Abstract
Nipah virus (NiV) is a highly pathogenic zoonotic paramyxovirus that continues to cause outbreaks in humans characterized by high mortality and significant clinical sequelae in survivors. Currently, no therapeutics are approved for use in humans against NiV infection. Here, we report that 4′-chloromethyl-2′-deoxy-2′-fluorocytidine (ALS-8112) inhibits NiV. ALS-8112 is the parent nucleoside of lumicitabine, which has been evaluated in phase I and II clinical trials to treat pediatric and adult respiratory syncytial virus infection. In this study, we tested ALS-8112 against NiV and other major human respiratory pneumo- and paramyxoviruses in 2 human lung epithelial cell lines, and demonstrated the ability of ALS-8112 to reduce infectious wild-type NiV yield by over 6 orders of magnitude with no apparent cytotoxicity. However, further cytotoxicity testing in primary cells and bone marrow progenitor cells indicated cytotoxicity at higher concentrations of ALS-8112. Our results warrant the evaluation of lumicitabine against NiV infection in relevant animal models.
Keywords: Nucleoside analog, RSV, Henipavirus, Nipah virus, Lumicitabine, ALS-8112
Nipah virus (NiV), a highly pathogenic zoonotic paramyxovirus, continues to cause sporadic but fatal human outbreaks of acute respiratory disease and encephalitis on a near-annual basis (Spiropoulou, 2019). The 2018 NiV outbreak in Kerala, India, exemplified the high case fatality rate of NiV infection as well as its potential for human-to-human transmission (Arunkumar et al., 2018). Although currently no vaccines or antivirals are licensed for human use against NiV infection, a potent neutralizing antibody (m102.4) and numerous henipavirus glycoprotein-based vaccines have demonstrated efficacy in animal models (Broder et al., 2016; Broder et al., 2013). Additionally, the nucleotide analog pro-drug remdesivir (GS-5734) and nucleoside precursor favipiravir (T-705) have both shown robust protection against NiV infection in the non-human primate and hamster models, respectively (Dawes et al., 2018; Lo et al., 2019). Our search for new classes of NiV inhibitors led us to characterize the broad-spectrum activity of 4′-azidocytidine (R1479) and its 2′-fluoro-modified analogs against NiV and other members of the family Paramyxoviridae (Hotard et al., 2017; Lo et al., 2018). Our results led us to hypothesize that a similar nucleoside analog, 4′-chloromethyl-2′-deoxy-2′-fluorocytidine (ALS-8112), would also inhibit NiV. ALS-8112 is the parent nucleoside of the orally bioavailable methyl propionate derivative lumicitabine (ALS-008176, JNJ-64041575), developed by Janssen Biopharmaceuticals for treatment of respiratory syncytial virus (RSV) infection and evaluated in multiple phase I and phase II clinical trials (ClinicalTrials.gov, 2019; Deval et al., 2015) (Figure 1A). We coincidentally identified ALS-8112 as an antiviral hit while conducting a blinded small molecule library screen against a recombinant reporter NiV expressing ZsGreen1 fluorescent protein (rNiV-ZsG; formerly named NiV-GFP2AM)(Lo et al., 2014). We synthesized ALS-8112 by following reported procedures (Wang et al., 2015) and confirmed compound purity (> 95%) by UPLC/MS, 1H, 19F, and 13C NMR analysis. Human cervical carcinoma (HeLa, ATCC CCL-2) cells were pretreated with half-log serial dilutions of ALS-8112 for 1 h before infection with rNiV-ZsG using a multiplicity of infection (MOI) of 0.2. At 72 h post infection (hpi), we measured total green fluorescence intensity as an indicator of viral replication using a Biotek HD1 Synergy instrument. Concentrations of ALS-8112 that inhibited 50% of the green fluorescence signal (EC50) were calculated from dose-response data fitted to a 4-parameter logistic curve generated using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). ALS-8112 inhibited rNiV-ZsG in a dose-dependent manner (Figure 1B) and showed minimal cytotoxicity in uninfected HeLa cells, with 50% cytotoxicity concentrations (CC50) > 100 μM (Figure 1C).
Figure 1.

Inhibition of recombinant Nipah virus (NiV) expressing ZsGreen1 fluorescent protein (rNiV-ZsG) by 4′-chloromethyl-2′-deoxy-2′-fluorocytidine (ALS-8112). (A) Chemical structures of ALS-8112 and its prodrug ALS-8176. (B) Representative dose response curve of ALS-8112 activity against rNiV-ZsG (multiplicity of infection [MOI] = 0.2) in HeLa cells, obtained by measuring green fluorescence protein (GFP) signal intensity 72 h post infection using a Biotek HD1 Synergy instrument. Fluorescence signal intensity assayed in DMSO-treated, virus-infected cells was set as 100% GFP. Fluorescence assay was performed in 384-well black opaque plates. (C) Representative dose response curve measuring cell viability of uninfected, ALS-8112-treated HeLa cells 72 h post treatment. Cell viability was assayed using CellTiter-Glo assay reagent (Promega), with total luminescence measured using a Biotek HD1 Synergy instrument. Luminescence levels (indicative of cellular ATP levels as a surrogate marker of cell viability) assayed in DMSO-treated, uninfected cells were set as 100% cell viability. Dose response curves were fitted to the mean value of experiments performed in quadruplicate for each concentration in the 8-point, 3-fold dilution series using a 4-parameter non-linear logistic regression curve with variable slope. Cell viability assays were performed in 384-well white opaque plates.
