensing deals, or other collaboration agreements in phases 2 or 3, earlier deals in which it was unclear whether all costs were captured in data extraction, and estimates requiring extrapolation of 2 to 3 years of data. We classified estimates as medium quality when other judgment calls regarding financial reporting, as agreed upon by the authors, had to be made.
Two investigators independently categorized the quality of estimates and resolved discrepancies through discussions.
Costs of Failed Trials
Accurate information on costs of failures, ie, research and development outlays on candidates being developed by companies but not ultimately approved, is essential to estimating the costs of drug development. We accounted for failures using data on aggregate clinical trial success rates from a recent study by Wong et al (Table 1).18
Table 1. Clinical Trial Success Rates by Phase (on Aggregate and by Therapeutic Area)a.
| Source | Phase 1 to Approval, %b | Phase 2 to Approval, %c | Phase 3 to Approval, %d | FDA Submission to Approval, %e |
|---|---|---|---|---|
| Aggregate rates | ||||
| Wong et al18 | 13.8 | 35.1 | 59.0 | 83.2 |
| Thomas et al19 | 9.6 | 15.3 | 49.6 | 85.3 |
| Hay et al20 | 10.4 | 16.2 | 50.0 | 83.2 |
| Therapeutic-area–specific rates18 | ||||
| Oncology | 3.4 | 6.7 | 35.5 | 81.7 |
| Metabolism and endocrinology | 19.6 | 24.1 | 51.6 | 80.4 |
| Cardiovascular | 25.5 | 32.3 | 62.2 | 84.5 |
| Central nervous system | 15.0 | 19.5 | 51.1 | 82.2 |
| Autoimmune and inflammation | 15.1 | 21.2 | 63.7 | 80.3 |
| Ophthalmologyf | 32.6 | 33.6 | 74.9 | 80.4 |
| Infectious disease | 25.2 | 35.1 | 75.3 | 84.9 |
| Otherg | 20.9 | 27.3 | 63.6 | 80.4 |
Abbreviation: FDA, US Food and Drug Administration.
Rates across all indications for individual therapeutic agents (as opposed to rates for lead indications, which were higher in all phases). Only the success rates used in this analysis were reported.
Phase 1 trials, which usually include as many as 100 healthy volunteers and may take several months to conduct, are primarily used to assess the tolerability and safety of a therapeutic agent in different doses; these are sometimes referred to as first-in-human trials.
Phase 2 trials, which can involve as many as a few hundred patients with a disease or condition and take several months to 2 years to complete, are typically used to gather data on the efficacy and safety of a therapeutic agent in different doses.
Phase 3 trials, which can involve several thousand participants with a disease or condition and may take 1 to 4 years to run, are generally used to confirm the efficacy and safety of the dose of the therapeutic agent believed to provide the best risk-benefit ratio.23
Indicates the proportion of new drug applications and biologics license applications approved by the FDA. Wong et al18 reported aggregate and therapeutic-area–specific rates through phase 3. These data were supplemented with estimates of FDA submission to approval rates from Hay et al; if a particular category from the study by Wong et al was not reported by Hay et al, the category Other was used.20
This category was applied to therapeutic agents classified as treating sensory organ diseases, ie, anatomical therapeutic chemical classification system code S.
Values in this category were based on the rates for “all [agents] without oncology” reported by Wong et al.18 These rates were applied to therapeutic agents that were outside the other categories.
Wong et al reported that the percentages of FDA approvals were 13.8% for therapeutic agents entering phase 1, 35.1% for those entering phase 2, and 59.0% for those entering phase 3.18
Wong et al18 provided success rates through phase 3. We supplemented these rates with a recent estimate of the proportion of biologics license applications and new drug applications that are approved by the FDA (83.2%).20
Costing Method
For each agent, we estimated the expected research and development investment to bring the drug to market in 3 steps.
First, we summed direct and indirect research and development spending on a therapeutic agent in each year. All sums were inflation adjusted to 2018 dollars using the US consumer price index.
Second, we accounted for failed projects by dividing total research and development expenditures on a drug in a particular year by the corresponding aggregate phase-specific probability of success, similar to what was done in previous studies of costs of drug development.3,4,5,6,7 For example, for each drug, we divided phase 1 costs in each year by 0.138, which accounted for spending on the other 6.2 phase 1 trials that would fail, on average, for each successful development program. We used phase 1 rates to adjust preclinical expenditures, and we used the proportion of biologics license applications and new drug applications that are approved by the FDA to adjust costs once these applications were submitted to the agency for regulatory approval. Licensing fees and milestone payments, where captured, were adjusted using the success rate for the trial phase that was ongoing when the payments were made. When a phase shift took place within the financial year, we allocated the cost proportionally to the time spent in each phase. For example, if development moved from phase 1 to phase 2 on July 1 of a given year, we divided the costs equally between each phase. Similarly, in the year of approval, we multiplied the total cost by the fraction of the year elapsed by the time of approval. Hence, if a drug was approved on July 1, we only counted 50% of the costs in the year of approval since firms often incurred postapproval costs related to pharmacovigilance or testing in other indications.
