Abstract
BACKGROUND
Opioids are effective postoperative analgesics. Disturbingly, we have previously reported that opioids such as morphine can worsen inflammatory pain and peripheral and central neuropathic pain. These deleterious effects are mediated by immune mediators that promote neuronal hyperexcitability in the spinal dorsal horn. Herein, we tested whether perioperative morphine could similarly prolong postoperative pain in male rats.
METHODS
Rats were treated with morphine for 7 days, beginning immediately after laparotomy, while the morphine was tapered in a second group. Expression of genes for inflammatory mediators was quantified in the spinal dorsal horn. In the final experiment, morphine was administered before laparotomy for 7 days.
RESULTS
We found that morphine treatment after laparotomy extended postoperative pain by more than 3 weeks (time × treatment: P < .001; time: P < .001; treatment: P < .05). Extension of postoperative pain was not related to morphine withdrawal, as it was not prevented by dose tapering (time × treatment: P = .8; time: P < .001; treatment: P = .9). Prolonged postsurgical pain was associated with increased expression of inflammatory genes, including those encoding Toll-like receptor 4, NOD like receptor protein 3 (NLRP3), nuclear factor kappa B (NFκB), caspase-1, interleukin-1β, and tumor necrosis factor (P < .05). Finally, we showed that of preoperative morphine, concluding immediately before laparotomy, similarly prolonged postoperative pain (time × treatment: P < .001; time: P < .001; treatment: P < .001). There is a critical window for morphine potentiation of pain, as a 7-day course of morphine that concluded 1 week before laparotomy did not prolong postsurgical pain.
CONCLUSIONS
These studies indicate the morphine can have a deleterious effect on postoperative pain. These studies further suggest that longitudinal studies could be performed to test whether opioids similarly prolong postoperative pain in the clinic.
As pain is a common complaint after abdominal surgery, opioids are widely administered in the postoperative period.1 Intraoperative opioids can prolong pain in the postoperative period,2 but less is known concerning opioid treatment after surgery. We have recently shown that repeated administration of morphine around the time of injury can exacerbate pain in inflammatory and neuropathic models.3–5 The interaction between injury and opioid administration on pain is believed to be due to immune “priming”; that is, a primary immune challenge (hit 1) confers a heightened neuroinflammatory response to secondary challenge (hit 2). As tissue injury and morphine treatment can be proinflammatory,6,7 we have shown that each can interchangeably serve as hit 1 or hit 2.3–5 Whether morphine prolongs postoperative pain and promotes inflammatory signaling in the spinal cord are not known.
Inflammatory signaling in the spinal cord can have profound consequences for pain. For example, proinflammatory cytokines like tumor necrosis factor and interleukin (IL)-1β enhance excitatory neurotransmission by inducing neurotransmitter exocytosis, increasing synaptic strength, and disrupting glutamate homeostasis.8–12 These immunederived molecules, together with growth factors such as brain-derived neurotrophic factor, further promote neuroexcitability in pain pathways by disinhibiting GABAergic and glycinergic control.8,13,14 Production of such mediators is initiated through a range of receptors, including Toll-like receptors (TLRs) and purinergic receptors (eg, P2X7R) that can be activated by substances released by cell stress, damage, and death that signal danger to the host (damage-associated molecular patterns [DAMPs]).15,16 Critically, these receptors can activate NOD like receptor protein 3 (NLRP3) inflammasomes, which are responsible for proteolytic activation of IL-1β via caspase-1, and contribute to morphine exacerbation of neuropathic pain.4
The aim of this study was to determine whether postoperative morphine could exacerbate postsurgical pain, and if so, whether it was associated with increased inflammatory signaling in the spinal cord. The second aim was to test whether preoperative morphine would similarly exacerbate postoperative pain, to establish whether morphine could serve as hit 1 and hit 2. Laparotomy was the chosen surgical model because it has high face validity,17 and induces spinal inflammatory signaling.18
METHODS
Study Design
In the first experiment, all animals received laparotomy, followed by daily morphine or vehicle treatment beginning immediately after surgery and concluding 7 days after surgery. Behavioral assessments were performed before and after treatments. In the second experiment, all rats received equal morphine doses for at least days 0–7 postsurgery. Rats in the “abrupt” group continued with the same morphine dose until day 10. In the “tapered” group, the morphine dose was reduced by half each day from days 8 to 10. Behavioral assessments were performed before and after treatments. In the third experiment, all animals received laparotomy, followed by daily morphine or vehicle treatment beginning immediately after surgery and concluding 7 days after surgery. Tissues were collected 14 days after laparotomy. In the fourth experiment, 1 group received morphine immediately before laparotomy, and the other group received morphine with a 7-day washout before laparotomy; morphine was administered from day −14 to −8 presurgery. Each group received vehicle during the alternate 7 days of the 2-week period to counter balance the stress of injections. Behavioral assessments were performed before and after treatments.