To further confirm the activity of ALS-8112 against NiV and to assess its activity against related human respiratory viruses, we evaluated ALS-8112 inhibition of green fluorescent protein- (GFP) expressing recombinant reporter RSV (rgRSV224) (Hallak et al., 2000), measles virus (rMVEZ-GFP(3)) (Rennick et al., 2015), and human parainfluenza virus 3 (hPIV3-GFP) (Zhang et al., 2005) alongside rNiV-ZsG. To better model viral respiratory infection, we used 2 human respiratory epithelial cell lines: a bronchioalveolar carcinoma cell line (NCI-H358, ATCC CRL-5807) known to phosphorylate 4′-modified nucleosides at higher levels than HeLa cells (Lo et al., 2018), and a non-tumorigenic small airway epithelial cell line (HSAEC1-KT, ATCC CRL-4050) that exhibits phenotypic characteristics of normal primary cells (Ramirez et al., 2004) (Figure 2A–D). We pre-treated NCI-H358 and HSAEC1-KT cells with ALS-8112 for 1 h before infecting them with either rgRSV224 or hPIV3-GFP at MOI = 1, or with rMVEZGFP(3) or rNiV-ZsG at MOI = 0.25. For rgRSV224 and hPIV3-GFP, we used a Biotek Cytation5 to measure numbers of GFP-positive (GFP+) cells 96 hpi as an indicator of viral replication. Size parameters were set to exclude objects smaller than 5 μm and greater than 200 μm (to account for potential syncytia); a minimum criterion of 5,000 relative fluorescence units was required for any detected object to be classified as GFP+. EC50 values, indicating ALS-8112 concentrations required to reduce the number of virus-infected GFP+ cells by 50%, were calculated for each virus using the aforementioned method. For rMVEZ-GFP(3) and rNiV-ZsG, EC50 values were calculated based on total green fluorescence intensity measured 48 or 72 hpi. Whereas ALS-8112 expectedly inhibited rgRSV224 replication in both NCI-H358 and HSAEC1-KT cells with respective EC50 values of 1.23 and 0.36 μM (Figure 2A, Table 1), it was less potent against hPIV3-GFP or rMVEZ-GFP(3), with little to no effect in NCI-H358 cells and approximately 30-fold lower potency than against rgRSV224 in HSAEC1-KT cells (Figure 2B, 2C, Table 1). In contrast, ALS-8112 inhibited rNiV-ZsG in both cell lines at levels similar to those observed against rgRSV224, with EC50 values of 0.56 and 0.84 μM in NCI-H358 and HSAEC1-KT cells, respectively (Figure 2D, Table 1).
Figure 2.
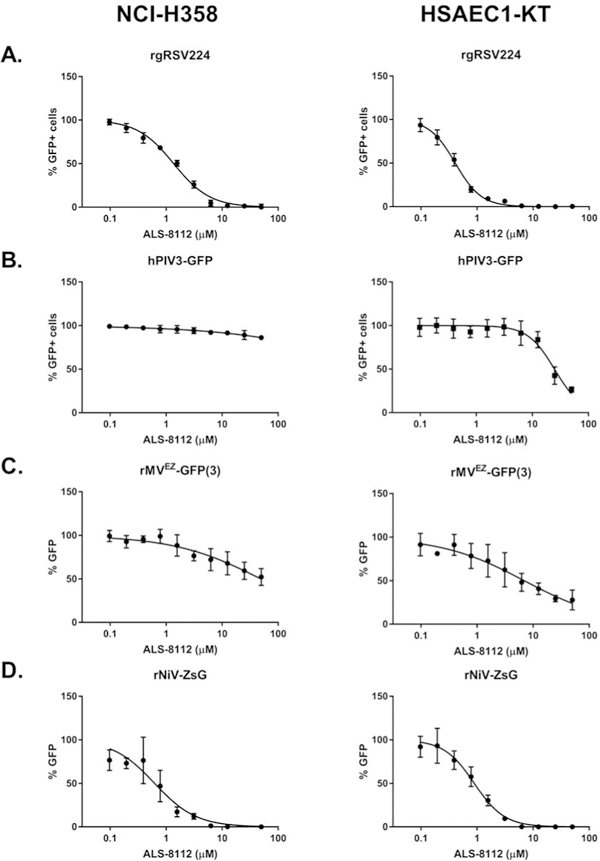
Antiviral activity of ALS-8112 against reporter pneumo- and paramyxoviruses in 2 distinct human respiratory epithelial cell lines. Representative dose response curves for ALS-8112 against recombinant reporter (A) respiratory syncytial virus (rgRSV224), (B) measles virus (rMVEZ(3)-GFP), (C) human parainfluenza virus 3 (hPIV3-GFP), and (D) rNiV-ZsG in human bronchioalveolar carcinoma (NCI-H358) and primary-like small airway epithelial cells (HSAEC1-KT), as denoted. Dose response curves for rgRSV224 and hPIV3-GFP were derived from the mean number of cells positive for green fluorescent protein (GFP+) by an image-based cell-counting assay measured using a Biotek Cytation5 instrument. GFP+ cell counts assayed in DMSO-treated, virus-infected cells were set as 100% GFP+ cells. Dose response curves for rMVEZ(3)-GFP and rNiV-ZsG were based on mean GFP signal intensity measured using a Biotek HD1 Synergy instrument. Fluorescence signal intensity assayed in DMSO-treated, virus-infected cells were set as 100% GFP. Data points and error bars for all reporter assays indicate the mean value and standard deviation of 4 biological replicates, and are representative of at least 4 or 6 independent experiments in NCI-H358 and HSAEC1-KT cells, respectively. Dose response curves were fitted to the mean value of experiments performed for each concentration in the 10-point, 2-fold dilution series using a 4-parameter non-linear logistic regression curve with variable slope. Image-based cell-counting assay was performed in 96-well black opaque plates with clear bottoms, while the fluorescence intensity assays were performed in 96-well black opaque plates.