Third, we applied a real cost of capital rate of 10.5% per year (ie, weighted average cost of capital in the pharmaceutical industry), as in the DiMasi et al study.4 Cost of capital is the required rate of return for an investor and encapsulates a risk-free rate (ie, opportunity cost) and premium based on the likelihood of business failure.24
Sensitivity and Subgroup Analyses
We ran 4 univariate sensitivity analyses. First, as the results were sensitive to the choice of aggregate clinical trial success rates (by phase), we recalculated the results using aggregate rates reported in 2 other studies (Table 1).19,20 Next, we calculated a second estimate of research and development costs using therapeutic-area–specific rates reported by Wong et al (Table 1), instead of aggregate rates. For example, oncology drugs in phase 1 have a 3.4% chance of ultimately receiving FDA approval, so we divided each year of phase 1 costs for these products by 0.034. Third, we performed a rerun of the analyses using a real cost of capital rate of 7% (as done by Prasad and Mailankody12) and 0% (to show noncapitalized outlays). Fourth, to account for potentially missing preclinical expenditures, we adopted the same assumption around preclinical costs as DiMasi et al, who reported that preclinical costs represented 42.9% of their total research and development estimate.4 Thus, for each product in our sample, we isolated clinical expenditures and imputed a preclinical cost that amounted to this percentage. No imputations were performed for products acquired through purchase after clinical development had begun since it was assumed that licensing fees and milestone payments reflected preclinical costs incurred by the company that sold the rights to the product. Additionally, we ran another sensitivity analysis but with imputations done for all products, including agents acquired through purchase.
As a subgroup analysis, we reported mean and median amounts by therapeutic area, using area-specific rates to adjust for costs of failure.
Statistical Analysis
We estimated the mean and median research and development investments across our sample in the base case and sensitivity analyses. We then restricted the sample to high-quality estimates and recalculated the mean and median amounts.
We conducted a nonparametric bootstrapped resampling with replacement (1000 iterations) to calculate 95% CIs around the estimated mean and median investments in research and development in our sample. We used χ2 tests to identify statistically significant differences in characteristics of the study sample vs therapeutic agents approved by the FDA between 2009 and 2018 that were excluded from our analysis. We used Kruskal-Wallis and Mann-Whitney U tests, as appropriate, to identify statistically significant differences in median estimated research and development investments across therapeutic areas and other drug characteristics.
All statistical tests were 2-tailed and used a type I error rate of 0.05. The data were analyzed using Stata version 15 (StataCorp).
Results
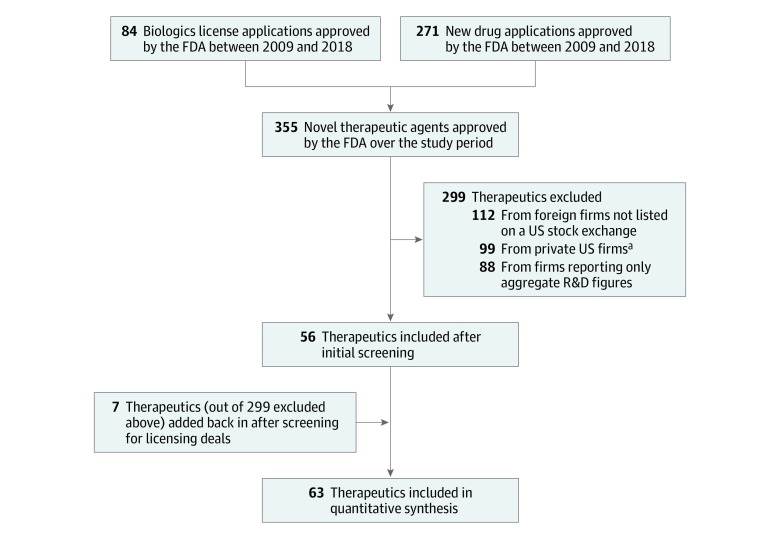
Between 2009 and 2018, the FDA approved 355 new drugs and biologics. Research and development expenditures from SEC government filings were available for 63 of these products, developed by 47 different companies (Figure 1). The sample covered 17.7% (63/355) of all new therapeutic agents approved by the FDA over this 10-year period. Twenty-three of the estimates were judged of high quality, 18 medium quality, and 22 low quality. eTable 2 in the Supplement provides the rationale for the quality categorization of each agent.