Subjects
Pathogen-free adult male Sprague Dawley rats (n = 6 rats/group for each experiment; 10–12 weeks old on arrival; Envigo Labs, Indianapolis, IN) were used in all experiments. Rats were housed in temperature-controlled (23°C ± 3°C) and light-controlled (12 hours light:dark cycle; lights on at 07:00 hours) rooms with standard rodent chow and water available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado Boulder.
Laparotomy Surgery
Laparotomy surgeries were performed using aseptic procedures under isoflurane anesthesia, as previously described.17 Briefly, the abdominal region was shaved and thoroughly cleaned with 70% ethanol and nolvasan surgical scrub. Approximately 0.5 cm below the left, caudal-most rib, a 3-cm diagonal incision was made, penetrating the peritoneal cavity. Wearing sterile latex gloves, the surgeon inserted the index finger up to the second knuckle into the opening and vigorously manipulated the viscera and musculature. Approximately 10 cm of the intestine was then exteriorized and vigorously rubbed between the surgeon’s thumb and index finger for 30 seconds. The intestines were then placed back into the peritoneal cavity. Sterile chromic gut sutures (cuticular 4–0 chromic gut; Ethicon, Comerville, NJ) were used to suture the peritoneal lining and abdominal muscle in 2 layers. The skin was closed with surgical staples. To prevent infection, the wound was dressed with Polysporin (Pfizer, Morris Plains, NJ) and 0.25 mL CombiPen (150,000 U/mL penicillin G procaine and penicillin G benzathine; Bimeda, Oakbrook Terrace, IL) was administered intramuscularly. Apart from morphine treatment included in the study designs, no other postoperative analgesics were administered, as they would have confounded interpretation.
Morphine Administration
Morphine was administered subcutaneously at 5 mg/kg per mL, twice daily. This approximate dose has previously been reported as the half maximal effective concentration (EC50) for analgesia in rats.19,20 Equivolume saline vehicle was used as the control. Morphine was gifted by the National Institute of Drug Abuse drug depository, and was prepared and reported as free base concentrations.
Mechanical Allodynia
Testing was conducted blind with respect to group assignment. Rats received at least three 60-minute habituations to the test environment before behavioral testing. The von Frey test21 was performed as previously described.22 A logarithmic series of 10 calibrated Semmes-Weinstein monofilaments (von Frey hairs; Stoelting, Wood Dale, IL) were applied randomly to the left versus right hind paws to define the threshold stimulus intensity required to elicit a paw withdrawal response. Log stiffness of the hairs ranged from manufacturer-designated 3.61 (0.40 g) to 5.18 (15.14 g) filaments. The behavioral responses were used to calculate absolute threshold (the 50% probability of response) by fitting a Gaussian integral psychometric function using a maximum-likelihood fitting method.23,24
Reverse Transcription-Polymerase Chain Reaction
Total RNA was isolated using a standard method of phenol:chloroform extraction.25 cDNA amplification was performed using Quantitect SYBR Green PCR kit (Qiagen, Germantown, MD) in iCycler iQ 96-well PCR plates (Bio-Rad, Hercules, CA) on a MyiQ single Color Real-Time PCR Detection System (Bio-Rad). Primer sequences (GenBank, National Center for Biotechnology Information; www.ncbi.nlm.nih.gov) are presented in Table 1. Each sample was measured in duplicate by using the MyiQ single Color Real-Time PCR Detection System (Bio-Rad). Threshold for detection of polymerase chain reaction product was set in the log-linear phase of amplification and the threshold cycle (CT) was determined for each reaction. The level of the target mRNA was quantified relative to the housekeeping gene (Gapdh) using the delta delta CT method.26 Gapdh was not significantly different between treatments.
Table 1.