Table 1.
Summary of ALS-8112 antiviral activity and cytotoxicity in NCI-H358 and HSAEC1-KT cells.
| Virus ID | Assay | EC50 (μM) | CC50 (μM) | SI (CC50/EC50) | |||
|---|---|---|---|---|---|---|---|
| NCI-H358 | HSAEC1-KT | NCI-H358 | HSAEC1-KT | NCI-H358 | HSAEC1-KT | ||
| NiV-M | CPE | 1.60 ± 0.07 | 2.49 ± 0.09 | > 50 | > 50 | > 31 | > 20 |
| NiV-B | CPE | 0.89 ± 0.14 | 1.29 ± 0.41 | > 56 | > 38 | ||
| VTR | ND | 0.30 | N/A | > 166 | |||
| rNiV-ZsG | REP | 0.56 ± 0.10 | 0.84 ± 0.21 | > 89 | > 59 | ||
| CPE | 2.04 ± 0.22 | 3.08 ± 0.67 | > 24 | > 14 | |||
| VTR | ND | 1.37 | N/A | > 36 | |||
| rgRSV224 | REP | 1.23 ± 0.11 | 0.36 ± 0.06 | > 40 | > 138 | ||
| rMVEZGFP(3) | REP | 41.9 ± 5.4 | 11.6 ± 8.3 | ~ 1.2 | ~ 4.3 | ||
| hPIV3-GFP | REP | > 50 | 10.8 ± 8.7 | N/A | ~ 4.6 | ||
SI- selectivity index; CPE- cytopathic effect; VTR- virus titer reduction; REP- reporter virus; ND- not determined; N/A- not applicable. EC50 values indicate the mean of at least 4 independent experiments ± standard deviation of the mean.
Since NiV causes profound in vitro cytopathic effect (CPE) , we evaluated the ability of ALS-8112 to inhibit CPE induced by wild-type NiV of both Malaysian (NiV-M) (Chua et al., 2000) and Bangladesh (NiV-B) genotypes (Harcourt et al., 2005), as well as by rNiV-ZsG (Figure 3A–C). NCI-H358 and HSAEC1-KT cells pre-treated for 1 h with ALS-8112 were infected with each virus (MOI = 0.25), and CPE inhibition was determined 72 hpi using CellTiterGlo 2.0 reagent (Promega). In this assay, levels of cellular ATP, measured by luminescence, inversely correlate with CPE levels. ALS-8112 consistently inhibited NiV-induced CPE, with EC50 values in the sub-micromolar to single-digit micromolar range (0.89–3.08 μM, Table 1). We used the same assay to measure cell viability and cytotoxicity in uninfected NCI-H358 and HSAEC1-KT cells treated with ALS-8112 for 72 and 96 h, respectively, and found CC50 values above 50 μM (Figure 3D, Table 1). We calculated the selectivity index (SI) for ALS-8112 against each virus used in this study by dividing the CC50 value for each cell line by the corresponding EC50 value (Table 1).
Figure 3.