Figure 1. Selection Process for Therapeutic Agents.
FDA indicates the US Food and Drug Administration; R&D, research and development.
aIncludes firms that went public while developing the therapeutic agent in question but for which US Securities and Exchange Commission (SEC) filings were missing for more than 3 years of the drug development period.
Sample Characteristics
Table 2 presents statistics for the 63 included therapeutic agents. The sample contained a larger proportion of orphan drugs, therapeutic agents that benefited from expedited development or approval pathways, and first-in-class drugs compared with all FDA-approved products between 2009 and 2018, although these differences were not statistically significant. Differences in the breakdown of products by therapeutic area, accelerated vs regular approval, and approval dates were statistically significant.
Table 2. Characteristics of New Therapeutic Agents Approved by the US Food and Drug Administration Between 2009 and 2018a.
| Characteristics | No. (%) | P Value | |
|---|---|---|---|
| Included Agents (n = 63) | Full Sample (n = 355) | ||
| Agent type | |||
| Pharmacologic | 47 (75) | 271 (76) | .72 |
| Biologic | 16 (25) | 84 (24) | |
| Therapeutic areab | |||
| Antineoplastic and immunomodulating agents | 20 (32) | 116 (33) | .02 |
| Alimentary tract and metabolism | 15 (24) | 44 (12) | |
| Nervous system | 8 (13) | 33 (9) | |
| Antiinfective agents for systemic use | 5 (8) | 40 (11) | |
| Other | 15 (24) | 122 (34) | |
| Orphan drug | 31 (49) | 145 (41) | .14 |
| Drug received accelerated approval | 14 (22) | 41 (12) | .003 |
| Drug benefited from any expedited development or approval pathwayc | 48 (76) | 234 (66) | .06 |
| Innovativeness | |||
| First in class | 27 (43) | 127 (36) | .20 |
| Next in class | 36 (57) | 228 (64) | |
| Route of administrationd | |||
| Oral | 28 (44) | 187 (53) | .20 |
| Injection | 20 (32) | 87 (25) | |
| Intravenous | 10 (16) | 41 (12) | |
| Other | 5 (8) | 40 (11) | |
| Approval dates | |||
| 2009-2013 | 17 (27) | 142 (40) | .02 |
| 2014-2018 | 46 (73) | 213 (60) | |
Analyses were carried out using χ2 tests comparing the data for included agents (n = 63) vs excluded ones (n = 292).
Other therapeutic areas included blood and blood-forming organs, cardiovascular system, dermatologicals, musculoskeletal system, sensory organs, and various.
Pathways included accelerated approval, breakthrough therapy, fast track, orphan drug, and priority review.
Injection included intramuscular and subcutaneous; other routes included multiple, ophthalmic, and topical.
Research and Development Investments
Without adjustments for costs of failed trials, no statistically significant differences in the median research and development investment required to bring a new drug to market were observed across any of the drug characteristics shown in Table 2, except median costs for biologic drugs that were higher than those for pharmacologic drugs (eTable 3 in the Supplement). For the 63 agents included in the analysis, outlays for research and development (ie, total noncapitalized direct and indirect expenses incurred during preclinical and clinical testing) were estimated at a median of $319.3 million (95% CI, $236.4 million-$351.4 million), and the estimated mean outlay was $374.1 million (95 CI, $301.9 million-$464.2 million), (eTable 4 in the Supplement). The mean (SD) number of years of data per drug was 8.3 (2.8) years. eTable 5 in the Supplement shows the dates of phase changes for clinical trials of each included agent.