PCR Primer Sequences
| Gene | Primer Sequence (5′–3′) |
|---|---|
| Gapdh | F: AGGGACAATCTCACACAGG R: GACTCAACCTTCCTCTCCA |
| Tlr4 | F: TCCCTGCATAGAGGTACTTC R: CACACCTGGATAAATCCAGC |
| P2rx7 | F: TTTCGGTTTGGCCACCGTGT R: ACTTTAACGTCGGCTTGGGC |
| RT1-Da | F: AGCACTGGGAGTTTGAAGAG R: AAGCCATCACCTCCTGGTAT |
| Nfkbia | F: CACCAACTACAATGGCCACA R: GCTCCTGAGCGTTGACATCA |
| Nlrp3 | F: AGAAGCTGGGGTTGGTGAATT R: GTTGTCTAACTCCAGCATCTG |
| Mir223 | F: TCTGGCCTTCTGCAGTGTTA R: CTGATAAGCATGAGCCACAC |
| Casp1 | F: ATGCCGTGGAGAGAAACAAG R: CCAGGACACATTATCTGGTG |
| Il1b | F: GAAGTCAAGACCAAAGTGG R: TGAAGTCAACTATGTCCCG |
| Tnf | F: CAAGGAGGAGAAGTTCCCA R: TTGGTGGTTTGCTACGACG |
| Hsp90 | F: TTATCACAGGTGAGACCAAG R: AAGTTCCAGTCCTTCTTTGG |
| Bgn | F: AACTGCATTGAGATGGGTGG R: TCAGGGAGATCTTTGGGGAT |
| Hmgb1 | F: GAGGTGGAAGACCATGTCTG R: AAGAAGAAGGCCGAAGGAGG |
Statistics
A power analysis was performed using G*Power 327 with power (1 − β) set at .95 and α = .05, which indicated that n = 5/group for aim 1, and n = 6/group for aim 2 were sufficient to reach statistical significance (P < .05). Data from the von Frey test were analyzed as the interpolated 50% thresholds (absolute threshold) in log10 of stimulus intensity (monofilament stiffness in milligrams × 10). Statistical methods, comparisons, and results are summarized in Table 2. Statistical comparisons are indicated on the figures for clarity and are presented as mean ± standard deviation. Statistical significance was set at P < .05.
Table 2.
Statistical Analyses
| Comparison | Statistical Test | P Value | Confidence Interval | Figure |
|---|---|---|---|---|
| Morphine versus saline baseline | 2-tailed paired t test | .22 | −0.306 to 0.090 | 1A |
| Morphine versus saline over time | 2-way repeated measures ANOVA | Time × treatment: <.001; time: <.001; treatment: .0102 | 1A | |
| Morphine versus saline: day 3 | Sidak post hoc | <.001 | −1.101 to −0.3287 | 1A |
| Morphine versus saline: day 7 | Sidak post hoc | <.001 | −1.141 to −0.369 | 1A |
| Morphine versus saline: day 10 | Sidak post hoc | <.001 | .3877–1.16 | 1A |
| Morphine versus saline: day 14 | Sidak post hoc | <.001 | 0.7374–1.51 | 1A |
| Morphine versus saline: day 21 | Sidak post hoc | <.001 | 0.5891–1.361 | 1A |
| Morphine versus saline: day 28 | Sidak post hoc | <.001 | 0.4243–1.196 | 1A |
| Morphine versus saline: day 35 | Sidak post hoc | >.9 | −0.3676 to 0.4046 | 1A |
| Morphine versus saline: day 42 | Sidak post hoc | >.9 | −0.3645 to 0.4077 | 1A |
| Abrupt versus tapered baseline | 2-tailed paired t test | .80 | −0.3515 to 0.2849 | 1B |
| Abrupt versus tapered over time | 2-way repeated measures ANOVA | Time × treatment: P = .8; P < .001; treatment: P = .9) | 1B | |
| Abrupt versus tapered: day 3 | Sidak post hoc | .99 | −0.562 to 0.3404 | 1B |
| Abrupt versus tapered: day 7 | Sidak post hoc | .99 | −0.5996 to 0.3034 | 1B |
| Abrupt versus tapered: day 10 | Sidak post hoc | .99 | −0.549 to 0.3538 | 1B |
| Abrupt versus tapered: week 2 | Sidak post hoc | >.99 | −0.531 to 0.3719 | 1B |
| Abrupt versus tapered: week 3 | Sidak post hoc | >.99 | −0.5172 to 0.3858 | 1B |
| Abrupt versus tapered: week 4 | Sidak post hoc | >.99 | −0.4515 to 0.4515 | 1B |
| Abrupt versus tapered: week 5 | Sidak post hoc | >.99 | −0.4515 to 0.4515 | 1B |
| Abrupt versus tapered: week 6 | Sidak post hoc | .99 | −0.3355 to 0.5675 | 1B |
| Abrupt versus tapered: week 7 | Sidak post hoc | .99 | −0.365 to 0.538 | 1B |
| Abrupt versus tapered: week 8 | Sidak post hoc | .98 | −0.292 to 0.6101 | 1B |
| Morphine versus saline | 2-tailed unpaired t test | <.0001 | 74.52–97.48 | 2A |