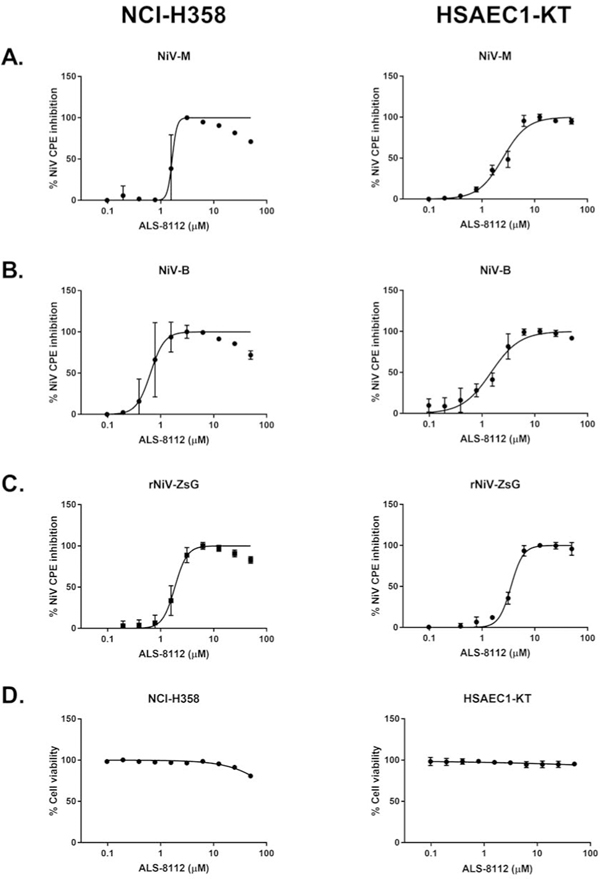
ALS-8112 potently reduces NiV cytopathic effect (CPE) and has minimal cytotoxicity in 2 distinct respiratory cell lines. Representative dose response curves of ALS-8112 against (A) wild-type NiV Malaysia (NiV-M), (B) wild-type NiV Bangladesh (NiV-B), and (C) reporter rNiV-ZsG as measured by CPE determination in human bronchioalveoloar carcinoma (NCI-H358) and primary-like small airway epithelial cells (HSAEC1-KT), as denoted. (D) Representative dose response curve measuring cell viability of uninfected NCI-H358 and HSAEC1-KT cells treated with ALS-8112 72 h (NCI-H358) and 96 h (HSAEC1-KT) post treatment. Data points and error bars for CPE assays indicate the mean value and standard deviation of 4 biological replicates, and are representative of at least 4 or 6 independent experiments in NCI-H358 and HSAEC1-KT cells, respectively. CPE and cell viability were assayed using CellTiter-Glo 2.0 assay reagent, with luminescence measured using a Biotek HD1 Synergy instrument. Luminescence levels (indicative of cellular ATP levels as a surrogate marker of cell viability) assayed in DMSO-treated, uninfected cells were set as 100% cell viability. Dose response curves were fitted to the mean value of experiments performed for each concentration in the 10-point, 2-fold dilution series using a 4-parameter non-linear logistic regression curve with variable slope. All CPE and cell viability assays were conducted in 96-well opaque white plates.
Having confirmed the anti-NiV activity of ALS-8112 in reporter and CPE assays, we tested its ability to reduce infectious virus titers. We infected HSAEC1-KT cells with 0.25 MOI of either wild-type NiV-B or rNiV-ZsG (to represent the NiV-M genotype) for 1 h, removed the virus inoculum, washed the cells once with phosphate-buffered saline (PBS), and then replenished cells with growth media containing ALS-8112. After 48 hpi, we harvested cell supernatants and quantitated viral titers by 50% tissue culture infective dose (TCID50) assays on African green monkey cells (Vero, ATCC CCL-81) using the Reed and Muench method as previously described (Lo et al., 2014; Reed and Muench, 1938). ALS-8112 significantly reduced titers of rNiV-ZsG and NiV-B by up to 6 or 7 orders of magnitude, respectively, in a dose-dependent manner (Figure 4A–B). Furthermore, fluorescent micrographs of rNiV-ZsG-infected HSAEC1-KT cells treated with ALS-8112 48 hpi demonstrated a dose-dependent decrease in rNiV-ZsG-infected cells and syncytia, with all visible evidence of infection ablated at a concentration of 12.5 μM (Figure 4C).
Figure 4.
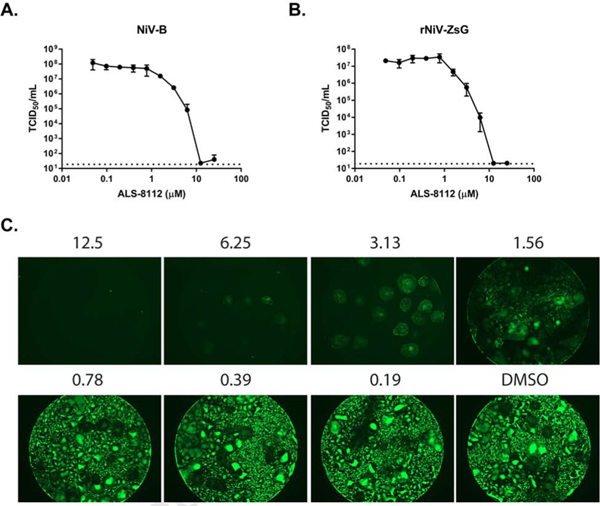
ALS-8112 reduces NiV infectious virus titer and syncytia formation in primary-like HSAEC1-KT cells. Reduction of infectious virus titer of (A) wild-type NiV-B and (B) rNiV-ZsG by ALS-8112. HSAEC1-KT cells were infected for 1 h (MOI = 0.25) before inoculum was removed and replaced with growth media containing ALS-8112. Cell supernatants were harvested 48 h post infection and were titrated in 10-fold dilutions onto Vero cells. Cells were monitored for CPE 7 days post infection to calculate virus titer. Infectious titer (TCID50/mL) values in log scale for each data point represent the mean observed for each concentration in the 10-point, 2-fold dilution series of each compound performed with 4 biological replicates. Dotted line indicates limit of detection. Error bars indicate standard deviation of the mean values.(C) Fluorescence micrographs of HSAEC1-KT cells infected with rNiV-ZsG treated with indicated concentrations of ALS-8112 (in μM) 48 h post infection. Micrographs were captured with the EVOS FL Cell Imaging System (Thermo Fisher) at 2× magnification using the GFP fluorescence filter. Infectious virus titer reduction and fluorescence micrographs were performed using 96-well plates with black opaque sides and clear bottoms.
