Abstract
Background:
The ventilation defect percent (VDP), measured from hyperpolarized (HP) 129Xe magnetic resonance imaging (MRI), is sensitive to functional changes in cystic fibrosis (CF) lung disease. The purpose of this study was to measure and compare VDP from HP 129Xe MRI acquired at two institutions in stable pediatric CF subjects with preserved lung function.
Methods:
This retrospective analysis included 26 participants from two institutions (18 CF, 8 healthy, age range 10–17). Pulmonary function tests, N2 multiple breath washout (to measure lung clearance index, LCI), and HP 129Xe MRI were performed. VDP measurements were compared between two trained analysts using mean-anchored linear binning. Correlations were investigated for VDP compared to the forced expiratory volume in one second (FEV1) and LCI.
Results:
VDP measurements agreed for the two analysts with an intraclass correlation coefficient of 0.99. In the combined dataset, VDP measured by Analyst 1 was 5.96±1.82% and 15.96±6.76% for the healthy and CF groups, respectively (p=0.0004). Analyst 2 showed similar differences between healthy and CF (p=0.0003). VDP measured by either analyst was shown to correlate with FEV1 (R2=0.33, p=0.003; and R2=0.26, p=0.009 for Analysts 1 and 2, respectively) and LCI (R2=0.76, p<0.0001; and R2=0.77, p<0.0001 for Analysts 1 and 2, respectively).
Conclusion:
HP 129Xe MRI provides a robust measurement of ventilation heterogeneity in stable pediatric CF subjects at two sites. Since measurements performed at two sites yielded similar VDP values with near-identical values between different analysts, implementation of the technique in multi-center trials in CF appears feasible.
Keywords: Hyperpolarized 129Xe, Magnetic Resonance Imaging, Ventilation Defect Percent, Cystic Fibrosis
INTRODUCTION
Hyperpolarized (HP) 3He or 129Xe magnetic resonance imaging (MRI) provides a sensitive measure of ventilation heterogeneity in pediatric cystic fibrosis (CF) patients with preserved lung function (1,2). HP gas MRI is typically performed during a breath-hold following inhalation of a dose of HP 3He or 129Xe, and the images reflect the distribution of the inhaled gas inside the lungs. In obstructive lung diseases such as CF, the HP gas does not fully equilibrate with the whole lung in a single breath, resulting in a heterogenous image with signal voids that represent unventilated or poorly ventilated regions of the lung. Early HP gas MRI studies in CF have assessed the ventilation distribution using reader scoring systems (3) and counting the ventilation defects (4). In recent years, HP gas MRI studies in CF have objectively quantified the spatial distribution of ventilation using a ventilation defect percent, or VDP (2,5), which is defined as the fraction of unventilated lung (i.e., the ventilation defect volume divided by the total lung volume, represented as a percentage).
While previous HP gas MRI studies in CF have used both 3He (6–8) and 129Xe (2,5,9), future studies will likely focus on using HP 129Xe due to the lower cost and greater availability of the gas. HP 3He and 129Xe VDP measurements have been successfully validated in other obstructive lung diseases, such as chronic obstructive pulmonary disease, with a slightly greater VDP expected from HP 129Xe MRI due to the greater density and lower diffusivity of the inhaled gas compared to 3He (10). VDP is potentially more sensitive for detecting functional changes in the lungs compared to pulmonary function test (PFT) indices, such as the forced expiratory volume in one second (FEV1) (2). VDP has been shown to be reproducible in stable CF lung disease (8), and sensitive to changes in ventilation distribution that may not be detectable using FEV1 alone (7). One study in pediatric CF reported an increased VDP in CF patients compared to healthy controls, whereas FEV1 did not differentiate between both groups (2). In addition, VDP is strongly correlated with the multiple breath washout (MBW) measurements of the lung clearance index (LCI), which is a measure of ventilation heterogeneity (5). Therefore, VDP has the potential to be used as an outcome measure for the management of CF lung disease and testing novel therapeutics, such as CF transmembrane conductance regulator (CFTR) modulators (6).
Before HP gas measures can be adopted clinically, multi-center prospective clinical trials will be required, which will require a harmonized VDP analysis approach. Therefore, standardized procedures will be required for consistent acquisition and analysis between centers, which will be especially important for sites that may be using different MRI platforms, different RF coil hardware, and different HP 129Xe dosing strategies. As a preliminary step in the pathway to clinical translation, we performed a retrospective analysis of HP 129Xe images from similar stable pediatric CF subjects obtained at two different institutions using slightly different MRI systems to assess the following: (i) the agreement between VDP measurements performed by two trained analysts using the same software, and (ii) the correlation between VDP and PFT measurements (i.e., FEV1 and LCI).
METHODS
Subjects
This study was a retrospective analysis that included previously reported image data acquired at the Hospital for Sick Children (Site #1) in Toronto, Ontario, Canada (5) and Cincinnati Children’s Hospital Medical Center (Site #2) in Cincinnati, OH, USA (2). The study at Site #1 was regulated by Health Canada and approved by the Hospital for Sick Children Research Ethics Board (clinicaltrials.gov registry number ). The study at Site #2 was regulated by an FDA investigational new drug approval (IND 123,577) and approved by the Cincinnati Children’s Institutional Review Board (clinicaltrials.gov registry number ). Table 1 summarizes the subject demographics for 16 pediatric participants at Site #1 and 10 pediatric participants at Site #2, all between the ages of 10 and 17. The combined dataset included 18 well-controlled stable CF participants and 8 age-matched healthy controls (p=0.73). Well-controlled stable CF was defined as having no changes in respiratory symptoms or medication for four weeks (Site #1) or one week (Site #2) prior to participation in the study.