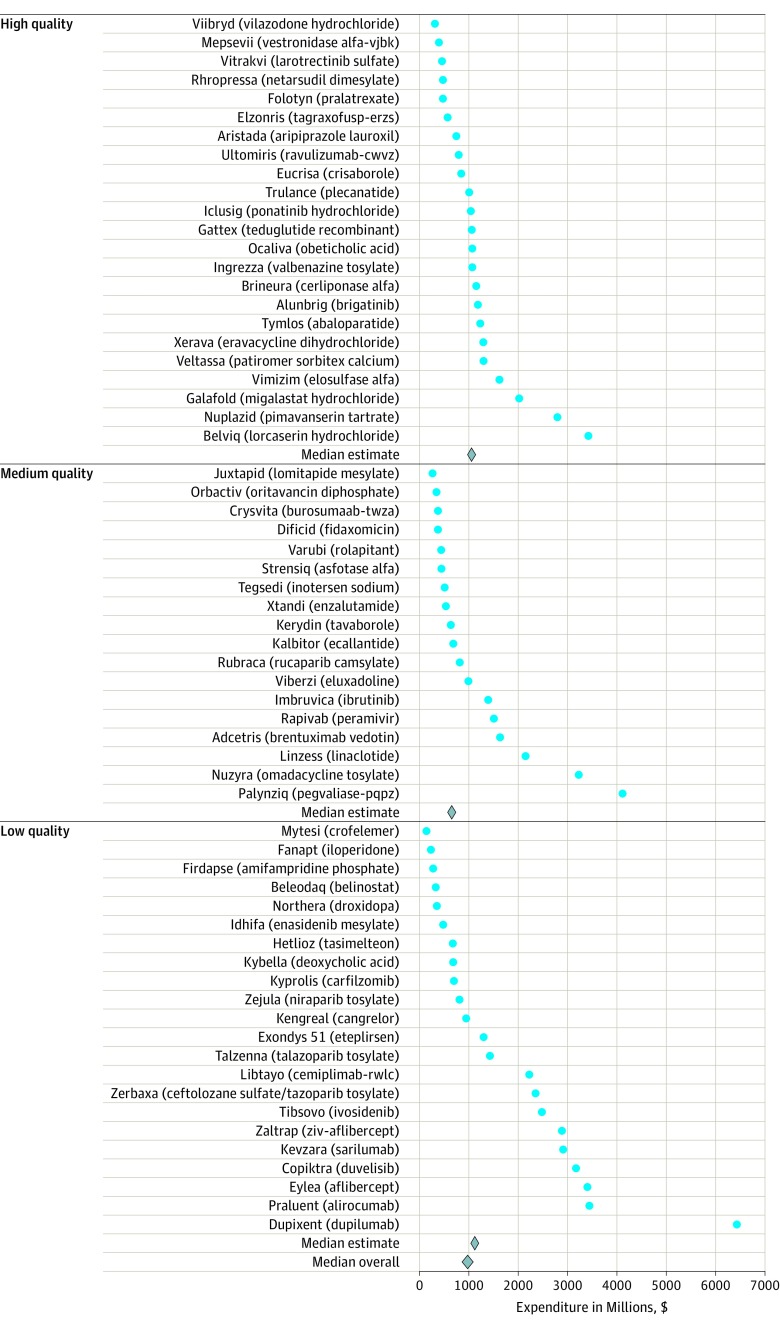
After accounting for costs of failed trials, the estimated median research and development investment required to bring a new drug to market, capitalized at a rate of 10.5% per year, was $985.3 million (95% CI, $683.6 million-$1228.9 million), and the estimated mean was $1335.9 million (95% CI, $1042.5 million-$1637.5 million) (Table 3). Figure 2 shows point estimates for each of the 63 agents, which ranged from $143.2 million for Mytesi (crofelemer) to $6419.0 million for Dupixent (dupilumab).
Table 3. Median Expected Research and Development Expenditure on New Therapeutic Agents Approved by the US Food and Drug Administration (2009-2018) in Main and Sensitivity Analyses.
| Parameter Varied in Sensitivity Analysis | Research and Development Costs in US$, Millions (95% CI) | |
|---|---|---|
| All Included Agents (n = 63) |
High-Quality Sample (n = 23) |
|
| Source of aggregate clinical trial success rates | ||
| Wong et al (base case)18 | 985.3 (683.6-1228.9) |
1048.1 (796.6-1180.6) |
| Hay et al20 | 1404.9 (1102.2-1773.4) |
1620.3 (1191.8-1773.4) |
| Thomas et al19 | 1465.8 (1121.5-1887.1) |
1678.4 (1259.7-1999.3) |
| Aggregate vs therapeutic-area–specific success rates | ||
| Aggregate rates (base case)18 | 985.3 (683.6-1228.9) |
1048.1 (796.6-1180.6) |
| Area-specific rates18 | 1385.2 (1053.9-1971.8) |
1220.1 (994.3-2118.3) |
| Cost of capital rate | ||
| 10.5% (base case) | 985.3 (683.6-1228.9) |
1048.1 (796.6-1180.6) |
| 7% | 848.9 (671.1-1076.6) |
930.0 (692.0-1018.1) |
| 0% | 688.2 (450.8-850.6) |
697.9 (567.9-850.6) |
| Adjustment for potential underreporting of spending on preclinical trials | ||
| No (base case) | 985.3 (683.6-1228.9) |
1048.1 (796.6-1180.6) |
| Yes, excluding agents acquired through purchase | 1228.9 (943.8-1900.9) |
1482.0 (1104.7-1860.9) |
| Yes, including all products | 1628.4 (1196.5-2072.2) |
1751.1 (1255.3-2246.9) |
Figure 2. Estimated Expenditures for Therapeutic Agents by Quality of the Estimates.