In parallel, we tested ALS-8112 cytotoxicity in a panel of cell types, including human epithelial lung (A549, ATCC CCL-185), primary human peripheral blood mononuclear (PBM), human lymphoblastoid (CEM, ATCC CCL-119), Vero, and human hepatocellular carcinoma cells (HepG2, ATCC HB-8065). Even though ALS-8112 induced no notable toxicity in A549, HepG2, or Vero cells, toxicity was observed in PBM (CC50 = 4.2 μM) and CEM cells (CC50 = 2.8 μM) (Table 2). Since in vivo administration of nucleoside analogues is often associated with side effects like anemia, neutropenia, pancreatitis, and lactic acidosis, these results prompted us to measure mitochondrial and bone marrow toxicity (Inoue et al., 1989; Lewis et al., 2003), as well as lactic acid production as a cellular stress marker associated with mitochondrial toxicity (Stuyver et al., 2006).
Table 2.
Cytotoxicity of ALS-8112 in various cell systems.
| CC50 (μM) | ||||
|---|---|---|---|---|
| PBM | CEM | Vero | A549 | HepG2 |
| 4.2 | 2.8 | > 100 | > 20 | 80.6 |
To determine mitochondrial toxicity, HepG2 cells were propagated in the presence of ALS-8112 (up to 50 μM) for 14 days prior to purification of total cellular nucleic acids (MagNa Pure LC Total Nucleic Acid Isolation kit, Roche Life Science) and quantification of mitochondrial COXII DNA (mtDNA) and nuclear ribosomal DNA by real-time PCR assays (LightCycler 480 II, Roche Life Science). Lamivudine (3TC; 10 μM) or β-D-2′,3′-dideoxycytidine (ddC; 1 μM) were used as negative and positive controls, respectively (Table 3). Lactic acid levels were also determined in the culture supernatant after 14 days of treatment with each drug. All samples were assayed in duplicate.
Table 3.
Effect of ALS-8112 on levels of mitochondrial and nuclear DNA and lactic acid production in HepG2 cells.
| Compound | Concentration (μM) | % Inhibition, mtDNA/nDNA | IC50 (μM) mtDNA/nDNA | mtDNA content: % of untreated control (range) | Lactic acid production: % untreated of control |
|---|---|---|---|---|---|
| ALS-8112 | 1 | 2.73/37.7 | > 50/> 50 | 166 (160–173) | 109 ± 4.2 |
| 10 | 28.72/23.4 | 99.0 (67.5–145) | 177 ± 6.0 | ||
| 50 | 43.6/48.7 | 117 (58–234) | 245 ± 14.7 | ||
| 3TC (negative control) | 10 | < 1/< 1 | > 10/> 10 | 117 (113–122) | 105 ± 1.8 |
| ddC (positive control) | 1 | 84.8/22.0 | > 1/>1 | 20.7 (18.5–23.0) | 138 ± 1.6 |
| Untreated control | 0 | 0/0 | N/A | 100 (69–145) | 100 ± 7.1 |
mtDNA - mitochondrial DNA; nDNA - nuclear DNA; N/A - not applicable.
Bone marrow toxicity was determined in primary human hematopoietic bone marrow progenitor cells obtained from Stemexpress and cultured as previously described in soft agar, resulting in colonies of erythroid (colony forming units-erythroid; CFU-E) or myeloid lineage bone marrow cells (colony forming units-granulocyte myeloid; CFU-GM) (Cretton et al., 1991; Sommadossi et al., 1992).Cells cultured in triplicate were treated with 0.1, 1.0, 10, or 100 μM of either ALS-8112, AZT (positive control for bone marrow toxicity), or DMSO (negative vehicle control). After 21 days, cells were placed at 4°C for 30 min to allow semi-solid agar to become liquid, followed by gentle washing with cold PBS. Suspensions were centrifuged for 10 min at 1500 rpm, and supernatants were removed. Cells were stained with fluorescent-tagged markers for CFU-E and CFU-GM lineages as previously described (Jafari et al., 2018; Li et al., 2014; Pelletier et al., 2017; Schundeln et al., 2014). CFU-E and CFU-GM colonies were quantified for all conditions, with at least 200,000 total events collected. Inhibition of colony growth was calculated as percent of DMSO control for AZT and ALS-8112, along with CC50 and CC90 values. ALS-8112 concentrations of up to 50 μM had no apparent effect on mtDNA or nuclear DNA, and only induced a 2.5-fold increase in lactic acid at 50 μM (Table 3). However, ALS-8112 CC50 values were 4.0 and 3.7 μM in CFU-E and CFU-GM, respectively, similar to AZT, a nucleoside analog known to cause anemia and neutropenia in humans (CC50 of 0.4 and 1.1 μM in CFU-E and CFU-GM) (Table 4).