Table 1:
Subject demographics and pulmonary function tests for all participants included in the retrospective study.
| Subject | Age | Sex | Height (cm) | Weight (kg) | FEV1 (% pred.) | LCI | Genotype | |
|---|---|---|---|---|---|---|---|---|
| Site #1 Healthy | 1 | 10 | F | 146 | 40.5 | 84 | 6.17 | - |
| 2 | 13 | F | 152 | 41.3 | 96 | 7.17 | - | |
| 3 | 11 | M | 145 | 32.6 | 94 | 6.74 | - | |
| 4 | 17 | M | 168 | 60.8 | 95 | 6.06 | - | |
| 5 | 11 | F | 132 | 42.6 | 137 | 7.11 | - | |
| 6 | 14 | M | 163 | 52.9 | 129 | - | - | |
| Site #1 CF | 7 | 17 | M | 170 | 55.3 | 74 | 16.87 | DeltaF508/DeltaF508 |
| 8 | 10 | F | 143 | 30.6 | 109 | 6.83 | DeltaF508/DeltaF508 | |
| 9 | 10 | F | 140 | 31.2 | 86 | 9.10 | DeltaF508/DeltaF508 | |
| 10 | 11 | F | 141 | 30.4 | 81 | 11.71 | DeltaF508, Duplications of exons 7–11 | |
| 11 | 14 | M | 175 | 57.0 | 71 | 11.98 | G480S/A559T | |
| 12 | 11 | F | 144 | 31.4 | 61 | 15.35 | C.1653_1655delCTT/c.1837T>G | |
| 13 | 16 | F | 152 | 37.4 | 78 | 15.98 | DeltaF508/DeltaF508 | |
| 14 | 16 | F | 148 | 43.5 | 83 | 10.88 | W1282X/1282X | |
| 15 | 14 | F | 145 | 38.5 | 96 | 9.08 | W1282X/1282X | |
| 16 | 11 | M | 156 | 38.2 | 99 | 9.56 | DeltaF508/DeltaF508 | |
| Site #2 Healthy | 17 | 14 | M | 177 | 64.8 | 106 | - | - |
| 18 | 12 | M | 141 | 34.8 | 103 | - | - | |
| Site #2 CF | 19 | 14 | M | 167 | 54.4 | 97 | 11.28 | DeltaF508/DeltaF508 |
| 20 | 12 | F | 162 | 43.6 | 77 | 15.28 | DeltaF508/DeltaF508 | |
| 21 | 13 | F | 161 | 48.9 | 96 | 8.49 | G551D, F508 | |
| 22 | 14 | F | 156 | 51.2 | 106 | 6.73 | F508, L206W | |
| 23 | 16 | M | 179 | 65.5 | 120 | 9.81 | DeltaF508/DeltaF508 | |
| 24 | 11 | M | 142 | 34.5 | 102 | - | F508, G178R | |
| 25 | 15 | F | 159 | 43.7 | 72 | - | DeltaF508/DeltaF508 | |
| 26 | 11 | F | 147 | 34.3 | 86 | 11.01 | DeltaF508/DeltaF508 |
Site #1 Data Acquisition
The study at Site #1 performed pulmonary function tests (PFTs), nitrogen multiple breath washout (N2 MBW) and HP 129Xe MRI in a single study visit. Spirometry and plethysmography were performed (Vmax, VIASYS CareFusion, San Diego, CA, USA) by qualified research team members according to ATS guidelines (11). N2 MBW was performed to determine LCI (Exhalyzer D, EcoMedics, Duernten, Switzerland) by qualified research team members, according to ERS/ATS standards (12). LCI was defined as the cumulative expired volume required to achieve 1/40th of the initial N2 concentration divided by the functional residual capacity (FRC).
HP 129Xe MRI was performed in the coronal plane at 1.5T (HDx, GE Healthcare, Waukesha, WI) using a flexible wrap-around quadrature transmit/receive RF coil tuned to the 129Xe resonance frequency (Clinical MR Solutions, Brookfield, WI, USA). Isotopically enriched 129Xe (83%) was polarized to approximately 15% (Model 9800, Polarean, Durham, NC, USA) and dispensed into a 1L Tedlar bag (Jensen Inert Products, Coral Springs, FL, USA). The HP 129Xe dose was set to 10% of total lung capacity (TLC), as measured using plethysmography, and balanced to 1L with medical grade N2. Throughout the imaging session, the subject’s oxygen saturation and heart rate were monitored.
Following inhalation of the dose bag from FRC, HP 129Xe images were acquired in a 16 second breath-hold using a fast spoiled gradient recalled echo (GRE) sequence (see the online supplement for a summary of all imaging parameters used in both studies). Most participants had two repeated HP 129Xe scans within a few minutes of each other. Following HP 129Xe imaging, the 129Xe vest coil was exchanged for an 8-channel torso array coil (GEHC) and conventional 1H lung images were acquired. For 1H imaging, subjects breathed in a 1L dose bag of N2 from FRC and GRE images were acquired in a 13 second breath-hold (see Table 2).
Table 2:
Comparison of subject demographics, pulmonary function tests, and VDP results for all participants included in the retrospective study. Results are shown separately for each institution and also as a combined dataset.
| Site #1 | Site #2 | Combined | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Healthy | CF | p Value | Healthy | CF | p Value | Healthy | CF | p Value | |
| n | 6 | 10 | - | 2 | 8 | - | 8 | 18 | - |
| Sex (males) | 3 | 3 | - | 2 | 3 | - | 5 | 6 | - |
| Age (mean ± SD) | 12.7 ± 2.6 | 13.0 ± 2.7 | 0.8945 | 13.0 ± 1.4 | 13.3 ± 1.8 | 1 | 12.7 ± 2.3 | 13.1 ± 2.3 | 0.7299 |
| FEV1 (% pred. ± SD) | 105.8 ± 21.6 | 83.8 ± 14.3 | 0.0593 | 104.5 ± 2.1 | 94.5 ± 15.7 | 0.2889 | 105.5 ± 18.3 | 85.6 ± 15.5 | 0.0636 |
| LCI (± SD) | 6.68 ± 0.47 | 11.52 ± 3.12 | 0.0017 | * | 10.39 ± 2.77 | * | 7.05 ± 0.99 | 11.10 ± 2.95 | 0.0012 |
| Analyst 1 VDP (% ± SD) | 6.55 ± 0.74 | 16.38 ± 6.15 | 0.0012 | 1.80** | 13.72 ± 7.25 | ** | 5.96 ± 1.82 | 15.96 ± 6.76 | 0.0004 |
| Analyst 2 VDP (% ± SD) | 5.76 ± 2.05 | 16.56 ± 6.90 | 0.0012 | 2.81** | 13.47 ± 6.58 | ** | 5.39 ± 2.17 | 15.48 ± 6.79 | 0.0003 |
LCI was not available for the healthy participants from Site #2.