Indicates the estimated research and development investment needed for an average company to bring one of these products to market based on aggregate clinical trial success rates. Individual companies may have spent more or less on their respective development pipelines, based on their individual success rates.
Restricting the analysis to high-quality estimates (n = 23), the estimated median research and development investment increased from $985.3 million to $1048.1 million (95% CI, $796.6 million-$1180.6 million), while the estimated mean declined from $1335.9 million to $1143.3 million (95% CI, $880.4 million-$1442.1 million).
Sensitivity Analyses
Table 3 shows the results of univariate sensitivity analyses. When the aggregate success rates reported by Hay et al20 were used instead of those reported by Wong et al,18 the estimated median research and development investment, capitalized at a rate of 10.5% per year, increased from $985.3 million to $1404.9 million (95% CI, $1102.2 million-$1773.4 million), while the estimated mean increased from $1335.9 million to $1976.6 million (95% CI, $1595.5 million-$2454.8 million). When the rates from Thomas et al19 were used, the estimated median research and development investment, capitalized at an annual rate of 10.5%, was $1465.8 million (95% CI, $1121.5 million-$1887.1 million), and the estimated mean was $2059.5 million (95% CI, $1639.9 million-$2511.7 million).
When therapeutic-area–specific rates from Wong et al,18 rather than aggregate rates, were used to account for costs of failed trials for each agent, the estimated median research and development investment, capitalized at a rate of 10.5% per year, increased from $985.3 million to $1385.2 million (95% CI, $1053.9 million-$1971.8 million), and the estimated mean rose from $1335.9 million to $2307.2 million (95% CI, $1726.9 million-$3013.0 million).
When the costs were capitalized at an annual rate of 7% instead of 10.5%, the median expected investment decreased from $985.3 million to $848.9 million (95% CI, $671.1 million-$1076.6 million), and the mean decreased from $1335.9 million to $1158.6 million (95% CI, $929.3 million-$1407.2 million). When costs were not capitalized, rather than capitalized at an annual rate of 10.5%, the median expected investment decreased from $985.3 million to $688.2 million (95% CI, $450.8 million-$850.6 million), and the mean decreased from $1335.9 million to $884.8 million (95% CI, $717.1 million-$1084.7 million).
With the adjustments for potentially missing preclinical costs done for 33 of 63 products (ie, excluding agents acquired through purchase), based on the DiMasi et al4 approach, the estimated median research and development investment increased from $985.3 million to $1228.9 million (95% CI, $943.8 million-$1900.9 million), while the estimated mean increased from $1335.9 million to $1800.7 million (95% CI, $1396.4 million-$2268.5 million). With the adjustments for potentially missing preclinical costs done for all 63 products, the estimated median research and development investment increased to $1628.4 million (95% CI, $1196.5 million-$2072.2 million), while the estimated mean increased to $2214.7 million (95% CI, $1734.1 million-$2719.9 million).
Restricting the sensitivity analyses to high-quality estimates (n = 23), the estimated median and mean research and development investments required to bring a new drug to market increased in most cases (Table 3). eTable 6 in the Supplement shows the estimates for each agent in the base case and sensitivity analyses.
Subgroup Analyses by Therapeutic Area
Median estimates by therapeutic area (for areas with ≥5 drugs), adjusted using area-specific rates and capitalized at 10.5% per year, ranged from $765.9 million (95% CI, $323.0 million-$1473.5 million) for nervous system agents to $2771.6 million (95% CI, $2051.8 million-$5366.2 million) for antineoplastic and immunomodulating agents. The corresponding mean estimates ranged from $1076.9 million (95% CI, $508.7 million-$1847.1 million) for nervous system agents to $4461.2 million (95% CI, $3114.0 million-$6001.3 million) for antineoplastic and immunomodulating agents (Table 4).
Table 4. Mean And Median Expected Research and Development Expenditure on New Therapeutic Agents Approved by the US Food and Drug Administration (2009-2018) by Therapeutic Area.
| Therapeutic Areaa | Sample Size | Expenditure in US$, Millions (95% CI)b |
|
|---|---|---|---|
| Median | Mean | ||
| Antineoplastic and immunomodulating agents | 20 | 2771.6 (2051.8-5366.2) | 4461.2 (3114.0-6001.3) |
| Alimentary tract and metabolism | 15 | 1217.6 (613.9-1792.4) | 1430.3 (920.8-2078.7) |
| Nervous system | 8 | 765.9 (323.0-1473.5) | 1076.9 (508.7-1847.1) |
| Antiinfectives for systemic use | 5 | 1259.9 (265.9-2128.3) | 1297.2 (672.5-1858.5) |
| Dermatologicals | 4 | 747.4 | 1998.3 |
| Cardiovascular system | 3 | 339.4 | 1152.4 |
| Musculoskeletal system | 3 | 1052.6 | 937.3 |
| Blood and blood-forming organs | 2 | 793.0 | 793.0 |
| Sensory organs | 2 | 1302.8 | 1302.8 |
| Otherc | 1 | 1121.0 | 1121.0 |
Therapeutic areas were obtained from the anatomical therapeutic chemical classification system database.17 Where agents were not yet classified, the categorization was based on the approved indication.