Table 4.
Cytotoxicity evaluation of ALS-8112 in erythroid and myeloid bone marrow progenitor cells.
| Compound | CC50/CC90 (μM) | |
|---|---|---|
| E-BM | GM-BM | |
| ALS-8112 | 4.0/11.4 | 3.7/8.8 |
| AZT (positive control) | 0.4/7.9 | 1.1/47.6 |
E-BM - erythroid bone marrow; GM-BM - granulocyte myeloid bone marrow.
In summary, we utilized reporter virus, CPE, virus titer reduction, and fluorescence microscopy assays to demonstrate the in vitro antiviral activity of ALS-8112 against NiV at low concentrations, confirming our initial hypothesis. We observed minor discrepancies in EC50 values for NiV-B and rNiV-ZsG viruses across the different assays (though all values were within a 5-fold range), which was expected due to different cellular or viral parameters used to measure antiviral potency. However, the inability of ALS-8112 to inhibit hPIV3-GFP and rMVEZGFP(3) viruses was unexpected given the phylogenetic relatedness of these viruses to both RSV and NiV and the high level of amino acid conservation in the large polymerase nucleotide binding domains across pneumo- and paramyxovirus families (Lo et al., 2017). The recent determinations of both RSV and human metapneumovirus (hPMV) polymerase structures have provided insight into the RNA-dependent RNA polymerase catalytic domain (Gilman et al., 2019; Pan et al., 2019). By mapping the positions of the RSV polymerase mutations conferring resistance against ALS-8112 (Deval et al., 2015) onto the hMPV polymerase in complex with lumicitabine, Pan et al showed that even relatively subtle conservative amino acid changes adjacent to the conserved catalytic domain motifs can be responsible for marked changes in antiviral potency (Pan et al., 2019). The relative lack of potency of ALS-8112 against both hPIV3-GFP and rMVEZGFP(3) viruses may be determined by such intrinsic variations. The difference in EC50 values for ALS-8112 against hPIV3-GFP from this study (~10 μM) compared to those from a prior study (1.3 μM) (Deval et al., 2015) is likely due to several factors, including the cell lines used and the parameters chosen to measure viral replication (e.g., measuring GFP fluorescence versus viral RNA). The in vitro potency of ALS-8112 against NiV is similar to that observed for RSV in this study and suggests that in vivo testing of lumicitabine or a related prodrug against NiV infection is warranted. However, despite the successful completion of numerous clinical trials (ClinicalTrials.gov, 2019) which included evidence of in vivo efficacy against human RSV infection (DeVincenzo et al., 2015), the development of lumicitabine was discontinued by Janssen Biopharma (Dunn, 2019; Johnson, 2019). Findings of a prior study documented both the moderate cytotoxicity of ALS-8112 in MT-4 cells and the incorporation of its nucleotide triphosphate form by mitochondrial DNA polymerase γ (Clarke et al., 2015). Provided these caveats, any future in vivo dosing regimens to evaluate the antiviral efficacy of ALS-8112/lumicitabine would require careful optimization to avoid adverse effects.
Highlights for “Potent in vitro activity of β-D-4′-chloromethyl-2′-deoxy-2′-fluorocytidine against Nipah virus”.
β-D-4′-chloromethyl-2′-deoxy-2′-fluorocytidine (ALS-8112) is a nucleoside analog.
ALS-8112 inhibits reporter recombinant and wild-type Nipah virus replication.
ALS-8112 reduces Nipah virus infectious virus titer by > 6 orders of magnitude.
Requirements for in vivo evaluation of ALS-8112 against Nipah virus are discussed.
Acknowledgements
The findings and conclusions in this report are those of the authors and do not necessarily represent those of the Centers for Disease Control and Prevention. We thank Tatyana Klimova for helpful edits and comments on the manuscript.
Funding: This work was financially supported by CDC Emerging Infectious Disease Research core funding.
This work was also supported in part by CFAR NIH Grant 5P30-AI-50409 (to RFS).