Only one healthy individual at Site #2 had HP 129Xe images that were acceptable for analysis (SNR > 8.5).
Site #2 Data Acquisition
The study at Site #2 used an approach that was similar to the Site #1 study, with some important differences. PFTs, N2 MBW and HP 129Xe MRI were not necessarily performed in a single study visit. Same-day spirometry was performed (Koko, nSpire, Longmont, CO) if clinical PFTs had not been performed in the last six months. Plethysmography was not performed, but instead the participant’s height was used to estimate TLC for HP 129Xe dosing. Unlike the Site #1 study, where dose bags used a fixed 1L volume, the Site #2 study used dose bags that were filled to 1/6th of the participant’s TLC. Dose bags were either 100% HP 129Xe, or a mixture of 50% HP 129Xe/ 50% N2, depending on the gas polarization (described below). N2 MBW was performed on the same day if possible, or up to 34 weeks after the MRI study visit (Exhalyzer D, EcoMedics, Duernten, Switzerland).
HP 129Xe MRI was performed in the axial plane at 3T (Philips Achieva, Best, Netherlands) using a home-built 129Xe saddle RF coil. 129Xe was polarized using either a commercially-built system (Polarean, Durham, NC, USA) or a home-built polarizer. Dose bags from the commercially-built polarizer were filled with 100% 129Xe, while dose bags from the home-built system were diluted with 50% N2. Either case resulted in a similar signal-to-noise ratio (SNR) since the home-built system provided up to double the polarization of the commercially built system (~30%). Similar to the Site #1 study, HP 129Xe and conventional 1H acquisitions used coached inhalations from FRC and breath-holds of up to 16 seconds. Conventional 1H imaging used the integrated 1H body coil without moving the participant or 129Xe coil. The 1H and HP 129Xe image acquisitions at Site #2 both used a GRE pulse sequence, and the parameters are shown in the online supplement.
Data Analysis
VDP was measured by two trained individuals using the mean-anchored linear binning technique described by Thomen et al. (2). Both analysts independently processed the images from all 26 participants. Analyst 1 had one year of experience in lung image analysis, while Analyst 2 had seven years of experience. The 1H images were segmented to create a lung mask using seeded region-growing in 3D Slicer (https://www.slicer.org), and then registered to HP 129Xe images using a landmark-based approach with affine transformations (1). The region-growing algorithm excluded large blood vessels from the thoracic cavity mask, but an explicit vesselness filter was not implemented to remove smaller blood vessels (13,14). If there were any registration errors, due to differences in lung inflation between the 1H and HP 129Xe acquisitions, the analyst performed manual corrections to the thoracic cavity mask.
After the preliminary segmentation and registration steps, VDP was measured in Matlab (Mathworks, Natick, MA, USA). A bias field correction was used to account for RF coil inhomogeneities, where each HP 129Xe image slice by the mean lung signal value in each dimension (anterior-posterior, left-right, apex-base). The HP 129Xe image volume was divided by the mean signal value inside the lung mask, and voxels with a signal less than 60% of the mean value were considered part of the defect region. The VDP threshold was set based on a study that included a similar age-matched population of healthy children and pediatric CF patients, where a 60% signal threshold was shown to provide the maximum difference between VDP values measured in healthy and diseased lungs (2). Before calculating the final VDP, a median filter (3×3 kernel) was applied to the defect mask obtained from linear binning (15).
The final VDP was calculated as the total volume of unventilated lung obtained from the 129Xe images divided by the total lung volume obtained from the 1H image masks. VDP was compared between analysts using a Bland-Altman analysis in GraphPad Prism (Graphpad Software, La Jolla, CA, USA). The inter-analyst reliability of VDP measurements was assessed by calculating the intraclass correlation coefficient (ICC) in Matlab. HP 129Xe images with a center slice SNR below 8.5 were excluded from the Bland-Altman and correlation analyses. The SNR threshold of 8.5 was determined by the trained analysts, based on the ability to reliably co-register the 1H and 129Xe images in a given subject. VDP was compared between groups using a Mann-Whitney test, with p<0.05 being considered significant. VDP was also correlated with FEV1 and LCI using a linear regression analysis in GraphPad Prism. Where possible, LCI was retrospectively analyzed by the MBW over-read center at Site #1. For eight subjects at Site #2, the MBW raw data was lost and the LCI could not be re-processed. In those cases, the MBW raw data was not over-read and the LCI value provided by Site #2 was used.
RESULTS
Figure 1 shows a comparison of segmented ventilation and defect maps obtained from HP 129Xe MRI performed by both analysts in two representative CF patients at Site #1 and Site #2, respectively. Both analysts identified qualitatively similar thoracic cavity masks, as well as similar segmented masks and VDP results. The HP 129Xe images included in this study (SNR>8.5) had a mean SNR of 15.1±3.8 and 18.2±4.7 for Sites 1 and 2, respectively (p=0.12). Table 2 summarizes the VDP results for the healthy and CF patient cohorts, separated by institution and analyst. Figures 2(a) and 2(b) show the VDP results as box and whisker plots separated by institution and also as a combined dataset. VDP was significantly different between the healthy and CF groups for the Site #1 data and also for the combined dataset (p<0.05 for either analyst). There were not enough healthy participants at Site #2 to reach a significant difference for that subset. The CF cohorts from the two sites had VDP values that were not significantly different from one another (p=0.44 and p=0.46 for Analysts 1 and 2, respectively).