| Morphine versus saline | 2-tailed unpaired t test | <.0001 | 26.86–38.46 | 2B |
| Morphine versus saline | 2-tailed unpaired t test | .0002 | 48.1–88.58 | 2C |
| Morphine versus saline | 2-tailed unpaired t test | .0006 | 46.34–93.96 | 2D |
| Morphine versus saline | 2-tailed unpaired t test | .0013 | 45.58–138.8 | 2E |
| Morphine versus saline | 2-tailed unpaired t test | .0185 | 15.53–107.8 | 2F |
| Morphine versus saline | 2-tailed unpaired t test | .0031 | 12.87–40.09 | 2G |
| Morphine versus saline | 2-tailed unpaired t test | .0005 | 30.87–70.21 | 2H |
| Morphine versus saline | 2-tailed unpaired t test | .0122 | 30.41–137.7 | 2I |
| Morphine versus saline | 2-tailed unpaired t test | .0002 | 30.73–63.01 | 2J |
| Morphine versus saline | 2-tailed unpaired t test | <.0001 | 54.59–67.71 | 2K |
| Morphine versus saline | 2-tailed unpaired t test | <.0001 | 65.96–108.1 | 2L |
| Morphine “−7” versus morphine “−14” baseline | 2-tailed paired t test | .93 | −0.24 to 0.2229 | 3 |
| Morphine “−7” versus morphine “−14” over time | 2-way repeated measures ANOVA | Time × treatment: <.001; time: <.001; treatment: .0004 | 3 | |
| Morphine “−7” versus morphine “−14”: day 0 | Sidak post hoc | .1596 | −0.6612 to 0.05669 | 3 |
| Morphine “−7” versus morphine “−14”: day 3 | Sidak post hoc | >.9999 | −0.3539 to 0.364 | 3 |
| Morphine “−7” versus morphine “−14”: day 7 | Sidak post hoc | .9997 | −0.291 to 0.4269 | 3 |
| Morphine “−7” versus morphine “−14”: day 10 | Sidak post hoc | .0025 | −0.8388 to −0.1209 | 3 |
| Morphine “−7” versus morphine “−14”: day 14 | Sidak post hoc | <.0001 | −1.147 to −0.4287 | 3 |
| Morphine “−7” versus morphine “−14”: day 21 | Sidak post hoc | <.0001 | −1.322 to −0.6043 | 3 |
| Morphine “−7” versus morphine “−14”: day 28 | Sidak post hoc | <.0001 | −1.127 to −0.4088 | 3 |
| Morphine “−7” versus morphine “−14”: day 35 | Sidak post hoc | .6351 | −0.5658 to 0.1521 | 3 |
| Morphine “−7” versus morphine “−14”: day 42 | Sidak post hoc | .9988 | −0.2788 to 0.4391 | 3 |
Abbreviation: ANOVA, analysis of variance.
RESULTS
Morphine Treatment After Laparotomy Prolongs Postoperative Pain
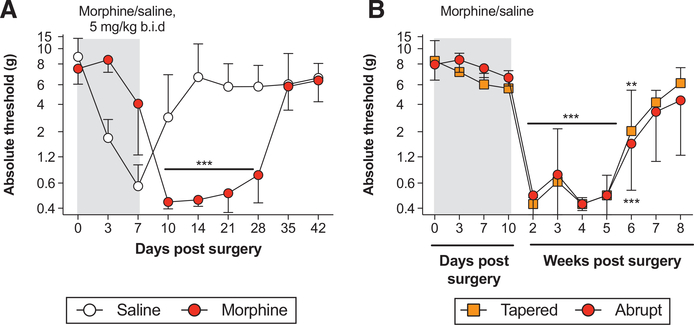
We aimed to test whether repeated morphine administration could prolong postoperative pain. In the first experiment, all animals received laparotomy, followed by daily morphine or vehicle treatment (5 mg/kg twice a day) beginning immediately after surgery and concluding 7 days after surgery. Morphine treatment prolonged postoperative pain by over 2 weeks (Figure 1A; time × treatment: F7,70 = 77.85, P < .001; time: F7,70 = 29.26, P < .001; treatment: F1,10 = 7.78, P < .05). The difference between the means at day 14 was 6.9 g.
Figure 1.
Morphine treatment after laparotomy prolongs postoperative pain. Rats received laparotomy, followed by vehicle or morphine treatment. A, Morphine was administered daily, beginning immediately after surgery (day 0), to day 7 postsurgery (5 mg/kg twice a day; grey shaded bar). B, Between days 0 and 7 after surgery, morphine (grey shaded bar) was administered at 5 mg/kg (twice a day). In the abrupt group, morphine treatment continued at 5 mg/kg (twice a day) until day 10. In the tapered group, the morphine dose was reduced daily, day 8: 2.5 mg/kg (twice a day); day 9: 1.25 mg/kg (twice a day); day 10: 0.625 mg/kg (twice a day). Von Frey thresholds were determined before laparotomy (day 0), and across a timecourse after surgery. Data are presented as mean ± standard error of the mean; n = 6/group; **P < .01, ***P < .001.