Bootstrapped CIs were not calculated for therapeutic areas with less than 5 samples. Estimates were based on therapeutic-area–specific success rates reported by Wong et al.18
The product Veltassa (patiromer sorbitex calcium) was assigned to the therapeutic area Various under the subgroup Drugs for treatment of hyperkalemia and hyperphosphatemia.
Discussion
Based on data for 63 therapeutic agents developed by 47 companies between 2009 and 2018, the median research and development investment required to bring a new drug to market was estimated to be $985 million, and the mean was estimated to be $1336 million. Estimates differed across therapeutic areas, with costs of developing cancer drugs the highest. The results included costs of failed clinical trials and varied in sensitivity analyses using different estimates of trial success, preclinical expenditures, and cost of capital.
These figures were higher than the median capitalized research and development cost of $780 million (in 2018 US dollars) reported by Prasad and Mailankody for oncology drugs.12 This may be because adjustments based on clinical trial success rates were applied in the present study to account for costs of failures, whereas Prasad and Mailankody restricted their analysis to companies bringing their first drug to market and then summed the total research and development expenditures of each company during the development periods of the drugs in their sample. Most of the companies included in their study appeared to be more successful than the average company.25,26,27 Moreover, their analysis was based on data for 10 oncology drugs, which limits the comparability of their results with the present study.
The mean estimate of $1.3 billion in the present study was lower than the $2.8 billion (in 2018 US dollars) reported by DiMasi et al, which was based on data for 106 products developed by 10 large firms.4 The estimate by DiMasi et al used confidential data on costs voluntarily submitted by anonymous companies without independent verification, making them difficult to validate.12,28,29,30 The higher estimate of DiMasi et al seems to reflect a combination of higher clinical costs incurred by larger drug developers, lower estimates of trial success for each stage of development compared to the more recent data presented by Wong et al, and different assumptions about preclinical expenditures as their data set did not permit allocating these expenditures to specific agents.
The results of the present study varied widely when subject to sensitivity analyses, especially using different success rates. The methods employed by Wong et al to handle missing data were an improvement on earlier studies of trial success rates, and their study was based on a larger sample.18 Wong et al also noted that the most cited studies of success rates19,20,31 originated from researchers with ties to the pharmaceutical industry, and elaborated that “previous estimates of drug development success rates [relied] on relatively small samples from databases curated by the pharmaceutical industry and [were] subject to potential selection biases.”18 Also, compared with these earlier studies of success rates,19,20,31 the timing of the work by Wong et al18 more closely aligned with that of the present study, thereby improving its internal validity.
There are challenges in isolating preclinical investments by drug companies. It is especially difficult to identify the exact date from which costs should start being allocated to individual agents during the early stages of preclinical research. The base case scenario in this study relied on preclinical costs reported by firms in SEC filings, which were likely underestimated since many companies did not attribute costs during the drug discovery stages to individual candidates. DiMasi et al estimated that preclinical costs accounted, on average, for 42.9% of total capitalized costs, based on aggregated data on preclinical spending and assumptions around the duration of preclinical testing.4 Although preclinical costs were variously estimated in the present study, including indirectly through license fees, preclinical data were directly captured for 19 products. For these products, preclinical costs generally accounted for a lower share of the total capitalized costs (ranging from 0.3% to 50.7%; median 12.4%) than what was estimated by DiMasi et al (eTable 4 in the Supplement). For comparison, however, the 42.9% estimate was used to impute preclinical costs in sensitivity analyses in this study. Further validation work is needed to establish the preclinical share of research and development estimates for individual products.
Greater transparency around research and development costs is essential for analysts to check the veracity of claims by companies that the steep prices of new drugs are driven by high development outlays. While these expenditures are undoubtedly high, as shown in this study, it is important for policy makers, regulators, and payers to know the exact scale of these investments. This knowledge can inform the design of pricing policies that give adequate rewards for innovative drugs that bring value to health care systems.
Limitations
This study has several limitations. First, data were unavailable for many products approved by the FDA during the study period. No data were available for products developed by non-US companies not listed on a US stock exchange and large drug firms that did not report research and development figures for individual drug candidates. Thus, there was likely an overrepresentation of smaller firms, which may have run leaner operations than larger ones. This limited the generalizability of the results to all products.