Figure 1:
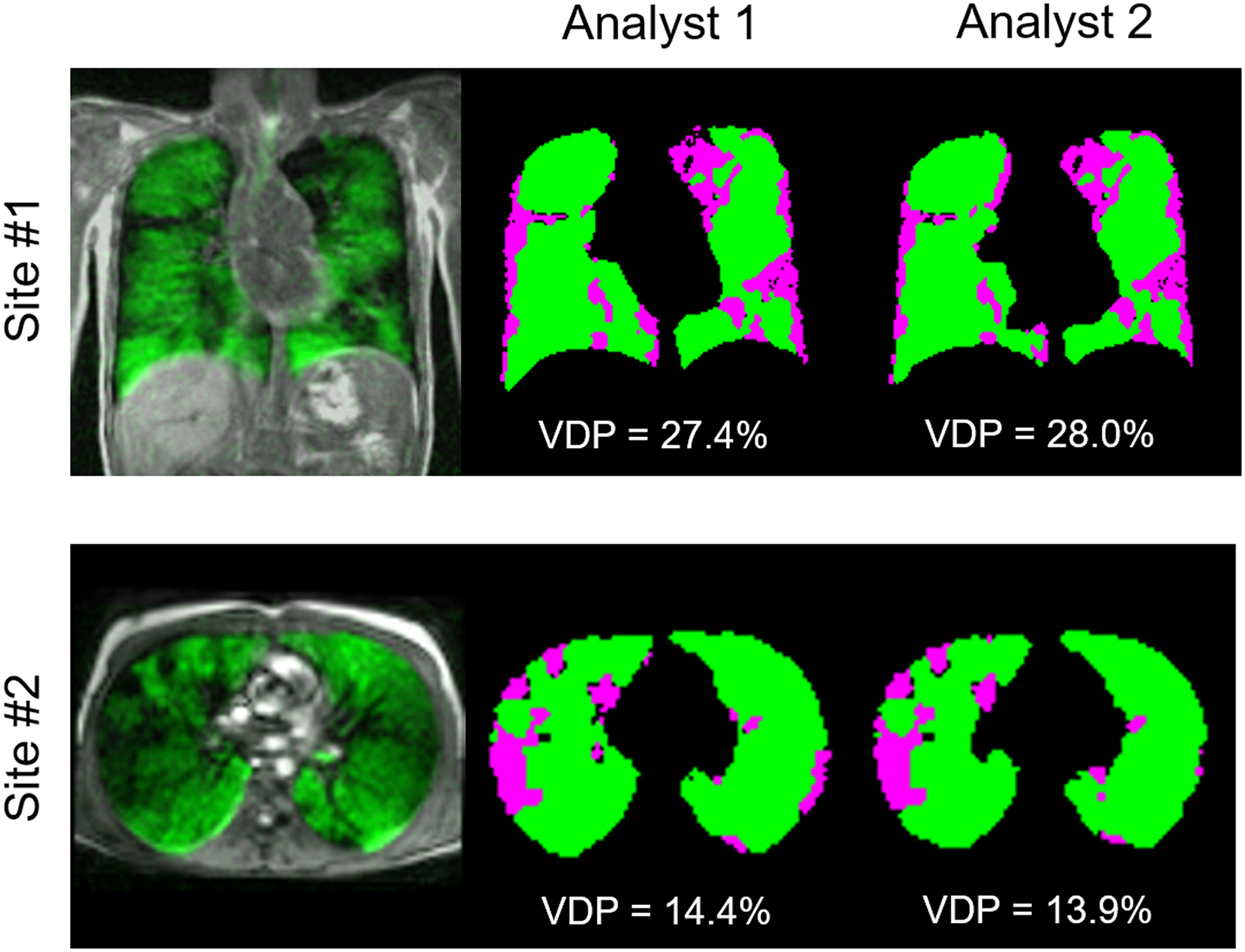
Comparison of segmented ventilation and defect maps obtained from two representative CF patients at Site #1 (top) and Site #2 (bottom). The leftmost images show an overlay of the center slice HP 129Xe image in green, with the 1H image in greyscale. VDP results and segmented maps are shown for both trained analysts, where ventilated areas are shown in green and defect areas are shown in purple.