Footnotes
Declaration of interests: All authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arunkumar G, Chandni R, Mourya DT, Singh SK, Sadanandan R, Sudan P, Bhargava B, People, N.N.I., Health, 2018. Outbreak investigation of Nipah Virus Disease in Kerala, India, 2018. J Infect Dis. [DOI] [PubMed] [Google Scholar]
- Broder CC, Weir DL, Reid PA, 2016. Hendra virus and Nipah virus animal vaccines. Vaccine 34, 3525–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder CC, Xu K, Nikolov DB, Zhu Z, Dimitrov DS, Middleton D, Pallister J, Geisbert TW, Bossart KN, Wang LF, 2013. A treatment for and vaccine against the deadly Hendra and Nipah viruses. Antiviral Res 100, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, Ksiazek TG, Rollin PE, Zaki SR, Shieh W, Goldsmith CS, Gubler DJ, Roehrig JT, Eaton B, Gould AR, Olson J, Field H, Daniels P, Ling AE, Peters CJ, Anderson LJ, Mahy BW, 2000. Nipah virus: a recently emergent deadly paramyxovirus. Science 288, 1432–1435. [DOI] [PubMed] [Google Scholar]
- Clarke MO, Mackman R, Byun D, Hui H, Barauskas O, Birkus G, Chun BK, Doerffler E, Feng J, Karki K, Lee G, Perron M, Siegel D, Swaminathan S, Lee W, 2015. Discovery of beta-D-2’-deoxy-2’alpha-fluoro-4’-alpha-cyano-5-aza-7,9-dideaza adenosine as a potent nucleoside inhibitor of respiratory syncytial virus with excellent selectivity over mitochondrial RNA and DNA polymerases. Bioorg Med Chem Lett 25, 2484–2487. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov, 2019.
- Cretton EM, Xie MY, Bevan RJ, Goudgaon NM, Schinazi RF, Sommadossi JP, 1991. Catabolism of 3’-azido-3’-deoxythymidine in hepatocytes and liver microsomes, with evidence of formation of 3’amino-3’-deoxythymidine, a highly toxic catabolite for human bone marrow cells. Mol Pharmacol 39, 258–266. [PubMed] [Google Scholar]
- Dawes BE, Kalveram B, Ikegami T, Juelich T, Smith JK, Zhang L, Park A, Lee B, Komeno T, Furuta Y, Freiberg AN, 2018. Favipiravir (T-705) protects against Nipah virus infection in the hamster model. Sci Rep 8, 7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval J, Hong J, Wang G, Taylor J, Smith LK, Fung A, Stevens SK, Liu H, Jin Z, Dyatkina N, Prhavc M, Stoycheva AD, Serebryany V, Liu J, Smith DB, Tam Y, Zhang Q, Moore ML, Fearns R, Chanda SM, Blatt LM, Symons JA, Beigelman L, 2015. Molecular Basis for the Selective Inhibition of Respiratory Syncytial Virus RNA Polymerase by 2’-Fluoro-4’-Chloromethyl-Cytidine Triphosphate. PLoS Pathog 11, e1004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVincenzo JP, McClure MW, Symons JA, Fathi H, Westland C, Chanda S, Lambkin-Williams R, Smith P, Zhang Q, Beigelman L, Blatt LM, Fry J, 2015. Activity of Oral ALS-008176 in a Respiratory Syncytial Virus Challenge Study. N Engl J Med 373, 2048–2058. [DOI] [PubMed] [Google Scholar]
- Dunn A, 2019. J&J bails on RSV drug, taking $900M impairment charge. [Google Scholar]
- Gilman MSA, Liu C, Fung A, Behera I, Jordan P, Rigaux P, Ysebaert N, Tcherniuk S, Sourimant J, Eleouet JF, Sutto-Ortiz P, Decroly E, Roymans D, Jin Z, McLellan JS, 2019. Structure of the Respiratory Syncytial Virus Polymerase Complex. Cell 179, 193–204 e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak LK, Spillmann D, Collins PL, Peeples ME, 2000. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol 74, 10508–10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt BH, Lowe L, Tamin A, Liu X, Bankamp B, Bowden N, Rollin PE, Comer JA, Ksiazek TG, Hossain MJ, Gurley ES, Breiman RF, Bellini WJ, Rota PA, 2005. Genetic characterization of Nipah virus, Bangladesh, 2004. Emerg Infect Dis 11, 1594–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotard AL, He B, Nichol ST, Spiropoulou CF, Lo MK, 2017. 4’-Azidocytidine (R1479) inhibits henipaviruses and other paramyxoviruses with high potency. Antiviral Res 144, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Tsushita K, Itoh T, Ogura M, Hotta T, Saneyoshi M, Yoshida S, Saitoh H, Tomoda Y, Nagai Y, 1989. In vitro bone marrow toxicity of nucleoside analogs against human immunodeficiency virus. Antimicrob Agents Chemother 33, 576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari K, Tierens A, Rajab A, Musani R, Schuh A, Porwit A, 2018. Visualization of Cell Composition and Maturation in the Bone Marrow Using 10-Color Flow Cytometry and Radar Plots. Cytometry B Clin Cytom 94, 219–229. [DOI] [PubMed] [Google Scholar]
- Johnson, J., 2019. Washington, DC.