In the second experiment, we aimed to test whether the prolonged allodynia could be explained by morphine withdrawal. After laparotomy, all rats received 5 mg/kg twice a day morphine for at least days 0–7 postsurgery (Figure 1B). Rats in the “abrupt” group continued with morphine (5 mg/kg twice a day) until day 10. In the “tapered” group, the morphine dose was reduced by half each day from days 8 to 10 (day 8: 2.5 mg/kg [twice a day]; day 9: 1.25 mg/kg [twice a day]; day 10: 0.625 mg/kg [twice a day]). Tapering the morphine dose down did not prevent the prolongation of allodynia, relative to abruptly stopping treatment (time × treatment: F9,90 = 0.6, P = .8; time: F9,90 = 55.65, P < .001; treatment: F1,10 = 0.02, P = .9). Both treatment paradigms still induced allodynia that persisted until 42 days after surgery (P < .01). Notably, 10 days of morphine treatment prolonged allodynia for a week longer than 7 days of morphine treatment (Figure 1A, B). The difference between the means at day 14 was 0.14 g.
Morphine Treatment After Laparotomy Increases Inflammatory Signaling
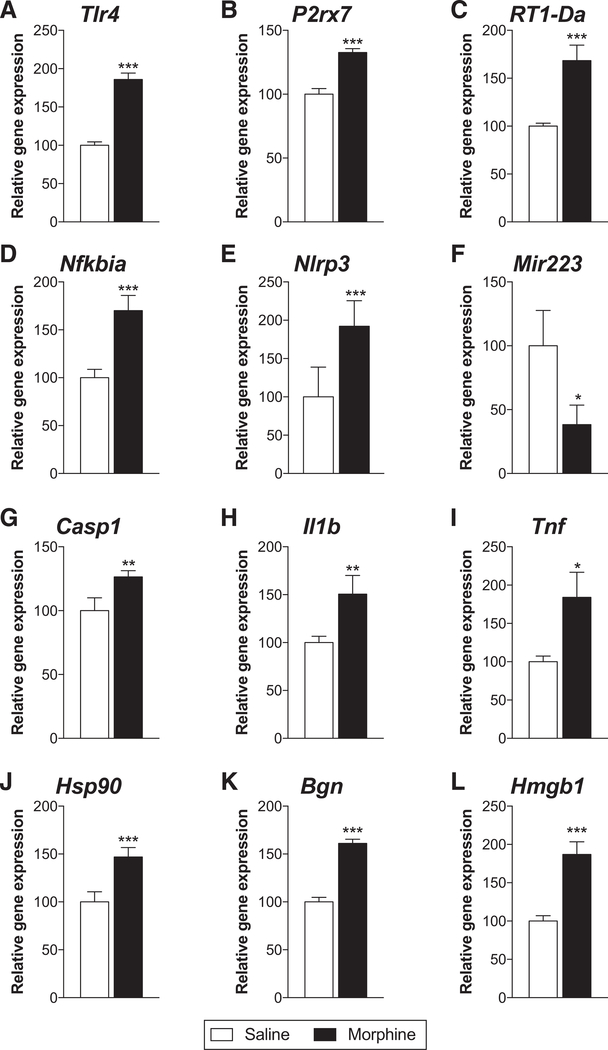
We have previously shown that morphine treatment exacerbates inflammatory signaling in various pain models, particularly via TLR4 and NLRP3 inflammasomes.3–5,28 Therefore, we tested whether inflammatory signaling was increased in the L1/2 dorsal spinal cord on day 14 after laparotomy, when morphine or vehicle was administered from days 0–7 (5 mg/kg twice a day). Genes for TLR4 and P2X7R, which prime and activate inflammasomes, as well as for the microglia activation marker major histocompatibility complex class II, were increased in expression by morphine treatment (Figure 2A–C; P < .001). Expression of the gene for IκBα, an inhibitory subunit of the transcription factor nuclear factor kappa B (NFκB) (increased expression is indicative of NFκB activation) that is responsible for NLRP3 and IL-1β transcription, as well as NLRP3, was increased by morphine treatment (Figure 2D, E; P < .001). Expression of a negative regulator of NLRP3, microRNA-223, was decreased by morphine treatment (Figure 2F; P < .05). The genes encoding the enzyme caspase-1 and the product IL-1β were elevated by morphine treatment (Figure 2G, H; P < .01). mRNA for the cytokine tumor necrosis factor was also increased by morphine treatment (Figure 2I; P < .05). Finally, we have previously shown that the DAMPs HSP-90, biglycan, and HMGB1 contribute to the persistence of neuropathic pain by morphine.28 Here, we similarly show that morphine treatment increases transcript expression for genes encoding these DAMPs (Figure 2J–L; P < .001).