Figure 2:
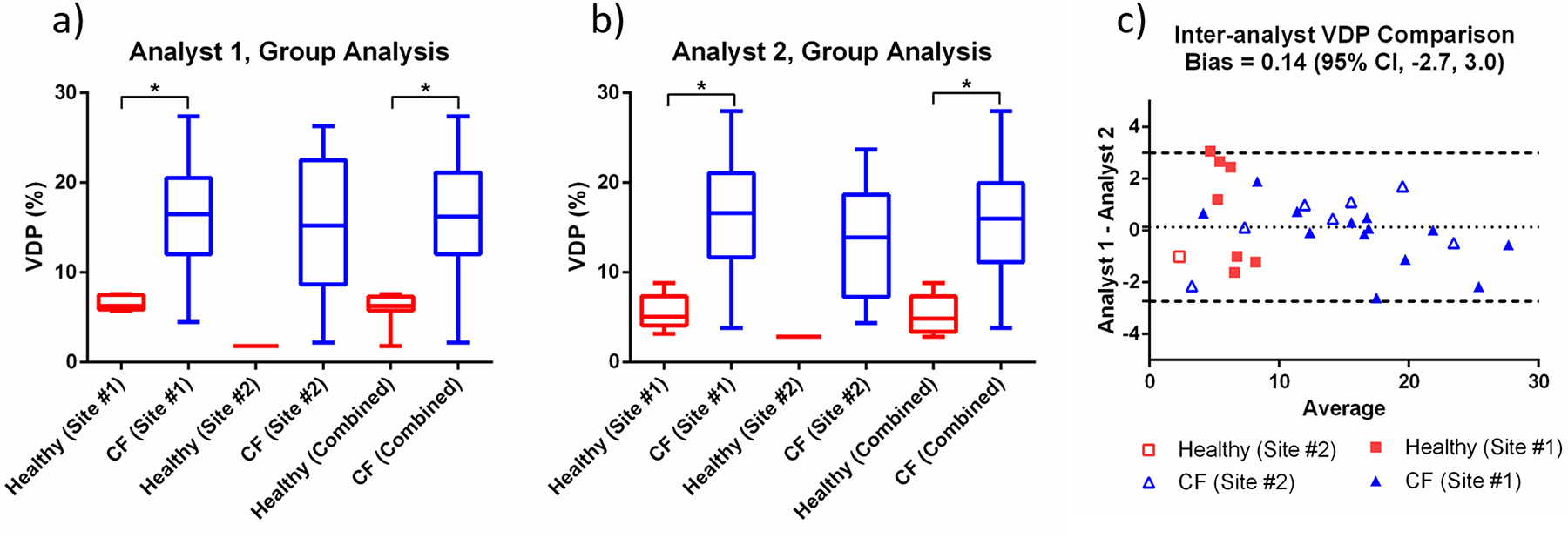
Box and whisker plots showing the VDP measured by (a) Analyst 1 and (b) Analyst 2 for healthy and CF cohorts. VDP results are shown separately for each institution and also as a combined cohort. (c) Bland-Altman analysis showing the inter-analyst agreement for all VDP measurements in the combined dataset.
Figure 2(c) shows a Bland-Altman analysis for VDP measurements performed by the two analysts. The mean bias (± standard deviation) in VDP between analysts was insignificant (0.14±1.46), and the inter-analyst ICC was 0.99 (p=0). The average absolute difference in VDP between Site #1 and Site #2 was 3.7 and 3.0 percentage points for Analysts 1 and 2, respectively. VDP measurements obtained by the two analysts were strongly correlated with one another (R2=0.96, p<0.0001). Figures 3(a) and 3(b) show a correlation analysis for FEV1 compared to VDP measured by Analyst 1 and Analyst 2, respectively. For both analysts, there was a weak to moderate negative correlation between VDP and FEV1, when either the Site #1 data or the combined dataset were considered. Figures 3(c) and 3(d) show the relationship between VDP and LCI for both analysts, confirming the expected strong positive correlations; however, there was a significant correlation between VDP and LCI only when either the Site #1 data or the combined dataset were considered, underlining the value added by combining data from the two sites.
Figure 3:
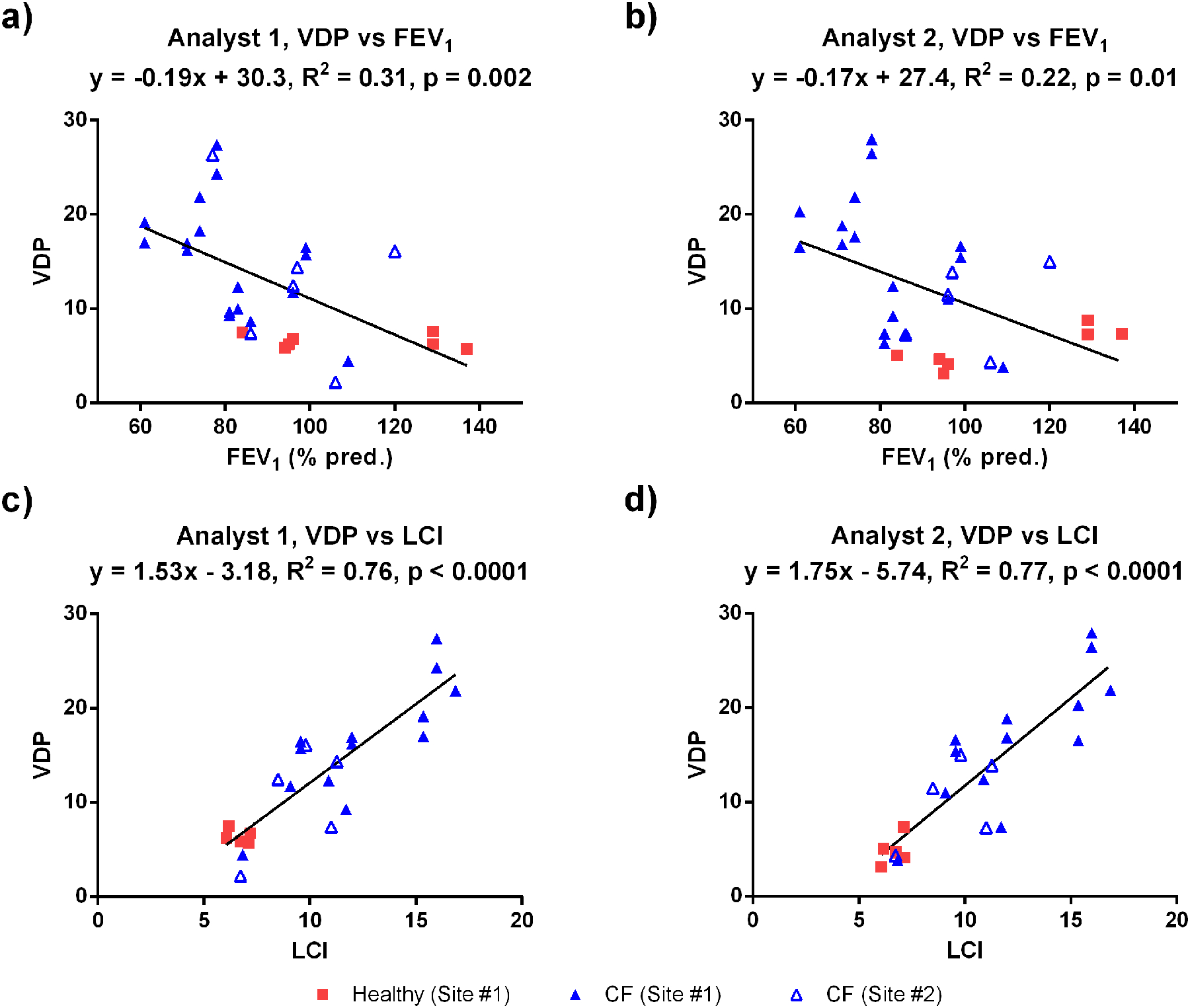
(a,b) Correlation between VDP and FEV1 for both analysts. (c,d) Correlation between VDP and LCI for both analysts. For each plot (a-d), the solid line represents the correlation for the combined dataset, while the plot title includes the separate correlations for each site.