Second, the included agents differed from other drugs approved by the FDA between 2009 and 2018, although not all differences were statistically significant. The sample included a larger proportion of orphan drugs, products in certain therapeutic areas, first-in-class drugs, therapeutic agents that received accelerated approval, and products approved between 2014 and 2018.
Third, there were inconsistencies in research and development reporting between companies, which made it difficult to ensure perfect comparability of research and development figures between firms. These inconsistencies may have been explained by differences in accounting policies. For instance, some firms allocated overhead and administrative costs to direct research and development figures, while others reported these costs separately. Some reported preclinical research costs as a separate line item, while others incorporated them in overhead costs. Companies also reported costs associated with licensing deals, drug acquisitions, and collaboration agreements differently, so it is likely that not all costs were fully reflected in some estimates.
Fourth, uncertainties in the analysis may have resulted in under- or overestimations of research and development expenditures for some products. It is difficult to attribute costs to individual drug candidates in the early stages of preclinical development, so only the costs reported by firms in SEC filings were considered in the base case analysis. However, since preclinical costs may have been underreported by some companies, sensitivity analyses were conducted to produce an upper-bound estimate of preclinical expenditures. Conversely, many drug firms conducted trials for a particular candidate for multiple indications, which may have led to overestimations of costs since trial expenditures were not broken down by indication but instead reported as annual lump sums for each agent. Also, the estimates did not reflect any public tax credits or subsidies, which may have led to further overestimations of costs incurred by companies.
Conclusions
This study provides an estimate of research and development costs for new therapeutic agents based on publicly available data. Differences from previous studies may reflect the spectrum of products analyzed, the restricted availability of data in the public domain, and differences in underlying assumptions in the cost calculations.
eTable 1. Characteristics of Therapeutic Agents Included in the Analysis
eTable 2. Quality Score of the Research and Development Estimate for Each Therapeutic Agent
eTable 3. Median Research and Development Outlays (2018 US $, Millions) by Product Category, Without Adjustment for Costs of Failures
eTable 4. Research and Development Costs (2018 US $, Millions), Broken Down by Phase of Clinical Development, for All Therapeutic Agents Included in the Analysis
eTable 5. Dates of Clinical Trial Phase Changes for all Therapeutic Agents Included in the Analysis
eTable 6. Estimated Research and Development Costs (2018 US $, Millions) for All Therapeutic Agents Included in the Analysis
References
- 1.Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA. 2016;316(8):858-871. doi: 10.1001/jama.2016.11237 [DOI] [PubMed] [Google Scholar]
- 2.Morgan S, Grootendorst P, Lexchin J, Cunningham C, Greyson D. The cost of drug development: a systematic review. Health Policy. 2011;100(1):4-17. doi: 10.1016/j.healthpol.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 3.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185. doi: 10.1016/S0167-6296(02)00126-1 [DOI] [PubMed] [Google Scholar]
- 4.DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20-33. doi: 10.1016/j.jhealeco.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 5.Jayasundara K, Hollis A, Krahn M, Mamdani M, Hoch JS, Grootendorst P. Estimating the clinical cost of drug development for orphan versus non-orphan drugs. Orphanet J Rare Dis. 2019;14(1):12. doi: 10.1186/s13023-018-0990-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mestre-Ferrandiz J, Sussex J, Towse A. The R&D Cost of a New Medicine. London, UK: Office of Health Economics; 2012. [Google Scholar]
- 7.Adams CP, Brantner VV. Estimating the cost of new drug development: is it really 802 million dollars? Health Aff (Millwood). 2006;25(2):420-428. doi: 10.1377/hlthaff.25.2.420 [DOI] [PubMed] [Google Scholar]
- 8.Adams CP, Brantner VV. Spending on new drug development. Health Econ. 2010;19(2):130-141. doi: 10.1002/hec.1454 [DOI] [PubMed] [Google Scholar]
- 9.DiMasi JA, Grabowski HG. The cost of biopharmaceutical R&D: is biotech different? Manage Decis Econ. 2007;28(4-5):469-479. doi: 10.1002/mde.1360 [DOI] [Google Scholar]
- 10.DiMasi JA, Grabowski HG, Vernon J.. R&D costs and returns by therapeutic category. Drug Inf J. 2004;38(3):211-223. doi: 10.1177/009286150403800301 [DOI] [Google Scholar]
- 11.Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9(3):203-214. doi: 10.1038/nrd3078 [DOI] [PubMed] [Google Scholar]
- 12.Prasad V, Mailankody S. Research and development spending to bring a single cancer drug to market and revenues after approval. JAMA Intern Med. 2017;177(11):1569-1575. doi: 10.1001/jamainternmed.2017.3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration Drugs@FDA: FDA-approved drugs. https://www.accessdata.fda.gov/scripts/cder/daf/. Published 2019. Accessed March 3, 2019.