Figure 2.
Morphine treatment increases inflammatory signaling after laparotomy. Rats received laparotomy, followed by vehicle or morphine treatment on days 0–7 after surgery (5 mg/kg twice a day). Two weeks after surgery, L1/2 dorsal spinal cords were collected and mRNA expression was quantified via real-time reverse transcription-polymerase chain reaction. Expression of genes was compared between saline and morphine treatment for (A) toll-like receptor 4 (TLR4), (B) P2X7R, (C) major histocompatibility complex (MHC) class II, (D) IκBα, (E) NLRP3, (F) microRNA (miR)-223, (G) caspase-1, (H) interleukin (IL)-1β, (I) tumor necrosis factor (TNF), (J) heat shock protein (HSP-90), (K) biglycan, and (L) high mobility group box 1 (HMGB1). Data are presented as mean ± standard error of the mean; n = 6/group; *P < .05, **P < .01, ***P < .001.
Morphine Pretreatment Exacerbates Subsequent Postoperative Pain
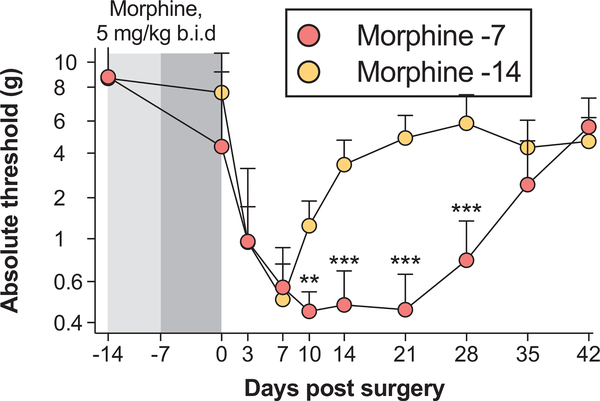
To test whether the prolongation of postoperative pain depends on the timing of morphine administration, we dosed morphine before laparotomy (Figure 3). One group received morphine immediately before laparotomy (days −7 to −1 presurgery; “morphine −7”). The other group received morphine with a 7-day washout before laparotomy; morphine was administered from days −14 to −8 presurgery (“morphine −14”). Each group received vehicle during the alternate 7 days of the 2-week period to counter balance the stress of injections. Morphine treatment immediately before laparotomy prolonged the duration of postoperative pain, relative to treatment that had washed out for 7 days before surgery (time × treatment: F8,80 = 12.18, P < .001; time: F8,80 = 34.43, P < .001; treatment: F1,10 = 30.68, P < .001). The difference between the means at day 14 was 2.7 g.
Figure 3.
Morphine pretreatment exacerbates postoperative pain. Rats were pretreated with morphine according to 2 different schedules: morphine administration from days −14 to −8 (5 mg/kg twice a day), followed by vehicle twice daily from days −7 to −1 (“morphine −14”); vehicle administration twice daily from days −14 to −8 followed by morphine from days −7 to −1 (5 mg/kg twice a day; “morphine −7”). All rats then received laparotomy. Von Frey thresholds were determined before morphine/vehicle (day −14), and across a timecourse after surgery. Data are presented as mean ± standard error of the mean; n = 6/group; **P < .01, ***P < .001.
DISCUSSION
We show that postoperative morphine treatment prolongs postsurgical pain. This prolongation is likely independent of opioid withdrawal as tapered morphine dosing still exaggerated the allodynia. We further show that opioid treatment increased expression of genes that encode components of NLRP3 inflammasomes, proinflammatory cytokines, and DAMPs that signal through TLR4 and P2X7R. Finally, we demonstrated that preoperative morphine treatment similarly prolongs postoperative pain, but only when morphine immediately precedes surgery; prolonged pain did not ensue when morphine was allowed to washout for a week before surgery.