DISCUSSION
To our knowledge, this study is the first to compare VDP measurements obtained from HP 129Xe MRI performed in stable pediatric CF subjects at two institutions. In a combined 129Xe image dataset consisting of 28 pediatric participants (8 healthy, 18 CF), good inter-observer agreement was demonstrated for VDP values measured by two trained analysts, and the VDP results were similar for the two populations of mild pediatric CF lung disease (Table 1). VDP was significantly different between the healthy and CF groups in the combined dataset, while data from the individual sites may lack statistical power. Therefore, there is strong potential for the use of HP 129Xe MRI in future multi-center trials.
This study showed the expected correlations with physiological parameters; VDP showed a weak to moderate correlation with FEV1 and a strong correlation with LCI. Although stronger correlations have been previously reported between VDP and LCI in stable pediatric CF subjects (5), the slightly reduced correlations in the present study likely reflect the variability introduced by the inclusion of multi-center data combined with differences in the data acquisition. Although there was good agreement in VDP between analysts, some small differences in VDP results can likely be attributed to the 1H/129Xe registration and thoracic cavity segmentation. While the VDP calculation was largely automated, the initial segmentation and registration steps required some manual input and adjustments. As the VDP increased, the analysts noted that it became more difficult to find matching landmarks between 1H and HP 129Xe images, especially when the defects were large and encompassed an entire lung lobe. To avoid potential mis-registration issues, future work will explore the simultaneous acquisition of 1H and HP 129Xe lung images in the same breath-hold (6,16). Without the need to register the 1H and HP 129Xe images, the VDP analysis could potentially be fully automated.
There are a few limitations in this study, which stem from the retrospective nature of the analysis. That is, images were acquired at two institutions that used different hardware, magnetic field strengths, scanner vendors, and RF coil designs. In addition, the two sites used similar pulse sequences, but with slightly different acquisition parameters. Even though the two sites had different configurations, both were able to acquire good quality HP 129Xe images, and the VDP appears to be a robust measure of ventilation heterogeneity in these healthy children and stable pediatric CF subjects. The SNR was expected to be slightly higher at 3T compared to 1.5T (17); however, the SNR difference in this retrospective study was too small to be statistically significant. Therefore, SNR was not expected to significantly influence the VDP. The original studies at both institutions only acquired low resolution 1H images to facilitate VDP calculation; however, future studies will also acquire high resolution 1H images for morphological scoring. Previous studies have shown that 1H MRI morphological scores are able to detect structural abnormalities in CF (18,19). In addition, 1H morphological scores are correlated with LCI and are sensitive to CF treatment response (20).
Another limitation of this study was the difference in dosing strategies used by the two institutions. Since the Site #1 cohorts used a 1L bag volume, the total lung volume for imaging breath-holds (i.e., FRC+1L) was likely close to TLC in some of the younger pediatric subjects. On the other hand, the Site #2 cohorts used a smaller bag volume (i.e., 1/6th of TLC), which would have been closer to a comfortable inspiratory tidal volume. It has been shown that the inhaled dose volume affects VDP results, with larger inspiratory volumes leading to a reduced VDP (21). A larger inhaled volume can fill areas of the lung that would otherwise be obstructed at smaller volumes; however, it has also been shown that both dosing approaches lead to similar correlations between VDP and LCI (21). Despite the different dosing strategies used at the two sites, VDP appears to provide a robust measure of ventilation heterogeneity. This result might be explained by the use of mean-anchored linear binning to calculate VDP, which uses a fixed signal threshold that includes some partially ventilated areas of the lung. Since Site #1 used a larger dose volume, it is possible that some partially ventilated areas of the lung might have appeared as defect had the dosing used a smaller volume. Nevertheless, the inclusion of partially ventilated regions in mean-anchored linear binning results in a similar VDP for the two CF populations. Future work will continue to investigate the sensitivity of other VDP calculation methods in multi-center trials, such as k-means (22,23), spatial fuzzy c-means clustering (24) or variations of the linear binning approach (25,26).
HP 129Xe MRI is a relatively new and sensitive technique, and although more experience is required to fully implement in multi-center clinical trials (27,28), this study presents a step forward in assessing multi-site variability in methods and endpoints. This comparative work demonstrates strong agreement using HP 129Xe MRI between two pediatric CF settings and we conclude that multi-center clinical trials using this approach are feasible. Future multi-center studies will focus on standardizing the image acquisition protocols and parameters. A recent multi-center study has shown that standardizing 1H-based MRI is feasible in pediatric CF (29). Based on the somewhat limited availability of HP 129Xe MRI technology at the current time, it is likely that 1H-based imaging techniques will see more widespread clinical adoption; however, HP 129Xe MRI is anticipated to be used in specialized centers upon regulatory approval. HP 129Xe MRI will likely play a role in providing outcome measures for interventional trials involving CFTR modulators, and may also play a role in future clinical management via precision medicine. Since HP 129Xe MRI can potentially detect CF lung disease before traditional PFTs (2), its sensitivity may allow for interventional trials with fewer subject numbers than previous studies using FEV1 or LCI as outcome measures (30).