- Lewis W, Day BJ, Copeland WC, 2003. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nature reviews. Drug discovery 2, 812–822. [DOI] [PubMed] [Google Scholar]
- Li J, Hale J, Bhagia P, Xue F, Chen L, Jaffray J, Yan H, Lane J, Gallagher PG, Mohandas N, Liu J, An X, 2014. Isolation and transcriptome analyses of human erythroid progenitors: BFU-E and CFU-E. Blood 124, 3636–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo MK, Feldmann F, Gary JM, Jordan R, Bannister R, Cronin J, Patel NR, Klena JD, Nichol ST, Cihlar T, Zaki SR, Feldmann H, Spiropoulou CF, de Wit E, 2019. Remdesivir (GS-5734) protects African green monkeys from Nipah virus challenge. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo MK, Jordan PC, Stevens S, Tam Y, Deval J, Nichol ST, Spiropoulou CF, 2018. Susceptibility of paramyxoviruses and filoviruses to inhibition by 2’-monofluoro- and 2’-difluoro-4’-azidocytidine analogs. Antiviral Res 153, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo MK, Jordan R, Arvey A, Sudhamsu J, Shrivastava-Ranjan P, Hotard AL, Flint M, McMullan LK, Siegel D, Clarke MO, Mackman RL, Hui HC, Perron M, Ray AS, Cihlar T, Nichol ST, Spiropoulou CF, 2017. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci Rep 7, 43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo MK, Nichol ST, Spiropoulou CF, 2014. Evaluation of luciferase and GFP-expressing Nipah viruses for rapid quantitative antiviral screening. Antiviral Res 106, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Qian X, Lattmann S, El Sahili A, Yeo TH, Jia H, Cressey T, Ludeke B, Noton S, Kalocsay M, Fearns R, Lescar J, 2019. Structure of the human metapneumovirus polymerase phosphoprotein complex. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier MG, Szymczak K, Barbeau AM, Prata GN, O’Fallon KS, Gaines P, 2017. Characterization of neutrophils and macrophages from ex vivo-cultured murine bone marrow for morphologic maturation and functional responses by imaging flow cytometry. Methods 112, 124–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, Dimaio JM, Milchgrub S, Smith AL, Souza RF, Gilbey L, Zhang X, Gandia K, Vaughan MB, Wright WE, Gazdar AF, Shay JW, Minna JD, 2004. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer research 64, 9027–9034. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench H, 1938. A simple method of estimating fifty percent endpoints. Am J Hygiene 27, 493–497. [Google Scholar]
- Rennick LJ, de Vries RD, Carsillo TJ, Lemon K, van Amerongen G, Ludlow M, Nguyen DT, Yuksel S, Verburgh RJ, Haddock P, McQuaid S, Duprex WP, de Swart RL, 2015. Live-attenuated measles virus vaccine targets dendritic cells and macrophages in muscle of nonhuman primates. J Virol 89, 2192–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schundeln MM, Walde G, Basu O, Havers W, Kremens B, 2014. Quantification of nucleated cells, CD34-positive cells and CFU-GM colonies in single bone marrow samples and bone marrow harvests derived from healthy children. Pediatr Hematol Oncol 31, 340–348. [DOI] [PubMed] [Google Scholar]
- Sommadossi JP, Schinazi RF, Chu CK, Xie MY, 1992. Comparison of cytotoxicity of the (−)- and (+)enantiomer of 2’,3’-dideoxy-3’-thiacytidine in normal human bone marrow progenitor cells. Biochem Pharmacol 44, 1921–1925. [DOI] [PubMed] [Google Scholar]
- Spiropoulou CF, 2019. Nipah Virus Outbreaks: Still Small but Extremely Lethal. J Infect Dis 219, 18551857. [DOI] [PubMed] [Google Scholar]
- Stuyver LJ, McBrayer TR, Tharnish PM, Clark J, Hollecker L, Lostia S, Nachman T, Grier J, Bennett MA, Xie MY, Schinazi RF, Morrey JD, Julander JL, Furman PA, Otto MJ, 2006. Inhibition of hepatitis C replicon RNA synthesis by beta-D-2’-deoxy-2’-fluoro-2’-C-methylcytidine: a specific inhibitor of hepatitis C virus replication. Antiviral chemistry & chemotherapy 17, 79–87. [DOI] [PubMed] [Google Scholar]
- Wang G, Deval J, Hong J, Dyatkina N, Prhavc M, Taylor J, Fung A, Jin Z, Stevens SK, Serebryany V, Liu J, Zhang Q, Tam Y, Chanda SM, Smith DB, Symons JA, Blatt LM, Beigelman L, 2015. Discovery of 4’-chloromethyl-2’-deoxy-3’,5’-di-O-isobutyryl-2’-fluorocytidine (ALS-8176), a first-in-class RSV polymerase inhibitor for treatment of human respiratory syncytial virus infection. J Med Chem 58, 1862–1878. [DOI] [PubMed] [Google Scholar]
- Zhang L, Bukreyev A, Thompson CI, Watson B, Peeples ME, Collins PL, Pickles RJ, 2005. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol 79, 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]