Accumulating evidence shows that opioids and their metabolites induce inflammatory signaling in the spinal cord.20,29–34 Such signaling may be engaged through opioid receptors and/or TLRs on microglia,30,35,36 though both concepts have been challenged.37,38 Nonetheless, many groups have shown that opioids increase spinal production of inflammatory mediators such as cytokines and chemokines, growth factors, nitroxidative species, and bioactive lipids (summarized in 7). These mediators are potently pronociceptive.6,39
We posited that the spinal inflammatory signaling induced by morphine would interact with that provoked by surgery, similar to that described previously.18,40,41 This is likely the case, as morphine treatment enhanced expression of inflammatory genes after laparotomy, as we have previously reported for peripheral and central neuropathic pain, as well as inflammatory pain.3–5 In particular, expression of genes in the NLRP3 inflammasome pathway—which is responsible for activation of IL-1β—was increased by morphine. This is consistent with our previous report that NLRP3 inflammasomes are responsible for initiation and maintenance of prolonged neuropathic pain by morphine.4 We also show that mRNA transcripts for several DAMPs (HMGB1, biglycan, HSP-90) are increased in expression by morphine. These DAMPs signal through TLR4 and P2X7R,15,42 and may be produced as a consequence of cell stress/death induced by the combined challenge of laparotomy and morphine.28 We have previously shown that these DAMPs causally contribute to the maintenance of morphine-induced persistent neuropathic pain.28
This study shows that opioids administered in the perioperative period can prolong postoperative pain. Much of the clinical study has focused on the impact of intraoperative opioids on postoperative pain, with a moderate detrimental effect reported.2,43 Preoperative opioids were associated with higher increased postoperative opioid use and self-reported pain after orthopedic surgery.44 These results are supported by our data showing that morphine administration immediately before laparotomy prolongs postoperative pain, in contrast to allowing morphine to washout. To the best of our knowledge, there are no longitudinal studies that have investigated whether postoperative opioid use is a risk factor for persistent postsurgical pain. Our data suggest that such studies are warranted, and that adjuvant therapies that attenuate central immune signaling could be tested as a possible intervention, in addition to reducing opioid administration before surgery.
KEY POINTS.
Question: Does perioperative morphine prolong postoperative pain in rats?
Findings: Both pre- and postoperative morphine prolonged postsurgical pain, and postoperative morphine treatment increased spinal inflammatory gene expression.
Meaning: Morphine can have a deleterious effect on postoperative pain, which warrants investigation in humans.
ACKNOWLEDGMENTS
The authors thank Lei Feng, MS (Department of Biostatistics, University of Texas MD Anderson Cancer Center, Houston, TX) and Jonathan Tuke, PhD (School of Mathematical Sciences, Computer Science and Mathematics, The University of Adelaide, Adelaide, Australia) for their biostatistical advice.
Funding: This work was supported by National Health and Medical Research Council CJ Martin Fellowship (ID 1054091) and American Australian Association Sir Keith Murdoch Fellowship (P. M. Grace); and NIH Grants DE021966, DA023132 (L. R. Watkins).
Footnotes
The authors declare no conflicts of interest.
DISCLOSURES
Name: Peter M. Grace, PhD.
Contribution: This author helped conceive the study, design the experiments, collect and analyze the data, and write the article.
Name: Erika L. Galer, BA.
Contribution: This author helped collect and analyze the data.
Name: Keith A. Strand, BA.
Contribution: This author helped collect and analyze the data.
Name: Kaci Corrigan, BA.
Contribution: This author helped collect and analyze the data.
Name: Debra Berkelhammer, BA.
Contribution: This author helped collect and analyze the data.
Name: Steven F. Maier, PhD.
Contribution: This author helped write the article.
Name: Linda R. Watkins, PhD.
Contribution: This author helped conceive the study, design the experiments, and write the article.
This manuscript was handled by: Jianren Mao, MD, PhD.
Reprints will not be available from the authors.