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge helpful discussions with members of the 129Xe MRI Clinical Trials Consortium. The authors would like to thank the following individuals at SickKids for their help with data collection: Yonni Friedlander, Michelle Klingel, Krzysztof Kowalik, Andras Lindenmaier, Tammy Rayner, Laura Seed, Elaine Stirrat, Ruth Weiss, David Wilson, and Brandon Zanette; in addition to the following at Cincinnati Children’s: Laura Walkup, Zackary Cleveland, Erin Watters, and Kelly Thornton. We would also like to thank the following sources of funding: The Hospital for Sick Children (Catalyst Grant from the Cystic Fibrosis Centre), Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant (RGPIN 217015-2013), Canadian Institutes of Health Research (CIHR) operating and project grants (MOP 123431, PJT 153099), the Cincinnati Children’s Research Foundation, and the National Institutes of Health (T32 HL007752, R01 HL131012). MJC was funded by a Research Training Competition (Restracomp) Fellowship from the Hospital for Sick Children and a Mitacs Elevate Postdoctoral Fellowship.
REFERENCES
- 1.Santyr G, Kanhere K, Morgado F, Rayment JH, Ratjen F, Couch MJ. Hyperpolarized Gas Magnetic Resonance Imaging of Pediatric Cystic Fibrosis Lung Disease. Acad Radiol 2018;DOI: 10.1016/j.acra.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 2.Thomen RP, Walkup LL, Roach DJ, Cleveland ZI, Clancy JP, Woods JC. Hyperpolarized 129Xe for investigation of mild cystic fibrosis lung disease in pediatric patients. J Cyst Fibros 2017;16(2):275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnelly LF, MacFall JR, McAdams HP, Majure JM, Smith J, Frush DP, Bogonad P, Charles HC, Ravin CE. Cystic fibrosis: combined hyperpolarized 3He-enhanced and conventional proton MR imaging in the lung -preliminary observations. Radiology 1999;212(3):885–889. [DOI] [PubMed] [Google Scholar]
- 4.Mentore K, Froh DK, de Lange EE, Brookeman JR, Paget-Brown AO, Altes TA. Hyperpolarized HHe 3 MRI of the lung in cystic fibrosis: assessment at baseline and after bronchodilator and airway clearance treatment. Acad Radiol 2005;12(11):1423–1429. [DOI] [PubMed] [Google Scholar]
- 5.Kanhere N, Couch MJ, Kowalik K, Zanette B, Rayment JH, Manson D, Subbarao P, Ratjen F, Santyr G. Correlation of Lung Clearance Index with Hyperpolarized 129Xe Magnetic Resonance Imaging in Pediatric Subjects with Cystic Fibrosis. Am J Respir Crit Care Med 2017;196(8):1073–1075. [DOI] [PubMed] [Google Scholar]
- 6.Altes TA, Johnson M, Fidler M, Botfield M, Tustison NJ, Leiva-Salinas C, de Lange EE, Froh D, Mugler JP 3rd. Use of hyperpolarized helium-3 MRI to assess response to ivacaftor treatment in patients with cystic fibrosis. J Cyst Fibros 2017;16(2):267–274. [DOI] [PubMed] [Google Scholar]
- 7.Kirby M, Svenningsen S, Ahmed H, Wheatley A, Etemad-Rezai R, Paterson NA, Parraga G. Quantitative evaluation of hyperpolarized helium-3 magnetic resonance imaging of lung function variability in cystic fibrosis. Acad Radiol 2011;18(8):1006–1013. [DOI] [PubMed] [Google Scholar]
- 8.O’Sullivan B, Couch M, Roche JP, Walvick R, Zheng S, Baker D, Johnson M, Botfield M, Albert MS. Assessment of repeatability of hyperpolarized gas MR ventilation functional imaging in cystic fibrosis. Acad Radiol 2014;21(12):1524–1529. [DOI] [PubMed] [Google Scholar]
- 9.Walkup LL, Thomen RP, Akinyi TG, Watters E, Ruppert K, Clancy JP, Woods JC, Cleveland ZI. Feasibility, tolerability and safety of pediatric hyperpolarized 129Xe magnetic resonance imaging in healthy volunteers and children with cystic fibrosis. Pediatr Radiol 2016;46(12):1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirby M, Svenningsen S, Owrangi A, Wheatley A, Farag A, Ouriadov A, Santyr GE, Etemad-Rezai R, Coxson HO, McCormack DG, Parraga G. Hyperpolarized 3He and 129Xe MR imaging in healthy volunteers and patients with chronic obstructive pulmonary disease. Radiology 2012;265(2):600–610. [DOI] [PubMed] [Google Scholar]
- 11.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J 2005;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- 12.Robinson PD, Latzin P, Verbanck S, Hall GL, Horsley A, Gappa M, Thamrin C, Arets HG, Aurora P, Fuchs SI, King GG, Lum S, Macleod K, Paiva M, Pillow JJ, Ranganathan S, Ratjen F, Singer F, Sonnappa S, Stocks J, Subbarao P, Thompson BR, Gustafsson PM. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur Respir J 2013;41(3):507–522. [DOI] [PubMed] [Google Scholar]
- 13.Tustison NJ, Avants BB, Flors L, Altes TA, de Lange EE, Mugler JP 3rd, Gee JC. Ventilation-based segmentation of the lungs using hyperpolarized (3)He MRI. J Magn Reson Imaging 2011;34(4):831–841. [DOI] [PubMed] [Google Scholar]