- 14.Lanthier M, Miller KL, Nardinelli C, Woodcock J. An improved approach to measuring drug innovation finds steady rates of first-in-class pharmaceuticals, 1987-2011. Health Aff (Millwood). 2013;32(8):1433-1439. doi: 10.1377/hlthaff.2012.0541 [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration Novel drug approvals (2011-2018). https://www.fda.gov. Accessed March 2, 2019.
- 16.Darrow JJ, Kesselheim AS. Drug development and FDA approval, 1938-2013. N Engl J Med. 2014;370(26):e39. doi: 10.1056/NEJMp1402114 [DOI] [PubMed] [Google Scholar]
- 17.WHO Collaborating Centre for Drug Statistics Methodology ATC/DDD Index 2020. https://www.whocc.no/atc_ddd_index/. Published 2020. Accessed January 27, 2020.
- 18.Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20(2):273-286. doi: 10.1093/biostatistics/kxx069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas DW, Burns J, Audette J, Carroll A, Dow-Hygelund C, Hay M. Clinical Development Success Rates 2006-2015. Washington, DC: Biotechnology Innovation Organization, Amplion, and Biomedtracker; 2016. [Google Scholar]
- 20.Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32(1):40-51. doi: 10.1038/nbt.2786 [DOI] [PubMed] [Google Scholar]
- 21.US Securities and Exchange Commission EDGAR company filings. https://www.sec.gov/edgar/searchedgar/companysearch.html. Published 2019. Accessed April 2, 2019.
- 22.Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programme. 3rd ed Oxford, UK: Oxford University Press; 2005, https://pure.york.ac.uk/portal/en/publications/methods-for-the-economic-evaluation-of-health-care-programme-third-edition(e43f24cd-099a-4d56-97e6-6524afaa37d1)/export.html. Accessed March 2, 2019. [Google Scholar]
- 23.US Food and Drug Administration Step 3: Clinical research phase studies. https://www.fda.gov/patients/drug-development-process/step-3-clinical-research#Clinical_Research_Phase_Studies. Published 2018. Accessed January 27, 2020.
- 24.Chit A, Chit A, Papadimitropoulos M, Krahn M, Parker J, Grootendorst P. The opportunity cost of capital: development of new pharmaceuticals. Inquiry. 2015;52:pii:004695801558464. doi: 10.1177/0046958015584641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Gronde T, Pieters T. Assessing pharmaceutical research and development costs. JAMA Intern Med. 2018;178(4):587-588. doi: 10.1001/jamainternmed.2017.8706 [DOI] [PubMed] [Google Scholar]
- 26.DiMasi JA. Assessing pharmaceutical research and development costs. JAMA Intern Med. 2018;178(4):587. doi: 10.1001/jamainternmed.2017.8703 [DOI] [PubMed] [Google Scholar]
- 27.Prasad V, Mailankody S. Assessing pharmaceutical research and development costs—reply. JAMA Intern Med. 2018;178(4):588-589. doi: 10.1001/jamainternmed.2017.8737 [DOI] [PubMed] [Google Scholar]
- 28.Light DW, Warburton RN. Extraordinary claims require extraordinary evidence. J Health Econ. 2005;24(5):1030-1033. doi: 10.1016/j.jhealeco.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 29.Goozner M. A much-needed corrective on drug development costs. JAMA Intern Med. 2017;177(11):1575-1576. doi: 10.1001/jamainternmed.2017.4997 [DOI] [PubMed] [Google Scholar]
- 30.Avorn J. The $2.6 billion pill—methodologic and policy considerations. N Engl J Med. 2015;372(20):1877-1879. doi: 10.1056/NEJMp1500848 [DOI] [PubMed] [Google Scholar]
- 31.DiMasi JA, Feldman L, Seckler A, Wilson A. Trends in risks associated with new drug development: success rates for investigational drugs. Clin Pharmacol Ther. 2010;87(3):272-277. doi: 10.1038/clpt.2009.295 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of Therapeutic Agents Included in the Analysis
eTable 2. Quality Score of the Research and Development Estimate for Each Therapeutic Agent
eTable 3. Median Research and Development Outlays (2018 US $, Millions) by Product Category, Without Adjustment for Costs of Failures
eTable 4. Research and Development Costs (2018 US $, Millions), Broken Down by Phase of Clinical Development, for All Therapeutic Agents Included in the Analysis
eTable 5. Dates of Clinical Trial Phase Changes for all Therapeutic Agents Included in the Analysis
eTable 6. Estimated Research and Development Costs (2018 US $, Millions) for All Therapeutic Agents Included in the Analysis