REFERENCES
- 1.Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377:2215–2225. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis. Br J Anaesth. 2014;112:991–1004. [DOI] [PubMed] [Google Scholar]
- 3.Ellis A, Grace PM, Wieseler J, et al. Morphine amplifies mechanical allodynia via TLR4 in a rat model of spinal cord injury. Brain Behav Immun. 2016;58:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grace PM, Strand KA, Galer EL, et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A. 2016;113:E3441–E3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loram LC, Grace PM, Strand KA, et al. Prior exposure to repeated morphine potentiates mechanical allodynia induced by peripheral inflammation and neuropathy. Brain Behav Immun. 2012;26:1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grace PM, Maier SF, Watkins LR. Opioid-induced central immune signaling: implications for opioid analgesia. Headache. 2015;55:475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kronschläger MT, Drdla-Schutting R, Gassner M, Honsek SD, Teuchmann HL, Sandkühler J. Gliogenic LTP spreads widely in nociceptive pathways. Science. 2016;354:1144–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain. 2000;4:247–257. [DOI] [PubMed] [Google Scholar]
- 11.Yan X, Weng HR. Endogenous interleukin-1β in neuropathic rats enhances glutamate release from the primary afferents in the spinal dorsal horn through coupling with presynaptic N-methyl-D-aspartic acid receptors. J Biol Chem. 2013;288:30544–30557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan X, Yadav R, Gao M, Weng HR. Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia. 2014;62:1093–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coull JA, Beggs S, Boudreau D, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Nei H, Dougherty PM. A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factor-alpha. J Neurosci. 2010;30:12844–12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacagnina MJ, Watkins LR, Grace PM. Toll-like receptors and their role in persistent pain. Pharmacol Ther. 2017;184:145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trang T, Beggs S, Salter MW. ATP receptors gate microglia signaling in neuropathic pain. Exp Neurol. 2012;234:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101:191–203. [DOI] [PubMed] [Google Scholar]
- 18.Hains LE, Loram LC, Weiseler JL, et al. Pain intensity and duration can be enhanced by prior challenge: initial evidence suggestive of a role of microglial priming. J Pain. 2010;11:1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutchinson MR, Lewis SS, Coats BD, et al. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav Immun. 2009;23:240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchinson MR, Zhang Y, Shridhar M, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- 22.Grace PM, Hutchinson MR, Manavis J, Somogyi AA, Rolan PE. A novel animal model of graded neuropathic pain: utility to investigate mechanisms of population heterogeneity. J Neurosci Methods. 2010;193:47–53. [DOI] [PubMed] [Google Scholar]
- 23.Harvey LO. Efficient estimation of sensory thresholds. Behav Res Methods Instrum Comput. 1986;18:623–632. [Google Scholar]
- 24.Treutwein B, Strasburger H. Fitting the psychometric function. Percept Psychophys. 1999;61:87–106. [DOI] [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 27.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 28.Grace PM, Strand KA, Galer EL, Rice KC, Maier SF, Watkins LR. Protraction of neuropathic pain by morphine is mediated by spinal damage associated molecular patterns (DAMPs) in male rats. Brain Behav Immun. 2017. August 30 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burma NE, Bonin RP, Leduc-Pessah H, et al. Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nat Med. 2017;23:355–360. [DOI] [PubMed] [Google Scholar]
- 30.Ferrini F, Trang T, Mattioli TA, et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl− homeostasis. Nat Neurosci. 2013;16:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grace PM, Ramos KM, Rodgers KM, et al. Activation of adult rat CNS endothelial cells by opioid-induced toll-like receptor 4 (TLR4) signaling induces proinflammatory, biochemical, morphological, and behavioral sequelae. Neuroscience. 2014;280:299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis SS, Hutchinson MR, Rezvani N, et al. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience. 2010;165:569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. [DOI] [PubMed] [Google Scholar]
- 34.Xie N, Gomes FP, Deora V, et al. Activation of μ-opioid receptor and Toll-like receptor 4 by plasma from morphine-treated mice. Brain Behav Immun. 2017;61:244–258. [DOI] [PubMed] [Google Scholar]
- 35.Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal. 2007;7:98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leduc-Pessah H, Weilinger NL, Fan CY, Burma NE, Thompson RJ, Trang T. Site-specific regulation of P2X7 receptor function in microglia gates morphine analgesic tolerance. J Neurosci. 2017;37:10154–10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corder G, Tawfik VL, Wang D, et al. Loss of μ opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat Med. 2017;23:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattioli TA, Leduc-Pessah H, Skelhorne-Gross G, et al. Toll-like receptor 4 mutant and null mice retain morphine-induced tolerance, hyperalgesia, and physical dependence. PLoS One. 2014;9:e97361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354:572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu D, Guo Q, Ai Y, Cai H, Yan J, Dai R. Glial activation and segmental upregulation of interleukin-1beta (IL-1beta) in the rat spinal cord after surgical incision. Neurochem Res. 2006;31:333–340. [DOI] [PubMed] [Google Scholar]
- 41.Obata H, Eisenach JC, Hussain H, Bynum T, Vincler M. Spinal glial activation contributes to postoperative mechanical hypersensitivity in the rat. J Pain. 2006;7:816–822. [DOI] [PubMed] [Google Scholar]
- 42.Babelova A, Moreth K, Tsalastra-Greul W, et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284:24035–24048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang L, Ye F, Luo Q, Tao Y, Shu H. Increased hyperalgesia and proinflammatory cytokines in the spinal cord and dorsal root ganglion after surgery and/or fentanyl administration in rats. Anesth Analg. 2018;126:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hina N, Fletcher D, Poindessous-Jazat F, Martinez V. Hyperalgesia induced by low-dose opioid treatment before orthopaedic surgery: an observational case-control study. Eur J Anaesthesiol. 2015;32:255–261. [DOI] [PubMed] [Google Scholar]