- 14.He M, Kaushik SS, Robertson SH, Freeman MS, Virgincar RS, McAdams HP, Driehuys B. Extending semiautomatic ventilation defect analysis for hyperpolarized (129)Xe ventilation MRI. Acad Radiol 2014;21(12):1530–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomen RP, Sheshadri A, Quirk JD, Kozlowski J, Ellison HD, Szczesniak RD, Castro M, Woods JC. Regional ventilation changes in severe asthma after bronchial thermoplasty with (3)He MR imaging and CT. Radiology 2015;274(1):250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wild JM, Ajraoui S, Deppe MH, Parnell SR, Marshall H, Parra-Robles J, Ireland RH. Synchronous acquisition of hyperpolarised 3He and 1H MR images of the lungs - maximising mutual anatomical and functional information. NMR Biomed 2011;24(2):130–134. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, Norquay G, Parnell SR, Deppe MH, Ajraoui S, Hashoian R, Marshall H, Griffiths PD, Parra-Robles J, Wild JM. Hyperpolarized 129Xe gas lung MRI-SNR and T2* comparisons at 1.5 T and 3 T. Magn Reson Med 2012;68(6):1900–1904. [DOI] [PubMed] [Google Scholar]
- 18.Wielputz MO, Puderbach M, Kopp-Schneider A, Stahl M, Fritzsching E, Sommerburg O, Ley S, Sumkauskaite M, Biederer J, Kauczor HU, Eichinger M, Mall MA. Magnetic resonance imaging detects changes in structure and perfusion, and response to therapy in early cystic fibrosis lung disease. Am J Respir Crit Care Med 2014;189(8):956–965. [DOI] [PubMed] [Google Scholar]
- 19.Roach DJ, Cremillieux Y, Fleck RJ, Brody AS, Serai SD, Szczesniak RD, Kerlakian S, Clancy JP, Woods JC. Ultrashort Echo-Time Magnetic Resonance Imaging Is a Sensitive Method for the Evaluation of Early Cystic Fibrosis Lung Disease. Ann Am Thorac Soc 2016;13(11):1923–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stahl M, Wielputz MO, Graeber SY, Joachim C, Sommerburg O, Kauczor HU, Puderbach M, Eichinger M, Mall MA. Comparison of Lung Clearance Index and Magnetic Resonance Imaging for Assessment of Lung Disease in Children with Cystic Fibrosis. Am J Respir Crit Care Med 2017;195(3):349–359. [DOI] [PubMed] [Google Scholar]
- 21.Smith L, Hughes PJC, Marshall H, Collier G, West N, Horsley A, Wild J. Hyperpolarised gas MRI shows a decrease in lung ventilation defects at increased inspiratory lung volumes in Cystic Fibrosis. In: Proceedings of the 26th Annual Meeting of ISMRM, 2018; 1083. [Google Scholar]
- 22.Zha W, Niles DJ, Kruger SJ, Dardzinski BJ, Cadman RV, Mummy DG, Nagle SK, Fain SB. Semiautomated Ventilation Defect Quantification in Exercise-induced Bronchoconstriction Using Hyperpolarized Helium-3 Magnetic Resonance Imaging: A Repeatability Study. Acad Radiol 2016;23(9):1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirby M, Heydarian M, Svenningsen S, Wheatley A, McCormack DG, Etemad-Rezai R, Parraga G. Hyperpolarized 3He magnetic resonance functional imaging semiautomated segmentation. Acad Radiol 2012;19(2):141–152. [DOI] [PubMed] [Google Scholar]
- 24.Hughes PJC, Horn FC, Collier GJ, Biancardi A, Marshall H, Wild JM. Spatial fuzzy c-means thresholding for semiautomated calculation of percentage lung ventilated volume from hyperpolarized gas and 1 H MRI. J Magn Reson Imaging 2017. [DOI] [PubMed] [Google Scholar]
- 25.He M, Driehuys B, Que LG, Huang YT. Using Hyperpolarized 129Xe MRI to Quantify the Pulmonary Ventilation Distribution. Acad Radiol 2016;23(12):1521–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collier GJ, Acunzo L, Smith LJ, Hughes PJ, Norquay G, Chan H, Biancardi AM, Marshall H, Wild JM. Linear binning maps for image analysis of pulmonary ventilation with hyperpolarized gas MRI: transferability and clinical applications. In: Proceedings of the 26th Annual Meeting of ISMRM, 2018; 4482. [Google Scholar]
- 27.Verbanck S, Vanderhelst E. The Respective Roles of Lung Clearance Index and Magnetic Resonance Imaging in the Clinical Management of Patients with Cystic Fibrosis. Am J Respir Crit Care Med 2018;197(3):409. [DOI] [PubMed] [Google Scholar]
- 28.Kanhere N, Couch MJ, Rayment JH, Ratjen F, Santyr G. Reply to Verbanck and Vanderhelst: The Respective Roles of Lung Clearance Index and Magnetic Resonance Imaging in the Clinical Management of Patients with Cystic Fibrosis. Am J Respir Crit Care Med 2018;197(3):411–412. [DOI] [PubMed] [Google Scholar]
- 29.Wielputz MO, von Stackelberg O, Stahl M, Jobst BJ, Eichinger M, Puderbach MU, Nahrlich L, Barth S, Schneider C, Kopp MV, Ricklefs I, Buchholz M, Tummler B, Dopfer C, Vogel-Claussen J, Kauczor HU, Mall MA. Multicentre standardisation of chest MRI as radiation-free outcome measure of lung disease in young children with cystic fibrosis. J Cyst Fibros 2018;17(4):518–527. [DOI] [PubMed] [Google Scholar]
- 30.Subbarao P, Stanojevic S, Brown M, Jensen R, Rosenfeld M, Davis S, Brumback L, Gustafsson P, Ratjen F. Lung clearance index as an outcome measure for clinical trials in young children with cystic fibrosis. A pilot study using inhaled hypertonic saline. Am J Respir Crit Care Med 2013;188(4):456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


